Introduction
In vitro fertilization and embryo transfer
(IVF-ET) has become the most effective treatment for infertile
couples in the past three decades (1). Embryo quality is one of the most
important factors affecting the results of IVF-ET. Several studies
have attempted to improve the quality of embryos cultured in
vitro by determining the potential mechanism of embryonic
development and adjusting the in vitro culture conditions
(2–5). A zygote undergoes a series of
cleavages and subsequently enters the blastocyst stage. Numerous
physiological cellular processes, including apoptosis, cell-cell
adhesion, cell polarity differentiation and proliferation, occur
between the zygote and the blastocyst stages, and are vital for
early embryonic development (6,7).
Rho-associated protein kinase (ROCK)-LIM domain
kinase 1/2-cofilin-actin is one of the most important signaling
pathways regulating actin assembly and actin depolymerization.
Previous studies (8,9) have demonstrated the importance of
actin filaments for oocyte and embryo development. Y-27632 is a
specific inhibitor of ROCKs, which are downstream effectors of Rho
GTPase that function in numerous physiological cellular processes,
including contraction, adhesion, migration and proliferation
(10). Y-2763 has been shown to
have different effects on mammalian embryonic development. For
example, Y-27632 has been found to inhibit mouse blastocyst cavity
formation (11) and prevent early
mouse embryo development (12).
However, Y-27632 has also been found to stimulate the revivability
of in vitro-produced bovine blastocysts following
vitrification (13). In another
study, Y-27632 was demonstrated to increase the post-thaw survival
rates of embryos (14). Until
recently, the precise effect of Y-27632 on human embryos and single
blastomeres, remained to be fully elucidated (8,15–17).
Previous experiments have shown that single
blastomeres from human cleavage-stage embryos are pluripotent and
can differentiate into human embryonic stem cells (hESCs) (18,19).
Several studies have found that Y-27632 markedly reduces the
apoptosis of dissociative hESCs and improves the recovery of hESCs
from cryopreservation (20–23).
It has also been shown to effectively improve the survival of other
pluripotent cell, including induced pluripotent stem (iPS) cells
(24). These findings indicate the
utility of Y-27632 in a variety of applications across a number of
different cell types (22,25,26).
However, the direct effect of Y-27632 alone on single blastomeres
from cleavage-stage embryos remains to be elucidated.
Therefore, the present study aimed to determine the
effect of Y-27632 on the development of fresh human embryos and
single blastomeres obtained from human embryos. In order to
overcome the ethical problems associated with experimentation on
human embryos, poor-quality embryos and embryos in which abnormal
fertilization occurred were used.
Materials and methods
Ethical statement
Ethical approval for the present study was granted
by the Institutional Ethics Committee of the First Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China). Couples
agreed to donate their discarded embryos for the present study,
following being clearly informed about the details, and written
informed consent was obtained from these couples.
Patients
All the discarded embryos were donated for use in
the present study by patients undergoing fresh in vitro
fertilization (IVF)/intracytoplasmic sperm injection cycles at the
Reproductive Medical Center, The First Affiliated Hospital of Sun
Yet-sen University. Between March 2010 and May 2010, and between
November 2010 and April 2011, a total of 224 couples agreed to
donate their discarded embryos for the present study following
being clearly informed of the details. A total of 784 embryos were
donated by these patients, of which 526 were allocated to the first
part of the present study, and the remaining 258 were allocated to
the second part. The experimental protocol was approved of the
First Affiliated Hospital of Sun Yat-sen University.
All the patients received a long protocol for
stimulation. Gonadotropin with follicle-stimulating hormone (FSH;
Gonal-F; Merck-Serono Company, Corsier-sur-Vevey, Switzerland) was
administered at 150–300 IU/day between days 3 and 5. The patient
received 10,000 IU human chorionic gonadotropin (hCG; Livzon
Pharmaceutical Group Co., Ltd., Guangdong, China), and transvaginal
ultrasound-guided oocyte retrieval was performed 36 h later (Aloka
SSD-3500; Hitachi-Aloka Medical, Ltd., Tokyo, Japan). All oocytes
were inseminated 3 h following retrieval.
Day 3 embryo assessment
The day 3 embryos were graded, as described
previously (27) using an Olympus
IX71 microscope (Olympus Corp, Tokyo, Japan). Briefly, grade 1,
equal-sized symmetrical blastomeres; grade 2, uneven blastomeres
with <10% fragmentation; grade 3, 10–50% fragmentation; grade 4,
>50% fragmentation. Grade 3 or 4 embryos, or embryos with fewer
than four blastomeres were considered to be of poor quality, and
those embryos demonstrating poor-quality were used for the present
study.
Blastomere isolation
In the second part of the experiment, 258 of the
collected day 3 embryos were used. Blastomeres from the discarded
embryos were isolated by micromanipulation. Briefly, the zona
pellucida was removed with 0.5% pronase (Sigma-Aldrich, St. Louis,
MO, USA), and the embryos were washed twice in calcium- and
magnesium-free phosphate-buffered solution (PBS). Single
blastomeres were isolated with a 50-µm diameter micropipette
by gentle repeated aspiration.
Blastocyst formation
Following morphological assessment of the day 3
embryos, for the first part of the experiment, 526 embryos were
collected and allocated to blastocyst culture with or without
Y27632 (Sigma-Aldrich). In the second part of the experiment,
following isolation of the blastomeres, sibling blastomeres of
similar size, derived from the same embryo, were paired and
allocated to blastocyst culture, with or without, Y-27632. The
remaining unpaired blastomeres were abandoned.
The embryos and single blastomeres were cultured in
conventional culture medium comprised of blastocyst medium (Sage)
supplemented with 10% serum protein substitute (Sage) for an
additional 5 days at 37°C and 5% CO2. In the
experimental group, 10 µM Y-27632 was added to the
blastocyst culture medium. The concentration of 10 µM has
been a commonly used working concentration in previous human embryo
and hESC studies (20–26).
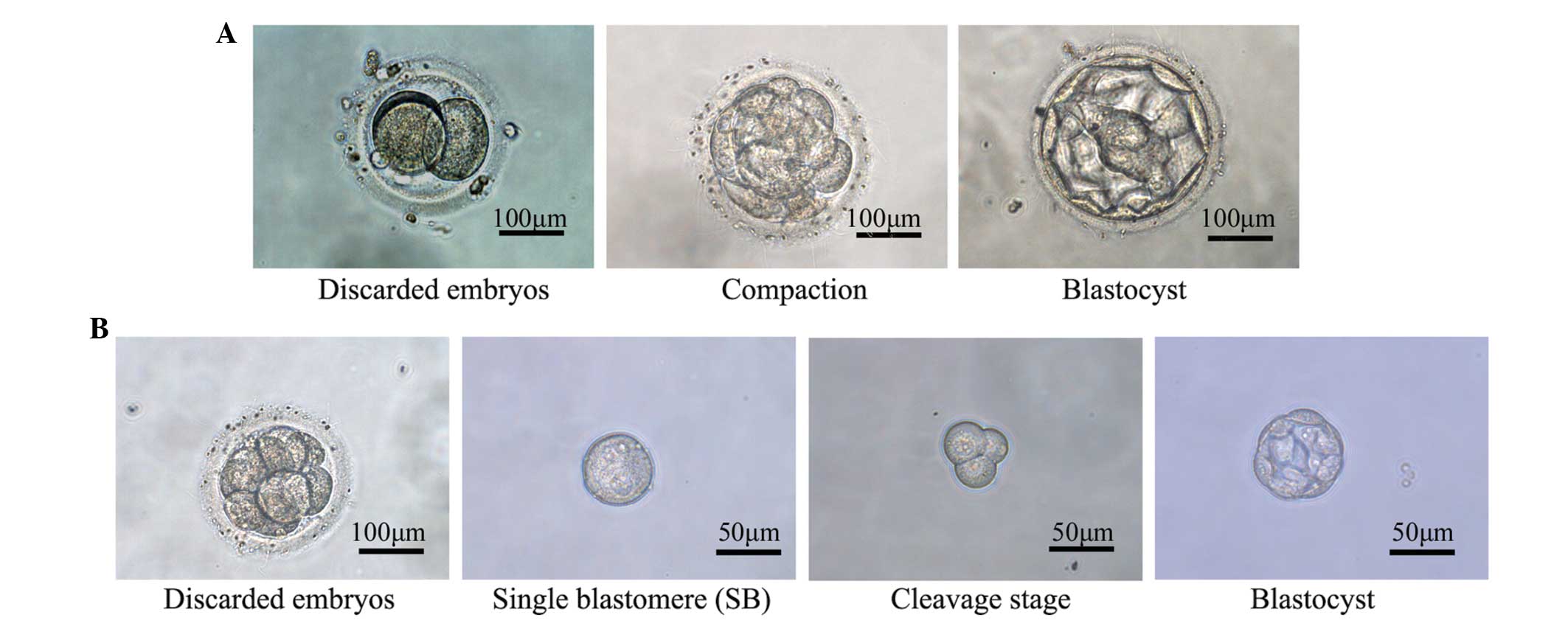
From day 5, embryonic development was recorded
daily, including the time of blastocyst formation and the
blastocyst grade, as described by Gardner et al (28). The development of single
blastomeres was also recorded daily from day 5 onwards (Fig. 1).
Immunofluorescence
On day 6, a number of the blastocysts from the
single blastomeres were stained with 2 µg/ml Hoechst-33342
(Sigma-Aldrich) to count the numbers of cells. On day 7,
immunofluorescence analysis was performed to detect the expression
levels of the pluripotency marker octamer-binding transcription
factor (Oct)3/4 and E-cadherin in the blastocysts cultured from
single blastomeres. Blastocysts were fixed with 4% formaldehyde at
room temperature (RT) and permeabilized with 0.2% Triton-X100
(Sigma-Aldrich) in PBS for 20 min, prior to blocking with 10% goat
serum (Wuhan Boster Biological Technology, Ltd., Wuhan, China) in
PBS for 1 h. Subsequently, the blastocysts were incubated with the
following primary antibodies overnight at 4°C: Polyclonal rabbit
anti-human OCT3/4 (1:200; 18976; Abcam, Cambridge, UK) and
polyclonal rabbit anti-human E-cadherin antibody (1:100; 15148;
Abcam). The blastocysts were then incubated with a
fluorescent-conjugated secondary antibody (goat anti-rabbit IgG
fluorescein isothiocyanate; 1:40; 6717; Abcam) for 1 h at RT. The
cell nuclei were stained with 4′,6-diamidino-2-phenylindole
(Sigma-Aldrich). Specimens were observed using Zeiss LSM 510 Meta
Confocal Microscope (Carl Zeiss AG, Oberkochen, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) to measure the mRNA expression
levels of E-cadherin
Total RNA was extracted from blastocysts (20
blastocysts per group) on day 7 using the TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
accordance with the manufacturer's instructions. A total of 1
µg total RNA was reverse transcribed using the Moloney
murine leukemia virus reverse transcriptase (Promega Corp, Madison,
WI, USA). Subsequently, cDNA samples were used as the template for
PCR. RT-qPCR was performed using SYBR Green and an ABI 7300
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. An initial DNA
denaturation step at 95°C for 10 min was followed by 40 cycles of
denaturation at 95°C for 15 sec, primer annealing at 60°C for 1
min, and an extension step at 72°C for 15 sec. RT-qPCR was
performed in triplicate on blastocyst samples to quantify the mRNA
expression of E-cadherin. The housekeeping gene, glyceraldehyde
3-phosphate dehydrogenase (GAPDH), was used as an internal control.
The primer sequences were as follows: GAPDH, forward
5′-CGGAGTCAACGGATTTGGTCG-3′ and reverse 5′-CCTGGAAGATGGTGATGGG-3′);
and E-cadherin, forward 5′-GGCACTTGACCCTGATACG-3′ and reverse
5′-GCTGGACCGAGAGAGTTAC-3′. Data were analyzed with Sequence
Detector software (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Relative expression of E-cadherin was calculated using the
2−ΔΔCq method.
Statistical analysis
Statistical comparisons between groups were
performed using analysis of variance, χ2 test, Fisher's
exact test, Student's t-test and Breslow survival analysis,
using SPSS software version 13.0 (SPSS, Inc., Chicao, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
In the first part of the present study (Fig. 2), 526 embryos were alternately
allocated to blastocyst culture, with or without Y-27632 (n=263
each). Following confirmation that 75 of these 526 fertilized eggs
had not cleaved, of the remaining embryos, 228 and 223 were
cultured with and without Y-27632, respectively. Basal conditions,
including embryonic grade, were comparable between the two groups
(Table I). In total, 61 (26.8%)
and 64 (28.7%) embryos of the Y-27632 and control groups reached
the blastocyst stage, although this difference was not
statistically significant (P=0.644; Table II). Comparison of the day 5
blastocyst grades between the two groups revealed no statistically
significant difference (Table
III). The duration of blastocyst formation was also compared
between the two groups. On day 5, the blastocyst formation ratio in
the experimental group was 11.4% (26/228), which was significantly
lower (P=0.015), compared with the corresponding rate in the
control group (19.7%; 44/223; Table
II). Breslow survival analysis of the blastocyst formation time
was performed. The median formation time in the experimental group
was 6.18 days, which was significantly higher than that of the
control group (5.73 days; P=0.005; Table IV).
 | Table IBasal conditions between the
experimental and control groups in the first part of the present
study. |
Table I
Basal conditions between the
experimental and control groups in the first part of the present
study.
| Conditions | Y-27632 | Control | P-value |
|---|
| Age (years) | 30.92±4.84 | 30.90±4.67 | 0.686 |
| Embyo grade; n
(%) |
| 1 | 11 (4.8) | 15 (6.7) | 0.386 |
| 2 | 137 (60.1) | 120 (53.8) | 0.178 |
| 3 and 4 | 80 (35.1) | 88 (39.5) | 0.337 |
| Cell number; n
(%) |
| 2–3 | 51 (22.4) | 51 (22.9) | 0.899 |
| 4–5 | 101 (44.3) | 97 (43.9) | 0.864 |
| ≥ 6 | 73 (32.0) | 74 (32.6) | 0.792 |
| Compact | 3 (1.3) | 1 (0.4) | 0.326 |
 | Table IIBlastocyst formation between the
experimental and control groups. |
Table II
Blastocyst formation between the
experimental and control groups.
| Day | Y-27632 (n=228) n
(%) | Control (n=223) n
(%) | P-value |
|---|
| 5 | 26 (11.4) | 44 (19.7) | 0.015a |
| 6 | 25 (11.0) | 14 (6.3) | 0.077 |
| 7 | 8 (3.5) | 5 (2.2) | 0.422 |
| 8 | 2 (0.9) | 1 (0.4) | 0.575 |
| Total | 61 (26.8) | 64 (28.7) | 0.644 |
 | Table IIIBlastocyst grade between the
experimental and control groups. |
Table III
Blastocyst grade between the
experimental and control groups.
| Grade | Y-27632 (n=26) n
(%) | Control (n=44) n
(%) | P-value |
|---|
| Blastocyst |
| 3 | 10 (38.5) | 10 (22.7) | 0.159 |
| 4 | 14 (53.8) | 28 (63.6) | 0.419 |
| 5 | 2 (7.7) | 6 (13.6) | 0.714 |
| 6 | 0 | 0 | – |
| Inner cell
mass |
| A | 4 (15.4) | 4 (9.1) | 0.681 |
| B | 9 (34.6) | 16 (36.5) | 0.882 |
| C | 13 (50.0) | 24 (54.6) | 0.712 |
| Trophectoderm |
| A | 4 (15.4) | 10 (22.7) | 0.458 |
| B | 12 (46.2) | 19 (43.2) | 0.809 |
| C | 10 (38.5) | 15 (34.1) | 0.712 |
 | Table IVSurvival analysis of blastocyst
formation time. |
Table IV
Survival analysis of blastocyst
formation time.
| Group | Median formation
time (days) | P-value |
|---|
| Y-27632 | 6.18 | 0.005a |
| Control | 5.73 |
Following confirmation that Y-27632 increased the
ratio of formation of blastocysts from single human blastomeres,
the present study obtained 1,192 blastomeres from 258 discarded day
3 embryos, and sibling blastomeres of similar sizes were equally
allocated into experimental and control groups (n=596 each;
Fig. 1). On day 4, 213 (35.7%)
blastomeres in the Y-27632 group, and 203 (34.1%) blastomeres in
the control group had cleaved to form two or more cells; however,
no statistically significant difference was observed in the number
of cleaved cells between the two groups (P=0.543; Table V).
 | Table VBlastocyst formation rate of human
individual blastomeres. |
Table V
Blastocyst formation rate of human
individual blastomeres.
| Stage | Y-27632 (n=596) n
(%) | Control (n=596) n
(%) |
|---|
| Cleavage (day
4) | 213 (35.7) | 203 (34.1) |
| Blastocyst | 82 (13.8)a | 51 (8.6) |
Treatment with Y-27632 increased the blastocyst
formation ratio of human individual blastomeres. The results
revealed that 82 blastocysts of 596 blastomeres (13.8%) and 51
blastocysts of 596 blastomeres (8.6%) were formed in the presence
and absence of Y-27632, respectively, and this difference was
statistically significant between the two groups (P=0.004; Table V).
On day 6, a total of 33 and 14 blastocysts in the
experimental and control groups, respectively, were stained to
count the cell number. The average numbers of blastocysts were
24.7±13.3 and 24.6±8.8, in the presence and absence of Y-27632,
respectively. However, this difference was not statistically
significant (P=0.973; Fig.
3A).
On day 7, the expression of Oct3/4 was detected in
13 and 8 blastocysts in the experimental and control groups,
respectively. Only a small percentage of the blastocysts expressed
Oct3/4, and the number of cells, which stained positive for Oct3/4
ranged between 2 and 5 per blastocyst. The Oct3/4(+) blastocyst
rates were 30.8% (4/13) and 25.0% (2/8) in the experimental and
control groups, respectively, which was not a statistically
significant difference (P=0.776; Fig.
3B).
The protein expression of E-cadherin was detected in
eight blastocysts in both the experimental and control groups using
indirect immunofluorescence. The culture of blastocysts in the
presence of Y-27632 resulted in an upregulation in the expression
of E-cadherin (Fig. 3C). The mRNA
expression of E-cadherin was detected in 20 blastocysts in both the
experimental and control groups using RT-qPCR. There was a
significant increase in the expression of E-cadherin in the Y-27632
group (P=0.022; Fig. 3D).
Discussion
The present study initially investigated the effect
of the ROCK inhibitor, Y-27632, on fresh human cleavage-stage
embryos, and on the development of single blastomeres. The results
of these investigations demonstrated that Y-27632 increased the
ratio of formation of blastocysts from single human blastomeres.
However, Y-27632 inhibited the formation of day 5 blastocysts and
delayed the total formation of blastocysts from discarded human
embryos.
In contrast to previous reports, the data of the
present study suggested that Y-27632 did not affect the total ratio
of blastocyst formation or the quality of the human embryos. Cortes
et al reported that Y-27632 increases post-thaw embryo
survival and development up to the blastocyst stage (14). However, in the experiments in the
present study used discarded fresh embryos from fresh IVF-ET
cycles. This suggests that Y-27632 may have a positive effect on
post-thaw embryos, rather than on fresh embryos. This is similar to
another study, which reported that Y-27632 improves the
revivability of bovine blastocysts following vitrification and
warming (13). By contrast, other
studies have suggested that ROCK inhibition prevents early mouse
embryo development and blastocyst cavity formation (12,17,26),
and in these studies it was concluded that ROCK is involved in
early embryonic development. However, in these previous studies,
the precise time of blastocyst formation was not recorded. In the
present study, the precise time of blastocyst formation was
recorded every day. On day 5, the blastocyst formation ratio of the
embryos in the Y-27632 group was lower than that in the control
group, and this difference was statistically significant. The
survival analysis in the present study indicated that ROCK
inhibition delayed the formation of blastocysts by almost half a
day. Thus, the results of the present study led to the hypothesis
that Y-27632 negatively affects fresh human embryos by delaying the
time of development. However, due to the ethical issues associated
with experimentation on human embryos, no further investigations on
clinical embryo transfer trials can be performed to prove the
conclusions of the present study.
To further investigate the involvement of ROCK in
blastomere development, the present study investigated the effect
of Y-27632 on single blastomeres. Of note, Y-27632 increased the
blastocyst formation ratio of single blastomeres in the discarded
human embryos. Taei et al found that, when combined with the
pluripotency-enhancing small molecule, CHIR99021, Y-27632 enhanced
the generation of hESCs from single blastomeres of fair and
poor-quality embryos (29). Other
studies have also demonstrated the roles of Y-27632 in pluripotent
cells, including iPS cells and hESCs. In addition, Y-27632 improves
the survival of dissociative hESCs and cryopreserved single hESCs.
It has been suggested that ROCK inhibition increases the survival
of hESCs as a result of increased cell-cell interactions, reduced
dissociation-induced apoptosis, maintenance of pluripotent
characteristics and enhanced cell propagation. In the present
study, the expression level of OCT3/4 express did not differ
significantly between the experimental and control groups.
Therefore, it was concluded that Y-27632 had no effect on
maintaining pluripotent characteristics during the development of
single blastomeres. Furthermore, the present study counted the
number of blastocysts, and found that Y-27632 had no effect on cell
propagation. However, the results of the experiment confirmed that
Y-27632 enhanced the expression of E-cadherin at the protein and
mRNA levels. Cell-cell connections were improved by Y-27632, which
may be one of mechanisms by which Y-27632 increases the blastocyst
formation ratio of single blastomeres. However, the mechanism is
complex and further investigations are required to clarify this.
However, based on the limited sample size in the present study, no
further experiments were performed to investigate the mechanism in
detail.
Notably, Y-27632 had different effects on the
cleavage-stage embryo and single blastomeres. Several previous
animal studies have demonstrated that Y-27632 has a negative effect
on embryonic development (12,17,26).
Y-27632 suppressed embryo cleavage, compaction and blastocyst
formation, and these results suggest that ROCK is involved in
polarization, cleavage and blastocoel cavity formation in early
cleavage-stage embryos. In the present study, the inhibition of
ROCK by Y-27632 resulted in delayed blastocyst formation. The
possible underlying mechanism may be that interblastomere adhesion
was enhanced by Y-27632, and different blastomeres with different
fates were tightly bound together, leading to low efficiency of
development. In contrast to the results with whole embryos, single
blastomeres cultured with Y-27632 produced more blastocysts. The
possible underlying mechanism for this many be that Y-27632
enhanced cell-cell adhesion and markedly reduced
dissociation-induced apoptosis. Y-27632 has already been shown to
permit the survival of dissociated pluripotent cells, including iPS
cells and hESCs. However, the different effects of Y-27632 on
cleavage-stage embryos and single blastomeres observed in the
present study, together with the results of previous studies,
indicate that ROCK has different roles in embryos of different
species, and at different developmental stages. The mechanism is
complex and more well-designed experiments are required for
clarification.
In conclusion, the present study demonstrated that
Y-27632 increased the rate of formation of blastocysts from single
human blastomeres, but delayed the formation of blastocysts from
discarded human embryos. Further studies are required to
investigate the various roles of ROCK inhibition on the development
of early human embryos and blastomeres, such as how this cytokine
may be interacting with additional regulatory molecules.
Acknowledgments
This study was supported by grants from the Key
Laboratory of Guangdong Province and the National Natural Science
Foundation of China (grant nos. 81100472 and 81270750).
References
|
1
|
Sutcliffe AG and Ludwig M: Outcome of
assisted reproduction. Lancet. 370:351–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantikou E, Youssef MA, van Wely M, van
der Veen F, Al-Inany HG, Repping S and Mastenbroek S: Embryo
culture media and IVF/ICSI success rates: a systematic review. Hum
Reprod Update. 19:210–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bontekoe S, Blake D, Heineman MJ, Williams
EC and Johnson N: Adherence compounds in embryo transfer media for
assisted reproductive technologies. Cochrane Database Syst Rev.
7:CD0074212010.PubMed/NCBI
|
|
4
|
Carney SK, Das S, Blake D, Farquhar C,
Seif MM and Nelson L: Assisted hatching on assisted conception (in
vitro fertilization (IVF) and intracytoplasmic sperm injection
(ICSI). Cochrane Database Syst Rev. 12:CD0018942012.
|
|
5
|
Bontekoe S, Mantikou E, van Wely M,
Seshadri S, Repping S and Mastenbroek S: Low oxygen concentrations
for embryo culture in assisted reproductive technologies. Cochrane
Database Syst Rev. 7:CD0089502012.PubMed/NCBI
|
|
6
|
Watson AJ and Barcroft LC: Regulation of
blastocyst formation. Front Biosci. 6:D708–D730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dard N, Breuer M, Maro B and Louvet-Vallée
S: Morphogenesis of the mammalian blastocyst. Mol Cell Endocrinol.
282:70–77. 2008. View Article : Google Scholar
|
|
8
|
Gumus E, Bulut HE and Kaloglu C:
Cytoskeletal changes in oocytes and early embryos during in vitro
fertilization process in mice. Anat Histol Embryol. 39:51–58. 2010.
View Article : Google Scholar
|
|
9
|
Rawe VY, Payne C and Schatten G: Profilin
and actin-related proteins regulate microfilament dynamics during
early mammalian embryogenesis. Hum Reprod. 21:1143–1153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishizaki T, Uehata M, Tamechika I, Keel J,
Nonomura K, Maekawa M and Narumiya S: Pharmacological properties of
Y-27632, a specific inhibitor of rho-associated kinases. Mol
Pharmacol. 57:976–983. 2000.PubMed/NCBI
|
|
11
|
Kawagishi R, Tahara M, Sawada K, Ikebuchi
Y, Morishige K, Sakata M, Tasaka K and Murata Y: Rho-kinase is
involved in mouse blastocyst cavity formation. Biochem Biophys Res
Commun. 319:643–648. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan X, Chen KL, Zhang Y, Cui XS, Kim NH
and Sun SC: ROCK inhibition prevents early mouse embryo
development. Histochem Cell Biol. 142:227–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hochi S, Abdalla H, Hara H, Shimoda M,
Morita H, Kuwayama M and Hirabayashi M: Stimulatory effect of
Rho-associated coiled-coil kinase (ROCK) inhibitor on revivability
of in vitro-produced bovine blastocysts after vitrification.
Theriogenology. 73:1139–1145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cortes JL, Sanchez L, Ligero G,
Gutierrez-Aranda I, Catalina P, Elosua C, Leone PE, Montes R, Bueno
C, Ramos-Mejía V, et al: Mesenchymal stem cells facilitate the
derivation of human embryonic stem cells from cryopreserved
poor-quality embryos. Hum Reprod. 24:1844–1851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clayton L, Hall A and Johnson MH: A role
for Rho-like GTPases in the polarisation of mouse eight-cell
blastomeres. Dev Biol. 205:322–331. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koike S, Keino-Masu K and Masu M:
Deficiency of autotaxin/lysophospholipase D results in head cavity
formation in mouse embryos through the LPA receptor-Rho-ROCK
pathway. Biochem Biophys Res Commun. 400:66–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laeno AM, Tamashiro DA and Alarcon VB:
Rho-associated kinase activity is required for proper morphogenesis
of the inner cell mass in the mouse blastocyst. Biol Reprod.
89:1222013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geens M, Mateizel I, Sermon K, De Rycke M,
Spits C, Cauffman G, Devroey P, Tournaye H, Liebaers I and Van de
Velde H: Human embryonic stem cell lines derived from single
blastomeres of two 4-cell stage embryos. Hum Reprod. 24:2709–2717.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van de Velde H, Cauffman G, Tournaye H,
Devroey P and Liebaers I: The four blastomeres of a 4-cell stage
human embryo are able to develop individually into blastocysts with
inner cell mass and trophectoderm. Hum Reprod. 23:1742–1747. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe K, Ueno M, Kamiya D, Nishiyama A,
Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S,
Muguruma K and Sasai Y: A ROCK inhibitor permits survival of
dissociated human embryonic stem cells. Nat Biotechnol. 25:681–686.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pakzad M, Totonchi M, Taei A, Seifinejad
A, Hassani SN and Baharvand H: Presence of a ROCK inhibitor in
extracellular matrix supports more undifferentiated growth of
feeder-free human embryonic and induced pluripotent stem cells upon
passaging. Stem Cell Rev. 6:96–107. 2010. View Article : Google Scholar
|
|
22
|
Rizzino A: Stimulating progress in
regenerative medicine: Improving the cloning and recovery of
cryopreserved human pluripotent stem cells with ROCK inhibitors.
Regen Med. 5:799–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang P, Wu X, Hu C, Wang P and Li X: Rho
kinase inhibitor Y-27632 and Accutase dramatically increase mouse
embryonic stem cell derivation. In Vitro Cell Dev Biol Anim.
48:30–36. 2012. View Article : Google Scholar
|
|
24
|
Horiguchi A, Yazaki K, Aoyagi M, Ohnuki Y
and Kurosawa H: Effective Rho-associated protein kinase inhibitor
treatment to dissociate human iPS cells for suspension culture to
form embryoid body-like cell aggregates. J Biosci Bioeng.
118:588–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sivasubramaiyan K, Totey S, Bhat V, Totey
SM and Deb K: Y-27632 enhances differentiation of blastocyst like
cystic human embryoid bodies to endocrinologically active
trophoblast cells on a biomimetic platform. J Biomed Sci.
16:882009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Duan X, Xiong B, Cui XS, Kim NH,
Rui R and Sun SC: ROCK inhibitor Y-27632 prevents porcine oocyte
maturation. Theriogenology. 82:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steer CV, Mills CL, Tan SL, Campbell S and
Edwards RG: The cumulative embryo score: A predictive embryo
scoring technique to select the optimal number of embryos to
transfer in an in-vitro fertilization and embryo transfer
programme. Hum Reprod. 7:117–119. 1992.PubMed/NCBI
|
|
28
|
Gardner DK, Lane M and Schoolcraft WB:
Culture and transfer of viable blastocysts: A feasible proposition
for human IVF. Hum Reprod. 15(Suppl 6): S9–S23. 2000.
|
|
29
|
Taei A, Hassani SN, Eftekhari-Yazdi P,
Rezazadeh Valojerdi M, Nokhbatolfoghahai M, Masoudi NS, Pakzad M,
Gourabi H and Baharvand H: Enhanced generation of human embryonic
stem cells from single blastomeres of fair and poor-quality
cleavage embryos via inhibition of glycogen synthase kinase β and
Rho-associated kinase signaling. Hum Reprod. 28:2661–2671. 2013.
View Article : Google Scholar : PubMed/NCBI
|