Introduction
The brain is one of the body's most important
organs, and as such has both a specific role and occupies a primary
position in the organism (1). The
brain is of high energy consumption (2,3) and
low energy capacity (4,5). In addition, the brain also exhibits
substrate specificity with the preference of lactate, ketones and
glucose (6,7). All of the above features contribute
to the mechanisms underlying energy absorption and utilization.
Numerous studies investigated the mechanism underlying the brain's
ability for selfishness, the combined results of which formed the
'selfish brain' theory (8–12). As previous results have
demonstrated, in order to satisfy its energy requirements, the
brain prioritizes the adjustment of its own ATP concentration when
regulating the allocation of energy from food sources (9). The brain activates its stress system
to compete for energy resources with other organs (allocation), and
alters appetite (food intake) in order to alleviate the stress
system and return to a state of rest (10). Under conditions of stress or
nutrient deficiency, the brain safeguards its own energy supply
even if this requires sacrificing the energy requirements of other
organs.
Two basic hypotheses have been proposed to explain
how the brain uses its regulatory methods to compete with other
organs under harsh conditions: The 'lipostatic' theory and the
'glucostatic' theory. The former was originally proposed by Kennedy
(11), and proposed that the brain
relied on the leptin hormones in fat and muscle tissue as feedback
signals (12–14). The latter proposed that blood
glucose levels were used as an indicator, an important factor in
the central regulatory system (15), and the brain's so called
'selfishness' would therefore be based on cerebral insulin
suppression (10,16). However, the adequate supply of
energy to the brain is the result of both lipid conversion and the
continuous supply of glucose, which combine elements of both the
glucose and the lipostatic theory. Previous studies on the brain
revealed that the mechanism underlying the physiological regulation
and feedback signaling pathways of energy predominantly focus on
absorbing energy from peripheral neurons and other organs (8,9).
Whether the brain uses its own substances as part of its energy
supply source, and the role of water content in the maintenance of
brain mass remain to be fully elucidated. Alterations in water
content and the mechanisms underlying its regulation may further
clarify the 'selfish brain' theory. The present study aimed to
investigate the self-regulation of the brain under food and/or
water shortage by examining autophagy and water control by AQPs.
AQPs have a well-established role in water balance (17), and 6 of the 13 AQP family members
have been identified in the brain. AQP-1 and 4 were observed to be
the most representative proteins in the regulation of brain water
content, and were demonstrated to be associated with cerebral edema
(18–20). In addition, energy regulation and
autophagy were also important for the brain to acquire sufficient
energy. In the present study, LC3 was introduced as a protein
marker to evaluate brain autophagy (21). LC3I can be phosphorylated to LC3II
during autophagy. Therefore, brain autophagy is reflected by
LC3II/LC3I (22). The results of
the present study may further the understanding of the 'selfish
brain' theory. In addition, it may provide strategies against
nutrient deficiency in humans and animals, predominantly based on
the target genes of AQPs and autophagy.
Materials and methods
Animal grouping and tissue sample
preparation
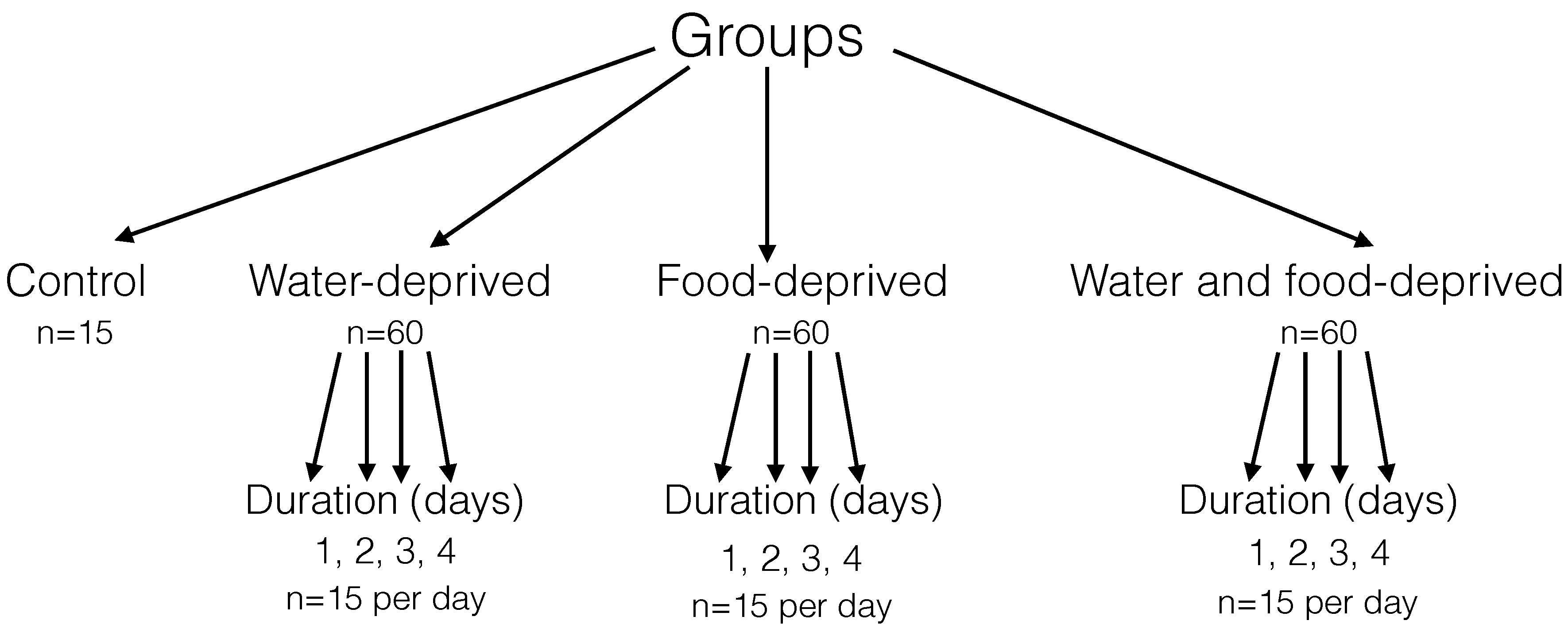
A total of 195 female Balb/c mice (18–20 g, 4-weeks
old) were provided by Vital River Laboratories Co., Ltd. (Beijing,
China). All the mice were raised in the Experimental Animal Center
of Beijing Institute of Radiation Medicine (Beijing, China) and
maintained in specific pathogen free grade animal facility. All
experiments were performed between 08:00 and 15:00 h. A maximum of
five mice were housed in one cage during an experiment. The feeding
room was maintained at a constant temperature of 25°C with normal
ventilation and a natural light/dark cycle. The mice were randomly
divided into four primary groups: A control group (n=15), a
water-deprived group (n=60), a food-deprived group (n=60) and a
water and food-deprived group (n=60). Then animals in each
experimental primary group (except the control group) were the
further divided into four secondary groups (n=15) with deprivation
durations of 1, 2, 3, 4 days. Among the mice of each secondary
group, five were used for histological observation, five for the
water content assay, and the remaining five mice were used for RNA
and protein detection (Fig. 1).
However, prior to dissection, all mice were sacrificed by cervical
dislocation. Mice in each secondary group were dissected and the
brain was harvested. The brain tissue samples (5 brains from each
subgroup) were washed with normal saline and then fixed in formalin
(Sinopharm Chemical Reagent Co. Ltd, Beijing, China) prior to
hematoxylin and eosin (H&E) staining. The remaining 10 brains
were frozen at −80°C for RNA and protein extraction.
All animal experiments were conducted in accordance
with guidance for the use of experimental animals following
approval by the Committee of Animal Care and Use Committee of
Beijing Institute of Radiation Medicine (Beijing, China).
Body weight and blood glucose
detection
Mice were weighed everyday during the experiment.
The data were collected to construct a growth curve. In addition,
blood glucose concentrations were detected each day as basic
physiological indexes among the three experimental groups. Blood
glucose was measured using blood glucose test strips (Roche
Diagnostics, Basel, Switzerland) according to the manufacturer's
protocol.
Analysis of water content
Lyophilization was used to detect the water content
of the brain tissue samples. The tissue samples of the five mice
from all secondary groups were pre-frozen in a −80°C freezer for 12
h, lyophilized in a −50°C vacuum freeze-drying instrument (model
FD-1A-50; Beijing Boyikang Laboratory Instruments Co., Ltd.,
Beijing, China) for 24 h, and then weighed to calculate the water
content.
H&E staining
Tissue samples were fixed in 4% formaldehyde
solution (pH 7.0; Sinopharm Chemical Reagent Co., Ltd.) for two
days, and then processed for paraffin sectioning using a paraffin
slicer (RM2235, Leica Microsystems, Inc., Buffalo Grove, IL, USA).
The sections (5 µm) were stained with hematoxylin (Sinopharm
Chemical Reagent Co. Ltd.) for 3 min, washed in tap water for 30
min, and de-stained in warm water for 10 sec. The sections were
then washed again in running water for 15 min, and stained with
eosin (Sinopharm Chemical Reagent Co., Ltd.) for 15 sec prior to
washing for 20 min. The brain tissue sections were finally
dehydrated using alcohol gradients, prior to xylene (Sinopharm
Chemical Reagent Co. Ltd.) clearance and cover slipping. The
stained sections were observed under a light microscope (DM2500,
Leica Microsystems, Inc.).
RNA and protein extraction
The −80°C preserved brain samples (100 mg/sample)
were homogenized using a handheld grinder (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 1 min on ice. Total RNA
from the mouse brains was extracted using Total RNA kits II (Omega
Bio-Tek, Inc., Norcross, GA, USA) according to the manufacturer's
protocol. Then, the extracted RNA (1 µg) was reverse
transcribed into cDNA using a Reverse Transcription system
(Sigma-Aldrich, St. Louis, MO, USA).
Total protein of the animal tissue samples preserved
at -80°C was extracted using a cell lysis buffer (Applygen
Technologies, Inc., Beijing, China) containing 50 mM Tris (pH 7.4),
150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1
mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM EDTA and 1 mM
protease inhibitor, prior to western blotting.
Quantitative polymerase chain reaction
(qPCR) analysis of AQP in the brain
qPCR was performed using an Illustra Ready-to-Go
RT-qPCR kit (GE Healthcare Life Sciences, Chalfont, UK). All
reactions were performed in a MasterCycler machine (Eppendorf,
Hauppauge, NY, USA) under an initial denaturing step at 94°C for 5
min, followed by 35 cycles of 94°C denaturation for 30 sec, 55°C
annealing for 30 sec, and 72°C extension for 1 min. The genes of
the AQP family were amplified by PCR using the following primers
with β-actin as a reference gene: β-actin forward,
5′-ATGATGCCCCCAGGGCTGTGTT-3′ and reverse,
5′-TTGCTCTGGGCCTCATCACCCA-3′; Aqp1 forward,
5′-TTCTGGGTGGGGCCGTTCATTG-3′ and reverse,
5′-TCTGTGAAGTCGCTGCTGCGTG-3′; and Aqp4 forward,
5′-AGGAAGCCTTCAGCAAAGCCGC-3′ and reverse,
5′-ACTTGGCTCCGGTTGTCCTCCA-3′. The primers were designed by the
multiplex PCR primer designing web server (https://sourceforge.net/projects/mpprimer/) (23). PCR products were identified by 2%
agarose gel electrophoresis (Sigma-Aldrich), and expression levels
were quantified by image analysis using Image J for integrated
optical density analysis (version 2x; National Institutes of
Health, Bethesda, MA, USA), and then plotted using Origin 8.1
software (OriginLab Corporation, Northampton, MA, USA). All
experiments were repeated in triplicate.
Western blot analysis of brain autophagy
marker protein LC3I/II
Total proteins (30 pg) extracted from the tissue
samples from the various groups were separated by 12.5% SDS-PAGE at
60 V for 2.5 h. The proteins were then transferred to a
polyvinylidene difluoride membrane (PVDF; GE Healthcare Life
Sciences) following the manufacturer's protocol. Then, the PVDF
membranes were blocked with 5% non-fat milk (Sigma-Aldrich) for 1
h. The membrane was probed with mouse-derived anti-LC3 primary
antibody [1:2,000 in 0.1% phosphate-buffered saline with Tween 20
(PBST; Sigma-Aldrich), and mouse-derived anti-β-actin primary
antibody (1:3,000 in PBST; Sigma-Adrich)] overnight at 4°C. The
membranes were washed for 10 min, 3 times with PBST resolution
prior to incubation with horseradish peroxidase-conjugated goat
anti-mouse secondary antibody (1:5,000 in PBST, Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 1 h at
room temperature. Chemiluminescence substrate luminal reagent (GE
Healthcare Bio-Sciences) was used to detect the immunolabeled bands
by exposure to X-ray films (Kodak, Rochester, NY, USA). Protein
bands were also analyzed using the above-mentioned ImageJ software.
All experiments were repeated at least three times.
Statistical analysis
All data are presented as mean ± standard error.
One-repeated measure analysis of two factors factorial design was
using SAS (version 9.4; SAS Institure, Cary, NC, USA). One-way
analysis of variance was performed in order to test for significant
differences between the groups. Multiple comparisons were performed
using the Student-Newman-Keuls post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Brain mass was maintained with reducing
body weight and blood glucose levels
Body weight (g) and blood glucose levels (mmol/l)
were measured throughout the duration of the experiment. The body
weight of the mice reduced in the water and/or food deprivation
groups in a time-dependent manner, and specifically in the group
subjected to both food and water deprivation (Fig. 2A). The levels of blood glucose were
markedly reduced on the first day of deprivation. In both the food
deprivation group or the food and water deprivation group, the
levels of blood glucose were rapidly reduced from the second to the
fourth day. As expected, food was important for the maintenance of
blood glucose levels. Mice in the water deprivation group also
presented a decline in the blood glucose levels, although this
reduction was not as marked as the other two groups (Fig. 2B). Conversely to body weight, the
brain weight of the mice in the three primary experimental groups
remained relatively stable (Fig.
2C). Therefore, the ratios of brain weight to whole body weight
(%) increased with body weight loss (Fig. 2D).
Brains of the mice are the last organ to
suffer cell injury
The brains of the mice were the last organ to suffer
cell injury. To examine the cell morphology of the murine tissue
samples under experimental conditions, the paraffin tissue sections
were stained with H&E. As the duration of water or food
shortage increased, no significant injuries in the brain structure
were observed among the four groups (Fig. 3). Compared with other organs, the
brain was the last organ to suffer cell injury in the body, as
determined by histological observation (Fig. 3)
AQPs regulated the water content
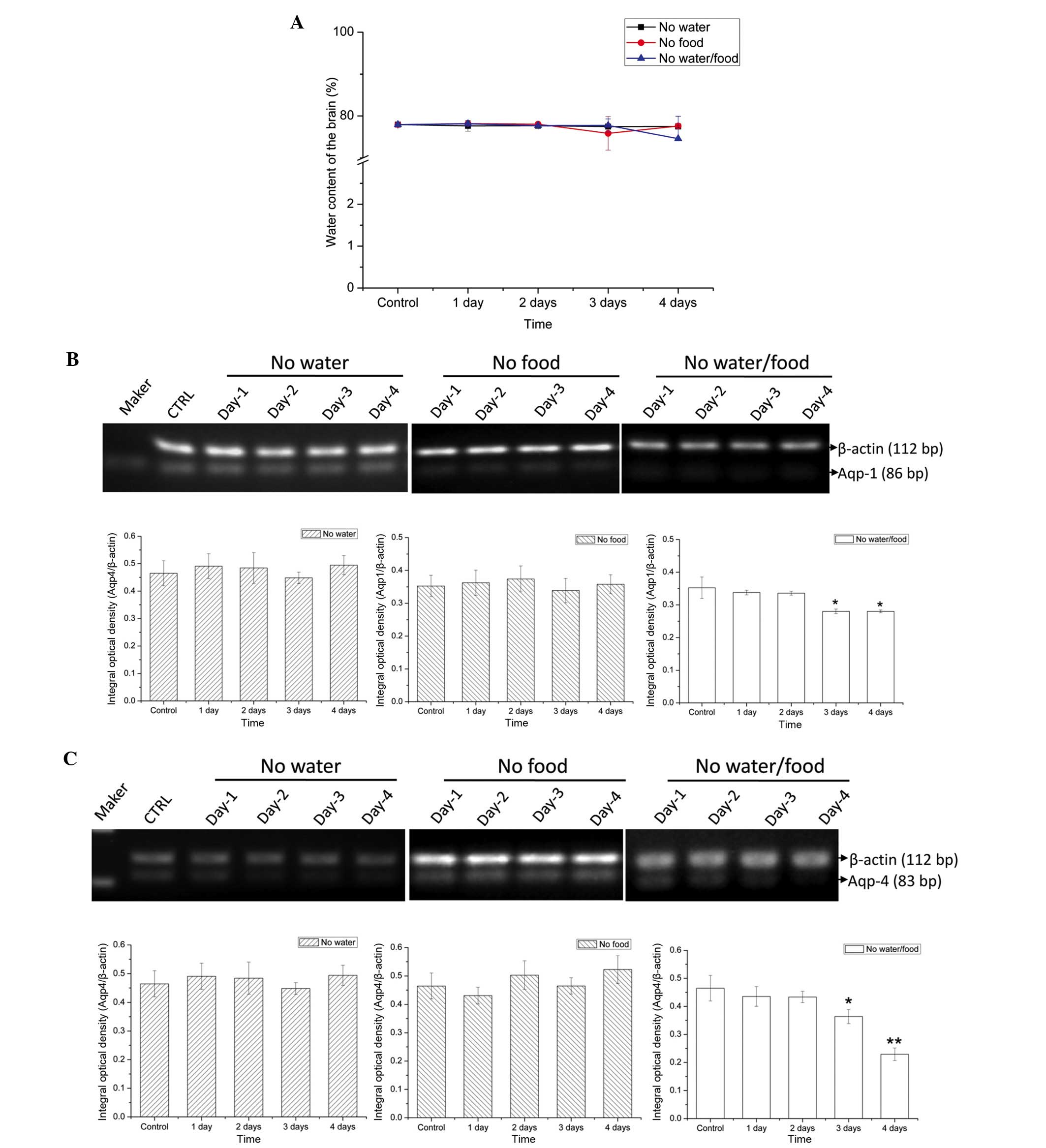
To further explore the ability of the brain to
maintain its own function under severe conditions, brain tissue
samples were examined for water content at each experimental time
point. Compared with the other organs, the water content of the
brain (%) showed no significant reduction (Fig. 4A), suggesting that the brain may
have the ability to sequester water under harsh conditions.
Furthermore, to investigate the mechanism underlying water
regulation, the expression levels of two important proteins of the
AQP family, AQP 1 and 4, were examined. Compared with the control
group, there were no significant alterations in AQP 1 and 4
expression levels in the water-deprived and food-deprived groups
(Fig. 4B and C). However, in the
water and food deprivation group, the expression levels of both AQP
1 and 4 decreased significantly on the third day of deprivation
(AQP1, P=0.03671; AQP4, P=0.02854) and the fourth day (AQP1,
P=0.03637; AQP4, P=0.00231; Fig. 4B
and C), suggesting that the brain may have a regulatory role in
maintaining water balance through AQPs.
Autophagy is involved in brain survival
during lack of water and food
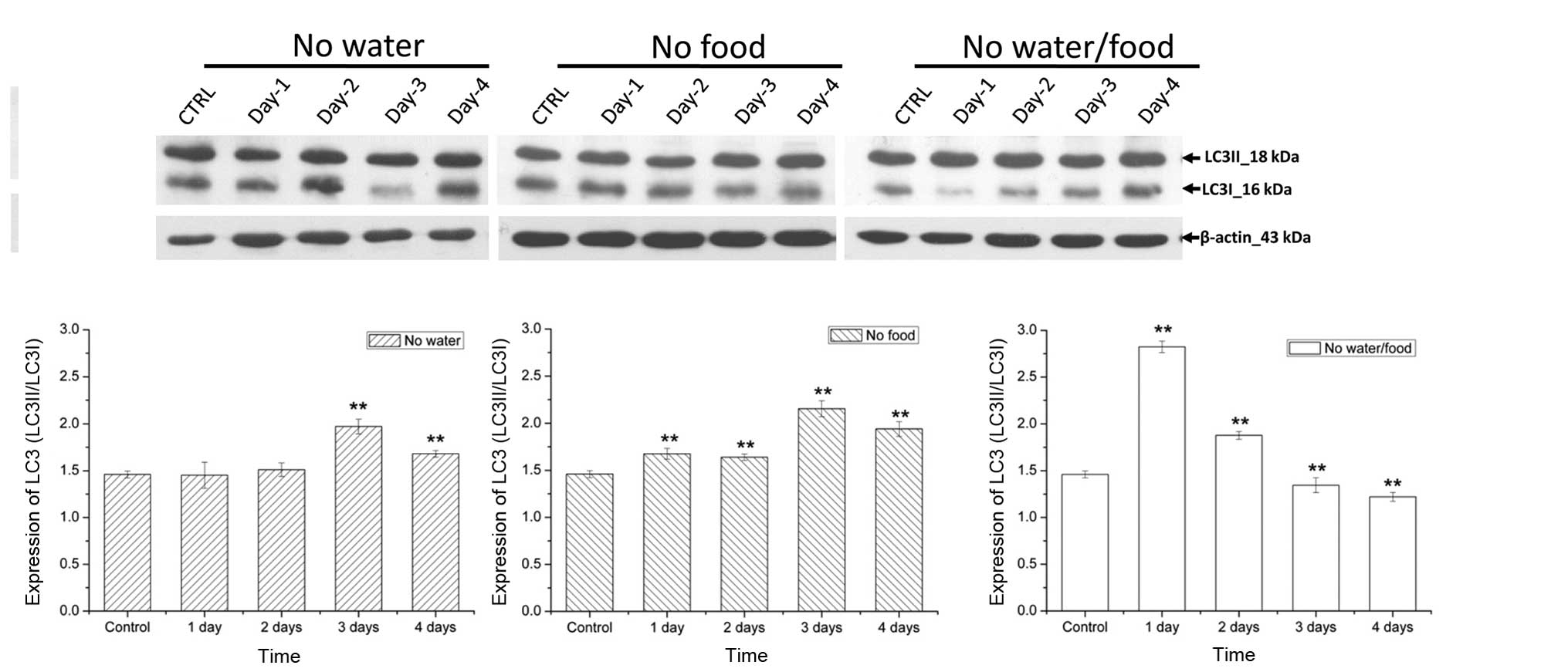
Energy supply was also investigated to further
elucidate the mechanism underlying the ability of the brain to
function under harsh conditions of deprivation. LC3II/I served as
marker proteins examine the activity levels of autophagy in brain.
The expression levels of LC3II/LC3I (%) in the food deprivation
group increased significantly compared with those in the control
group on the first day of deprivation (P=0.00812), indicating that
autophagy was maintained at a high level during food deprivation
(Fig. 5). However, in the water
deprivation group, the expression levels of LC3II/LC3I increased
significantly from the third day (P= 0.00592). In addition, the
LC3II/LC3I expression levels increased significantly from the first
day in the food and water deprivation group, indicating that the
activity levels of autophagy appeared early and increased under
simultaneous deprivation conditions (Fig. 5).
Discussion
In the present study, mice were deprived of water
and/or food. Under each experimental condition of nutritional
deficiency, the body weight was reduced whereas brain weight was
maintained whether under water or food shortage. These results were
consistent with the 'selfish brain' theory (10,24).
In addition, food shortage markedly reduced blood glucose
concentrations, however, water deprivation did not cause such
effects. Furthermore, both water and food deprivation caused energy
deficiency, which may activate the energy regulation system. Under
severe energy shortage, the brain may be able to protect itself by
becoming the last unaffected organ with steady water content and
normal physiological structures. Therefore, it is important to
elucidate the mechanism underlying the brain's unique selfish
ability under harsh conditions. Considering the important role of
the brain, the present study focused on water regulatory proteins
and autophagy-associated proteins in the brains of mice.
In the brain, AQPs have important roles in
controlling water content (17,25).
Six types of AQP have been revealed to be expressed in the brain,
of which AQP4 was the most extensively studied, and proved to be
predominantly associated with cerebral edema (18–20).
To verify whether the brain mass was maintained by energy
adaptation or by increasing the water content in the cerebral
cells, the water content and expression levels of edema marker
proteins, AQP1 and 4, were detected (26). Whether water or food-deprived,
water content was not significantly affected. Therefore, cerebral
edema was not present during the course of water or/and food
shortage. Furthermore, compared with the control group, the
expression levels of AQP1 and 4 in the group deprived of both water
and food were only reduced on the third day. Alterations in AQP1
and 4 expression levels were similar, and were associated with
brain water content. On the fourth day, the expression levels of
both AQP1 and 4 reduced in the water and food deprivation group. In
addition, brain water content was also reduced in the water and
food deprivation group. Therefore, the mouse brain maintained its
normal water content via regulated expression of AQP1 and 4. The
brain may not only compete for energy with peripheral tissues in a
selfish way, but also compete for water via AQPs.
The mice brain maintained its water supply under
water shortage conditions in the same manner as energy regulation,
by selfishly obtaining the energy of other organs (24). However, could the brain draw energy
from its own cellular substances in an unselfish way? To answer the
question, the autophagy levels in the mice brain were determined
under the deprivation of water and/or food. The protein expression
levels of LC3 were used as a biomarker to measure the mouse brain
autophagic levels (22,27). The results in the present study
demonstrated that, both water and food shortage were able to
activate autophagy in cerebral cells. Among the deprivation
factors, food had an important role in activating autophagy in the
early period. As previous studies noted, autophagy is the major
mechanism underlying the degradation of long-lived proteins and the
only known signaling pathway responsible for the degradation of
organelles (21,28). In addition, autophagy was regulated
by nutritional status, hormonal factors and other factors including
temperature, oxygen concentrations and ultraviolet radiation
(29,30). Autophagy is a process associated
with energy re-usage in cells, and therefore food shortage may
affect autophagy more directly and apparently compared with water
shortage. Although the water content is less important for energy
metabolism compared with food intake, long-term water shortage also
caused functional disorders of the mice and the degradation of
proteins in cerebral cells, which finally lead to the activation of
autophagy. Therefore, in addition to competing with the energy of
other organs, the cerebral cells may also use their own substance
and energy resources through autophagy under harsh conditions,
which complement the traditional 'selfish brain' theory (10).
In conclusion, the present study further elucidated
the 'selfish brain' theory of water content maintenance via AQP1
and 4. In addition, unlike the traditional selfish theory, the
mouse brains were also demonstrated to obtain energy through
autophagy, which may be considered as an unselfish method. AQPs as
well as their regulatory signaling pathways merit further research
to clarify the role of water content in the brain.
Acknowledgments
The present study was supported by grants from the
General Program of the National Natural Science Foundation of China
(grant nos. 31300703 and 81271206), the National Basic Research
Project (program 973; grant no. 2012CB518200), the High-Technology
Program of China (program 863; grant no. 2012AA022402), the
National Key Technologies R&D Program for New Drugs of China
(grant no. 2012ZX09102301-016), The General Program of Basic
Research Project of Science and Technology of Jiangsu Province
(grant no. BK2012640), the Special Program of Science and
Technology Development of Suzhou of Jiangsu Province (grant no.
ZXY2012017) and the State Key Laboratory of Proteomics of China
(grant nos. SKLP-K201004, SKLP-Y201105, SKLP-0201104 and
SKLP-0201002).
References
|
1
|
Peters A, Pellerin L, Dallman M, Oltmanns
KM, Schweiger U, Born J and Fehm HL: Causes of obesity: Looking
beyond the hypothalamus. Prog Neurobiol. 81:61–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shulman RG, Rothman DL, Behar KL and Hyder
F: Energetic basis of brain activity: Implications for
neuroimaging. Trends Neurosci. 27:489–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bélanger M, Allaman I and Magistretti PJ:
Brain energy metabolism: Focus on astrocyte-neuron metabolic
cooperation. Cell Metab. 14:724–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown AM and Ransom BR: Astrocyte glycogen
and brain energy metabolism. Glia. 55:1263–1271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenblatt F: The perceptron: A
probabilistic model for information storage and organization in the
brain. Psychol Rev. 65:386–408. 1958. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Erecinska M, Cherian S and Silver IA:
Energy metabolism in mammalian brain during development. Prog
Neurobiol. 73:397–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebert D, Haller RG and Walton ME: Energy
contribution of octanoate to intact rat brain metabolism measured
by 13C nuclear magnetic resonance spectroscopy. J Neurosci.
23:5928–5935. 2003.PubMed/NCBI
|
|
8
|
Peters A, Schweiger U, Pellerin L, Hubold
C, Oltmanns KM, Conrad M, Schultes B, Born J and Fehm HL: The
selfish brain: Competition for energy resources. Neurosci Biobehav
Rev. 28:143–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters A, Schweiger U, Fruhwald-Schultes
B, Born J and Fehm HL: The neuroendocrine control of glucose
allocation. Exp Clin Endocrinol Diabetes. 110:199–211. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hitze B, Hubold C, Van Dyken R,
Schlichting K, Lehnert H, Entringer S and Peters A: How the selfish
brain organizes its supply and demand. Fron Neuroenergetics.
2(7)2010.
|
|
11
|
Kennedy GC: The role of depot fat in the
hypothalamic control of food intake in the rat. Proc R Soc Lond B
Biol Sci. 140:578–596. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahima RS, Prabakaran D, Mantzoros C, Qu D,
Lowell B, Maratos-Flier E and Flier JS: Role of leptin in the
neuroendocrine response to fasting. Nature. 382:250–252. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lord GM, Matarese G, Howard JK, Baker RJ,
Bloom SR and Lechler RI: Leptin modulates the T-cell immune
response and reverses starvation-induced immunosuppression. Nature.
394:897–901. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahima RS, Saper CB, Flier JS and Elmquist
JK: Leptin regulation of neuroendocrine systems. Front
Neuroendocrinol. 21:263–307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayer J: Regulation of energy intake and
the body weight: The glucostatic theory and the lipostatic
hypothesis. Ann N Y Acad Sci. 63:15–43. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubera B, Hubold C, Zug S, Wischnath H,
Wilhelm I, Hallschmid M, Entringer S, Langemann D and Peters A: The
brain's supply and demand in obesity. Front Neuroenergetics.
4(4)2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badaut J, Fukuda AM, Jullienne A and Petry
KG: Aquaporin and brain diseases. Biochim Biophys Acta.
1840.1554–1565. 2014.
|
|
18
|
Badaut J, Lasbennes F, Magistretti PJ and
Regli L: Aquaporins in brain: Distribution, physiology and
pathophysiology. J Cereb Blood Flow Metab. 22:367–378. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papadopoulos MC and Verkman AS:
Aquaporin-4 and brain edema. Pediatr Nephrol. 22:778–784. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki R, Okuda M, Asai J, Nagashima G,
Itokawa H, Matsunaga A, Fujimoto T and Suzuki T: Astrocytes
co-express aquaporin-1,-4 and vascular endothelial growth factor in
brain edema tissue associated with brain contusion. Acta Neurochir
Suppl. 96:398–401. 2006. View Article : Google Scholar
|
|
21
|
Henell F, Berkenstam A, Ahlberg J and
Glaumann H: Degradation of short-and long-lived proteins in
perfused liver and in isolated autophagic vacuoles-lysosomes. Exp
Mol Pathol. 46:1–14. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanida I, Minematsu-Ikeguchi N, Ueno T and
Kominami E: Lysosomal turnover, but not a cellular level, of
endogenous LC3 is a marker for autophagy. Autophagy. 1:84–91. 2005.
View Article : Google Scholar
|
|
23
|
Shen Z, Qu W, Wang W, Lu Y, Wu Y, Li Z,
Hang X, Wang X, Zhao D and Zhang C: MPprimer: A program for
reliable multiplex PCR primer design. BMC Bioinformatics.
11(143)2010. View Article : Google Scholar
|
|
24
|
Fehm HL, Kern W and Peters A: The selfish
brain: Competition for energy resources. Prog Brain Res.
153:129–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tait MJ, Saadoun S, Bell BA and
Papadopoulos MC: Water movements in the brain: Role of aquaporins.
Trends Neurosci. 31:37–43. 2008. View Article : Google Scholar
|
|
26
|
Griesdale DE and Honey CR: Aquaporins and
brain edema. Surg Neurol. 61:418–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuma A, Matsui M and Mizushima N: LC3, an
autophagosome marker, can be incorporated into protein aggregates
independent of autophagy: Caution in the interpretation of LC3
localization. Autophagy. 3:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, McMillan-Ward E, Kong J, Israels S
and Gibson S: Oxidative stress induces autophagic cell death
independent of apoptosis in transformed and cancer cells. Cell
Death Differ. 15:171–182. 2007. View Article : Google Scholar : PubMed/NCBI
|