Introduction
Ovarian cancer is the second most common
gynecological malignancy and the fifth most frequent cause of
cancer-associated mortality in females worldwide, with >2
million new cases and 1 million mortalities in 2013 (1,2). At
present, surgery followed by chemotherapy is the current standard
treatment for localized ovarian cancer. However, the highly
invasive property and difficulty of detection of early-stage
ovarian cancer leads to a poor prognosis (3). As a result, only 40% of patients in
all stages survive and 70–80% of patients with distant metastases
succumb to the disease within 5 years of diagnosis (4,5).
Thus, the therapeutic options for metastatic ovarian cancer remain
limited. Therefore, there is an urgent need to elucidate the
molecular mechanisms and to identify novel agents for ovarian
cancer patients to obtain an improved treatment outcome.
Increasing evidence has demonstrated the pivotal
role of the extracellular matrix (ECM) and matrix
metalloproteinases (MMPs) in cancer invasion and metastasis
(6,7). As a family of structurally conserved,
zinc-dependent endopeptidases, the MMPs are important in
proteolysis of the ECM. Among them, MMP-2/9 can degrade components
of the ECM and thus eliminate the barrier that restrains cancer
invasion (8,9). Mitogen-activated protein kinase
(MAPK) family members, including extracellular signal-regulated
kinase (ERK), p38 and c-Jun N-terminal kinase (JNK), have been
demonstrated to be crucial in mediating MMP2/9 production (10,11).
In previous years, natural products have obtained
increasing attention as new anti-tumor therapeutic drugs due to
their relatively few side effects (12). Among them, nitidine chloride (NC;
Fig. 1A) is a natural bioactive
phytochemical alkaloid derived from the root of Zanthoxylum
nitidum (Roxb). Previous studies have demonstrated that NC has
anti-inflammatory, anti-oxidant, anti-fungal and anti-HIV functions
(13,14). NC has been demonstrated to have
anti-tumor activity in different types of cancer. NC can induce
apoptosis and inhibit the growth and metastasis of renal cancer
(15,16). NC was also found to suppress the
proliferation of hepatocellular carcinoma (17). Another study demonstrated that NC
is able to inhibit the growth of gastric cancer via the signal
transducer and activator of transcription 3 signaling pathway
(18). In addition, NC is able to
suppress the growth of breast cancer and inhibit its metastasis
through the c-Src/focal adhesion kinase pathway (19,20).
However, to the best of our knowledge, no studies have investigated
whether NC has a direct effect on ovarian cancer migration and
invasion or the mechanisms of this effect.
 | Figure 1Chemical structure of NC and the
effect of NC on A2780 ovarian cancer cell proliferation. (A)
Chemical structure of NC. (B) Following treatment with NC for 24 h
at different concentrations (0, 1, 2.5, 5 and 10 µM), the
viability of A2780 cells was determined by an MTT assay. (C)
Following treatment with 0, 5 and 10 µM NC for different
time periods (0, 6, 12, 24 and 48 h), an MTT assay was used to
detect the viability of A2780 cells. The results are presented as
the mean ± standard deviation from three independent experiments.
*P<0.05, **P<0.01 vs. the control group
(0 µM NC). NC, nitidine chloride; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide. |
In the present study, the effects of NC on ovarian
cancer cell migration and invasion, as well as the underlying
molecular mechanisms were evaluated.
Materials and methods
Cell lines and reagents
The human A2780 ovarian cancer cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cells were routinely cultured in RPMI-1640 containing 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin (Macgene Biotechnology Ltd., Beijing, China) in 5%
CO2 at 37°C. Monoclonal rabbit anti-human ERK1/2 (cat.
no. 4695; 1:1,000 dilution), monoclonal rabbit anti-human
phospho-ERK1/2 (cat. no. 4370; 1:1,000 dilution), monoclonal rabbit
anti-human p38 (cat. no. 8690; 1:1000 dilution), monoclonal rabbit
anti-human phospho-p38 (cat. no. 4631; 1:1,000 dilution),
monoclonal rabbit anti-human JNK (cat. no. 9252; 1:1,000 dilution),
monoclonal rabbit anti-human phospho-JNK (cat. no. 4668; 1:1,000
dilution), monoclonal rabbit anti-human MMP-2 (cat. no. 13132;
1:1,000 dilution) and monoclonal rabbit anti-human MMP-9 (cat. no.
13667; 1:1,000 dilution) antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). NC was purchased
from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China) and
dissolved in dimethyl sulfoxide (DMSO). U0126, a selective
inhibitor of ERK, was purchased from Sigma-Aldrich (St. Louis, MO,
USA).
MTT assay of cell viability and
proliferation
Cell viability and proliferation were assessed using
a 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT; Beyotime Institute of Biotechnology, Haimen, China) assay.
A2780 cells (5,000 cells/well) in 100 µl medium were seeded
into 96-well plates. Following stimulation with NC of various
concentrations (0, 1, 2.5, 5 and 10 µM) for various times
(0, 6, 12, 24 and 48 h), 20 µl MTT (5 mg/ml) was added into
each well. Following incubation for 4 h at 37°C, 100 µl of
DMSO was added to each well for another 15 min. Finally, the
absorbance values were determined using a microplate luminometer
(iMark; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm.
A2780 cells stimulated with 0 µM NC at various time points
(0, 6, 12, 24 and 48 h) were used as the control group.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A2780 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and then reverse transcribed to cDNA using a
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
qPCR analysis was performed using a LightCycler (Bio-Rad
Laboratories Inc.). The primer sequences used for qPCR were as
follows: MMP-2, forward: 5′-TTG ATG GCA TCG CTC AGA TC-3′ and
reverse: 5′-TTG TCA CGT GGC GTC ACA GT-3′; MMP-9, forward: 5′-GAC
GCA GAC ATC GTC ATC CA-3′ and reverse: 5′-CAC AAC TCG TCA TCG TCG
AAA-3′. The cycling conditions were as follows: 95°C for 10 sec,
followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and a
final extension at 72°C for 3 min. SYBR Green I was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.) Melting curves were
assessed to confirm the specificity of the products generated for
each set of primers. Following amplification, the ΔΔCq comparative
method was then used to normalize the relative levels of gene
expression to GAPDH (21).
Experiments were performed in triplicate.
Western blot analysis
Following treatment, the cells were harvested and
then the protein was extracted using protein lysis buffer (Beyotime
Institute of Biotechnology). Centrifugation was performed at 10,000
× g for 15 min at 4°C. Total cell protein concentrations were
determined using the bicinchoninic acid protein assay kit (Pierce
Biotechnology Inc., Rockford, IL, USA). Equal protein from cell
lysates was loaded onto 12% SDS-PAGE gels (Bio-Rad Laboratories,
Inc.). Following electrophoresis, proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA) and then blocked with 5% fat-free milk at room temperature
for 1 h, and incubated with the indicated primary antibodies
overnight at 4°C. Subsequently, the membranes were washed with
Tris-buffered saline and Tween 20 and incubated with polyclonal
goat anti-rabbit horseradish peroxidase-conjugated IgG (cat. no.
ZDR-5306; 1:10,000 dilution; ZSGB-BIO, Beijing, China) secondary
antibody for 1 h at room temperature. Immune complexes were
detected with enhanced chemiluminescence reagents and the blots
were quantified by densitometric analysis using the Alpha Imager
2200 (Alpha Innotech Corp., Santa Clara, CA, USA). Reactions were
performed once per PVDF membrane.
Scratch wound healing assay
A scratch wound healing assay was used to assess the
migration ability of the A2780 cells. In brief, the A2780 cells
(1×106/well) were seeded in 6-well plates and cultured
with RPMI-1640 supplemented with 10% FBS. When reaching confluency,
each well was scratched with a 200 µl pipette tip. To assess
the effects of NC on the migration of A2780 cells, 5 µM NC
was added to the plates. After 24 h of incubation, images of the
wound healing areas were captured and then the distance between two
cell edges was analyzed by ImageJ software (version 1.48; National
Institutes of Health, Bethesda, MD, USA).
In vitro invasion assay
To evaluate the effect of NC on the invasive ability
of A2780 cells, the transwell system was used. A2780 cells were
cultured in Boyden chambers, with 8-µm pore filter inserts,
in 24-well plates (Corning Life Sciences, Corning, NY, USA). The
pore inserts were pre-coated with Matrigel (BD Biosciences, San
Jose, CA, USA) overnight. A2780 cells (1×105 cells/well)
were suspended in 100 µl RPMI-1640 supplemented with 1% FBS
and added to the upper chamber. RPMI-1640 with 10% FBS and 5
µM NC were added to the lower chamber. The cells on the top
of the membrane were gently scraped using a swab. After 24 h of
incubation at 37°C, the cells attaching to the lower surface were
fixed with methanol and stained with 0.1% crystal violet (Beyotime
Institute of Biotechnology) for 15 min at room temperature. A total
of five random high-power fields (magnification, ×200; Nikon E100;
Nikon Corp, Tokyo, Japan) of each sample were selected and counted
to evaluate the average number of invasive cells.
In vitro migration assay
For the migration assay, cells were added to upper
chambers without coated Matrigel. The assay was performed as
described above for the invasion assay. The cells on the lower
surface were also counted in five randomly selected fields and then
the cell number was analyzed statistically.
Statistical analysis
The data are expressed as the mean ± standard
deviation. All experiments were repeated at least three times.
Comparisons among values for all groups were performed by one-way
analysis of variance. Holm's t-test was used for analysis of
differences between different groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
NC inhibits the cell viability of ovarian
cancer cells
The structure of NC is shown in Fig. 1A. To determine the specific role of
NC on the cell viability of A2780 cells, an MTT assay was used as
described in Materials and methods. As shown in Fig. 1B, various concentrations (0, 1,
2.5, 5 and 10 µM) of NC were added to the cultured A2780
cells. At a dose of 10 µM, NC significantly inhibited the
viability of A2780 cells after 24 h incubation. However, at
concentrations <10 µM (1, 2.5 and 5 µM), the
inhibitory effect was not significant. Furthermore, 5 and 10
µM NC were selected to stimulate the cells at different time
points (0, 6, 12, 24 and 48 h). As shown in Fig. 1C, after 24 h stimulation, NC
significantly inhibited the cell viability at a dose of 10
µM, while 5 µM NC did not significantly inhibit cell
viability. However, after 48 h stimulation, 5 and 10 µM NC
significantly inhibited cell viability. As a result, 5 µM NC
for 24 h was selected in the subsequent migration and invasion
experiments in order to exclude the effect of cell viability.
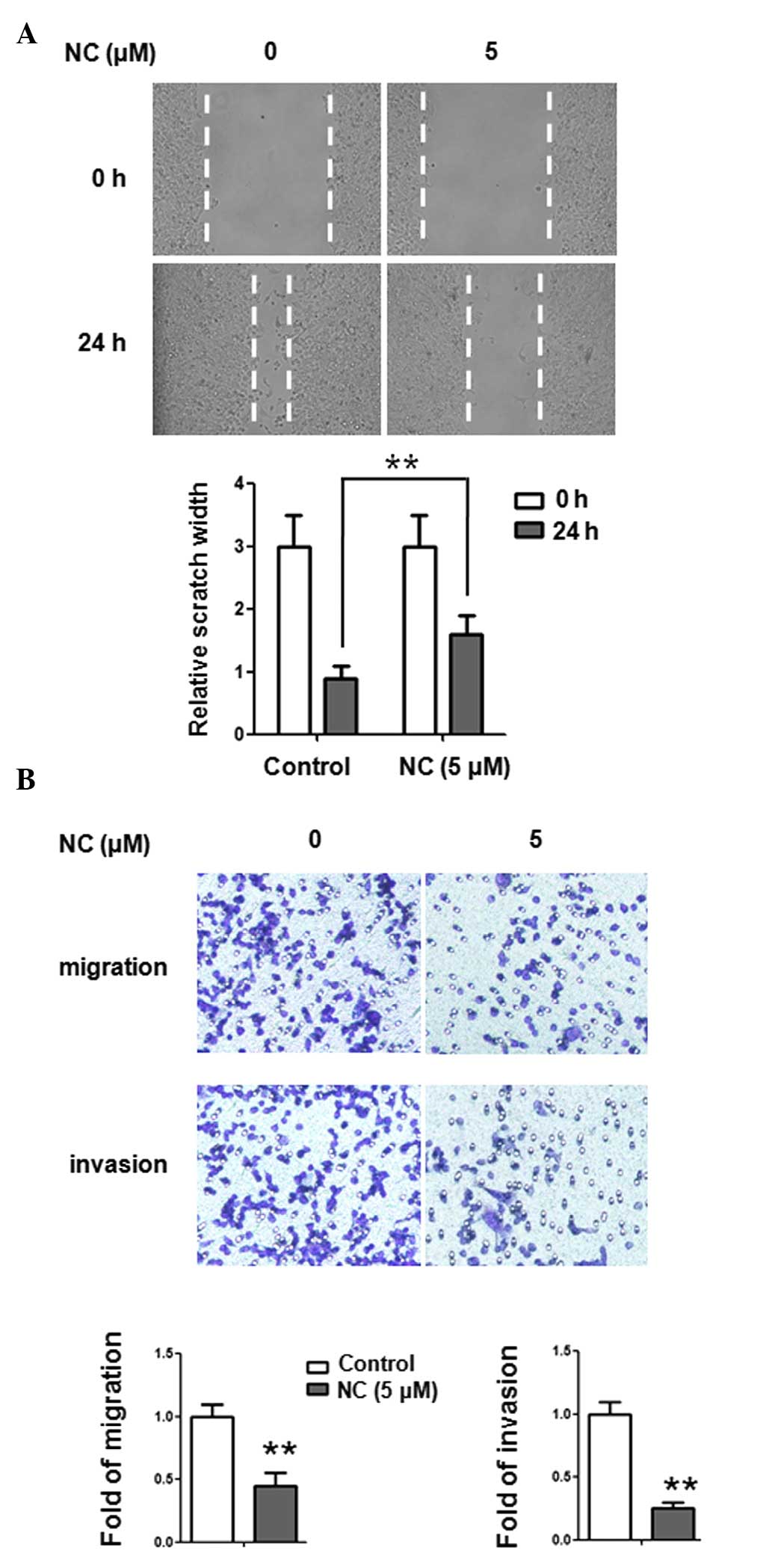
NC inhibits the migration and invasion of
ovarian cancer cells
The wound healing assay was used to investigate the
effect of NC on the migration of ovarian cancer cells. A2780
ovarian cancer cells were treated with NC at a concentration of 5
µM for 24 h. As shown in Fig.
2A, migration of A2780 cells was inhibited by NC (5 µM).
The results of the wound healing assay demonstrated that healing
over the scratch was significantly deceased following treatment
with NC. In order to further examine the effect of NC on cell
migration and invasion, a Transwell assay was performed. A2780
cells were treated with NC at a concentration of 5 µM for 24
h. As shown in Fig. 2B, the
migration and invasion of A2780 cells were significantly inhibited
by NC (5 µM). The results of the Transwell assay indicated
that NC could inhibit the migratory and invasive ability of ovarian
cancer cells.
NC downregulates the expression of
MMP-2/9 in ovarian cancer cells
Numerous evidence has supported that MMPs,
particularly MMP-2 and MMP-9, are important in the process of
cancer cell migration and invasion (22,23).
The present study thus determined the effect of NC on MMP-2 and
MMP-9 expression in A2780 ovarian cancer cells. Various
concentrations of NC (0, 2.5, 5 and 10 µM) were added to the
A2780 cells, respectively, and the cells were then cultured for
another 24 h. As shown in Fig. 3A,
NC treatment significantly decreased the expression of MMP-2 and
MMP-9 in A2780 cells at a concentration of 5 and 10 µM. The
A2780 cells were then stimulated with 5 µM NC for different
time periods (0, 6, 12 and 24 h). As shown in Fig. 3B, following stimulation with 5
µM NC for 6 h, the expression of MMP-2 and MMP-9 was not
altered. However, when the stimulating time was extended to 12 and
24 h, the expression of MMP-2 and MMP-9 was significantly decreased
compared with the control group. In addition, identical treatments
were performed and qPCR was applied to detect the mRNA levels of
MMP-2 and MMP-9. As shown in Fig.
3C, the relative mRNA expression of MMP-2 and MMP-9 was
significantly decreased when treated with 5 and 10 µM NC.
Similar to the protein level, it also decreased gradually following
stimulation with 5 µM for 6, 12 and 24 h (Fig. 3D). The results above suggest that
NC inhibited the production of MMP-2 and MMP-9 in a dose- and
time-dependent manner at the mRNA and protein levels. The results
above also indicate that the inhibitory effect of NC on A2780 cell
invasion and migration may be associated with MMP-2 and MMP-9
production.
ERK1/2 signaling pathway mediates
inhibition of MMP-2/9 production following treatment with NC
The MAPK signaling pathway is important in the
development of various types of cancer. Inhibition of MAPK
activation has been demonstrated to suppress tumor cell migration
and invasion (24). To determine
the association between NC and the production of MMP-2 and MMP-9,
the relative expression of three members of the MAPK family,
including phospho-JNK, phospho-p38 and phospho-ERK1/2 were
investigated following treatment of A2780 cells with 5 µM NC
for 0, 5, 15, 30 and 60 min. As shown in Fig. 4A and B, the relative expression of
phospho-ERK1/2 was significantly downregulated compared with the
control group. The activity of phospho-ERK1/2 rapidly decreased
after 5–30 min stimulation and gradually increased after 1 h
stimulation. However, the expression of phospho-JNK and phospho-p38
was not altered. The results above suggested that the ERK pathway
may be involved in MMP-2 and MMP-9 production of ovarian cancer
cells.
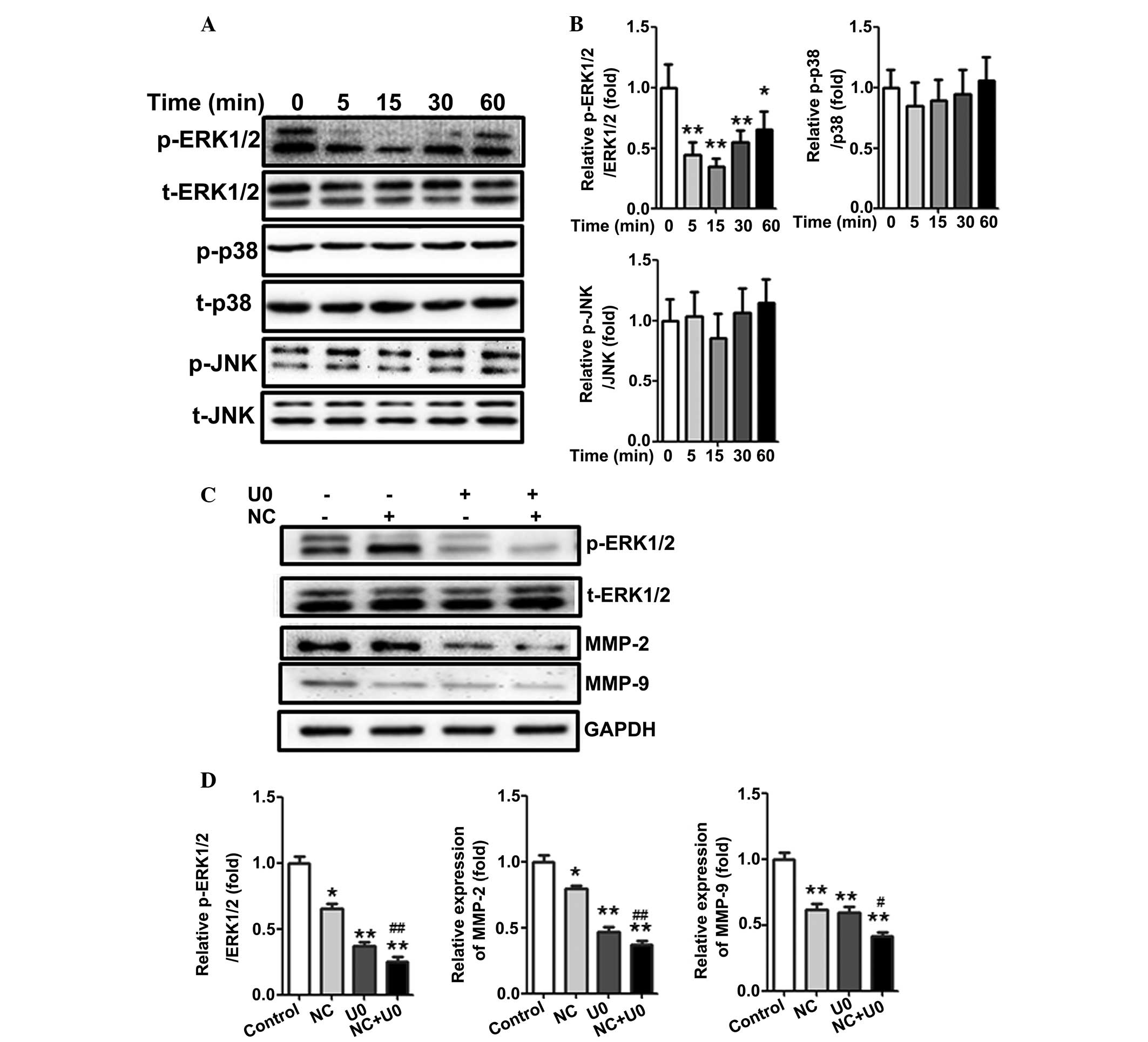 | Figure 4ERK1/2, but not p38 or JNK, regulates
NC-mediated production of MMP-2 and MMP-9 in A2780 ovarian cancer
cells. (A) A2780 cells were stimulated with 5 µM NC for
different time periods (0, 5, 15, 30 and 60 min) and the levels of
p-ERK1/2, ERK1/2, p-p38, p38, p-JNK and JNK were analyzed by
western blotting. (B) Statistical analysis of the western blotting
results. *P<0.05, **P<0.01 vs. the
control group. (C) A2780 cells were treated with NC (5 µM)
for 24 h and U0126 (10 µM) was added to A2780 cells 1 h
prior to NC treatment. The levels of p-ERK1/2, ERK1/2, MMP-2 and
MMP-9 were analyzed by western blot analysis with GAPDH as a
control. (D) Statistical analysis of the western blotting results.
*P<0.05, **P<0.01 vs. the control
group; #P<0.05, ##P<0.01, compared with the cells
treated with NC. Data are presented as the mean ± standard
deviation from three independent experiments. NC, nitidine
chloride; MMP, matrix metalloproteinase; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; t-, total. |
To further investigate whether the effect of NC is
ERK-dependent, U0126 (ERK inhibitor) was applied to inhibit ERK
activation. As shown in Fig. 4C and
D, inhibition of ERK activity with U0126 could further
downregulate the expression of MMP-2 and MMP-9 compared with NC
treatment. These results further demonstrated that NC-inhibited
MMP-2 and MMP-9 production may be ERK dependent.
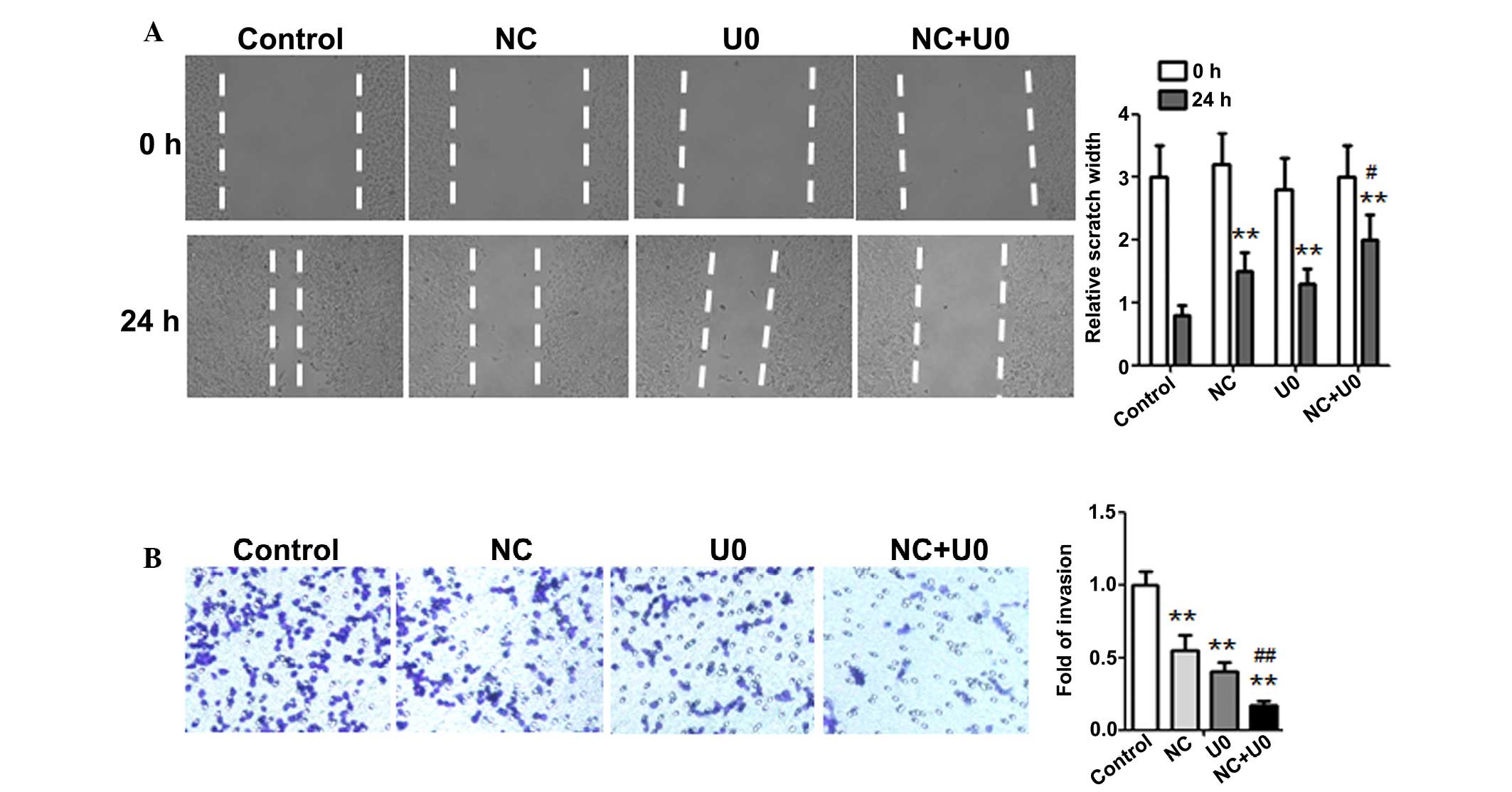
Inhibition of the ERK pathway enhanced
the anti-metastatic effect of NC in ovarian cancer cells
To further investigate whether the anti-metastatic
effect of NC was attributed to ERK signaling suppression, the
effect of U0126 on A2780 cell migration in the presence or absence
of NC was examined. As shown in Fig.
5A, the results of the wound healing assay demonstrated that
the NC-induced inhibition of wound healing was significantly
enhanced by using an ERK inhibitor. Similar results were observed
using the Transwell assay (Fig.
5B), which also demonstrated that inhibition of ERK activation
could significantly enhance the NC-induced inhibition of ovarian
cancer cell invasion. These results above support the theory that
suppression of metastasis by NC is regulated by inhibition of
MMP-2/9 production though the ERK signaling pathway in ovarian
cancer cells.
Discussion
Metastasis is considered the primary cause of
mortality in the majority of cancer patients and is one of the most
crucial issues in cancer research. Therefore, it is important to
examine the molecular mechanisms of metastasis and to identify
effective agents to inhibit cancer metastasis. However, traditional
chemotherapeutics are restricted in long-term application due to
their toxicity and side effects. There has been increasing
attention on natural drugs in tumor therapy with fewer side effects
(25,26). Accumulating evidence has
demonstrated that NC possesses the capacity to inhibit the
proliferation, migration and invasion and/or induce the apoptosis
of breast, renal, gastric and hepatocellular cancer cells (15–20).
Although previous studies have revealed the anti-tumor efficacy of
NC, whether NC has any effect on ovarian cancer cell migration and
invasion, as well as the detailed molecular mechanisms have not
been elucidated. The present study initially evaluated the effect
of NC on the viability of cultured ovarian cancer cells and the
results revealed that 5 µM NC significantly inhibited cell
proliferation after 48 h of incubation, while no difference was
observed before 24 h. As a result, 24 h was selected as the
stimulation time period and 5 µM as the stimulation
concentration for the following experiments involving cell
migration and invasion, in order to exclude the effect of
proliferation. To the best of our knowledge, the present study
demonstrated for the first time that NC could effectively inhibit
ovarian cancer cell migration and invasion. Furthermore, NC
inhibited ovarian cancer cell migration and invasion by suppressing
MMP-2 and MMP-9 production via the ERK signaling pathway. The
present study provided a novel molecular mechanism explaining how
NC exhibits its anti-tumor effect on ovarian cancer cells.
The metastasis and invasion of ovarian cancer is a
complicated and multi-step process that is mediated by numerous
factors. MMPs are proteinases that are crucial for malignant cells
in the proteolytic degradation of the basement membrane and ECM, in
order for malignant cells to migrate and invade into surrounding
tissues (22,23). Among these, the role of MMP-2 and
MMP-9 has been underlined; their type IV collagenase activity is
essential during the initial period of metastasis and invasion
(8,9). Increased expression of MMP-2 and
MMP-9 was associated with a poor prognosis in ovarian cancer
patients (27,28). The suppressive role of NC on the
migration and invasion of A2780 ovarian cancer cells was then
evaluated via scratch wound healing and Transwell assays.
Furthermore, the decreased expression of MMP-2 and MMP-9 observed
following NC treatment was associated with the inhibition of
migration and invasion in A2780 ovarian cancer cells. Therefore,
the finding that NC downregulated the expression of MMP-2 and
MMP-9, may show a potential association between the inhibition of
metastasis by NC and MMP activity.
Several studies have reported that MAPK family
members, including ERK, p38 and JNK, can lead to MMP2/9 production
and potentially promote the proliferation and metastasis of tumors
(10,11,29).
As one of the crucial signal transduction pathways, the ERK
signaling pathway is involved in the regulation of proliferation,
apoptosis, migration and invasion of cancer cells (30–32).
In the present study, the ERK activity in ovarian cancer cells was
assessed by western blot analysis. The results demonstrated that
the expression of phospho-ERK was downregulated following treatment
with NC. To verify that the ERK signaling pathway was involved in
the inhibition of ovarian cancer cell migration and invasion by NC,
ERK activity was inhibited by applying the ERK inhibitor U0126. It
was found that suppression of ERK phosphorylation by U0126 enhanced
MMP2/9 downregulation induced by NC. In addition, inhibition of ERK
activity further inhibited cell migration and invasion. These
results suggested that NC suppressed ovarian cancer cell migration
and invasion through inhibiting ERK activity and decreasing the
production of MMP-2/9. The results demonstrated that applying the
ERK inhibitor U0126 enhanced NC-induced downregulation of MMP-2/9,
further demonstrating that ERK was upstream of MMP-2/9.
In conclusion, to the best of our knowledge, the
present study indicated for the first time that NC inhibited the
migration and invasion of ovarian cancer cells by suppressing
MMP-2/9 production. Furthermore, the effect of NC was regulated by
the ERK signaling pathway. Therefore, our findings suggested that
NC is a possible drug for ovarian cancer therapy. However, further
in vivo research is required.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun ZL, Tang YJ, Wu WG, Xing J, He YF, Xin
DM, Yu YL, Yang Y and Han P: AZD1480 can inhibit the biological
behavior of ovarian cancer SKOV3 cells in vitro. Asian Pac J Cancer
Prev. 14:4823–4827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stallings-Mann M and Radisky D: Matrix
metalloproteinase-induced malignancy in mammary epithelial cells.
Cells Tissues Organs. 185:104–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liotta LA and Stetler-Stevenson WG:
Metalloproteinases and cancer invasion. Semin Cancer Biol.
1:99–106. 1990.PubMed/NCBI
|
|
9
|
Himelstein BP, Canete-Soler R, Bernhard
EJ, Dilks DW and Muschel RJ: Metalloproteinases in tumor
progression: The contribution of MMP-9. Invasion Metastasis.
14:246–258. 1994.PubMed/NCBI
|
|
10
|
Kim BS, Park JY, Kang HJ, Kim HJ and Lee
J: Fucoidan/FGF-2 induces angiogenesis through JNK- and
p38-mediated activation of AKT/MMP-2 signalling. Biochem Biophys
Res Commun. 450:1333–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin YJ, Park I, Hong IK, Byun HJ, Choi J,
Kim YM and Lee H: Fibronectin and vitronectin induce AP-1-mediated
matrix metal-loproteinase-9 expression through integrin alpha
α(5)β(1)/α(v) β(3)-dependent Akt, ERK and JNK signaling pathways in
human umbilical vein endothelial cells. Cell Signal. 23:125–134.
2011. View Article : Google Scholar
|
|
12
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Jiang W, Zhang Z, Qian M and Du B:
Nitidine chloride inhibits LPS-induced inflammatory cytokines
production via MAPK and NF-kappab pathway in RAW 264.7 cells. J
Ethnopharmacol. 144:145–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Del Poeta M, Chen SF, Von Hoff D, Dykstra
CC, Wani MC, Manikumar G, Heitman J, Wall ME and Perfect JR:
Comparison of in vitro activities of camptothecin and nitidine
derivatives against fungal and cancer cells. Antimicrob Agents
Chemother. 43:2862–2868. 1999.PubMed/NCBI
|
|
15
|
Fang Z, Tang Y, Jiao W, Xing Z, Guo Z,
Wang W, Xu Z and Liu Z: Nitidine chloride induces apoptosis and
inhibits tumor cell proliferation via suppressing ERK signaling
pathway in renal cancer. Food Chem Toxicol. 66:210–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang Z, Tang Y, Jiao W, Xing Z, Guo Z,
Wang W, Shi B, Xu Z and Liu Z: Nitidine chloride inhibits renal
cancer cell metastasis via suppressing AKT signaling pathway. Food
Chem Toxicol. 60:246–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao J, Xu T, Zheng JX, Lin JM, Cai QY, Yu
DB and Peng J: Nitidine chloride inhibits hepatocellular carcinoma
cell growth in vivo through the suppression of the JAK1/STAT3
signaling pathway. Int J Mol Med. 32:79–84. 2013.PubMed/NCBI
|
|
18
|
Chen J, Wang J, Lin L, He L, Wu Y, Zhang
L, Yi Z, Chen Y, Pang X and Liu M: Inhibition of STAT3 signaling
pathway by nitidine chloride suppressed the angiogenesis and growth
of human gastric cancer. Mol Cancer Ther. 11:277–287. 2012.
View Article : Google Scholar
|
|
19
|
Pan X, Han H, Wang L, Yang L, Li R, Li Z,
Liu J, Zhao Q, Qian M, Liu M and Du B: Nitidine Chloride inhibits
breast cancer cells migration and invasion by suppressing c-Src/FAK
associated signaling pathway. Cancer Lett. 313:181–191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun M, Zhang N, Wang X, Cai C, Cun J, Li
Y, Lv S and Yang Q: Nitidine chloride induces apoptosis, cell cycle
arrest, and synergistic cytotoxicity with doxorubicin in breast
cancer cells. Tumour Biol. 35:10201–10212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Li R, Meng Y, Li S, Donelan W,
Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, et al: Irisin
stimulates browning of white adipocytes through mitogen-activated
protein kinase p38 MAP kinase and ERK MAP kinase signaling.
Diabetes. 63:514–525. 2014. View Article : Google Scholar
|
|
22
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
23
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
24
|
Rajoria S, Suriano R, Wilson YL, Schantz
SP, Moscatello A, Geliebter J and Tiwari RK: 3,3′-diindolylmethane
inhibits migration and invasion of human cancer cells through
combined suppression of ERK and AKT pathways. Oncol Rep.
25:491–497. 2011.
|
|
25
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic
phytochemicals-promising cancer chemopreventive agents in humans? A
review of their clinical properties. Int J Cancer. 120:451–458.
2007. View Article : Google Scholar
|
|
27
|
Li LN, Zhou X, Gu Y and Yan J: Prognostic
value of MMP-9 in ovarian cancer: A meta-analysis. Asian Pac J
Cancer Prev. 14:4107–4113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu Z, Xu S, Xu Y, Ma J, Li J and Xu P: The
expression of tumor-derived and stromal-derived matrix
metalloproteinase 2 predicted prognosis of ovarian cancer. Int J
Gynecol Cancer. 25:356–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thompson N and Lyons J: Recent progress in
targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug
discovery. Curr Opin Pharmacol. 5:350–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gendron S, Couture J and Aoudjit F:
Integrin alpha2beta1 inhibits Fas-mediated apoptosis in T
lymphocytes by protein phosphatase 2A-dependent activation of the
MAPK/ERK pathway. J Biol Chem. 278:48633–48643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shelton JG, Steelman LS, White ER and
McCubrey JA: Synergy between PI3K/Akt and Raf/MEK/ERK pathways in
IGF-1R mediated cell cycle progression and prevention of apoptosis
in hematopoietic cells. Cell Cycle. 3:372–379. 2004.PubMed/NCBI
|
|
32
|
Ono H, Basson MD and Ito H: PTK6 promotes
cancer migration and invasion in pancreatic cancer cells dependent
on ERK signaling. PloS One. 9:e960602014. View Article : Google Scholar : PubMed/NCBI
|