1. Introduction
Extramedullary hematopoiesis (EMH) is characterized
by the formation and development of blood cells, external to the
bone marrow medullary spaces. However, this condition has not been
identified physiologically in adults. Therefore, the
pathophysiological variations in hematopoietic stem cells (HSCs)
and their microenvironment may be a component of the homing,
proliferation and maturation processes associated with
hematopoietic cells in the extramedullary organs of adults;
although, there is currently a dearth of studies addressing the
prevalence and fundamental processes of EMH (1). This may be explained by the fact that
EMH is generally identified as a secondary or accessory event to
another disorder, including benign hematologic disorder (2) or cancer (3), in certain cases developing
independently or lacking apparent trigger and without clinical or
diagnostic implications (4,5).
In bone marrow, the HSC niche, a distinct
microenvironment, maintains the characteristics of HSCs (6). It was recently demonstrated that
niche-constituting cells within human bone marrow include critical
elements, for example mesenchymal stromal cells (MSCs), blood
vessels, perivascular cells, macrophages and endosteal cells
(7). Investigations into the
various mechanisms underlying the interaction between these
elements and HSCs have also been conducted. HSCs occur in the
vicinity of, but not necessarily in direct contact with,
osteoblastic bone-lining cells that constitute the endosteal niche
regions. However, the sinusoidal endothelium also interacts with
HSCs, and form the vascular niche (6). Initially it was suggested that the
endosteal and vascular niches may control distinct HSC populations;
however, more recent data suggests that their effects may be more
complicated due to functional crosstalk between cells within the
two regions (8,9). Furthermore, the vascular niche has
been suggested to regulate HSCs in a distinct mechanism or
potentially harbor HSCs with various self-renewable properties
(10–12).
Certain mesenchymal cells, including chemokine
(C-X-C motif) ligand 12 (CXCL12)-abundant reticular cells (CAR
cells) (13), nestin-expressing
cells (14) and non-myelinating
Schwann cells derived from the neural crest (15), have been reported to function as
bone marrow niches. Of particular significance in localizing and
retaining HSCs in the vicinity of niches are the interactions
between CXCR4 and HSCs or CXCL12 (SDF-1). In addition, upregulation
of vascular cell adhesion molecule-1 (VCAM-1) and very late
antigen-4 (VLA-4) expression (16)
may be induced by chemokine interactions through CXCL12.
Furthermore, CXCL12-deficient embryos exhibited a marked decrease
in HSC populations and hematopoietic function was inhibited;
therefore indicating that CXC12 has a critical role during the
early stages of bone marrow hematopoietic cell colonization
(17).
CXCL12 may potentially be involved, not only in
hematopoietic cell colonization, but also in the migration and
maintenance of HSCs during EMH. For example, it was demonstrated
that stromal cells express CXCL12 and induce B-cell migration in
the spleen (18). Recently, in a
study by our group, whether CXCL12-positive cells were able to be
identified and if they had a role in hematopoietic regions of the
spleen during EMH was determined by investigating CXCL12 messenger
(m)RNA expression levels and localization of CXCL12-positive cells
in the spleen (19). A previous
study suggested that hematopoiesis in bone marrow cannot be
maintained by a vascular niche alone, as indicated by osteoblast
deficient mice (20). Since
splenic EMH exhibits neither osteoblastic nor endosteal niches,
elucidation of the details of crosstalk between HSCs and the niche
and/or microenvironments in splenic EMH may be significant in
understanding the overall biology of hematopoiesis in terms of
niche.
2. Sites of EMH
Spleen
The spleen is a frequent site of EMH during the
postnatal period; although, it is only a minor factor in the
embryonic stages of hematopoiesis. Despite the fact that the
splenic microenvironment is characterized by hypoxic/acidic
conditions and numerous macrophages that are harsh and inhospitable
for HSCs, EMH usually occurs within the red pulp (21). The mechanisms underlying the
interaction between HSCs and the stromal cells of the spleen during
EMH will be discussed subsequently.
Liver
Hepatic EMH is normal in infants up to ~5 weeks of
age (22). Conversely, hepatic EMH
in adults is associated with various pathological states, including
hepatic disorders, sepsis, transplantation, hepatic tumors, for
example hepatoblastoma; adenomas and hepatocellular carcinomas
(2,23,24).
EMH is most often observed within the hepatic sinusoids.
Lymph nodes
As with the spleen or liver, EMH may occasionally
occur in the lymph nodes and is frequently associated with
underlying hematopoietic neoplasms, for example myeloproliferative
neoplasms (MPNs) (25). Therefore,
in adults and infants; a hematologic evaluation, including blood
cell counts, peripheral blood smear and potentially a bone marrow
aspiration/biopsy should be performed if EMH is identified in the
lymph nodes.
Other sites
Other tissues that may be associated with EMH
include the heart, fatty tissue, adrenal glands, kidney,
periosteum, pleural cavity, para-vertebral regions, intra-spinal
tissue, pre-sacral region, nasopharyngeal region, para-nasal
sinuses (5) and numerous types of
benign/malignant neoplasm (26).
3. Embryonic/developmental EMH
The high clinical significance of bone marrow
transplantation has attracted researchers to focus on the
developmental origin of HSCs and niches (27). The yolk sac, in the initial stages
of embryonic development, is the initial site of primordial
development in the human hematopoietic system, and occurs in the
third week of development (28).
Vascular elements are initially formed within the yolk sac, as the
mesodermal cells begin to accumulate (29). Subsequently, clusters of primitive
mesodermal cells emerge in close association with these vascular
structures, termed blood islands, and the mesenchymal cells in
these blood islands develop into hematopoietic cells and begin to
circulate on approximately day 20 of development (30). The development of the rudimentary
liver occurs simultaneously to these events (29). HSCs are not generated directly, but
instead circulating hematopoietic precursor cells populate the
fetal liver (29). These cells
proliferate and differentiate in the hepatic parenchyma, forming
the primary site of hematopoiesis on approximately day 30 (29).
The aorta-gonads-mesonephros region also produces
HSCs. Bone marrow hematopoietic elements commence formation at
approximately eight weeks of development, which is relatively late
in embryogenesis (30). By week
11, HSCs and the bone marrow niche environment are established in
the marrow cavity (30). By
contrast, the spleen performs a minor function as a site of
erythropoiesis (26). At each
stage of development, stromal cell/HSC interactions via cytokines,
chemokines and growth factors have significant roles in the
maintenance of normal hematopoiesis (31).
4. EMH associated with hematological
disorders
Expansion of the hematopoietic space outside of the
bone marrow has been observed in numerous benign hematological
disorders, including thalassemia, sickle cell anemia, hereditary
spherocytosis, congenital dyserythroblastic anemia and idiopathic
thrombocytopenic purpura (26).
In response to reduced red blood cell (RBC) numbers,
a homeostatic mechanism induces an increase in the synthesis of
RBCs, typically via the production of erythropoietin (26). If the reduction in RBCs is
sufficiently severe, hematopoiesis will occur in the extramedullary
spaces (26). If granulocyte or
platelet production is also insufficient, a similar state occurs
(26).
Not only benign conditions, but also neoplastic
disorders, have been described by the term EMH (32–34).
Neoplastic myeloid proliferation in the extramedullary spaces has
been identified in association with MPNs, myelodysplastic
syndromes, granulocytic sarcoma and other myeloid neoplasms
(32–34). Cellular proliferation may consist
of trilineage marrow elements similar to those of benign EMH
(32–34). The most common sites of EMH
associated with neoplastic disorder are the spleen, lymph nodes,
skin, bone, small intestine, orbit, breast, cervix, nasal sinus,
mediastinum and brain (26,28,35).
5. EMH associated with stromal
abnormalities
In the infrequent case of stromal disorders of the
bone, irregularities in the bone marrow environment trigger EMH
(1). Osteopetrosis is an example
of such a case, where the marrow cavity is compromised due to
excess bone formation and proliferation, resulting in insufficient
space for the required hematopoietic element (36). In severe renal osteodystrophy and
Paget's disease similar transformations have also been observed
(26).
Additionally, EMH is prompted by the replacement of
bone marrow cavity spaces with non-hematopoietic and nonosteoid
tissues (35). For example, EMH
occasionally occurs as a result of extensive involvement of
infectious diseases, marrow fibrosis following inflammation,
storage diseases including Gaucher and Niemann-Pick diseases or
tumor metastasis (37).
6. Microenvironment of EMH
Of the various organs associated with EMH, the
spleen offers a unique site for evaluating HSC/niche interactions
as it is one of the most common sites of EMH, but does not have a
major role in embryonic/developmental hematopoiesis. In the human
spleen, it was verified that the expression levels of CXCL12, one
of the candidate markers of bone marrow niche-constituting cells,
were significantly higher in EMH-positive cases, compared with
those in EMH-negative cases (Fig.
1) (19). Furthermore, CXCL12
was demonstrated to be localized to the sinus endothelial cells of
the red pulp in EMH-positive spleens; whereas, CXCL12 was expressed
throughout the vascular endothelial cells of the white pulp in
spleens of EMH-negative and -positive cases (19). EMH frequently occurs in the red
pulp, and the data presented in the current review suggest that
splenic sinus endothelial cells expressing CXCL12 may contribute to
the attachment and recruitment of circulating hematopoietic
precursor cells, forming bone marrow niche-like regions of EMH in
the human spleen (19).
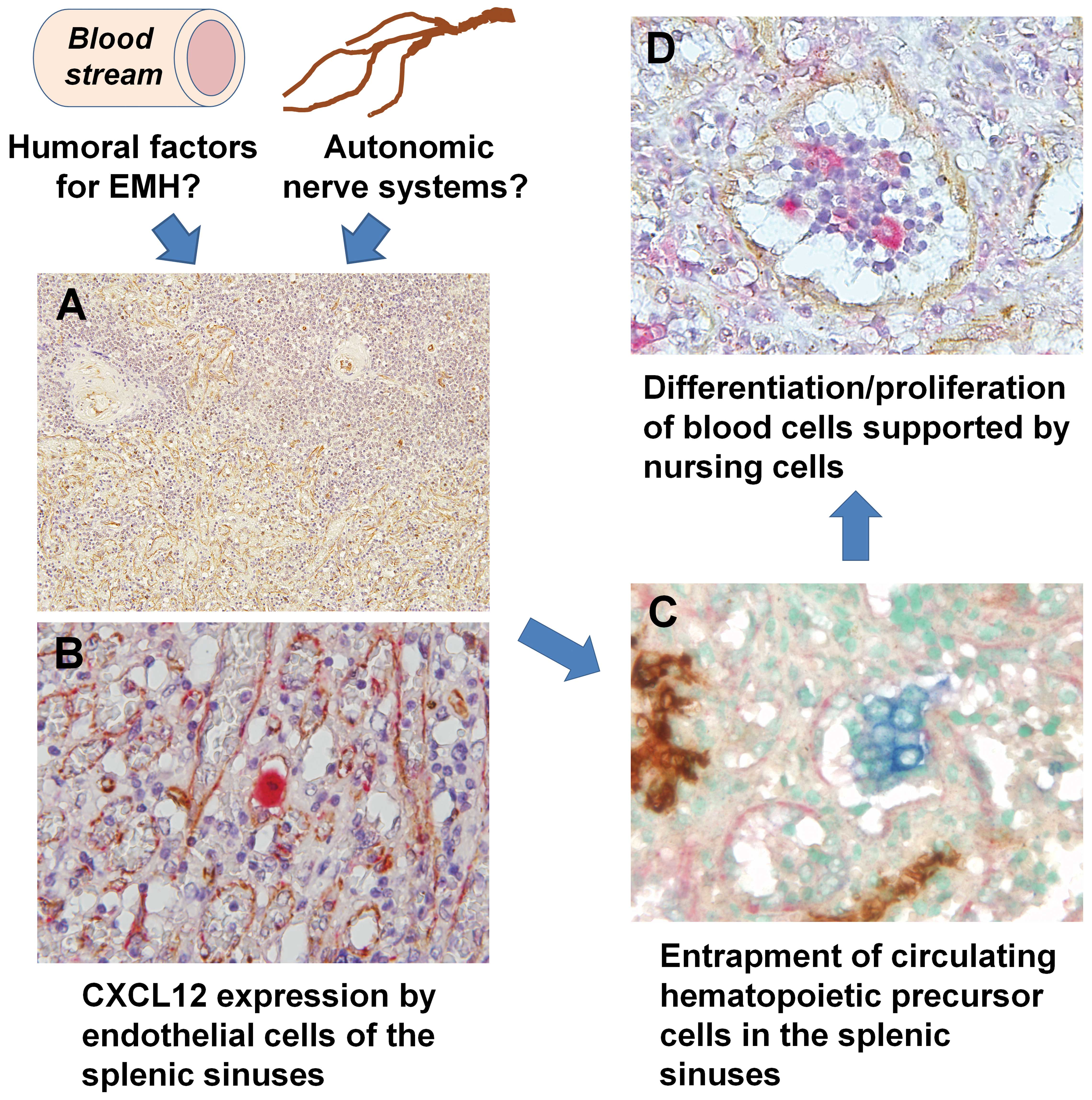 | Figure 1Summary of the distribution of
EMH-associated molecules in the spleen. Humoral factors and/or
autonomic nerve systems may induce the expression of CXCL12 in
splenic sinus endothelial cells. These cells may maintain HSCs, and
certain HSCs are able to differentiate into various lineage cells,
which are subsequently maintained by macrophages in the sinuses.
(A) Localization of CXCL12 (brown) in EMH-positive spleens (single
immunohistochemistry; magnification, ×50). Note the positive
reaction in the margin of the sinuses of the red pulp and
arterioles of the white pulp. (B) CXCL12-positive (brown) cells
were also positive for factor VIII (red), which was used as a sinus
endothelial cell marker, indicating that these cells were sinus
endothelial cells (double immunostaining; magnification, ×200). (C)
c-kit-positive (blue) and CD34-negative (brown) hematopoietic
precursor cells were observed adjacent to the CXCL12-positive (red)
sinus endothelial cells (triple immunostaining; magnification,
×200). (D) Erythroblasts and CD68-positive (red) macrophage-lineage
cells were identified in the sinuses of EMH-positive spleens.
CXCL12-positive endothelial cells (brown) existed separately from
the focus of erythroblastic accumulation (double immunostaining;
magnification, ×200). CXCL12, chemokine (C-X-C motif) ligand 12;
EMH, extramedullary hematopoiesis; HSCs, hematopoietic stem
cells. |
Splenic EMH, in particular erythroid EMH, is
suggested to occur within the red pulp of the spleen (39). A study confirmed that
erythroblasts, myeloid cells and megakaryocytes were identifiable
within the splenic cords and intrasinusoidal spaces in the red pulp
of the spleen in EMH-positive cases (21). The identification of multiple
stages of differentiation and mitotic erythroblast figures further
supported the hypothesis that erythroid EMH occurred in the red
pulp (21).
Erythroblastic islands of bone marrow constitute the
basic structure required for erythropoiesis, and include central
macrophages that form the microenvironment required for
erythroblast development and enucleation, as well as phagocytosis
of extruded nuclei (40,41). Following the release of HSCs from
bone marrow niches, erythroid cell maturation is facilitated and
the resulting erythroid colony-stimulating units may adhere to the
central macrophages forming distinct erythroblastic islands that
are separate from the existing niches (41). This hypothesis was found to be
applicable to EMH-positive spleens, where CXCL12-positive signals
on endothelial cells were identified distant from clusters of
mature erythroblasts (21).
A small population of c-kit-positive/CD34-negative
cells have been identified adjacent to CXCL12-positive sinus
endothelial cells in the EMH-positive spleen (21). The CD34 antigen has previously been
used to identify immature HSCs; however,
c-kit-positive/CD34-negative cells have also been shown to comprise
a fraction of HSCs (42) or
hematopoietic precursor cells, including erythroid progenitors
(43). For this reason,
c-kit-positive/CD34-negative cells in the EMH-positive spleen may
potentially be a type of hematopoietic precursor cell. Bone
marrow-derived HSCs in the arterial blood should enter into an open
blood system comprised of the red pulp of the splenic cords, so
that maturing cells may infiltrate into the venous sinuses
(26,44–48).
Previous studies have reported the presence of bone marrow-derived
HSCs within the sinuses and adjacent to endothelial cells of the
spleen (5,49). These results indicate that
circulating HSCs may be trapped by CXCL12-positive cells at the
sinus margins proximal to CXCL12-positive endothelial cells, in
order to initiate the first stage of EMH.
In order for EMH to develop, a niche environment
must be activated and expanded by multiple factors, including bone
marrow failure, excessive hematopoietic stimulation and tissue
inflammation, injury and repair, as well as abnormal cytokine
production (7,26). It has been demonstrated that
treatment with granulocyte-colony stimulating factor and
granulocyte macrophage colony-stimulating factor induces an
increase in spleen size associated with EMH (50,51).
The underlying causes of EMH in the spleen were not able to be
distinctly classified into the categories, described above;
however, further study is required in order to elucidate the
mechanisms underlying the upregulation of CXCL12 expression in the
spleen.
In addition, distinct tissue niches may induce the
development of various subsets of hematopoietic cells from HSCs. A
study has suggested that splenic niches may support the development
of certain distinct, dendritic-like antigen-presenting cells
(52). Although such experiments
utilizing animal models have provided useful results and
hypotheses, further studies should be conducted in order to
identify specific differences in the characteristics and
differentiation patterns of HSCs between human bone marrow and
splenic EMH.
Bone marrow-derived mesenchymal stromal/stem cells
(MSCs) are non-hematopoietic cells with the ability to
differentiate into various types of cell, including osteoblasts,
adipocytes and chondrocytes. MSCs are known to participate in the
formation of niches for HSC development in the bone marrow. Several
markers, including CD106, CD166, CD105, CD73 and CD44, are known to
be useful in the isolation of MSCs from various organs, including
bone marrow, placenta, adipose tissue, synovial membrane,
endometrium and cartilage (53).
The construction of niche-like regions during EMH may be attributed
to the MSC-derived cells of EMH organs, although CXCL12-positive
cells were only identified in the red pulp of EMH-positive sinus
endothelial cells (19).
7. Conclusion
In contrast to how endothelial and perivascular
cells and autonomic nerve systems maintain HSCs in the bone marrow
(11,54) and how endosteal niches may maintain
lymphoid progenitor cells (55),
CXCL12-positive endothelial cells in the EMH-positive spleen may
only contribute to the localization of HSCs to EMH-positive organs
(5,56,57).
As the results of a previous study indicated, CXCL12 is expressed
in the sinus endothelial cells of the EMH-positive spleen, which
may function in the entrapment of circulating hematopoietic
precursor cells, whereas the cluster of macrophage/differentiated
hematopoietic cells is separated from the CXCL12-positive
endothelial cells (19). Other
types of cell, including macrophages, as well as nerve, adipose,
and dendritic cells, may contribute to the maintenance and
differentiation of hematopoietic cells in EMH-positive spleens.
CXCL12 was therefore suggested to have a restricted role during
EMH, trapping the hematopoietic precursor cells in the sinuses of
the splenic red pulp. The function of CXCL12 in bone marrow
niche-constituting cells, for example CAR cells, may differ from
its function in the EMH-positive spleen. Further studies are
required in order to elucidate the mechanisms controlling the EMH
niche-like function of various cells in comparison with those of
the perivascular and/or endosteal cells of bone marrow niches.
References
|
1
|
Sohawon D, Lau KK, Lau T and Bowden DK:
Extra medullary haematopoiesis: a pictorial review of its typical
and atypical locations. J Med Imaging Radiat Oncol. 56:534–538.
2012. View Article : Google Scholar
|
|
2
|
Tsamandas AC, Jain AB, Raikow RB, Demetris
AJ, Nalesnik MA and Randhawa PS: Extramedullary hematopoiesis in
the allograft liver. Mod Pathol. 8:671–674. 1995.PubMed/NCBI
|
|
3
|
Vassiliou V, Papamichael D, Lutz S,
Eracleous E, Kountourakis P, Polyviou P, Michaelides I, Shoukris M
and Andreopoulos D: Presacral extramedullary hematopoiesis in a
patient with eectal adenocarcinoma: report of a case and literature
review. J Gastrointest Cancer Feb. 10:2012.Epub ahead of print.
|
|
4
|
Macki M, Bydon M, Papademetriou K,
Gokaslan Z and Bydon A: Presacral extramedullary hematopoiesis: an
alternative hypothesis. J Clin Neurosci. 20:1664–1668. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johns JL and Christopher MM:
Extramedullary hematopoiesis: a new look at the underlying stem
cell niche, theories of development and occurrence in animals. Vet
Pathol. 49:508–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mendelson A and Frenette PS: Hematopoietic
stem cell niche maintenance during homeostasis and regeneration.
Nat Med. 20:833–846. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flores-Figueroa E, Varma S, Montgomery K,
et al: Distinctive contact between CD34+hematopoietic progenitors
and CXCL12+CD271+mesenchymal stromal cells in benign and
myelodysplastic bone marrow. Lab Invest. 92:1330–1341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lilly AJ, Johnson WE and Bunce CM: The
haematopoietic stem cell niche: new insights into the mechanisms
regulating haematopoietic stem cell behaviour. Stem Cells Int.
274564:2011.
|
|
9
|
Lo Celso C, Fleming HE, Wu JW, et al:
Live-animal tracking of individual haematopoietic stem/progenitor
cells in their niche. Nature. 457:92–96. 2009. View Article : Google Scholar
|
|
10
|
Kiel MJ and Morrison SJ: Uncertainty in
the niches that maintain haematopoietic stem cells. Nat Rev
Immunol. 8:290–301. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding L, Saunders TL, Enikolopov G and
Morrison SJ: Endothelial and perivascular cells maintain
haematopoietic stem cells. Nature. 481:457–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levesque JP and Winkler IG: Hierarchy of
immature hematopoietic cells related to blood flow and niche. Curr
Opin Hematol. 18:220–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagasawa T, Omatsu Y and Sugiyama T:
Control of hematopoietic stem cells by the bone marrow stromal
niche: the role of reticular cells. Trends Immunol. 32:315–320.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mendez-Ferrer S, Michurina TV, Ferraro F,
et al: Mesenchymal and haematopoietic stem cells form a unique bone
marrow niche. Nature. 466:829–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamazaki S, Ema H, Karlsson G, et al:
Nonmyelinating Schwann cells maintain hematopoietic stem cell
hibernation in the bone marrow niche. Cell. 147:1146–1158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Avecilla ST, Hattori K, Heissig B, et al:
Chemokine-mediated interaction of hematopoietic progenitors with
the bone marrow vascular niche is required for thrombopoiesis. Nat
Med. 10:64–71. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ara T, Tokoyoda K, Sugiyama T, et al:
Long-term hematopoietic stem cells require stromal cell-derived
factor-1 for colonizing bone marrow during ontogeny. Immunity.
19:257–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellyard JI, Avery DT, Mackay CR, et al:
Contribution of stromal cells to the migration, function and
retention of plasma cells in human spleen: potential roles of
CXCL12, IL-6 and CD54. Eur J Immunol. 35:699–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miwa Y, Hayashi T, Suzuki S, Abe S, Onishi
I, Kirimura S, Kitagawa M and Kurata M: Up-regulated expression of
CXCL12 in human spleens with extramedullary haematopoiesis.
Pathology. 45:408–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visnjic D, Kalajzic Z, Rowe DW, et al:
Hematopoiesis is severely altered in mice with an induced
osteoblast deficiency. Blood. 103:3258–3264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolf BC and Neiman RS: Hypothesis: splenic
filtration and the pathogenesis of extramedullary hematopoiesis in
agnogenic myeloid metaplasia. Hematol Pathol. 1:77–80.
1987.PubMed/NCBI
|
|
22
|
MacSween RMN, Burt AD, Portmann BC, et al:
Pathology of the Liver, 4th ed. Diagn Cytopathol. 29:432003.
View Article : Google Scholar
|
|
23
|
Schlitt HJ, Schäfers S, Deiwick A, Eckardt
KU, Pietsch T, Ebell W, Nashan B, Ringe B, Wonigeit K and Pichlmayr
R: Extramedullary erythropoiesis in human liver grafts. Hepatology.
21:689–696. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsamandas AC, Jain AB, Raikow RB, Demetris
AJ, Nalesnik MA and Randhawa PS: Extramedullary hematopoiesis in
the allograft liver. Mod Pathol. 8:671–674. 1995.PubMed/NCBI
|
|
25
|
Craig CE, Quaglia A and Dhillon AP:
Extramedullary haematopoiesis in massive hepatic necrosis.
Histopathology. 45:518–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sohawon D, Lau KK, Lau T and Bowden DK:
Extra-medullary haematopoiesis: a pictorial review of its typical
and atypical locations. J Med Imaging Radiat Oncol. 56:538–534.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Malley DP: Benign extramedullary myeloid
proliferations. Mod Pathol. 20:405–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tavian M, Biasch K, Sinka L, Vallet J and
Péault B: Embryonic origin of human hematopoiesis. Int J Dev Biol.
54:1061–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tavian M, Cortés F, Charbord P, Labastie
MC and Péault B: Emergence of the haematopoietic system in the
human embryo and foetus. Haematologica. 84(Suppl EHA-4): 1–3.
1999.
|
|
30
|
Georgiades CS, Neyman EG, Francis IR,
Sneider MB and Fishman EK: Typical and atypical presentations of
extramedullary hemopoiesis. AJR Am J Roentgenol. 179:1239–1243.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tavian M and Péault B: The changing
cellular environments of hematopoiesis in human development in
utero. Exp Hematol. 33:1062–1069. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palatnik A, Narayan R and Walters M:
Extramedullary hematopoiesis involving uterus, fallopian tubes, and
ovaries, mimicking bilateral tuboovarian abscesses. Int J Gynecol
Pathol. 31:584–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akbulut S, Yavuz R, Akansu B, Sogutcu N,
Arikanoglu Z and Basbug M: Ectopic bone formation and
extramedullary hematopoiesis in the thyroid gland: report of a case
and literature review. Int Surg. 96:260–265. 2011. View Article : Google Scholar
|
|
34
|
Radopoulos D, Tzakas K and Tahmatzopoulos
A: A rare case of renal oncocytoma associated with erythrocytosis:
case report. BMC Urol. 23:262006. View Article : Google Scholar
|
|
35
|
Tavian M and Péault B: Embryonic
development of the human hematopoietic system. Int J Dev Biol.
49:243–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Neiman RS, Barcos M, Berard C, Bonner H,
Mann R, Rydell RE and Bennett JM: Granulocytic sarcoma: a
clinicopathologic study of 61 biopsied cases. Cancer. 48:1426–1437.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tavassoli M and Weiss L: An electron
microscopic study of spleen in myelofibrosis with myeloid
metaplasia. Blood. 42:267–279. 1973.PubMed/NCBI
|
|
38
|
Yoshida H, Kawane K, Koike M, et al:
Phosphatidylserine-dependent engulfment by macrophages of nuclei
from erythroid precursor cells. Nature. 437:754–758. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chasis JA and Mohandas N: Erythroblastic
islands: niches for erythropoiesis. Blood. 112:470–478. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sadahira Y and Mori M: Role of the
macrophage in erythropoiesis. Pathol Int. 49:841–848. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sonoda Y and Sasaki K: Surface morphology
of the central macrophages of erythroblastic islets in the spleen
of aged and pregnant mice: an immunohistochemical light microscopic
study. Arch Histol Cytol. 71:155–161. 2008. View Article : Google Scholar
|
|
42
|
Sonoda Y: Immunophenotype and functional
characteristics of human primitive CD34-negative hematopoietic stem
cells: the significance of the intra-bone marrow injection. J
Autoimmun. 30:136–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De Jong MO, Wagemaker G and Wognum AW:
Separation of myeloid and erythroid progenitors based on expression
of CD34 and c-kit. Blood. 86:4076–4085. 1995.PubMed/NCBI
|
|
44
|
Cesta MF: Normal structure, function and
histology of the spleen. Toxicol Pathol. 34:455–465. 2006.
View Article : Google Scholar
|
|
45
|
Mebius RE and Kraal G: Structure and
function of the spleen. Nat Rev Immunol. 5:606–616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mueller SN and Ahmed R: Lymphoid stroma in
the initiation and control of immune responses. Immunol Rev.
224:284–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asakura A and Rudnicki MA: Side population
cells from diverse adult tissues are capable of in vitro
hematopoietic differentiation. Exp Hematol. 30:1339–1345. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saito H, Yokoi Y, Watanabe S, et al:
Reticular meshwork of the spleen in rats studied by electron
microscopy. Am J Anat. 181:235–252. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wright DE, Wagers AJ, Gulati AP, et al:
Physiological migration of hematopoietic stem and progenitor cells.
Science. 294:1933–1936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stroncek D, Shawker T, Follmann D and
Leitman SF: G-CSF-induced spleen size changes in peripheral blood
progenitor cell donors. Transfusion. 43:609–613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Picardi M, De Rosa G, Selleri C, Scarpato
N, Soscia E, Martinelli V, Ciancia R and Rotoli B: Spleen
enlargement following recombinant human granulocyte
colony-stimulating factor administration for peripheral blood stem
cell mobilization. Haematologica. 88:794–800. 2003.PubMed/NCBI
|
|
52
|
O'Neill HC, Griffiths KL, Periasamy P, et
al: Spleen as a site for hematopoiesis of a distinct antigen
presenting cell type. Stem Cells Int. 954275:2011.
|
|
53
|
Sivasubramaniyan K, Lehnen D, Ghazanfari
R, Sobiesiak M, Harichandan A, Mortha E, Petkova N, Grimm S,
Cerabona F, de Zwart P, et al: Phenotypic and functional
heterogeneity of human bone marrow- and amnion-derived MSC subsets.
Ann NY Acad Sci. 1266:94–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lucas D, Scheiermann C, Chow A, Kunisaki
Y, Bruns I, Barrick C, Tessarollo L and Frenette PS:
Chemotherapy-induced bone marrow nerve injury impairs hematopoietic
regeneration. Nat Med. 19:695–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ding L and Morrison SJ: Haematopoietic
stem cells and early lymphoid progenitors occupy distinct bone
marrow niches. Nature. 495:231–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chute JP: Stem cell homing. Curr Opin
Hematol. 13:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jamieson CH, Barroga CF and Vainchenker
WP: Miscreant myeloproliferative disorder stem cells. Leukemia.
22:2011–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|















