Introduction
Ototoxicity is the tendency of a drug or chemical
agent to cause inner ear dysfunction, producing symptoms of hearing
loss and/or dizziness. Auditory cells can be injured by infection,
inflammation, loud noise and ototoxic drugs. The inflammatory
response to injury must be accompanied by tissue repair,
remodeling, induction of repair and remodeling capacity may inhibit
ototoxicity.
Prostaglandin-endoperoxide synthases, also termed
cyclooxygenases (COX), are the rate-limiting enzymes that catalyze
the production of prostanoids from arachidonic acid. COX enzymes
are categorized into two subtypes, COX-1 and COX-2. COX-2
expression is induced during inflammatory processes (1–3). A
previous study demonstrated that auditory cells produce
prostaglandin E2 (PGE2) in response to lipopolysaccharide (LPS)
stimulation via increased COX2 expression. PGE2 production may be
involved in tissue repair and remodeling in the organ of Corti
(4).
Rottlerin is a pigmented plant compound isolated
from the gland hair covering the fruit of Mallotus
philippensis. Gschwendt et al (5) and other studies have demonstrated
that rottlerin is a specific inhibitor of protein kinase C (PKC)δ
(6–8). However, certain previous studies
observed that rottlerin has a variety of PKCδ-independent actions
(9–12). The current study aimed to
investigate whether rottlerin affects the gene expression levels of
COX-2. The present study demonstrated that rottlerin-induced COX-2
upregulation is associated with the activation of p38
mitogen-activated protein kinase (MAPK) and increased expression of
activating transcription factor (ATF) 4, an endoplasmic reticulum
(ER) stress-associated transcription factor. Furthermore, the
ability of rottlerin to induce COX-2 expression was independent of
reactive oxygen species (ROS) generation.
Materials and methods
Cells and materials
Mouse auditory HEI-OC1 cells were purchased from the
House Ear Institute (Los Angeles, CA, USA) and cultured in sodium
pyruvate-free Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100
mg/ml streptomycin (all obtained from Welgene, Inc., Gyeongsan,
Korea) at 37°C in a humidified 5% CO2 atmosphere.
Recombinant interleukin (IL)-1β was purchased from R&D Systems,
Inc. Minneapolis, MN, USA). Phorbol-12-myristate-13-acetate (PMA)
was obtained from EMD Millipore (Billerica, MA, USA). Rottlerin,
PD98059, SB203580 and SP600125 were purchased from Enzo Life
Sciences, Inc. (Farmingdale, NY, USA). Glutathione (GSH),
N-acetylcysteine (NAC) and trolox were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Polyclonal mouse anti-COX-2 antibody
(1:1,000, cat. no. 160106) was obtained from the Cayman Chemical
Company (Ann Arbor, MI, USA). Polyclonal rabbit anti-p46/54 c-Jun
N-terminal kinase (JNK, 1:700, cat. no. 9252), polyclonal rabbit
anti-phospho (p)-p46/54 JNK (1:700, cat. no. 9251), polyclonal
rabbit anti-p38MAPK (1:700, cat. no. 9211), polyclonal rabbit
anti-p-p38MAPK (1:700, cat. no. 9212), polyclonal rabbit
anti-extracellular signal-regulated kinase (ERK1/2, 1:700, cat. no.
9102) and polyclonal rabbit anti-p-ERK1/2 (1:700, cat. no. 9101)
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Polyclonal rabbit anti-ATF4 (1:1,000, cat. no.
sc-200) and polyclonal rabbit anti-ATF3 (1:700, cat. no. sc-188)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Polyclonal rabbit anti-regulated in development
and DNA damage responses 1 (REDD1) anti body (1:1,000, cat. no.
10638-1-AP) was obtained from ProteinTech Group, Inc. (Chicago, IL,
USA). Monoclonal mouse anti-actin antibody (1:15,000, cat. no.
A5441) was purchased from Sigma-Aldrich.
Western blot analysis
For the western blotting, cells were washed with
cold phosphate-buffered saline (PBS) and lysed on ice in modified
radioimmunoprecipitation assay buffer (50 mM Tris-HCl pH 7.4, 1%
NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM
Na3VO4 and 1 mM NaF) containing protease
inhibitors (100 µM phenylmethylsulfonyl fluoride, 10
µg/ml leupeptin, 10 µg/ml pepstatin, and 2 mM EDTA).
The lysates were centrifuged at 10,000 × g for 10 min at 4°C and
the supernatant fractions were collected. The protein concentration
was determined using Micro BCA protein assay kit was from Pierce
Chemical (Rockford, IL, USA), according to the manufacturer's
protocol. An equal amount of cell lysate (60 µg) was
dissolved in sample buffer, and samples were boiled for 5 min. The
proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
Immobilon-P membranes (Amersham, Uppsala, Sweden) and were
subsequently blocked for 1 h at room temperature in 5% (w/v)
non-fat dried milk, and then were incubated for overnight at room
temperature with the aforementioned primary antibodies, followed by
an incubation with biotinylated secondary antibodies. The specific
proteins were detected using an enhanced chemiluminescence western
blotting kit (Merck Millipore, Darmstadt, Germany) according to the
manufacturer's instructions. The band intensity of COX-2 protein
was measured using Image J (National Institutes of Health,
Bethesda, MA, USA), and expressed as a ratio to actin band
intensity.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1 µg of
total RNA was used to synthesize cDNA using M-MLV RT (Gibco; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Then, 2 µl of cDNA was amplified with Taq
(Solgent, GeNetBio, South Korea) kits in a total volume of 25
µl according to the manufacturer's protocol. The PCR primers
were purchased from Macrogen (Seoul, South Korea). The following
primers were used for amplification: Sense
5′-ACACACTCTATCACTGGCACC-3′ and antisense 5′-TTCAGGGAGAAGCGTTTGC-3′
for COX-2; and sense 5′-ACGACATGGAGAAGATCTGG-3′ and antisense
5′-TCAGGCAGCTCATAGCTCTT-3′ for actin. The PCR amplification was
performed using the following cycling conditions: 94°C for 3 min;
17 (actin) or 25 cycles (COX-2) of 94°C for 45 sec, 58°C for 45
sec, 72°C for 1 min; and a final extension step at 72°C for 10 min.
The amplified products were separated by electrophoresis on a 1.5%
agarose gel (Invitrogen; Thermo Fisher Scientific, Inc.) and
detected under UV light.
Cell viability assay
MTT assay was used to determine the cell viability
using WelCount Cell Viability Assay kit (WelGENE, South Korea).
Briefly, 24 h after drug treatment the reagent was added to each
well and then measured with multi-well plate reader (at 450/690
nm).
Transfection and small interfering RNA
(siRNA)
HEI-OC1 cells were seeded at a density of
1×105 cells/well in 6-well culture plates one day prior
to transfection to achieve 50–60% confluency. The ATF4 and green
fluorescent protein (GFP) control siRNA duplexes used in the
present study were purchased from Santa Cruz Biotechnology, Inc.
and Invitrogen; Thermo Fisher Scientific, Inc., respectively. Cells
were transfected with siRNA oligonucleotides using Oligofectamine
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol.
Measurement of ROS
Intracellular accumulation of ROS was determined
using the fluorescent probe 2′, 7′-dichlorodihydrofluorescein
diacetate (H2DCFDA). H2DCFDA is commonly used to measure
ROS generation (13). Cells were
seeded in a 6-well plate for 24 h prior to treatment with 5
µM rottlerin, and treated with 5 µM H2DCFDA for 30
min. Cells were then trypsinized (Welgene, Inc., Gyeongsan, South
Korea) and resuspended in PBS. Fluorescence was measured at the
desired time intervals using a flow cytometer (BD FACSCanto II; BD
Biosciences, Franklin Lakes, NJ, USA) or detected using a
fluorescence microscope (Axiovert200; Zeiss GmbH, Jena,
Germany).
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent experiments. Data were
analyzed with one-way analysis of variance and Student-Newman-Keuls
post hoc comparisons using the Statistical Package for Social
Sciences software version 22.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rottlerin induces COX-2 expression in
HEI-OC1 cells
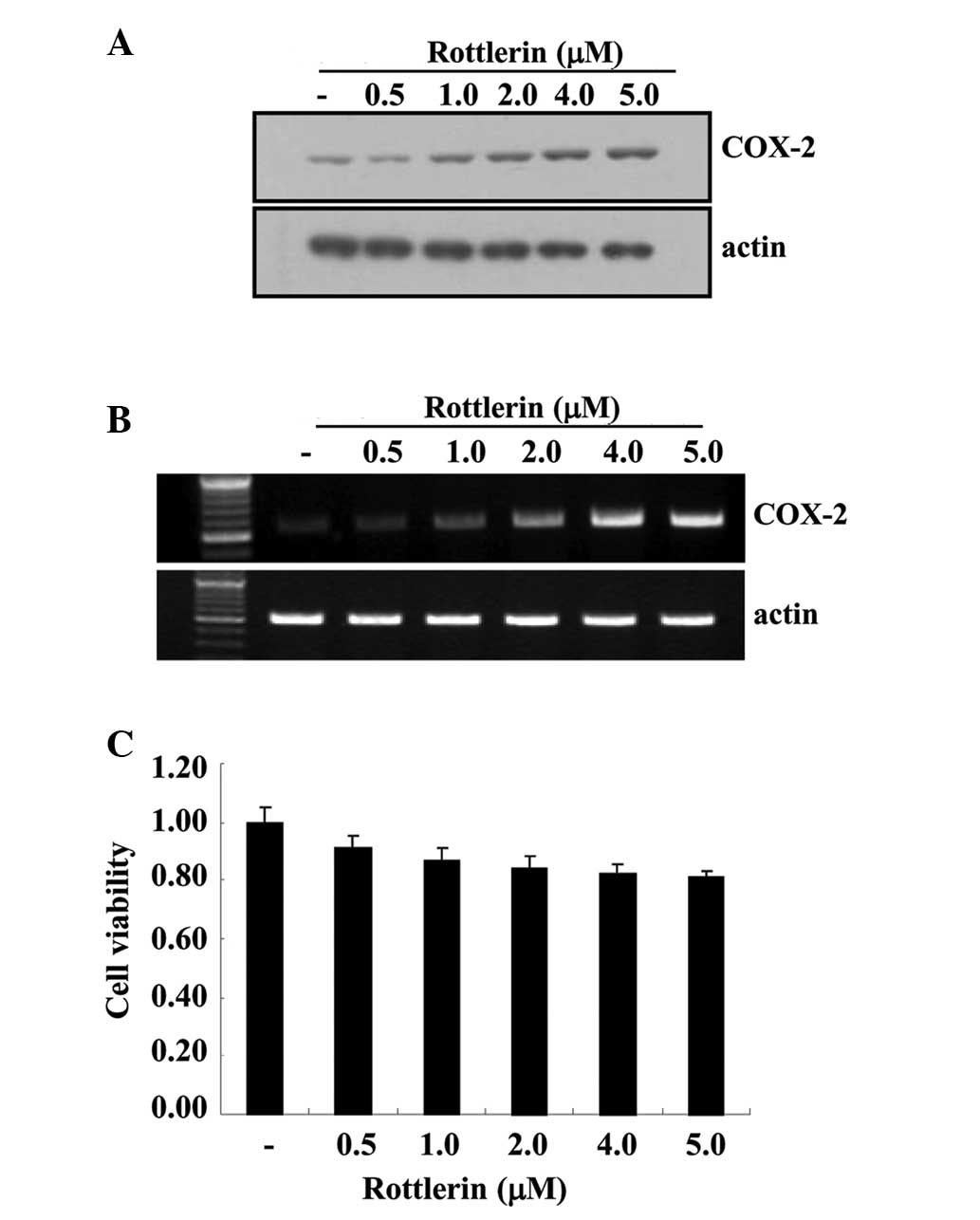
To determine whether rottlerin affects the mRNA and
protein expression levels of COX-2, HEI-OC1 cells were treated with
various rottlerin concentrations (0.5–5.0 µM) for 12 h. As
demonstrated in Fig. 1A, COX-2
protein levels were increased by rottlerin in a dose-dependent
manner. To elucidate the correlation between COX-2 protein and
COX-2 mRNA in HEI-OC1 cells, RT-PCR was performed. The levels of
COX-2 mRNA were also increased by rottlerin in a dose-dependent
manner. Additionally, the cytotoxic effects of rottlerin were
evaluated by MTT assay. Cell viability following rottlerin
treatment was determined to be >90% using the MTT assay
(Fig. 1C).
ROS generation did not affect
rottlerin-induced COX-2 expression in HEI-OC1 cells
Several previous studies have suggested that ROS are
associated with signaling mechanisms that induce COX-2 expression
(14,15). Therefore, the present study
examined whether rottlerin induces ROS production using
H2DCFDA-derived fluorescence and fluorescence-activated cells
sorting detection in HEI-OC1 cells. As presented in Fig. 2A and B, compared with untreated
cells, rottlerin significantly increased the intracellular ROS
levels within 10 min of treatment (P<0.0001). Additionally, the
present study investigated whether blockade of rottlerin-induced
ROS generation affects COX-2 expression levels in HEI-OC1 cells.
Compared with rottlerin treatment, pretreatment with the
antioxidants NAC, GSH and trolox did not inhibit rottlerin-induced
COX-2 protein expression (Fig.
2C). These results suggest that ROS are not involved in
rottlerin-induced COX-2 expression.
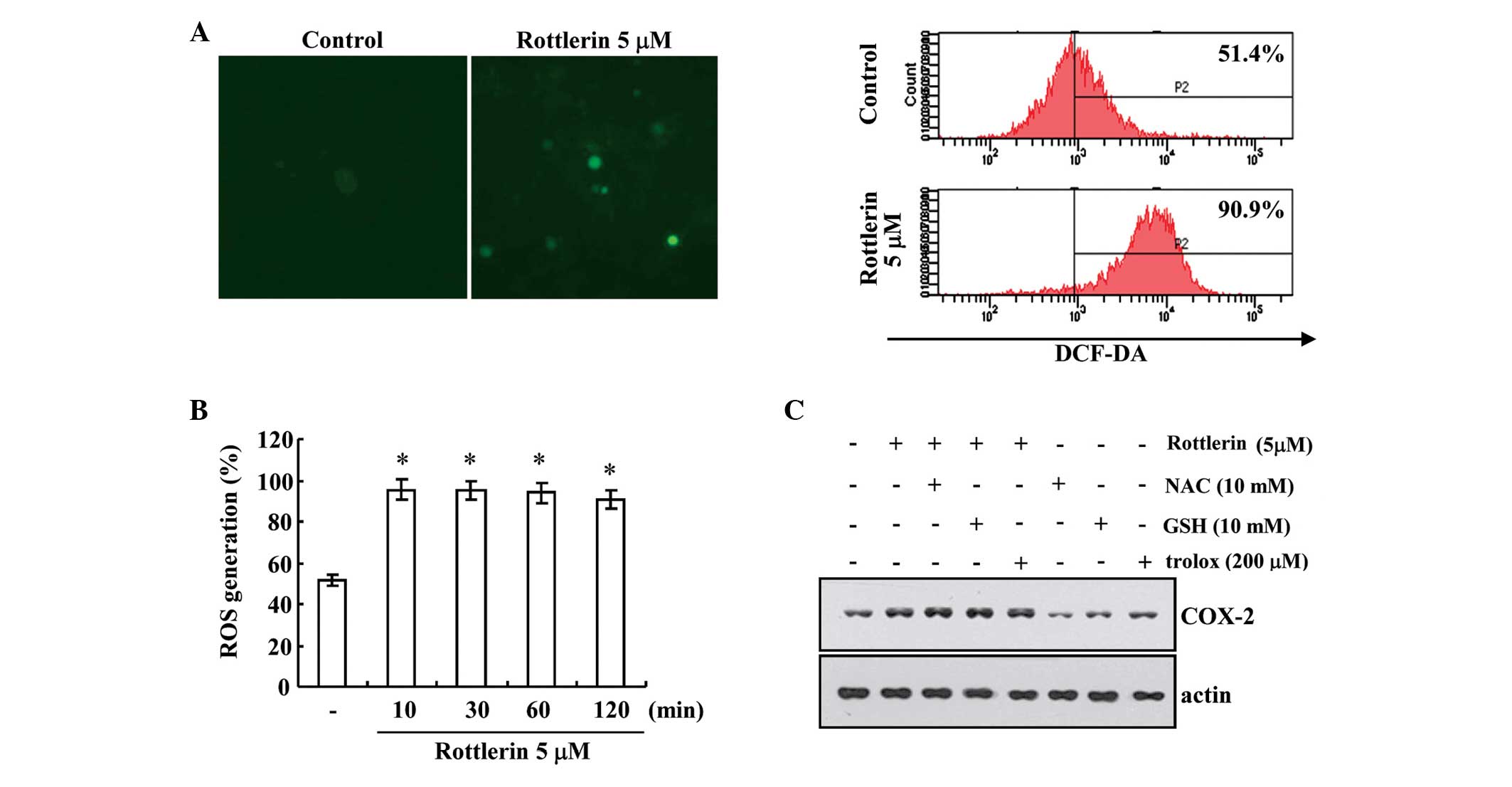 | Figure 2Effect of ROS generation on COX-2
expression in rottlerin treated HEI-OC1 cells. HEI-OC1 cells were
treated with 5 µM rottlerin for 10, 30, 60 and 120 min, and
then loaded with a DCF-DA fluorescent dye. DCF-DA fluorescence
intensity was detected by (A) fluorescence microscopy (left panel)
and flow cytometry (right panel) at 2 h. Magnificatio, ×200. (B)
Rottlerin (5 µM) increased ROS generation following 10, 20,
60 and 120 min treatment, as measured by flow cytometry. (C)
HEI-OC1 cells were pretreated with 10 mM NAC, 10 mM GSH and 200
µM trolox, and then 5 µM rottlerin for a further 12
h. The protein expression levels of COX-2 and actin were determined
by western blotting. The level of actin was used as a loading
control. *P<0.001 vs. control. DCF-DA,
dichloro-dihydro-fluorescein diacetate; ROS, reactive oxygen
species; NAC, N-acetylcysteine; GSH, glutathione; COX-2,
cyclooxygenase-2. |
Rottlerin-induced COX-2 expression is
associated with activation of p38MAPK
The current study investigated the effect of
rottlerin on MAPK activity in order to determine whether this
signaling pathway is involved in rottlerin-induced COX-2
expression. As shown in Fig. 3A,
rottlerin markedly increased the phosphorylation of p38MAPK, but
not of ERK and JNK. p38MAPK activation was detected at 3 h and
subsequently increased in a time-dependent manner (Fig. 3A). To determine the importance of
individual MAPK pathways in rottlerin-induced COX-2 expression, the
present study examined the effects of PD98059 (a potent ERK
inhibitor), SB203580 (a specific p38MAPK inhibitor) and SP600125 (a
potent JNK inhibitor) on rottlerin-induced COX-2 expression. As
presented in Fig. 3B, pretreatment
with SB203580 in the presence of rottlerin resulted in a 0.57-fold
decrease in COX-2 protein expression levels compared with rottlerin
treatment, but SP600125 and PD98059 did not affect COX-2 protein
expression. To further investigate the correlation between the
p38MAPK pathway and rottlerin-induced COX-2 expression, SB203580
dose-response experiments were conducted in rottlerin-treated
HEI-OC1 cells. As demonstrated in Fig.
3C, SB203580 markedly reduced COX-2 protein expression levels
in a dose-dependent manner. Together these results indicate that
the activation of p38MAPK pathway is important for the regulation
of rottlerin-induced COX-2 expression in HEI-OC1 cells.
 | Figure 3p38MAPK is associated with
rottlerin-induced COX-2 expression in HEI-OC1 cells. (A) HEI-OC1
cells were treated with 5 µM rottlerin for the indicated
time periods. The protein expression levels of phopho (p)-p38 MAPK,
p38, p-JNK, JNK, p-ERK, ERK and actin were determined by western
blotting. (B) HEI-OC1 cells were pretreated with the ERK inhibitor
PD98059 (50 µM), the p38MAPK inhibitor SB203580 (10
µM) or a JNK inhibitor SP600125 (20 µM), and treated
with 5 µM rottlerin for 12 h. The protein expression levels
of COX-2 and actin were determined by western blotting. (C) HEI-OC1
cells were pretreated with the indicated concentrations with
SB203580, and treated with 5 µM rottlerin for 12 h. The
protein expression levels of COX-2 and actin were determined by
western blotting. Actin served as a loading control. p-, phospho;
p38MAPK, p38 mitogen-activated protein kinase; JNK, c-Jun
N-terminal kinase; ERK, extracellular signal-regulated kinase;
COX-2, cyclooxygenase-2. |
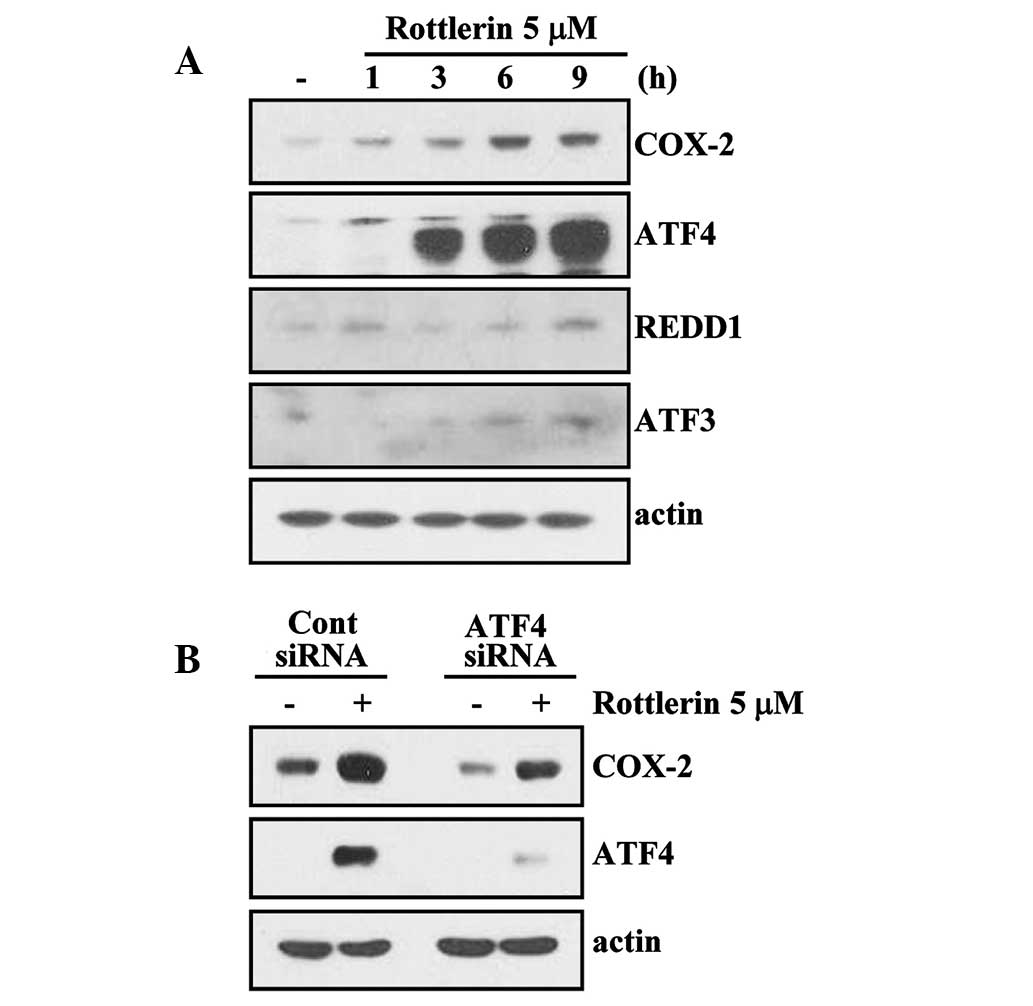
Rottlerin induces COX-2 expression via
activation of the ATF4 transcription factor
A previous study demonstrated that ER
stress-inducing agents can induce COX-2 expression (16). Therefore, kinetic studies were
performed on rottlerin-treated HEI-OC1 cells to determine the
effects of ER stress-associated transcription factors (REDD, ATF 3
and 4) on rottlerin-induced COX-2 expression. Incubation with
rottlerin caused a time-dependent increase in COX-2 protein
expression (Fig. 4). Notably, ATF4
protein expression levels were markedly increased by treatment with
rottlerin compared with untreated cells. However, ATF3 and REDD1
levels were not identified to be increased in response to rottlerin
(Fig. 4A). To examine whether
rottlerin-induced COX-2 expression is dependent on ATF4 activity,
an siRNA duplex targeting AFT4 mRNA was used. HEI-OC1 cells
transfected with the control (GFP) or ATF4 siRNA were treated with
rottlerin for 12 h. Suppression of ATF4 expression by transfection
with siRNA inhibited rottlerin-induced upregulation of COX-2
protein levels compared with control siRNA (Fig. 4B). These results suggest that the
expression of ATF4 is critical for rottlerin-induced COX-2
expression.
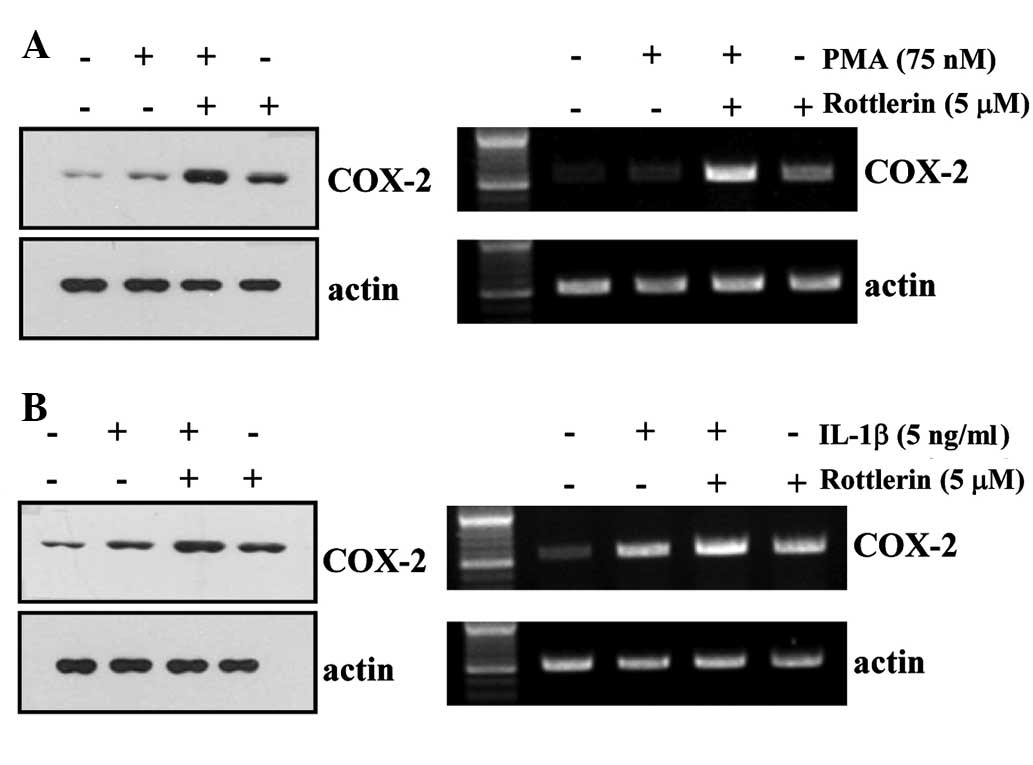
Rottlerin enhances PMA- and IL-1β-induced
COX-2 expression in HEI-OC1 cells
PMA and IL-1β have previously been demonstrated to
induce COX-2 expression (17-23).
Western blotting and RT-PCR were performed to determine the effect
of rottlerin on PMA- and IL-1β-induced COX-2 expression in HEI-OC1
cells. As demonstrated in Fig. 5,
treatment with rottlerin enhances PMA and IL-1β-induced COX-2
protein and mRNA expression compared with treatment with PMA/IL-1β
alone.
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that treatment of HEI-OC1 cells with
rottlerin markedly increases COX-2 protein and mRNA expression
levels. COX-2 expression is regulated differently depending on the
stimulus, cellular environment and cell type (24). Previous mechanistic studies of
COX-2 transcriptional regulation have demonstrated that the
transcription factors nuclear factor (NF)-κB, NF-IL-6, cAMP
response element and activator protein-1 are critical for COX-2
expression (24–26). Additionally, post-transcriptional
regulation of COX-2 expression has been predominantly attributed to
stabilization of the COX-2 mRNA by p38MAPK signaling and RNA
binding proteins (including ELAV-like RNA binding protein 1 and
ZFP36 ring finger protein) (27).
Studies using pharmacological MAPK inhibitors demonstrated the
importance of p38MAPK in rottlerin-induced COX-2 upregulation in
HEI-OC1cells.
Previous studies demonstrated that rottlerin induces
several characteristic ER stress markers, including upregulation of
heat shock protein family A member 5 and DNA damage inducible
transcript 3, phosphorylation of eukaryotic translation initiation
factor 2α and ER stress-specific X-box binding protein-1 splicing
(28). ER stress-inducing agents
enhance COX-2 expression in various cell types (16,29).
The present study demonstrated that suppression of ATF4 using siRNA
attenuated rottlerin-induced COX-2 expression (Fig. 4B), suggesting that ATF4 may be
critical for rottlerin-induced COX-2 expression. Rottlerin enhances
IL-1β-induced COX-2 expression through sustained p38MAPK activation
in breast cancer cells (30).
Rottlerin had been previously demonstrated as a specific inhibitor
of PKCδ (5). However in a previous
study, suppression of PKCδ expression with siRNA did not upregulate
IL-1β-induced COX-2 expression (30). Furthermore, rottlerin has been
demonstrated to induce pro-apoptotic ER stress via the
PKCδ-independent pathway (28).
Thus, PKCδ may be not involved in rottlerin- and IL-1β-induced
COX-2 upregulation in HEI-OC1 cells.
Feng et al (31) reported that reactive oxygen
intermediates are a specific and important regulator COX-2
expression induced by IL-1, tumor necrosis factor-α and LPS. A
previous study demonstrated that rottlerin increases heme
oxygenase-1 (HO-1) expression through ROS generation.
Rottlerin-induced HO-1 upregulation was abrogated in the presence
of NAC antioxidant (12). However,
in the present study, pretreatment with a ROS scavenger did not
suppress rottlerin-induced COX-2 upregulation. The precise
mechanisms underlying ROS-mediated regulation of rottlerin-induced
COX-2 upregulation remain to be fully elucidated. Furthermore,
COX-2 expression was enhanced when HEI-OC1 cells were concurrently
treated with rottlerin and IL-1β or PKC activator, PMA. The
findings of the present study suggest that other signaling pathways
are involved in rottlerin-induced COX-2 expression in human
auditory cells, which require further investigation. Additional
research is expected to be beneficial for the treatment of cochlear
inflammation-associated hearing loss.
Acknowledgments
The present study was supported by an NRF grant
funded by the Korean Government (MSIP; grant no. 2014R1A5A2010008)
and a 2015 Scholar Research Grant from Keimyung University (Daegu,
South Korea).
References
|
1
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: Structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsatsanis C, Androulidaki A, Venihaki M
and Margioris AN: Signalling networks regulating cyclooxygenase-2.
Int J Biochem Cell Biol. 38:1654–1661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Griswold DE and Adams JL: Constitutive
cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2):
Rationale for selective inhibition and progress to date. Med Res
Rev. 16:181–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanigawa T, Odkhuu E, Morikawa A, Hayashi
K, Sato T, Shibata R, Goto F, Ueda H and Yokochi T: Immunological
role of prostaglandin E2 production in mouse auditory cells in
response to LPS. Innate Immun. 20:639–646. 2014. View Article : Google Scholar
|
|
5
|
Gschwendt M, Müller HJ, Kielbassa K, Zang
R, Kittstein W, Rincke G and Marks F: Rottlerin, a novel protein
kinase inhibitor. Biochem Biophys Res Commun. 199:93–98. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cross T, Griffiths G, Deacon E, Sallis R,
Gough M, Watters D and Lord JM: PKC-delta is an apoptotic lamin
kinase. Oncogene. 9:2331–2337. 2000. View Article : Google Scholar
|
|
7
|
Kontny E, Kurowska M, Szczepańska K and
Maśliński W: Rottlerin, a PKC isozyme-selective inhibitor, affects
signaling events and cytokine production in human monocytes. J
Leukoc Biol. 67:249–258. 2000.PubMed/NCBI
|
|
8
|
Hsieh HL, Wang HH, Wu CY, Jou MJ, Yen MH,
Parker P and Yang CM: BK-induced COX-2 expression via
PKC-delta-dependent activation of p42/p44 MAPK and NF-kappaB in
astrocytes. Cell Signal. 19:330–340. 2007. View Article : Google Scholar
|
|
9
|
Soltoff SP: Rottlerin: An inappropriate
and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci.
8:453–458. 2007. View Article : Google Scholar
|
|
10
|
Song KS, Kim JS, Yun EJ, Kim YR, Seo KS,
Park JH, Jung YJ, Park JI, Kweon GR, Yoon WH, et al: Rottlerin
induces autophagy and apoptotic cell death through a
PKC-delta-independent pathway in HT1080 human fibrosarcoma cells:
The protective role of autophagy in apoptosis. Autophagy.
4:650–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim JH, Park JW, Choi KS, Park YB and Kwon
TK: Rottlerin induces apoptosis via death receptor 5 (DR5)
upregulation through CHOP-dependent and PKC delta-independent
mechanism in human malignant tumor cells. Carcinogenesis.
30:729–736. 2009. View Article : Google Scholar
|
|
12
|
Park EJ, Lim JH, Nam SI, Park JW and Kwon
TK: Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through
reactive oxygen species (ROS) dependent and PKC delta-independent
pathway in human colon cancer HT29 cells. Biochimie. 92:110–115.
2010. View Article : Google Scholar
|
|
13
|
LeBel CP, Ischiropoulos H and Bondy SC:
Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of
reactive oxygen species formation and oxidative stress. Chem Res
Toxicol. 5:227–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y and Wahl LM: Oxidative stress
augments the production of matrix metalloproteinase-1,
cyclooxygenase-2, and prostaglandin E2 through enhancement of
NF-kappa B activity in lipopolysaccharide-activated human primary
monocytes. J Immunol. 175:5423–5429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JJ, Huang WC and Chen CC:
Transcriptional regulation of cyclooxygenase-2 in response to
proteasome inhibitors involves reactive oxygen species-mediated
signaling pathway and recruitment of CCAAT/enhancer-binding protein
delta and CREB-binding protein. Mol Biol Cell. 16:5579–5591. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hung JH, Su IJ, Lei HY, Wang HC, Lin WC,
Chang WT, Huang W, Chang WC, Chang YS, Chen CC and Lai MD:
Endoplasmic reticulum stress stimulates the expression of
cyclooxygenase-2 through activation of NF-kappaB and pp38
mitogen-activated protein kinase. J Biol Chem. 279:46384–46392.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang ZF, Massey JB and Via DP:
Differential regulation of cyclooxygenase-2 (COX-2) mRNA stability
by interleukin-1 beta (IL-1 beta) and tumor necrosis factor-alpha
(TNF-alpha) in human in vitro differentiated macrophages. Biochem
Pharmacol. 59:187–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakao S, Ogtata Y, Shimizu E, Yamazaki M,
Furuyama S and Sugiya H: Tumor necrosis factor alpha
(TNF-alpha)-induced prostaglandin E2 release is mediated by the
activation of cyclo-oxygenase-2 (COX-2) transcription via NFkappaB
in human gingival fibroblasts. Mol Cell Biochem. 238:11–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molina-Holgado E, Ortiz S, Molina-Holgado
F and Guaza C: Induction of COX-2 and PGE(2) biosynthesis by
IL-1beta is mediated by PKC and mitogen-activated protein kinases
in murine astrocytes. Br J Pharmacol. 131:152–159. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miralpeix M, Camacho M, López-Belmonte J,
Canalías F, Beleta J, Palacios JM and Vila L: Selective induction
of cyclo-oxygenase-2 activity in the permanent human endothelial
cell line HUV-EC-C: Biochemical and pharmacological
characterization. Br J Pharmacol. 121:171–180. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shishodia S, Koul D and Aggarwal BB:
Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced
NF-kappa B activation through inhibition of activation of I kappa B
alpha kinase and Akt in human non-small cell lung carcinoma:
Correlation with suppression of COX-2 synthesis. J Immunol.
73:2011–2022. 2004. View Article : Google Scholar
|
|
22
|
Mitchell JA, Belvisi MG, Akarasereenont P,
Robbins RA, Kwon OJ, Croxtall J, Barnes PJ and Vane JR: Induction
of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial
cells: Regulation by dexamethasone. Br J Pharmacol. 113:1008–1014.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang YJ, Lu B, Choy PC and Hatch GM:
Regulation of cytosolic phospholipase A2, cyclooxygenase-1 and -2
expression by PMA, TNFalpha, LPS and M-CSF in human monocytes and
macrophages. Mol Cell Biochem. 246:31–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang YJ, Mbonye UR, DeLong CJ, Wada M and
Smith WL: Regulation of intracellular cyclooxygenase levels by gene
transcription and protein degradation. Prog Lipid Res. 46:108–125.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamura M, Sebastian S, Yang S, Gurates B,
Fang Z, Okamura K and Bulun SE: Induction of cyclooxygenase-2 in
human endometrial stromal cells by malignant endometrial epithelial
cells: Evidence for the involvement of extracellularly regulated
kinases and CCAAT/enhancer binding proteins. J Mol Endocrinol.
31:95–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wardlaw SA, Zhang N and Belinsky SA:
Transcriptional regulation of basal cyclooxygenase-2 expression in
murine lung tumor-derived cell lines by CCAAT/enhancer-binding
protein and activating transcription factor/cAMP response
element-binding protein. Mol Pharmacol. 62:326–333. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lasa M, Mahtani KR, Finch A, Brewer G,
Saklatvala J and Clark AR: Regulation of cyclooxygenase 2 mRNA
stability by the mitogen-activated protein kinase p38 signaling
cascade. Mol Cell Biol. 20:4265–4274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim JH, Park JW, Kim SH, Choi YH, Choi KS
and Kwon TK: Rottlerin induces pro-apoptotic endoplasmic reticulum
stress through the protein kinase C-delta-independent pathway in
human colon cancer cells. Apoptosis. 13:1378–1385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rasheed Z and Haqqi TM: Endoplasmic
reticulum stress induces the expression of COX-2 through activation
of eIF2α, p38-MAPK and NF-κB in advanced glycation end products
stimulated human chondrocytes. Biochim Biophys Acta.
1823:2179–2189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park EJ and Kwon TK: Rottlerin enhances
IL-1β-induced COX-2 expression through sustained p38 MAPK
activation in MDA-MB-231 human breast cancer cells. Exp Mol Med.
43:669–675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng L, Xia Y, Garcia GE, Hwang D and
Wilson CB: Involvement of reactive oxygen intermediates in
cyclooxygenase-2 expression induced by interleukin-1, tumor
necrosis factor-alpha, and lipopolysaccharide. J Clin Invest.
95:1669–1675. 1995. View Article : Google Scholar : PubMed/NCBI
|