Introduction
Laryngeal carcinoma is the second most common
neoplasm occurring in the head and neck region, with high levels of
invasion and metastasis (1,2). Due
to low survival rates, determining how to improve quality of life
and survival rates in patients with laryngeal cancer remains a
significant challenge (3). The
most frequently used treatment is chemotherapy, and
chemotherapeutic agents, including cisplatin, gefitinib, taxol and
doxorubicin, are used experimentally or clinically to improve the
prognosis of patients with laryngeal cancer (4,5).
However, due to multidrug resistance-induced therapeutic failure
and poor prognosis, more efficient chemotherapeutic agents and
novel agent combinations are required to improve the treatment of
laryngeal cancer.
Genistein, a soy-derived isoflavone, exhibits broad
tumor suppressive effects in various types of human cancer by
regulating several signaling pathways, including the
mitogen-actived protein kinase, Akt and Janus kinase/signal
transducers and activators of transcription (6–8).
However, current knowledge of its role in regulating laryngeal
cancer cell biofunction is limited to its inhibition of HEp-2 cell
growth (9), and more detailed
investigations of its function and mechanism are required.
As the combining of chemotherapeutic agents has been
a common and effective clinical strategy for cancer therapy, the
present study aimed to identify compounds, which can improve the
effects of genistein in preventing the progression of laryngeal
cancer. Epigenetic modifications have been found to be important in
cancer development and, correspondingly, compounds regulating this
event are currently considered as promising anticancer agents
(10,11). Tricho-statin A (TSA) is known to be
a specific inhibitor of histone deacetylases, and effectively
arrests cell growth and induces apoptosis in various types of human
cancer cell, including prostate, colorectal, breast and gastric
cancer (12,13). At present, there is a lack of
information regarding the effects of TSA on laryngeal carcinoma,
therefore, the present study aimed to investigate the combined
effects of genistein and TSA on laryngeal cancer treatment.
The results of the present study, demonstrated that
TSA improved genistein-induced tumor suppression in human laryngeal
cancer cells. The data obtained confirmed that TSA potentiated the
anti-laryngeal cancer action of genistein via promoting apoptosis
and reversing epithelial-mesenchymal transition (EMT).
Materials and methods
Antibodies and growth factors
The antibodies used in the present study included
monoclonal rabbit anti-E-cadherin (cat. no. 24E10), monoclonal
rabbit anti-vimentin (cat. no. 5741S), monoclonal rabbit anti-B
cell lymphoma (Bcl)-2 (cat. no. 2870) and monoclonal rabbit
anti-Bcl-2-associated X protein (Bax; cat. no. 14796) primary
antibodies obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Monoclonal mouse anti-PARP (1:1,000; cat. no sc-8007;
Santa Cruz Biotechnology, Inc.), monoclonal rabbit Akt (1:1,000;
cat. no. 4691; Cell Signaling Technology, Inc., Danvers, MA, USA),
monoclonal rabbit anti-pAkt (1:500; cat. no. 4060; Cell Signaling
Technology, Inc.), monoclonal mouse anti-S6 (1:1,000; cat. no.
2317; Cell Signaling Technology, Inc.), monoclonal rabbit anti-pS6
(1:200; cat. no. 4858; Cell Signaling Technology, Inc.), monoclonal
rabbit anti-4eBP (1:100; cat. no. 9452; Cell Signaling Technology,
Inc.), monoclonal rabbit anti-p4eBP (1:100; cat. no. 2855; Cell
Signaling Technology, Inc.) and monclonal rabbit anti-GAPDH
(1:2,000; cat. no. sc-25778; Santa Cruz Biotechnology, Inc.)
primary antibodies were also used. Polyclonal goat anti-rabbit
(cat. no. A21020) and goat anti-mouse (cat. no. A21010) secondary
antibodies were purchased from Abbkine, Inc. (Redlands, CA, USA).
Transforming growth factor (TGF)-β1 and epidermal growth factor
(EGF) were purchased from PeproTech, Inc. (Rocky Hill, NJ, USA).
Genistein and TSA were obtained from Sigma-Aldrich (St. Louis, MO,
USA) and were dissolved in dimethyl sulfoxide (DMSO; Sangon Biotech
Co., Ltd., Shanghai, China).
Cell culture
The HEp2 human laryngeal cancer cell line was
purchased from SIBS Cell Bank (Shanghai, China) and cultured in
high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
The medium was renewed every day and the cells were passaged prior
to reaching confluence.
EMT induction in the HEp-2 cells
EMT was achieved when the 1×104 HEp-2
cells were stimulated with EGF (30 ng/ml) for 48 h at 37°C.
Subsequently, the cells were treated with genistein (20 μM)
or genistein+TSA (20 μM: 100 nM), in the presence or absence
of EGF, for another 48 h at 37°C. Alterations in cell morphology
were documented by visualization using an inverted phase contrast
microscope (DFC500; Leica Microsystems GmbH, Wetzlar, Germany).
Western blotting
The HEp-2 cells were harvested and washed once with
phosphate-buffered saline (PBS), and further lysed in
radioimmunoprecipitation assay buffer with phenyl-methylsulfonyl
fluoride (Sangon Biotech Co., Ltd.). Protein concentrations were
determined using a Pierce BCA protein assay kit (Thermo Fisher
Scientific, Inc.). SDS-PAGE (12% gel) was utilized to separate
equal quantities (20 μg) of the proteins, which were then
electrically transferred onto nitrocellulose membrane (GE
Healthcare Life Sciences, Chalfont, UK), and blocked with 5% fat
free milk in Tris-buffered saline with 0.05% Tween (TBST) buffer
for 1 h at room temperature. Individual membranes were further
incubated with appropriately diluted primary antibodies (1:1,000)
overnight at 4°C. Horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:5,000) were then applied for 2 h at room temperature
following three intensive washes in TBST. The HRP substrate gives
rise to the final images using electrochemiluminescence (Western
Bright kit; cat. no. R-03031-D25; Advansta, Inc., Menlo Park, CA,
USA), visualized following X-film development [Fujifilm (China)
Investment Co., Ltd., Shanghai, China].
Invasion assay
Transwell inserts (Corning Incorporated, Corning,
NY, USA) were pre-coated with 50 μl Matrigel (BD
Biosciences, San Jose, CA, USA) and placed into wells of a 12-well
culture plate. Equal quantities of HEp-2 cells (2×105)
were seeded into the upper chamber in culture medium, and cell
invasion was determined at 24 h following the addition of the
chemo-attractant, of FBS, to the lower chamber. The invasive cells
were visualized using 0.1% crystal violet staining (Sangon Biotech
Co., Ltd.) following fixation with 4% para-formaldehyde (PFA) and
imaged using a DFC500 microscope (Leica Microsystems GmbH).
Subsequent quantification was performed using the mean numbers of
cells counted from five randomly selected fields (magnification,
×200). The data were summarized from three independent
experiments.
Wound healing assay
HEp-2 cells (2×105) were seeded in 6-well
plates. After 24 h, the cells were serum starved for 12 h. A linear
wound was created using a pipette tip and the cells were washed
three times using PBS. Subsequently, the cells were cultured in
medium supplemented with 1% FBS. Wounds were then assessed after 24
h. Images of 3 random fields were captured 24 h after the scratch
wound using a Leica DFC500 microscope (magnification, ×200). The
migration distance (arbitrary units) was determined as reduction in
the wounds gap using NIH Image J software (version 1.32; National
Institute of Health, Bethesda MD, USA).
Cell viability
Equal quantities of HEp-2 cells (2.0×104)
were seeded into wells of a 96-well plate and further cultured for
24 h. The cells were then treated with genistein (1, 10, 20, 50 and
100 μM) or TSA (10, 50, 100, 200 and 500 nM) for another 48
h at 37°C. This was followed by incubation for 4 h with MTT (Sangon
Biotech Co., Ltd.) at a final concentration of 0.5 mg/ml, following
which 100 μl DMSO was added following removal of the medium.
Cell viability was determined by measuring optical density (OD) at
490 nm (Bio-Rad Laboratories, Inc., Hercules, CA, USA). At least
three independent experiments were performed, and the final
inhibitory rates were determined according to the following
formula: Inhibitory rate (%) =
[1−(OD490compound/OD490solvent)] × 100%.
TUNEL assay
The HEp-2 cells were allowed to grow to 80%
confluence and fixed with 4% PFA for 30 min, followed by permeation
with 0.1% Triton X-100 (Sangon Biotech Co., Ltd.)/PBS on ice for 2
min. Following three washes with PBS, TUNEL reagent was applied for
the detection of apoptotic events, according to manufacturer's
protocol (Beyotian Biotechnology Co., Ltd., Shanghai,. China) and
4′,6-diamidino-2-phenylindole (Sangon Biotech Co., Ltd.) was used
to stain the nuclei. The staining results were visualized and
documented using an Olympus DP71X immunofluorescent microscope
(Olympus Corporation, Tokyo, Japan).
Annexin V-fluorescein isothiocyanate
(FITC) staining and fluorescence-activated cell sorting (FACS)
The staining protocol was performed, according to
the manufacturer's protocol (BD Biosciences). Briefly,
5×105 cells were harvested centrifugation at 1,000 × g
for 5 min at room temperature and suspended in 195 μl
binding buffer, followed by incubation for 10 min with 5 μl
Annexin V-FITC at room temperature in the dark. Following
additional centrifugation at 1,000 × g for 5 min at room
temperature, the cells were resuspended in 190 μl binding
buffer with 10 μl propidium iodide (Sangon Biotech Co.,
Ltd.) under gentle agitation. FACS was performed using the BD
Accuri C6 (BD Biosciences) and FlowJo software (version 7.6.2; Tree
Star, Inc., Ashland, OR, USA) to detect apoptotic events.
EdU assay
The EdU incorporation assay was performed using an
EdU assay kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China)
according to the manufacturer's instructions. Briefly, HEp-2 cells
(2×105) were incubated with DMEM containing 50 mM EdU
for 2 h. The nuclei were stained with Hoechst 33342 (5
μg/ml; Sigma-Aldrich) and the images were acquired with an
Olympus DP71X microscope.
Statistical analysis
SPSS software (version 16; SPSS, Inc., Chicago, IL,
USA) was used to perform statistical analysis. Data are expressed
as the mean ± standard error of the mean, and one-way analysis of
variance was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
TSA enhances the inhibitory effect of
genistein on HEp-2 cell growth
To evaluate whether genistein or TSA affect cell
viability, the HEp-2 cells were separately incubated with the
compounds, at a range of concentrations, for 2 days. Using an MTT
assay, the half maximal inhibitory concentration (IC50)
of genistein was determined as ~20 μM, and TSA as 100 nM
(Fig. 1A). The combined treatment
strategy of genistein (20 μM) and TSA (100 nM) led to a more
marked reduction in HEp-2 cell viability, compared with the
individual compound treatments. This occurred in a time-dependent
manner between 12 and 48 h (Fig.
1B–D). To confirm this, a 5-ethynyl-20-deoxyuridine
incorporation assay was performed. Cell proliferation was detected
following treatment with genistein and TSA, alone or in
combination, for 24 h. It was found that the combined treatment
with genistein (20 μM) and TSA (100 nM) resulted in a marked
decrease in cell proliferation (Fig.
1E and F). A similar result was obtained in the single colony
formation assay, indicating that the combination treatment had a
more marked effect in impairing the ability of HEp-2 cells to form
colonies, compared with any of the individual treatments. (Fig. 1G and H).
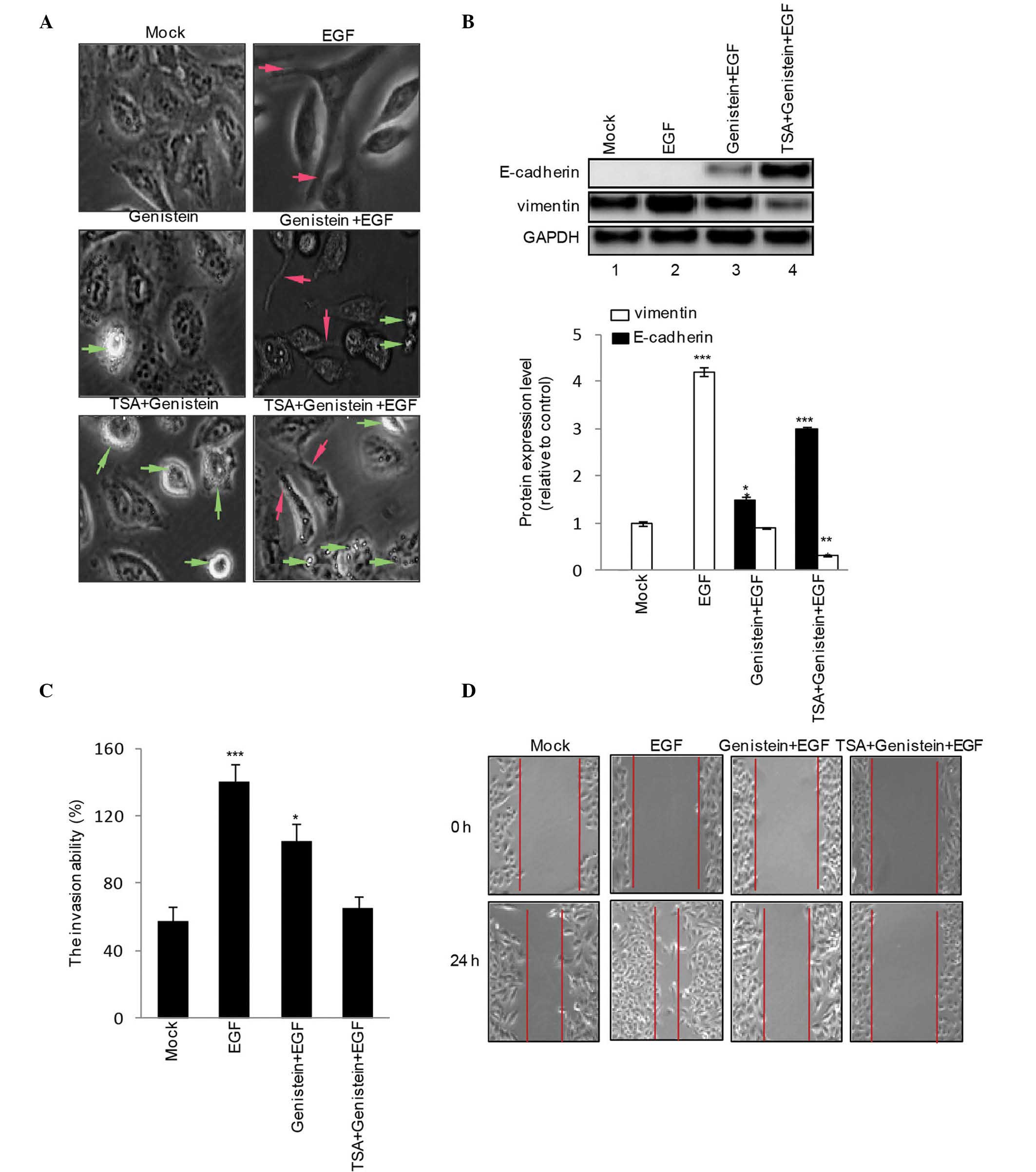
TSA has an additive effect on the
antagonistic effects of genistein on EGF-induced EMT in HEp-2
cells
To assess the role of genistein (20 μM), TSA
(100 nM) and genistein+TSA (20 μM:100 nM) on EGF-induced EMT
phenotype and mensenchymal gene expression levels, the HEp-2 cells
were treated with genistein (20 μM), TSA (100 nM) or
geinstein+TSA (20 μM:100 nM) in the presence or absence of
EGF for 48 h. EGF increased the numbers of cells with spindle-like
shapes and loss of cell-cell contact, a typical phenotype of EMT
(Fig. 2A; red arrow), associated
with an increase in the expression of vimentin (Fig. 2B). Treatment with genistein alone
led to EGF-induced EMT (Fig. 2A;
apoptotic cells indicated by green arrow), increased the expression
of E-cadherin and reduced the expression of vimentin (Fig. 2B), these effects were amplified by
treatment with genistein+TSA. In addition, the results of the
Transwell invasion assay revealed that genistein inhibited
EGF-induced HEp-2 cell invasion, although this was less marked,
compared with combined treatment with genistein and TSA (Fig. 2C). Subsequently, a scratch wound
migration assay was performed. A marked decrease in cell migration
was observed 48 h following treatment with genistein+TSA (Fig. 2D).
TSA promotes genistein-induced HEp2 cell
apoptosis
Following the observation of visible apoptotic
cellular morphology (Fig. 3A) and
peroxisome proliferator-activated receptor γ activation in the
genistein+TSA treated HEp2 cells (Fig.
3B), the present study investigated the extent to which
genistein and TSA induce HEp2 cells apoptosis. Following 48 h
treatment with genstein, TSA or geinstein+TSA, Annexin V/PI
staining demonstrated an elevation in the apoptotic population of
cells in the genistein- and the TSA-treated cells (14.5 and 11.2%,
respectively), however, a more marked increase of 40.3% was
detected in the cells in the combined treatment group (Fig. 3C and D). In addition, the TUNEL
assay revealed similar results to those observed in the Annexin
V/PI staining, with the most marked levels of apoptosis observed in
the combined treatment group (Fig. 3E
and F).
 | Figure 3Effects of combined TSA and genistein
treatment on HEp-2 cells apoptosis. Following treatment for 48 h
with TSA, genistein or TSA+genistein, the HEp-2 cells exhibited a
(A) pro-apoptotic phenotype (magnification, ×200) with (B) PPARγ
activation, detected using western blotting. Fluorescence-activated
cell sorting analysis with (C and D) PI and Annexin VI staining and
a (E and F) TUNEL assay (magnification, ×200) were utilized to
examine the combined effects of TSA+genistein on HEp-2 apoptosis.
The data were obtained from three independent experiments and
expressed as the mean ± standard error of the mean.
*P<0.05, **P<0.01 and
***P<0.001, vs. Mock. TSA, trichostatin A; PPARγ,
peroxisome proliferator-activated receptor γ; PI, propidium iodide;
FITC, fluorescein isothiocyanate. |
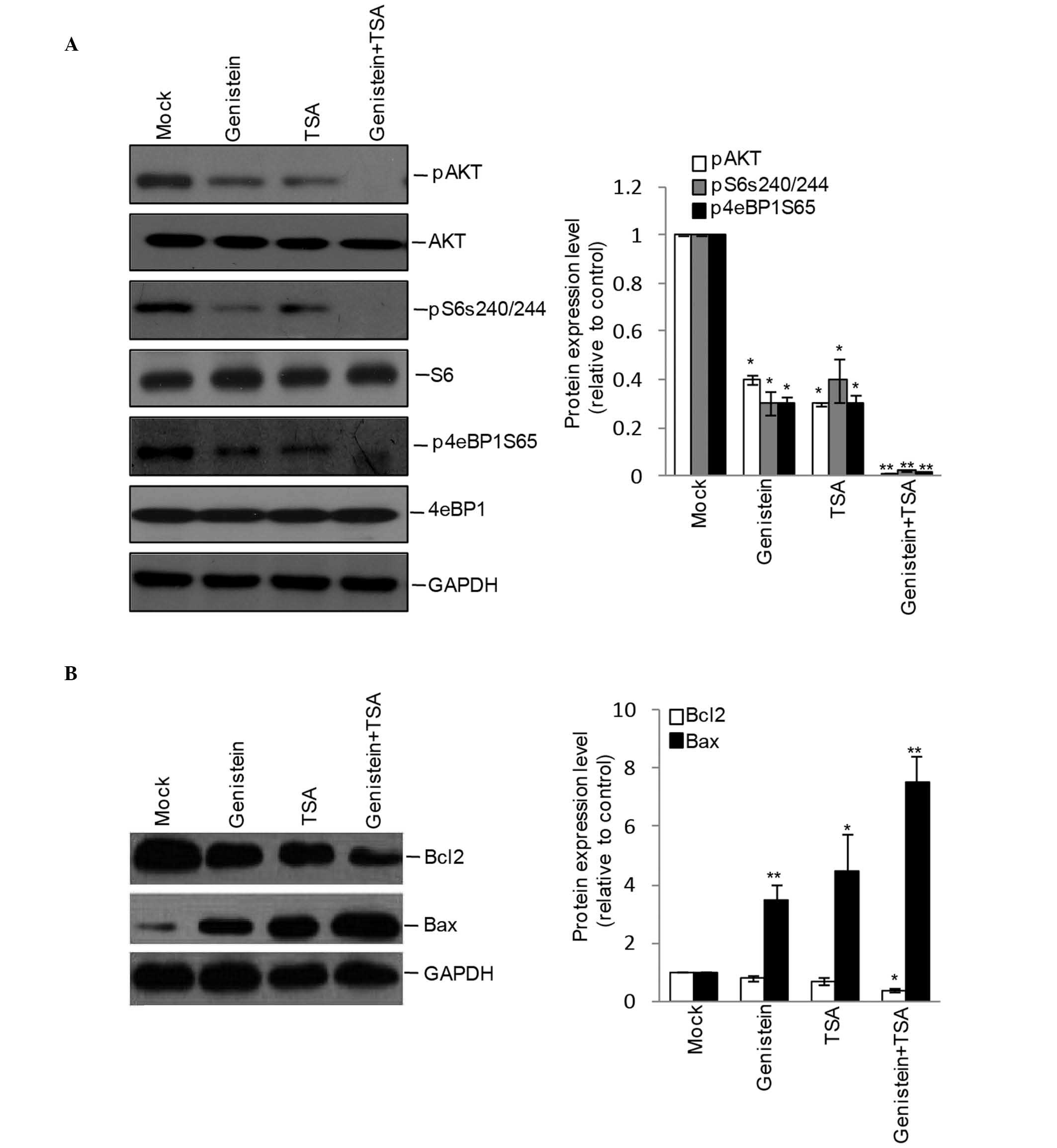
Combined treatment of TSA and genistein
inhibits Akt signaling in HEp2 cells
As the Akt pathway is central in controlling cell
apoptosis, the present study further detected the effects of
genistein, TSA and genistein+TSA on the activation of Akt and its
downstream molecules. Although the phosphorylation levels of Akt,
S6 and 4eBP1 were detectable in the genistein- and TSA-treated
cells, inhibition appeared more marked following combined treatment
with Genstein and TSA (Fig. 4A),
leading to an additive increase in the protein expression of
pro-apoptotic Bax and decrease in the protein expression of
anti-apoptotic Bcl-2 (Fig.
4B).
Discussion
Although currently used chemotherapeutic treatments
improve the prognosis of patients, the identification of improved
combined treatment strategies remains a requirement for improving
the survival rates of patients diagnosed with laryngeal cancer. The
present study aimed to identify efficient drugs for use in
laryngeal cancer therapy, and to evaluate whether or not the
combined effects of these drugs offer a novel therapeutic strategy
for the treatment of laryngeal cancer.
As common chemotherapeutic drugs, genistein and TSA
separately exert tumor suppressive effects in different types of
human cancer, including breast (8), gastric (13), lung (14,15)
and prostate cancer (16), through
various mechanisms. However, the combined consequence of the two
compounds, and their respective effects on the biological function
of laryngeal cancer cells remain to be fully elucidated. In the
present study, genistein and TSA were found to have significant,
although mild effects leading to reduced cell viability, colony
formation and invasion, and increased apoptosis. Compared with
other cancer cells, the tumor-repressing effects of Genstein and
TSA on the HEp2 cells differed, which may explained by the varied
genetic backgrounds of cells. The present study also investigated
the combined contribution of genistein and TSA on laryngeal cancer
growth. The concentrations of genistein and TSA used were 20 and
100 nM, respectively, determined as the IC50 values for
the individual compounds in the cell viability assay, to generate
this level of inhibition without any further additive cell toxicity
and avoid false positive effects caused by additive cell toxicity.
Compared with the effects of the individual drugs, the combined
treatment led to marked attenuation in HEp-2 cell viability, colony
formation and invasion, and marked promotion of apoptosis,
indicating an efficient strategy for suppressing HEp-2 cell
growth.
The present study then aimed to examine the
mechanism underlying genistein+TSA-induced suppression of laryngeal
cancer cells. Due to the marked reduction in invasive ability
observed in the HEp2 cells following genistein+TSA treatment, it
was hypothesized that this may link to HEp2 cell EMT, a crucial
process with a fundamental role in controlling cell migration,
invasion and metastasis (17). In
the present study, EGF was used to trigger HEp2 cell EMT, which led
to increased cell invasion accompanied by an upregulation in the
mensenchymal maker gene, vimentin. Furthermore, the data
experimentally confirmed that genistein alone was able to partially
antagonize the EGF-induced cell invasion and expression of vimentin
and induce the expression of the epithelial gene, E-cadherin, in
the laryngeal cancer HEp-2 cells. These effects have also been
observed in human hepatocellular carcinoma cells (18) and prostate cancer cells (16). In addition, combined treatment with
TSA, a compound previously showing EMT suppression in prostate
cancer PC3 cells (17) and breast
and gastric cancer cells, genistein completely reversed EGF-induced
EMT, indicating the possibility that genistein+TSA suppressed cell
growth and invasion through regulating cellular EMT. In addition,
although the induction of apoptosis by genistein or TSA has been
described in previous reports for other cancer cells, their effects
on laryngeal cancer cell apoptosis remain to be elucidated. In the
present study, it was observed that genistein or TSA had weak
effect on the promotion of cell apoptosis, however, when the two
compounds were applied in combination, a marked, but not additive,
acceleration in apoptotic rate was observed in the HEp2 cells. In
view of the signaling pathway, the present study confirmed that
genistein or TSA partially suppressed the activation of Akt and its
downstream molecules, however, their activation was completely
eliminated by the combined treatment of genistein with TSA. The
increased protein expression of pro-apoptotic Bax and a decreased
protein expression of anti-apoptotic Bcl-2 were also observed.
Therefore, genistein and TSA jointly promoted laryngeal cancer cell
apoptosis via silencing the activation of the Akt pathway.
Although the present study demonstrated that the
combination of genistein+TSA exerted anti-laryngeal tumor effects
in cultured HEp-2 cells, fundamental in vivo investigations
are required. For this purpose, future in vivo experiments
aim to evaluate the pharmacological efficiency of genistein+TSA on
laryngeal cancer therapy in experimental animals.
In conclusion, the present study was the first, to
the best of our knowledge, to report that genistein and TSA possess
anti-aryngeal cancer properties, and further identified the novel
drug combination of genistein+TSA for laryngeal cancer therapy,
which demonstrated marked advantages in suppressing cell growth and
invasion, and promoting apoptosis via the reversal of EMT and
quenching of Akt activation in HEp-2 cells. These results may
provide a basis for the clinical application of genistein and TSA
in the treatment of laryngeal cancer.
Acknowledgments
This study was supported by grants from the Liaoning
Provincial Department of Education Science Research Project (grant
no. L2014299).
References
|
1
|
Miao S, Mao X, Pei R, Miao S, Xiang C, Lv
Y, Yang X, Sun J, Jia S and Liu Y: Antitumor activity of
polysaccharides from Lepista sordida against laryngocarcinoma in
vitro and in vivo. Int J Biol Macromol. 60:235–240. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie J, Jin B, Li DW, Shen B, Cong N, Zhang
TZ and Dong P: ABCG2 regulated by MAPK pathways is associated with
cancer progression in laryngeal squamous cell carcinoma. Am J
Cancer Res. 4:698–709. 2014.PubMed/NCBI
|
|
3
|
Hoffman HT, Porter K, Karnell LH, Cooper
JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A and Robinson RA:
Laryngeal cancer in the United States: Changes in demographics,
patterns of care, and survival. Laryngoscope. (Suppl 111)116:1–13.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kraljevic Pavelic S, Cacev T and Kralj M:
A dual role of p21waf1/cip1 gene in apoptosis of HEp-2 treated with
cisplatin or methotrexate. Cancer Gene Ther. 15:576–590. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Jiang AC, Dong P, Wan Y and Yu ZW:
The characteristics of Hep-2 cell with multiple drug resistance
induced by Taxol. Otolaryngol Head Neck Surg. 137:659–664. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Groth-Pedersen L and Jäättelä M: Combating
apoptosis and multidrug resistant cancers by targeting lysosomes.
Cancer Lett. 332:265–274. 2013. View Article : Google Scholar
|
|
7
|
Suzuki R, Kang Y, Li X, Roife D, Zhang R
and Fleming JB: Genistein potentiates the antitumor effect of
5-Fluorouracil by inducing apoptosis and autophagy in human
pancreatic cancer cells. Anticancer Res. 34:4685–4692.
2014.PubMed/NCBI
|
|
8
|
Chen J, Lin C, Yong W, Ye Y and Huang Z:
Calycosin and genistein induce apoptosis by inactivation of
HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells.
Cell Physiol Biochem. 35:722–728. 2015.PubMed/NCBI
|
|
9
|
Beard N, Benghuzzi H, Tucci M and Cason Z:
The effects of genistein concentrations on Hep-2 cellular function.
Biomed Sci Instrum. 41:199–204. 2005.PubMed/NCBI
|
|
10
|
Dynek JN and Vucic D: Antagonists of IAP
proteins as cancer therapeutics. Cancer Lett. 332:206–214. 2013.
View Article : Google Scholar
|
|
11
|
Li YL, Yang TS, Ruan WM, Cui W, Jin Y and
Zou XM: Effect of trichostatin a on SGC-7901 gastric cancer cells.
Int J Clin Exp Med. 7:1958–1966. 2014.PubMed/NCBI
|
|
12
|
Han C, Gu H, Wang J, Lu W, Mei Y and Wu M:
Regulation of L-threonine dehydrogenase in somatic cell
reprogramming. Stem Cells. 31:953–965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruan WM, Li YL, Nie G, Zhou WX and Zou XM:
Differential expression of glycoprotein non-metastatic melanoma
protein B (GPNMB) involved in trichostatin A-induced apoptosis in
gastric cancer. Int J Clin Exp Med. 7:4857–4866. 2014.
|
|
14
|
Han C, Jin L, Mei Y and Wu M: Endoplasmic
reticulum stress inhibits cell cycle progression via induction of
p27 in melanoma cells. Cell Signal. 25:144–149. 2013. View Article : Google Scholar
|
|
15
|
Liu D, Yan L, Wang L, Tai W, Wang W and
Yang C: Genistein enhances the effect of cisplatin on the
inhibition of non-small cell lung cancer A549 cell growth in vitro
and in vivo. Oncol Lett. 8:2806–2810. 2014.PubMed/NCBI
|
|
16
|
Zhang LL, Li L, Wu DP, Fan JH, Li X, Wu
KJ, Wang XY and He DL: A novel anti-cancer effect of genistein:
Reversal of epithelial mesenchymal transition in prostate cancer
cells. Acta Pharmacol Sin. 29:1060–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Xu J, Wang H, Wu L, Yuan W, Du J
and Cai S: Trichostatin A, a histone deacetylase inhibitor,
reverses epithelial-mesenchymal transition in colorectal cancer
SW480 and prostate cancer PC3 cells. Biochem Biophys Res Commun.
456:320–326. 2015. View Article : Google Scholar
|
|
18
|
Dai W, Wang F, He L, Lin C, Wu S, Chen P,
Zhang Y, Shen M, Wu D, Wang C, et al: Genistein inhibits
hepatocellular carcinoma cell migration by reversing the
epithelial-mesenchymal transition: Partial mediation by the
transcription factor NFAT1. Mol Carcinog. 54:301–311. 2015.
View Article : Google Scholar
|