Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) are life-threatening diseases that present
with progressive dyspnea, refractory hypoxemia and a marked
increase in difficulties breathing (1,2).
Since the initial description of ARDS in 1967 (3), there have been advances in the
pathogenesis and treatment of this disease, however, challenges
remain. Epidemiological investigations suggest that ARDS remains a
significant health burden with substantial morbidity and mortality
(4). The majority of patients
require endotracheal intubation and positive pressure ventilation
(1,2) and account for millions of days spent
in intensive care units (5). In
recent years, treatments of ALI/ARDS with improved efficacy have
been sought, and studies have indicated that liquid ventilation
with perfluorocarbon (PFC) compounds is a promising therapeutic
approach (6–14). PFC is of interest for the treatment
of ALI/ARDS due to the unique physicochemical properties, such as
high solubility for oxygen, and the anti-inflammatory effects.
Liquid ventilation or aerosolized PFC has been demonstrated to
improve gas exchange and improve pulmonary compliance compared with
conventional mechanical ventilation in a variety of animal models
of ALI/ARDS (6–8). Specifically, PFC has been shown to
reduce levels of cytokines, chemokines and other mediators of
pulmonary inflammation in both in vivo and in vitro
models (9–14). However, the understanding of the
underlying mechanisms remains poor, in particular of the mechanisms
associated with the PFC-induced anti-inflammatory effects. A
limited number of mechanistic studies have indicated that PFC can
reduce the activation of nuclear factor-κB and/or the
Syk-phosphorylation pathway (15–17).
Considering that ARDS is a complex inflammatory disease, PFC may
serve an anti-inflammatory role through multiple mechanisms.
In the pathogenesis of ARDS, the early inflammatory
responses, including the expression of adhesion molecules and
cytokines in the lung with subsequent activation of neutrophils,
serve a pivotal role. Intercellular adhesion molecule-1 (ICAM-1) is
a cell surface glycoprotein that is expressed on alveolar
epithelial cells and vascular endothelium (18,19).
Injury to the alveolar epithelium and vascular endothelium is of
central importance in the pathogenesis of ALI/ARDS (20), and in lung injury, the expression
of ICAM-1 in lung tissue was increased which is thought to serve an
important role in neutrophil recruitment and trafficking into the
lung (21). ICAM-1 may be a useful
biomarker of ALI/ARDS. Clinical trials have demonstrated increased
ICAM-1 expression in the setting of acute lung injury (22,23),
and plasma and edema fluid levels of ICAM-1 are higher in patients
with ALI compared with patients with hydrostatic pulmonary edema.
Additionally, elevated plasma levels of ICAM-1 were associated with
poor outcomes in patients with acute lung injury (24,25).
In the present study, the effects of PFC on the expression of
ICAM-1 were investigated in an in vitro model of ARDS.
The anti-inflammatory mechanism of action of PFC
remains unclear. In recent years, the role of microRNAs (miRNAs) in
inflammation have received increased interest. miRNAa are
non-coding RNA molecules of approximately 22 nucleotides, which
modulate gene expression at the post-transcriptional level in
eukaryotic organisms. miRNAs control gene expression by pairing
with partially complementary target sites in mRNA 3′ untranslated
regions (UTRs), resulting in translational repression and/or mRNA
destabilization (26–28). With miRNA roles in developmental
timing, cell apoptosis and cell proliferation, evidence is mounting
that their regulatory effect is more prevalent than was previously
suspected (26). Recent studies
have indicated that miRNAs regulate inflammatory responses
(29), and serve critical roles in
inflammatory lung diseases including ALI/ARDS. Given their
particularly recognized role in the regulation of immune and
inflammatory responses, the present study hypothesized that PFC
attenuates the expression of ICAM-1 in injured alveolar epithelial
cells, a potential anti-inflammatory mechanism of PFC, through the
influence of one or a number of miRNAs. A previous study has
indicated that ICAM-1 was a target of tumor necrosis factor
(TNF)-induced miR-17-3p; with specific antagonism of miR-17-3p
increasing neutrophil adhesion to cultured endothelial cells.
Conversely, transfection with mimics of miR-17-3p reduced
neutrophil adhesion to endothelial cells (30). Therefore, the present study
speculates that miR-17-3p may serve a key role in the mechanism of
PFC attenuation of ICAM-1 expression in injured alveolar epithelial
cells. To evaluate the hypothesis, the present study used A549
cells stimulated with lipopolysaccharide (LPS) as an in
vitro model of ARDS. Due to the limited purity and viability of
primary human alveolar epithelial cells, and the alterations in the
morphological and biochemical characteristics of primary cells over
time, a human pulmonary alveolar cell carcinoma cell line (A549)
with epithelial type II cell properties was utilized instead of
primary human alveolar epithelial cells. Bacterial LPS is a
component of the outer envelope of all gram-negative bacteria, and
therefore is a highly proinflammatory molecule. LPS is able to
induce excessive inflammatory responses in tissue and cells,
resulting in a series of pathophysiological alterations, including
tissue functional disorders, disorganization, apoptosis and even
cell necrosis. The present study observed the effects of PFC on the
expression of ICAM-1 in LPS-induced A549 cells and aimed to
determine the potential mechanism associated with miRNAs.
Materials and methods
Cell culture
The A549 human pulmonary alveolar cell carcinoma
cell line with epithelial type II cell properties was obtained from
the American Type Culture Collection (Manassas, VA, USA). The cells
were grown as a monolayer on 6-well culture plates under conditions
of 100% humidity and 5% CO2 at 37°C. Cells were cultured
in high glucose-Dulbecco's modified Eagle's medium (HG-DMEM) (GE
Healthcare Life Sciences, Logan, UT, USA) with 10% fetal calf serum
(FCS; GE Healthcare Life Sciences), 100 U/ml penicillin, and 100
µg/ml streptomycin (both purchased from Beyotime Institute
of Biotechnology, Haimen, China). The cells were harvested with
0.25% trypsin-ethylenediaminetetraacetic acid (GE Healthcare Life
Sciences) to induce detachment. Following washing with DMEM
containing 10% FCS, cells were centrifuged at 200 x g for 4 min at
25°C and resuspended in fresh medium. The 293T human embryonic
kidney cell line (American Type Culture Collection) was also used,
and the culture conditions were the same as with the A549
cells.
PFC-in-DMEM suspension
Perfluorooctane (C8F18) (a
type of perfluorocarbon) was purchased from Huajieshi Medical
Treatment Facility Co., Ltd. (Shanghai, China). Perfluorooctane is
a clear, colorless and odorless liquid and has a molecular weight
of 438.06. At room temperature, its characteristics are the
following: vapor pressure is 61 mmHg; surface tension is 12 mN/m;
boiling point is 102.5°C; and density 1.75 g/ml (31). As PFCs are water insoluble and
cannot be used for cellular incubation, a PFC-DMEM suspension was
used for subsequent experiments (32). DMEM containing 10% FCS was mixed
with PFC at a ratio (v/v) of 9:1. The mixture was exposed to
ultrasonic energy for 10 sec on ice at 21 kHz and 350 W in a
transonic analogous ultrasonic unit (JY92-2D; Xinzhi Scientz
Biotechnology Co., Ltd., Ningbo, China). Following mixing, the
suspension appeared to be an emulsion. The number of droplets and
size distribution were stable in the PFC-DMEM suspension (data not
shown), however, two distinct liquid phases could be separated
after several hours. To avoid this phase separation, a mini shaker
was used (WuXiang Instrument and Meter Co., Ltd., Shanghai, China)
to shake the culture plate continuously, which was necessary to
guarantee the temporary mixing of the PFC-DMEM suspension and thus
contact of PFC and DMEM with the cells.
LPS
LPS was extracted from Escherichia coli
055:B5 (Sigma-Aldrich, St. Louis, MO, USA); the concentration in
the media and reagents used was 10 µg/ml.
Experimental protocol
A549 cells were divided into four groups as follows:
i) Untreated control group; ii) LPS group, incubated with LPS at a
final concentration of 10 µg/ml; iii) LPS+PFC group,
incubated with LPS and PFC-DMEM suspension at the above mentioned
concentrations; and iv) PFC group, incubated with the PFC-DMEM
suspension.
mR NA and miR NA quantification by
reverse transcription-quantitative polymerase chain reaction
(RT-qRCR) analysis
RT-qPCR was used to assess ICAM-1 mRNA and miR-17-3p
expression in A549 cells. Following treatment, the cells of each
group were harvested at 2, 4, 6 and 8 h (for analysis of mRNA
expression) or 1, 2, 3, 4, 5, 6, 7 and 8 h (for analysis of miRNA
expression). Total RNA was extracted from A549 cells using RNAiso
Plus (Takara Biotechnology Co., Ltd., Dalian, China). For mRNA
expression analysis, total RNA was reverse transcribed into cDNA
using the PrimeScript® RT Reagent kit with gDNA Eraser
(Takara Biotechnology Co., Ltd.). SYBR® Premix Ex TaqTM
II (Takara Biotechnology Co., Ltd.) was used for qPCR for the mRNA
quantification in A549 cells according to the manufacturer's
instructions. β-actin served as the housekeeping gene. The
following primers were used for RT-qPCR: β-actin (product size 215
bp), forward 5′-CAAAGACCTGTACGCCAACACAGT-3′ and reverse
5′-ACTCCTGCTTGCTGATCCACATCT-3′; ICAM-1 (product size 151 bp),
forward 5′-GCCCGAGCTCAAGTGTCT AA-3′ and reverse
5′-GGAGAGCACATTCACGGCA-3′. For miRNA analysis, total RNA was
reverse transcribed into cDNA using the miRcute miRNA First-Stand
cDNA Synthesis kit [poly(A) polymerase method] (Tiangen Biotech
Co., Ltd., Beijng, China). The miRcute miRNA qPCR Detection kit
(SYBR Green; Tiangen Biotech Co., Ltd.) was used for qPCR. U6
served as the small RNA reference housekeeping gene. The kits and
specific primers for miR-17-3p and U6 were purchased from Tiangen
Biotech Co., Ltd.. The primer sequences were as follows: miR-17-3p
(product size 22 bp), forward 5′-TGCGCACTGCAGTGAAGGCACT-3′ and
reverse 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTACA-3′; U6
(product size 101 bp), forward 5′-CGCTTCGGCAGCACATATAC-3′ and
reverse 5′-AATATGGAACGCTTCACGA-3′. The cDNA was amplified by PCR in
an iQ5 Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The thermal cycling conditions of the mRNA PCR
reaction were as follows: 5 Sec at 95° and 20 sec at 60°C for 40
cycles; the thermal cycling conditions of the miRNA PCR reaction
were as follows: 5 Sec at 95°C and 20 sec at 60°C for 45 cycles.
All reactions were performed in triplicate, and relative expression
of the RNAs was calculated using the 2−ΔΔCq method
(33,34).
Western blot analysis
Western blotting was used to assess ICAM-1 protein
expression in A549 cells. Following treatment, cells of each group
were harvested at 2, 4, 6 and 8 h for protein extraction. The
protein content of each sample was determined using a bicinchoninic
acid assay (Applygen Technologies, Inc., Beijing, China). A total
of 30 µg protein were mixed with loading buffer, heated at
95°C for 5 min for protein denaturation, and separated by 8% sodium
dodecyl sulfate-polyacrylamide gel (Beyotime Institute of
Biotechnology) electrophoresis. Separated proteins were transferred
onto polyvinylidene fluoride membranes (Beyotime Institute of
Biotechnology). Non-specific binding sites were blocked with 5%
skimmed milk in Tris-buffered saline with 0.1% Tween-20. Protein
expression was normalized to the housekeeping gene β-actin.
Monoclonal mouse anti-human ICAM-1 IgG antibody (dilution, 1:1,000;
sc-8439; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse
anti-human β-actin IgG antibody (dilution, 1:10,000; ab1801; Abcam,
Cambridge, MA, USA) were used for primary detection.
Horseradish-conjugated goat anti-mouse IgG antibody (dilution,
1:5,000; sc-2005; Santa Cruz Biotechnology, Inc.) was used for
secondary detection. Protein bands were visualized by enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA).
A549 cell transfection
A549 cells were transfected with 50 nM hsa-miR-17-3p
mimic or with 100 nM hsa-miR-17-3p inhibitor (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) using Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. Control samples were transfected with
a miRNA mimic negative control or miRNA inhibitor negative control
(Guangzhou RiboBio Co., Ltd.). The cells were transfected for 6 h
followed by two washes, then culturing continued with complete
growth medium for 48 h. The effects of transfection were assessed
by RT-qPCR. Following transfection, cells were divided into the
following 4 groups: i) Control group; ii) LPS group; iii) LPS+PFC;
and iv) PFC group, which were processed as detailed in the
experimental protocol and harvested at 2, 4, 6 and 8 h after
treatment for protein expression analysis.
Luciferase reporter gene assays
The pcDNA-LUC-ICAM-1 vector (containing the 3′UTR
region of ICAM-1 downstream of the luciferase gene), pcDNA-LUC
(empty vector) and Renilla vector (reference) were provided
by Dr Yajaira Suárez (New York University, New York, NY, USA).
Hsa-miR-17-3p mimic or miR negative control (50 nmol/l), and
pcDNA-LUC-ICAM-1 or pcDNA-LUC (200 ng) and Renilla vector
(10 ng) were co-transfected into 293T cells using Lipofectamine™
2000 for 6 h, then cultured with complete growth medium for 48 h.
The cells were divided into the following groups: i) Group A,
co-transfected with pcDNA-LUC, miR-17-3p and Renilla vector;
Group B, co-transfected with pcDNA-LUC, miR negative control and
Renilla vector; Group C, co-transfected with
pcDNA-LUC-ICAM-1, miR negative control and Renilla vector;
and Group D, co-transfected with pcDNA-LUC-ICAM-1, miR-17-3p and
Renilla vector. Luciferase activity analysis was performed
using the Dual-Luciferase® Assay system (Promega
Corporation, Madison, WI, USA). Relative luciferase activity was
obtained by normalizing the Renilla luciferase activity to
the firefly luciferase activity.
Statistics
Data are presented as the mean ± standard deviation.
Significant differences were analyzed by employing Student's
t-test; the comparison of multiple groups was performed
using one-way analysis of variance. SPSS Statistics 17.0.1 software
(SPSS, Inc., Chicago, IL, USA) was used for analysis. Data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of PFC on ICAM-1 expression in
LPS-induced A549 cells
Considering that ICAM-1 serves an important role in
ALI/ARDS, the effects of PFC on ICAM-1 expression were studied.
ICAM-1 mRNA expression levels in A549 cells from each group at 2,
4, 6 and 8 h following treatment were assessed by RT-qPCR (Fig. 1). The mRNA expression of the
control group at 2 h was used as a baseline to assess the relative
mRNA expression of ICAM-1 of the other time pointes. Following
stimulation with LPS, the mRNA expression of ICAM-1 was
significantly increased in the LPS group compared with the control
group (P<0.01). The expression of ICAM-1 mRNA peaked at 4 h
after stimulation and then gradually reduced in the LPS and LPS+PFC
groups. The LPS+PFC group exhibited significantly lower ICAM-1
expression levels than the LPS group at 2, 4, 6, (P<0.01) and 8
h (P<0.05) following treatment. There was no difference observed
between the PFC group and the control group (P>0.05) in ICAM-1
mRNA levels.
 | Figure 1Alterations in the relative ICAM-1
mRNA expression in A549 cells in each group. The expression of mRNA
in each group at 2, 4, 6 and 8 h following treatment was assessed
by reverse transcription-quantitative polymerase chain reaction.
The mRNA expression of the control group at 2 h was used as a
baseline to assess the relative mRNA expression of ICAM-1 of the
other groups. ICAM-1 mRNA expression was significantly increased in
the LPS group compared with the control group at the different time
points. The LPS+PFC group exhibited significantly lower ICAM-1 mRNA
expression than the LPS group at 2, 4, 6 and 8 h after treatment.
No effect of PFC alone was observed on the expression of ICAM-1.
Values are presented as the mean ± standard deviation, n=3.
*P<0.01 vs. the control group; #P<0.05,
##P<0.01 vs. the LPS group. ICAM-1, intercellular adhesion
molecule-1; LPS, lipopolysaccharide; PFC, perfluorocarbon; L+P, LPS
and PFC group. |
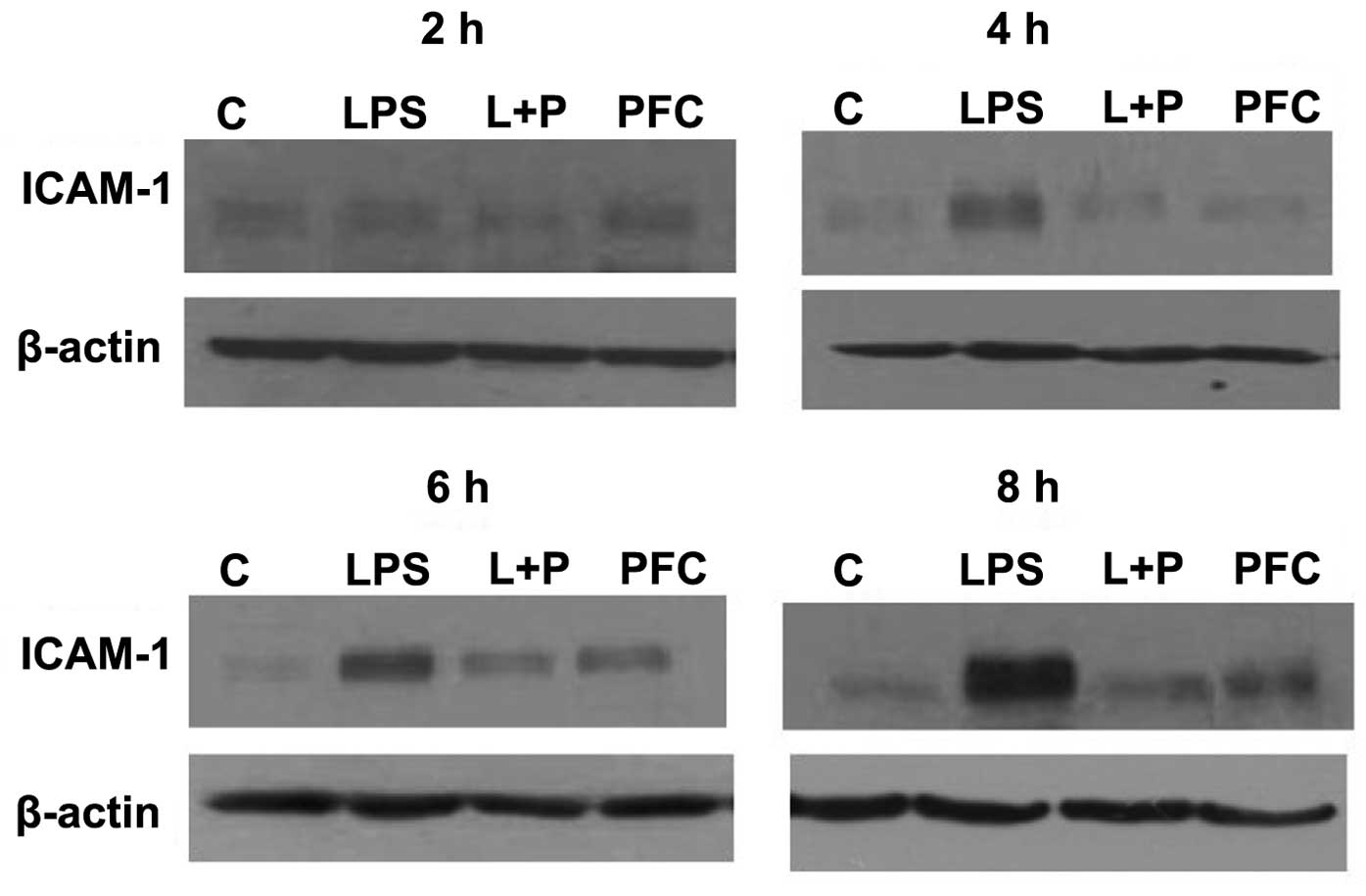
Western blotting was used to assess ICAM-1 protein
expression in A549 cells from each group at 2, 4, 6 and 8 h after
treatment (Fig. 2). ICAM-1 protein
expression was near undetectable in the control group, however,
following stimulation by LPS, the expression of ICAM-1 was
increased in the LPS group compared with the control group at 4, 6
and 8 h after treatment. The LPS+PFC group exhibited lower protein
expression of ICAM-1 than the LPS group at 4, 6 and 8 h after
treatment. There was no difference observed between the PFC group
and control group in ICAM-1 protein levels.
In summary, LPS induces the expression of ICAM-1 in
A549 cells. PFC attenuates ICAM-1 mRNA and protein expression
levels in LPS-induced A549 cells, with no significant effect on
untreated A549 cells.
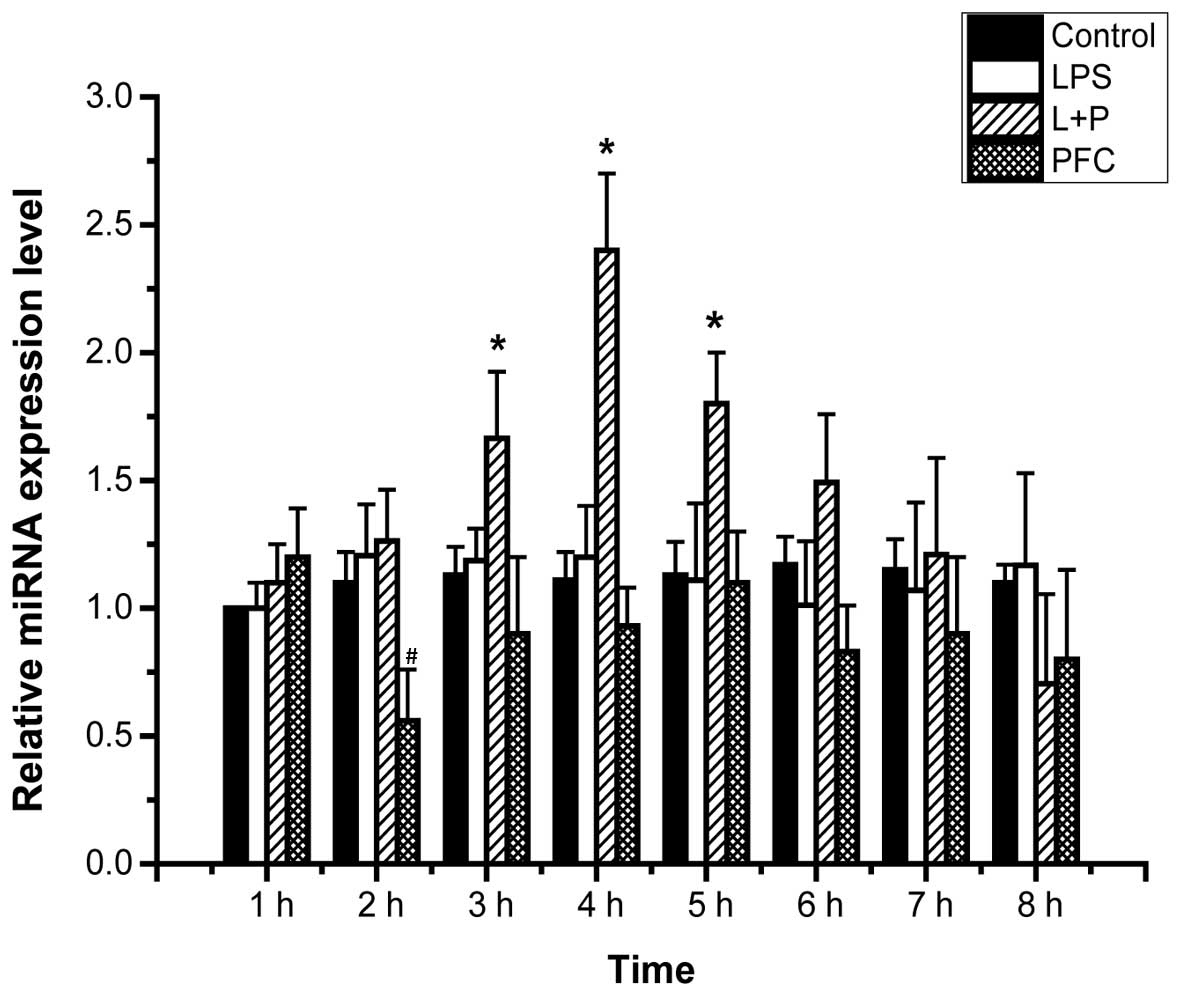
Effects of PFC on miR-17-3p expression in
LPS-induced A549 cells
A previous study reported that ICAM-1 was a target
of TNF-induced miR-17-3p (30),
therefore miR-17-3p may serve a key role in the mechanism of PFC
attenuation of ICAM-1 expression in LPS-induced A549 cells. To
evaluate this hypothesis, the effect of PFC on miR-17-3p expression
in LPS-induced A549 cells was investigated. The expression levels
of miRNA in A549 cells of each group from 1–8 h following treatment
were assessed by RT-qPCR (Fig. 3).
The expression level of miR-17-3p at 1 h in the control group
served as the baseline to assess the relative mRNA expression of
miR-17-3p of the other groups. The LPS+PFC group exhibited
significantly higher miR-17-3p expression compared with the LPS
group at 3, 4 and 5 h following treatment (P<0.05). This
indicates that PFC increases the expression of miR-17-3p in
LPS-induced A549 cells. There was no difference observed between
the PFC group and the control group in the expression levels of
miR-17-3p (P>0.05).
Effects of PFC on ICAM-1 expression in
LPS-induced A549 cells following miR-17-3p transfection
To determine the role of miR-17-3p in the
anti-inflammatory effects of PFC, the effects of PFC on ICAM-1
expression in LPS-induced A549 cells after miR-17-3p transfection
were investigated. A549 cells were transfected with 50 nM miR-17-3p
mimic or with 100 nM miR-17-3p inhibitor. Control samples were
transfected with miR mimic negative control or inhibitor negative
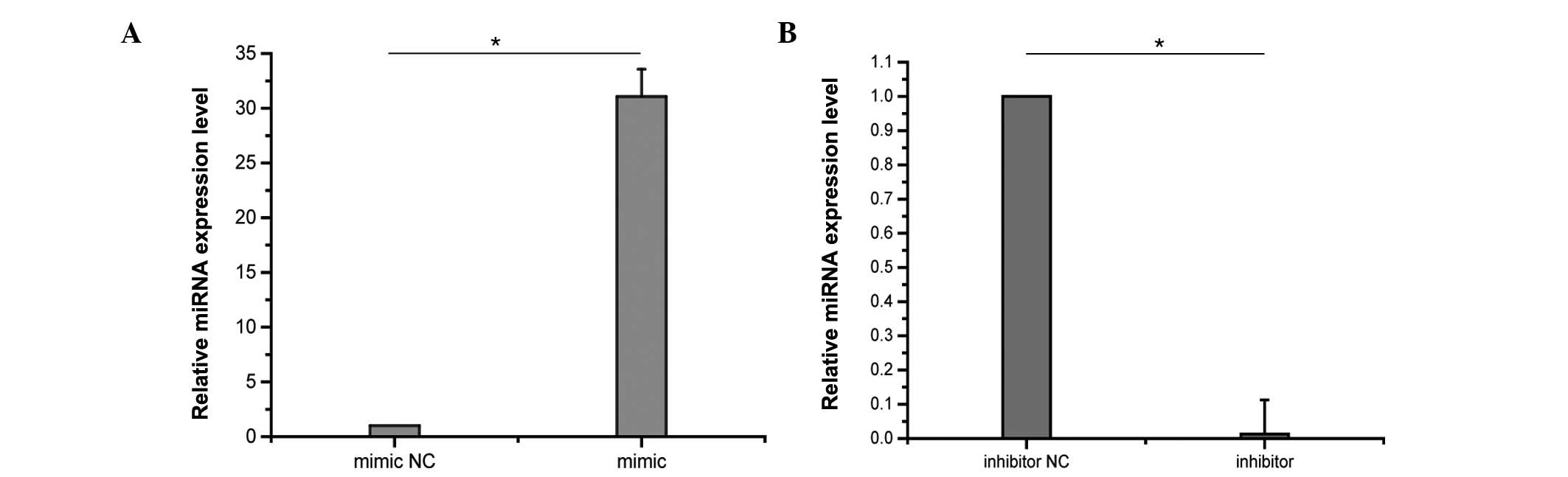
control. The effects of transfection on miRNA expression levels
were assessed by RT-qPCR. Following 48 h of transfection, miR-17-3p
levels were markedly increased in A549 cells transfected with the
miR-17-3p mimic compared with the negative control, and were
reduced by the transfection with the miR-17-3p inhibitor
(P<0.01; Fig. 4). Following
transfection, cells were divided into 4 groups as detailed in the
experimental protocol and were harvested at 2, 4, 6 and 8 h after
treatment for protein expression analysis.
Western blotting was used to assess ICAM-1 protein
expression in the A549 cells following transfection (Fig. 5). In the cells transfected with
miR-17-3p mimics or the mimic negative control, the
anti-inflammatory effects of PFC were greater in the mimic group
compared with the negative control group at each time point
(Fig. 5A–D). In the cells
transfected with the miR-17-3p inhibitor or the inhibitor negative
control, the anti-inflammatory effects of PFC were greater in the
inhibitor negative control group compared with the inhibitor group
at 2 and 4 h (Fig. 5E and F),
however, were not at 6 and 8 h (Fig.
5G and H). In summary, transfection of the miR-17-3p mimic in
A549 cells enhanced the anti-inflammatory effects of PFC, whereas
the miR-17-3p inhibitor weakened the anti-inflammatory effects of
PFC at early time points.
Interaction of miR-17-3p with the ICAM-1
3′UTR
Luciferase reporter gene assays were used to assess
the interaction between miR-17-3p and the ICAM-1 3′UTR. miR-17-3p
mimics or the mimic negative control, and pcDNA-LUC-ICAM-1 or
pcDNA-LUC, and Renilla vector were co-transfected into 293T
cells. Following 48 h of transfection, the relative luciferase
activity analysis of group A was 1.65±0.25; group B was 1.72±0.22;
group C was 0.7±0.15; and group D was 0.51±0.11. The relative
luciferase activity of group D was significantly lower than group C
(P<0.05), whereas there was no difference between groups A and B
(P>0.05). These data suggest an interaction between miR-17-3p
and the ICAM-1 3′UTR, and that ICAM-1 is a target gene of
miR-17-3p.
Discussion
In the present study, the effects of PFC on the
expression of ICAM in LPS-induced A549 cells were observed, with
the aim of determining the potential anti-inflammatory mechanism.
The significant observations include the following: i) As PFC is
insoluble in water, cell-culture experiments with A549 cells should
be performed using a PFC-DMEM suspension; ii) LPS induces ICAM-1
production in A549 cells, and PFC attenuates ICAM-1 mRNA and
protein expression levels in LPS-induced A549 cells, with no
significant effect on untreated cells; iii) PFC increased the
expression of miR-17-3p in LPS-induced A549 cells; iv) miR-17-3p
mimics enhanced the anti-inflammatory effects of PFC, whereas
miR-17-3p inhibitors weakened the anti-inflammatory effects of PFC
at early time points; and v) ICAM-1 is a target of miR-17-3p. Thus,
it may be concluded that PFC attenuates ICAM-1 expression in
LPS-induced A549 cells, and increased miR-17-3p expression may be
an associated mechanism.
ALI/ARDS is a critical clinical syndrome with
progressive dyspnea, refractory hypoxemia and high mortality. The
pathological features include inflammatory reaction and
alveolar-capillary membrane injury resulting from severe infection,
trauma and shock. Alveolar epithelial cells are an important cell
group associated with lung injury, and in addition to being the
target of inflammatory cells and mediators, are active inflammatory
cells and effector cells. They serve important roles in the
occurrence and progression of ALI/ARDS (35,36).
Previous studies have shown that alveolar epithelial cells are
stimulated to produce a number of inflammatory mediators such as
TNF-α, ICAM-1, monocyte chemotactic protein-1, interleukin (IL)-8
and IL-6 (35,36). Certain reports have indicated that
ICAM-1 serves an important role during the entire development and
progression of ALI/ARDS (37–40).
ICAM-1 is a cell surface glycoprotein that belongs to the
immunoglobulin superfamily of adhesion molecules. ICAM-1 is the
counter-receptor for the β2-integrins, lymphocyte
function-associated antigen and macrophage-1 antigen, and is
involved in leukocyte trafficking and lymphocyte activation
(41). Under physiological
conditions, ICAM-1 is expressed at low levels in epithelial cells
(42,43) and is induced by cytokines (TNF-α,
IL-1) and bacterial LPS (44). The
increased expression of ICAM-1 on epithelial cells is a
prerequisite for leukocyte trafficking through the endothelial and
epithelial barrier in ALI/ARDS, and facilitates the adhesion and
activation of leukocytes (mainly polymorphonuclear leukocytes) and
pulmonary vascular endothelial cells in addition to inducing the
“cascade effect” of inflammatory mediators in the lung (37–40).
Therefore, the measurement of soluble ICAM-1 levels may be useful
for identifying the patients with ALI at the highest risk of poor
outcomes (43,44). As ICAM-1 serves an important role
in ALI/ARDS, the inhibition of ICAM-1 expression may contribute to
the prevention and treatment of ALI.
In the current study, the aim was to observe the
effects of PFC on the expression of ICAM-1 in LPS-induced alveolar
epithelial cell and to determine the potential mechanism. The A549
cells (a human pulmonary alveolar cell carcinoma cell line with
epithelial type II cell properties) were stimulated with LPS as an
in vitro model of ARDS. From this model, PFC was observed to
have a protective effect on alveolar epithelial cells.
Additionally, PFC attenuates ICAM-1 mRNA and protein expression in
LPS-induced A549 cells. Further experiments indicated that PFC
increased the production of miR-17-3p in LPS-induced A549 cells,
and that transfection with a miR-17-3p mimic enhanced the
anti-inflammatory effects of PFC, whereas transfection with a
miR-17-3p inhibitor weakened the anti-inflammatory effects of PFC
at early time points. To conclude, miR-17-3p may be involved in the
anti-inflammatory effects of PFC by targeting ICAM-1. However, the
underlying mechanisms of how PFC affects miR-17-3p remain
unknown.
miR-17-3p has 22 nucleotides, and there are several
studies about its functions (45).
A study by Suárez et al (30) has shown that ICAM-1 is a target of
TNF-induced miR-17-3p in endothelial cells. Jiang and Li (46) confirmed that miR-17-3p expression
is regulated by TNF-α and LPS in HeLa cells. In addition, miR-17-3p
induces carcinoma by targeting vimentin (47), mediates stress responses by
targeting MDM2 (48), and inhibits
angiogenesis by downregulating fetal liver kinase-1 (49). In certain clinical studies,
circulating miR-17-3p in serum has been indicated to be a potential
non-invasive biomarker for colorectal cancer screening (50,51).
Other studies have shown that the miR-17-92 cluster, which encodes
miR-17-3p (and 6 other miRNAs), is expressed in numerous mammalian
tissues, and this cluster contributes to the development of the
heart, lungs, blood vessels and the immune system (52,53).
In addition, miR-17-3p is able to induce tumorigenesis (54,55)
and alters the expression of cell-cycle-related genes (56). As ARDS is a complex inflammatory
disease, and miRNAs serve critical roles in the inflammatory
response, it was suggested that influencing miR-17-3p may be an
anti-inflammatory mechanism of PFC.
Despite considerable investigation, new treatments
have not been discovered, and respiratory support remains the
predominant method of treating ALI/ARDS. Liquid ventilation with
PFC has been used clinically for over 50 years, and shows potential
for the treatment of ARDS. In recent years, the major studies
regarding liquid ventilation have focused on animal experiments or
in vitro experiments with few clinical trials. Based on
these animal studies and cell experiments, liquid ventilation holds
promise to improve low pulmonary compliance and gas exchange in
addition to reducing pulmonary inflammatory responses, and
ultimately improving the prognosis of animals with ARDS (6-14).
However, the results of clinical randomized controlled trials
(RCTs) and basic research have produced conflicting results, and
the outlook of liquid ventilation has been disappointing. Results
from clinical trials did not demonstrate an improved outcome of
partial liquid ventilation in patients with ARDS compared with
conventional mechanical ventilation (57–59).
In addition, no significant differences in the number of days free
from the ventilator, incidence of mortality or any
pulmonary-related parameter were observed (57–59).
Although these results may seem discouraging, it is too early to
conclude that there is no effect of PFCs in patients with ARDS.
Further multi-center clinical RCTs are required to provide the most
convincing evidence. A phase II clinical trial of perfluorocarbon
inhalation treatment of ALI/ARDS is underway at the Chinese
People's Liberation Army General Hospital (Beijing, China)
(ClinicalTrials.gov identifier: NCT
01391481), and the results are awaited. As a novel treatment, the
mechanism of action of liquid ventilation is not fully understood
and further basic and clinical research is required.
In summary, the present study observed that PFC
attenuates ICAM-1 mRNA and protein expression in LPS-induced A549
cells. Notably, for the first time, to the best of our knowledge,
miR-17-3p was reported to be involved in the anti-inflammatory
effects of PFC. Transfection with a miR-17-3p mimic enhanced the
anti-inflammatory effects of PFC, whereas transfection with a
miR-17-3p inhibitor weakened the anti-inflammatory effects of PFC
at early time points. In addition, miR-17-3p administration was
demonstrated to inhibit the expression of ICAM-1, and miR-17-3p was
shown to target ICAM-1. In the present study, evidence of the
biological anti-inflammatory effects are reported, which improve
the understanding of the protective effects of PFC. These findings
are likely to have important implications in the application of PFC
in the treatment of ALI/ARDS, and further investigation of miRNAs
and PFC will pave the way to developing a novel therapeutic
approach to the treatment of ALI/ARDS.
References
|
1
|
Dushianthan A, Grocott MP, Postle AD and
Cusack R: Acute respiratory distress syndrome and acute lung
injury. Postgrad Med J. 87:612–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar :
|
|
3
|
Ashbaugh DG, Bigelow DB, Petty TL and
Levine BE: Acute respiratory distress in adults. Lancet. 2:319–323.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blank R and Napolitano LM: Epidemiology of
ARDS and ALI. Crit Care Clin. 27:439–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakabayashi T, Tamura M and Nakamura T:
Partial liquid ventilation with low-dose perfluoro chemical and
high-frequency oscillation improves oxygenation and lung compliance
in a rabbit model of surfactant depletion. Biol Neonate.
89:177–182. 2006. View Article : Google Scholar
|
|
7
|
Bleyl JU, Ragaller M, Tschö U, Regner M,
Hübler M, Kanzow M, Vincent O and Albrecht M: Changes in pulmonary
function and oxygenation during application of perfluorocarbon
vapor in healthy and oleic acid-injured animals. Crit Care Med.
30:1340–1347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von der Hardt K, Kandler MA, Brenn G,
Scheuerer K, Schoof E, Dötsch J and Rascher W: Comparison of
aerosol therapy with different perfluorocarbons in surfactant
depleted animals. Crit Care Med. 32:1200–1206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schoof E, von der Hardt K, Kandler MA,
Abendroth F, Papadopoulos T, Rascher W and Dötsch J: Aerosolized
perfluorocarbon reduces adhesion molecule gene expression and
neutrophil sequestration in acute respiratory distress. Eur J
Pharmacol. 457:195–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von der Hardt K, Kandler MA, Fink L,
Schoof E, Dotsch J, Bohle RM and Rascher W: Laser-assisted
microdissection and real-time PCR detect anti-inflammatory effect
of perfluorocarbon. Am J Physiol Lung Cell Mol Physiol.
285:L55–L62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakstad B, Wolfson MR, Shaffer TH, Kähler
H, Lindemann R, Fugelseth D and Lyberg T: Perfluorochemical liquids
modulate cell-mediated inflammatory responses. Crit Care Med.
29:1731–1737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakata S, Yasui K, Nakamura T, Kubota N
and Baba A: Perf luorocarbon suppresses lipopolysaccharide- and
alpha-toxin-induced interleukin-8 release from alveolar epithelial
cells. Neonatology. 91:127–133. 2007. View Article : Google Scholar
|
|
13
|
Wissel H, Burkhardt W, Rupp J, Wauer RR
and Rüdiger M: Perfluorocarbons decrease Chlamydophila
pneumoniae-mediated inflammatory responses of rat type II
pneumocytes in vitro. Pediatr Res. 60:264–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu SF, Wang P, Liu RJ, Zhao J, Zhang XN,
Fu ZZ, Gao LM, Liang ZX, Sun JP and Chen LA: Perfluorocarbon
attenuates lipopolysaccharide-mediated inflammatory responses of
alveolar epithelial cells in vitro. Chin Med J (Engl).
124:2534–2539. 2011.
|
|
15
|
Haeberle HA, Nesti F, Dieterich HJ,
Gatalica Z and Garofalo RP: Perflubron reduces lung inflammation in
respiratory syncytial virus infection by inhibiting chemokines
expression and nuclear factor-kappa B activation. Am J Respir Crit
Care Med. 165:1433–1438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez R, Sarma V, Younkin E, Hirschl
RB, Ward PA and Younger JG: Exposure to perflubron is associated
with decreased Syk phosphorylation in human neutrophils. J Appl
Physiol (1985). 91:1941–1947. 2001.
|
|
17
|
Rossman JE, Caty MG, Rich GA,
Karamanoukian HL and Azizkhan RG: Neutrophil activation and
chemotaxis after in vitro treatment with perfluorocarbon. J Pediatr
Surg. 31:1147–1151; discussion 1150–1151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van de Stolpe A and van der Saag PT:
Intercellular adhesion molecule-1. J Mol Med (Berl). 74:13–33.
1996. View Article : Google Scholar
|
|
19
|
Kang BH, Crapo JD, Wegner CD, Letts LG and
Chang LY: Intercellular adhesion molecule-1 expression on the
alveolar epithelium and its modification by hyperoxia. Am J Respir
Cell Mol Biol. 9:350–355. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reutershan J and Ley K: Bench-to-bedside
review: Acute respiratory distress syndrome-how neutrophils migrate
into the lung. Crit Care. 8:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mendez MP, Morris SB, Wilcoxen S, Greeson
E, Moore B and Paine R III: Shedding of soluble ICAM-1into the
alveolar space in murine models of acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 290:L962–L970. 2006. View Article : Google Scholar
|
|
23
|
Beck-Schimmer B, Schimmer RC, Warner RL,
Schmal H, Nordblom G, Flory CM, Lesch ME, Friedl HP, Schrier DJ and
Ward PA: Expression of lung vascular and airway ICAM-1 after
exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol
Biol. 17:344–352. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agouridakis P, Kyriakou D, Alexandrakis
MG, Prekates A, Perisinakis K, Karkavitsas N and Bouros D: The
predictive role of serum and bronchoalveolar lavage cytokines and
adhesion molecules for acute respiratory distress syndrome
development and outcome. Respir Res. 3:252002. View Article : Google Scholar
|
|
25
|
Calfee CS, Eisner MD, Parsons PE, Thompson
BT, Conner ER Jr, Matthay MA and Ware LB; NHLBI acute respiratory
distress syndrome clinical trials network: Soluble intercellular
adhesion molecule-1 and clinical outcomes in patients with acute
lung injury. Intensive Care Med. 35:248–257. 2009. View Article : Google Scholar :
|
|
26
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suárez Y, Wang C, Manes TD and Pober JS:
Cutting Edge: TNF-induced micrornas regulate TNF-induced expression
of e-selectin and intercellular adhesion molecule-1 on human
endothelial cells: Feedback control of inflammation. J Immunol.
184:21–25. 2010. View Article : Google Scholar
|
|
31
|
Gabriel JL, Miller TF Jr, Wolfson MR and
Shaffer TH: Quantitative structure-activity relationships of
perfluorinated heterohydrocarbons as potential respiratory media.
Application to oxygen solubility, partition coefficient, viscosity,
vapor pressure and density. ASAIO J. 42:968–973. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wemhöner A, Hackspiel I, Hobi N, Ravasio
A, Haller T and Rüdiger M: Effects of perfluorocarbons on
surfactant exocytosis and membrane properties in isolated alveolar
type II cells. Resp Res. 11:522010. View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu M: Alveolar epithelium in host
defense: Cytokine production. Sepsis and Organ Dysfunction, from
Chaos to Rationale. Baue AE, Berlot G, Gullo A and Vincent JL:
Springer-Verlag Mailand; Milan, Italy: pp. 37–50. 2001
|
|
36
|
Simon RH and Paine R III: Participation of
pulmonary alveolar epithelial cells in lung inflammation. J Lab
Clin Med. 126:108–118. 1995.PubMed/NCBI
Zhang X, Wu D and Jiang X: Icam-1 and
acute pancreatitis complicated by acute lung injury. JOP. 10:8–14.
2009.PubMed/NCBI
|
|
37
|
Mendez MP, Morris SB, Wilcoxen S, Greeson
E, Moore B and Paine R III: Shedding of soluble ICAM-1 into the
alveolar space in murine models of acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 290:L962–L970. 2006. View Article : Google Scholar
|
|
38
|
Zhang XD, Hou JF, Qin XJ, Li WL, Chen HL,
Liu R, Liang X and Hai CX: Pentoxifylline inhibits intercellular
adhesion molecule-1 (ICAM-1) and lung injury in experimental
phosgene-exposure rats. Inhal Toxicol. 22:889–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sumagin R, Lomakina E and Sarelius IH:
Leukocyte endothelial cell interactions are linked to vascular
permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ
Physiol. 295:H969–H977. 2008. View Article : Google Scholar
|
|
40
|
van de Stolpe A and van der Saag PT:
Intercellular adhesion molecule-1. J Mol Med (Berl). 74:13–33.
1996. View Article : Google Scholar
|
|
41
|
Albelda SM, Smith CW and Ward PA: Adhesion
molecules and inflammatory injury. FASEB J. 8:504–512.
1994.PubMed/NCBI
|
|
42
|
Hopkins AM, Baird AW and Nusrat A: ICAM-1:
Targeted docking for exogenous as well as endogenous ligands. Adv
Drug Deliv Rev. 56:763–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ware LB, Koyama T, Billheimer DD, Wu W,
Bernard GR, Thompson BT, Brower RG, Standiford TJ and Martin TR:
Prognostic and pathogenetic value of combining clinical and
biochemical indices in patients with acute lung injury. Chest.
137:288–296. 2010. View Article : Google Scholar :
|
|
44
|
Calfee CS, Eisner MD, Parsons PE, Thompson
BT, Conner ER Jr, Matthay MA and Ware LB; NHLBI acute respiratory
distress syndrome clinical trials network: Soluble intercellular
adhesion molecule-1 and clinical outcomes in patients with acute
lung injury. Intensive Care Med. 35:248–257. 2009. View Article : Google Scholar :
|
|
45
|
Zhang X, Ladd A, Dragoescu E, Budd WT,
Ware JL and Zehner ZE: MicroRNA-17-3p is a prostate tumor
suppressor in vitro and in vivo and is decreased in high grade
prostate tumors analyzed by laser capture microdissection. Clin Exp
Metastasis. 26:965–979. 2009. View Article : Google Scholar
|
|
46
|
Jiang X and Li N: Induction of MiR-17-3p
and MiR-106a by TNFα and LPS. Cell Biochem Funct. 29:164–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shan SW, Fang L, Shatseva T, Rutnam ZJ,
Yang X, Du W, Lu WY, Xuan JW, Deng Z and Yang BB: Mature miR-17-5p
and passenger miR-17-3p induce hepatocellular carcinoma by
targeting PTEN, GalNT7 and vimentin in different signal pathways. J
Cell Sci. 126:1517–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li H and Yang BB: Stress response of
glioblastoma cells mediated by miR-17-5p targeting PTEN and the
passenger strand miR-17-3p targeting MDM2. Oncotarget. 3:1653–1668.
2012. View Article : Google Scholar
|
|
49
|
Yin R, Wang R, Guo L, Zhang W and Lu Y:
MiR-17-3p inhibits angiogenesis by downregulating Flk-1 in the cell
growth signal pathway. J Vasc Res. 50:157–166. 2013. View Article : Google Scholar
|
|
50
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Faltejskova P, Bocanek O, Sachlova M,
Svoboda M, Kiss I, Vyzula R and Slaby O: Circulating miR-17-3p,
miR-29a, miR-92a and miR-135b in serum: Evidence against their
usage as biomarkers in colorectal cancer. Cancer Biomark.
12:199–204. 2012.PubMed/NCBI
|
|
52
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mogilyansky E and Rigoutsos I: The
miR-17/92 cluster: A comprehensive update on its genomics,
genetics, functions and increasingly important and numerous roles
in health and disease. Cell Death Differ. 20:1603–1614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang ZW, An Y and Teng CB: The roles of
miR-17-92 cluster in mammal development and tumorigenesis. Yi
Chuan. 31:1094–1100. 2009.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takakura S, Mitsutake N, Nakashima M,
Namba H, Saenko VA, Rogounovitch TI, Nakazawa Y, Hayashi T, Ohtsuru
A and Yamashita S: Oncogenic role of miR-17-92 cluster in
anaplastic thyroid cancer cells. Cancer Sci. 99:1147–1154. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Attar M, Arefian E, Nabiuni M, Adegani FJ,
Bakhtiari SH, Karimi Z, Barzegar M and Soleimani M: MicroRNA 17-92
expressed by a transposone-based vector changes expression level of
cell-cycle-related genes. Cell Biol Int. 36:1005–1012. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hirschl RB, Croce M, Gore D, Wiedemann H,
Davis K, Zwischenberger J and Bartlett RH: Prospective, randomized,
controlled pilot study of partial liquid ventilation in adult acute
respiratory distress syndrome. Am J Respir Crit Care Med.
165:781–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kacmarek RM, Wiedemann HP, Lavin PT, Wedel
MK, Tütüncü AS and Slutsky AS: Partial liquid ventilation in adult
patients with acute respiratory distress syndrome. Am J Respir Crit
Care Med. 173:882–889. 2006. View Article : Google Scholar
|
|
59
|
Kaushal A, McDonnell CG and Davies MW:
Partial liquid ventilation for the prevention of mortality and
morbidity in paediatric acute lung injury and acute respiratory
distress syndrome. Cochrane Database Syst Rev.
2:CD0038452013.PubMed/NCBI
|