Introduction
Reactive oxygen species (ROS) are inevitably
produced during cellular energy metabolism (1), however, increased intracellular ROS
production causes oxidative damage to critical macromolecules,
including DNA, proteins and lipids (2–4).
Oxidative stress has been considered a major aspect of aging
(5–8), and is closely associated with an
onset of several neurodegenerative diseases (9,10).
Numerous antioxidant enzymes, such as superoxide
dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx),
thioredoxin (Trx) and peroxiredoxin (Prx) defend against oxidative
stress by maintaining a balanced intracellular redox state
(11,12); SOD converts superoxide radial
(O2•−) to hydrogen peroxide
(H2O2) and water, and CAT or GPx converts
H2O2 to water and oxygen (13).
Alterations in various molecular processes in the
hippocampus during aging is a great deal of interest as
hippocampus-dependent learning and memory is impaired in nearly
half of the healthy elderly population over 60 years of age
(14). In addition, the
hippocampus is vulnerable to neurological diseases, such as
cerebral ischemia, vascular dementia and Alzheimer's disease
(15–17). In this regard, numerous researchers
have demonstrated age-related changes in the levels of antioxidant
enzymes levels in the hippocampus. In particular, it has been
reported that CAT activity is unchanged or decreased in the aged
hippocampus of the mouse (18) and
rat (19,20).
Although certain studies have reported the
age-associated changes in CAT activity in the hippocampus, it has
not been fully elucidated. Therefore, in the present study, in
order to show fundamental data on the change in CAT levels during
normal aging, CAT expression was compared in the hippocampus among
the young, adult, and aged mice and rats, which are good animal
models in aging research (21,22),
using immunohistochemistry and western blot analysis.
Materials and methods
Experimental animals
Male ICR mice and Sprague Dawley rats were purchased
from Orient Bio Inc. (Seongnam, Korea). They were used at ages 1, 6
and 24 months for the young, adult and aged groups, respectively,
as the ages of mice and rats are similar (23–25).
The animals (n=14/group) were housed in a conventional state under
adequate temperature (23°C) and humidity (60%) control with a 12-h
light/dark cycle, and provided free access to food and water.
Animal handling and care followed the guidelines of current
international laws and policies (NIH Guide for the Care and Use of
Laboratory Animals, The National Academies Press, 8th Ed., 2011)
(26), and the experimental
protocols were approved by the Institutional Animal Care and Use
Committee of Kangwon National University (Chuncheon, Korea;
approval no. KW-130424-3). All experiments were conducted with the
aim of minimizing the number of animals used and avoiding animal
suffering.
Tissue processing for histology
The animals (n=7/group) were anesthetized
intraperitoneally 40 mg/kg pentobarbital sodium (JW Pharmaceutical
Co., Ltd., Seoul, Korea) and perfused transcardially with 0.1 M
phosphate-buffered saline (PBS, pH 7.4, Sigma-Aldrich, St. Louis,
MO, USA) followed by 4% paraformaldehyde (Sigma-Aldrich) in 0.1 M
PBS (pH 7.4, Sigma-Aldrich). The brains were removed and post-fixed
with the same solution for 6 h. The tissues were cryoprotected by
infiltration with 30% sucrose (Sigma-Aldrich) overnight. The brain
tissues were then frozen and sectioned (30 µm) with a
cryostat (CM1520, Leica Microsystems, Wetzlar, Germany), and
consecutive sections were collected in six-well plates containing
0.1 M PBS.
Immunohistochemistry
To examine age-related changes in NeuN and CAT
immunoreactivity in the hippocampi of the mice and rats,
immunohistochemical staining and quantitative analysis of
immunohistochemical data were performed according to a previous
method (27). Monoclonal mouse
anti-NeuN (1:800; EMD Millipore, Billerica, MA, USA; cat. no.
MAB377) and polyclonal rabbit anti-CAT antibody (1:250; Abcam,
Cambridge, MA, USA; cat. no. ab52477) used as primary antibodies
overnight at 4°C, followed by incubation with biotinylated horse
anti-mouse (1:200; cat. no. AI-2000) or goat anti-rabbit IgG
(1:200; cat. no. AI-1000) obtained from Vector Laboratories, Inc.,
Burlingame, CA, USA) secondary antibodies for 2 h at room
temperature and streptavidin peroxidase complex (1:200; Vector
Laboratories, Inc.). A negative control test was conducted using 5%
pre-immune serum (Vector Laboratories, Inc.) instead of primary
antibody in order to establish the specificity of the
immunostaining. The negative control resulted in the absence of
immunoreactivity in any neurons.
According to anatomical landmarks corresponding to
the mouse and rat brain atlas, seven sections with 120-µm
intervals per animal were selected to quantitatively analyze NeuN
and CAT immunoreactivity, respectively. As previously described
(28), digital images of the
hippocampus were captured with an AxioM1 light microscope (Carl
Zeiss, Oberkochen, Germany) equipped with a digital camera
(Axiocam, Carl Zeiss) connected to a PC monitor. NeuN- and
CAT-immunoreactive neurons were counted in a 250×250 µm
square in the hippocampi using an image analyzing system with
Optimas 6.5 (CyberMetrics, Scottsdale, AZ, USA) software. Cell
counts were obtained by averaging the counts from each animal. In
addition, the density of CAT-immunoreactive neurons was evaluated
on the basis of optical density (OD), which was obtained after the
transformation of the mean gray level using the formula: OD=log
(256/mean gray level). The OD of the background was taken from
areas adjacent to the measured area. After the background density
was subtracted, a ratio of the optical density of the image file
was calibrated as a % (relative optical density, ROD) using Adobe
Photoshop version 8.0 (Adobe Systems Inc., San Jose, CA, USA) and
then analyzed using NIH Image J software (version 1.46; NIH Image,
Bethesda, MD, USA). A ratio of the ROD was calibrated as a %, with
the young group designated as 100%.
Western blot analysis
To examine changes in the CAT levels in the mouse
and rat hippocampi between groups, animals at each age (n=7) were
used, and western blot analysis was performed according to a
previous method (27). In brief,
after sacrificing the animals by cervical dislocation, the striatum
was removed. The tissues were then homogenized in 50 mM PBS
containing 0.1 mM ethylene glycol bis (2-aminoethyl ether)-N, N,
N′, N′ tetraacetic acid (pH 8.0, Sigma-Aldrich), 0.2% Nonidet P-40
(Sigma-Aldrich), 10 mM ethylendiamine tetraacetic acid (pH 8.0,
Sigma-Aldrich), 15 mM sodium pyrophosphate (Sigma-Aldrich), 100 mM
β-glycerophosphate, (Sigma-Aldrich), 50 mM NaF (Sigma-Aldrich), 150
mM NaCl (Sigma-Aldrich), 2 mM sodium orthovanadate (Sigma-Aldrich),
1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich) and 1 mM
dithiothreitol (DTT, Sigma-Aldrich). After centrifugation at 10,000
× g for 20 min, the protein level in the supernatants was
determined using a Micro bicinchoninic acid protein assay kit
(Sigma-Aldrich) with bovine serum albumin as a standard (Pierce
Chemical, Rockford, IL, USA). Aliquots containing 20 µg
total protein were boiled in loading buffer containing 150 mM Tris
(pH 6.8, Sigma-Aldrich), 3 mM DTT, 6% SDS, 0.3% bromophenol blue
and 30% glycerol. The aliquots were then loaded onto a 12%
polyacrylamide gel. After electrophoresis, the gels were
transferred to nitrocellulose transfer membranes (Pall Corp., East
Hills, NY, USA). To reduce background staining, the membranes were
incubated with 5% non-fat dry milk (Sigma-Aldrich) in PBS
containing 0.1% Tween-20 (PBST; Sigma-Aldrich) for 45 min at room
temperature. Membranes were incubated with polyclonal rabbit
anti-CAT antibody (1:1,000) or monoclonal mouse β-actin (1:5,000;
Sigma-Aldrich; cat. no. A5316) overnight at 4°C. Following washing
with PBST three times, the membranes were incubated with
peroxidase-conjugated donkey anti-rabbit IgG (1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-2305) or goat
anti-mouse (1:1,000; Santa Cruz Biotechnology, Inc.; cat. no.
sc-2031) for 1 h at room temperature. An enhanced chemiluminescence
kit (Pierce ECL Western Blotting Substrate; Thermo Fisher
Scientific, Inc.) was used to detect the protein expression.
Western blot analysis was performed with three repetitions. The
result of the western blot analysis was scanned, and densitometric
analysis for the quantification of the bands was conducted using
Scion Image software (version 4.0.3.2; Scion Corp., Frederick, MD,
USA), which was used to count ROD. CAT levels were normalized to
β-actin, used as the internal control protein, respectively. A
ratio of the ROD was calibrated as %, with the young group
designated as 100%.
Statistical analysis
The data shown here represent the mean ± standard
error of the mean. Differences of the means among the groups were
statistically analyzed by analysis of variance with a post hoc
Bonferroni's multiple comparison test in order to elucidate
age-related differences among groups. Statistical analysis was
performed using GraphPad Instat (version 3.05; GraphPad Software,
Inc.; La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
NeuN immunoreactivity
Mice
Numerous NeuN-immunoreactive neurons were
distributed in the stratum pyramidale of the hippocampus proper
(CA1-3 regions) and the granule cell layer of the dentate gyrus in
the young, adult and aged mice. In addition, no significant
difference was identified in the mean number of NeuN-immunoreactive
neurons in the hippocampus proper and dentate gyrus among the
groups (Fig. 1A).
Rats
The distribution pattern of NeuN-immunoreactive
neurons in the rats was similar to that in the mice. In addition,
the number of NeuN-immunoreactive neurons in the hippocampus proper
and dentate gyrus was not significantly changed among the groups
(Fig. 1B).
CAT immunoreactivity
Mice
CAT immunoreactivity was then analyzed in the mice
(Fig. 2A). In the young mice, CAT
immunoreactivity was observed primarily in the pyramidal cells of
the stratum pyramidale and in certain non-pyramidal neurons in the
strata oriens and radiatum of the hippocampus proper (Fig. 2Ad and g). In addition, CAT
immunoreactivity was easily detected in granule cells of the
granule cell layer and hilar neurons of the polymorphic layer of
the dentate gyrus (Fig. 2Aj).
 | Figure 2CAT immunohistochemistryin the
hippocampus of the (A) mice and (B) rats in the young, adult and
aged groups. CAT immunoreactivity is highest in the adult group and
lowest in the aged group. GCL, granule cell layer; ML, molecular
layer; PL, polymorphic layer; SO, stratum oriens; SP, stratum
pyramidale; SR, stratum radiatum; DG, dentate gyrus. Scale bar, 400
µm (a–c) and 100 µm (d–l). (m) ROD as % of
CAT-immunoreactive structures in the hippocampus (n=7 per group).
*P<0.05, compared with the young group and
†P<0.05, compared with the adult group. The bars
indicate the mean ± standard error of the mean. ROD, relative
optical density. |
In the adult mice, the distribution pattern of
CAT-immunoreactive neurons was similar to that in the young mice;
however, the CAT immunoreactivity in all layers of the hippocampus
proper and dentate gyrus was significantly increased compared with
that in the young mice (Fig. 2Ae, h, k
and m).
In the aged mice, CAT immunoreactivity in all layers
of the hippocampus proper was significantly decreased compared with
that in the young and adult mice; however, CAT immunoreactivity in
the dentate gyrus was similar to the young mice (Fig. 2Af, i, 1 and m).
Rats
Generally, the distribution patter n of
CAT-immunoreactivity in the rats (Fig.
2B) was similar to that in mice (Fig. 2A). In the adult rats, the CAT
immunoreactivity was significantly increased in all layers of the
hippocampus proper and dentate gyrus compared with that in the
young rats (Fig. 2Be, h, k and m).
However, in the aged rats, CAT immunoreactivity was significantly
decreased in all layers of the hippocampus proper and the dentate
gyrus compared with that in the young and adult rats (Fig. 2Bf, i, l and m).
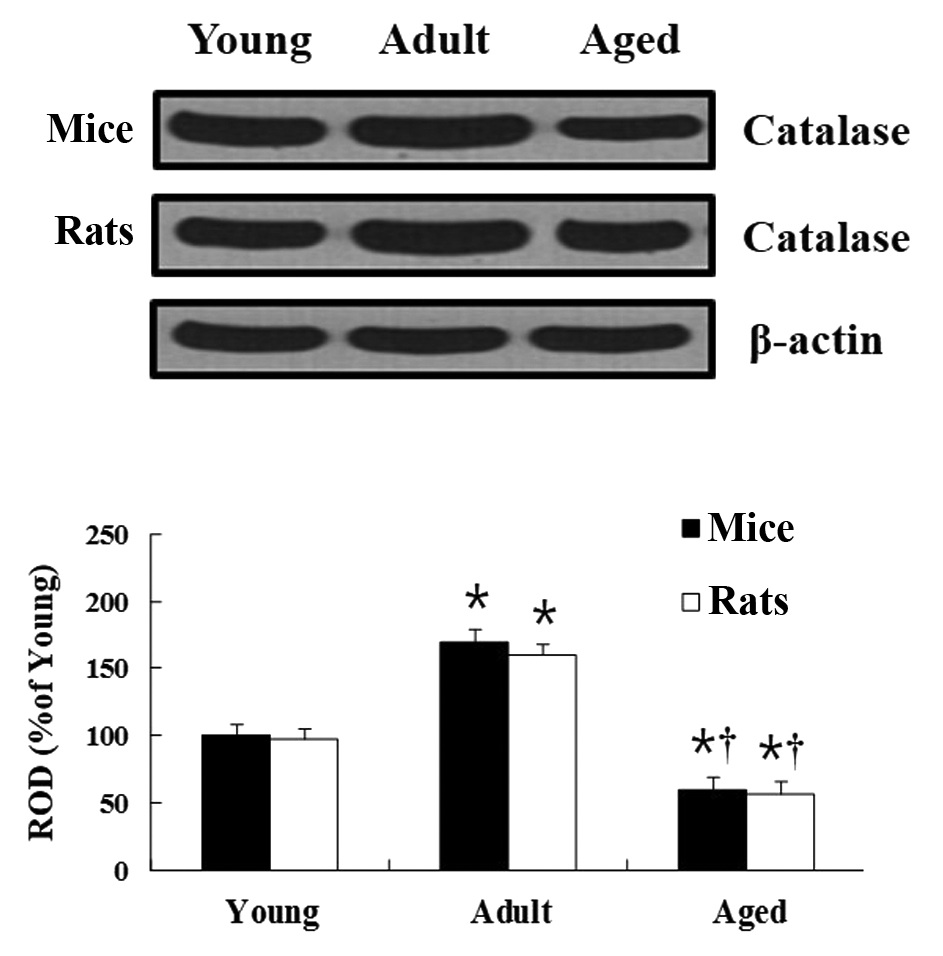
CAT protein level
Mice
In the western blot analysis, age-related changes of
CAT levels in the hippocampus were observed to be similar to those
identified in the immunohistochemical results. In the hippocampus
of the adult group, the CAT protein level was significantly
increased compared with that in the young group. In the hippocampus
of the aged group, CAT protein level was significantly decreased
compared with that in the young group (Fig. 3).
Rats
Age-associated changes to the CAT protein levels in
the rats were similar to that in the mice (Fig. 3).
Discussion
The present study aimed to investigate the
age-related changes in neuronal distribution in the hippocampi of
young, adult and aged mice and rats using NeuN
immunohistochemistry. No significant changes in cell morphology and
distribution patterns were identified during normal aging among all
of the groups, although the mean number of NeuN-immunoreactive
neurons was marginally decreased in all hippocampal subregions of
the mice and rats during aging. Previously, the presence of
age-related neuronal loss was reported, however, no significant
difference was identified in the number of cresyl violet-positive
neurons in the hippocampal CA1 region in aged gerbils compared with
that in young and adult gerbils (29). In addition, the results of the
present study are supported by a previous study that showed that
neuronal loss was found in the hippocampi of aged rodents (30).
In the present study, CAT expression in the
hippocampus was compared among young, adult, and aged mice and
rats, and it was demonstrated that CAT immunoreactivity was highest
in the pyramidal and granule neurons in the adult hippocampi and
lowest in the aged hippocampi. In addition, CAT protein levels in
the hippocampi were lowest in the aged mice and rats. The results
regarding the aged animals are consistent with those of previous
studies, which reported that CAT activity was significantly
diminished in the rat hippocampus during the normal aging process
(20,31). However, other studies have shown
inconsistent results; CAT activity in the hippocampus was unchanged
in aged Fischer-344 rats (19) and
C57BL/6 N mice (18). Furthermore,
Sohal et al (32) reported
that CAT was increased in the hippocampi of mice during aging. The
present study hypothesized that the levels of CAT in the
hippocampus from aged animals is lower than that those from young
and adult animals. However, the exact mechanism underlying the
decreased CAT levels in the aged hippocampus remains to be
determined. It is likely that the decreased CAT expression may be
associated with age-related functional changes in the
hippocampus.
It is well known that CAT, an important antioxidant
enzyme, protects cells from oxidative damage by ROS (33,34).
In the brain, CAT exerts numerous important functions and shows a
strong correlation between normal aging and increased oxidative
damage (31). For example, Lee
et al (35) reported that
overexpressing CAT using a viral vector delivery system in aging
rats decreased oxidative damage in the hippocampus. Furthermore,
CAT is crucial in modulating synaptic plasticity. Olsen et
al (36) demonstrated that
overexpressing mitochondrial CAT in mice resulted in improvements
in spatial learning and memory tested with a water maze test. In
addition, a recent study showed that exogenous treatment with a
synthetic CAT mimetic in aged mice ameliorated the age-related
decline in cognitive impairment (37).
In conclusion, the present study shows that CAT
expression was significantly decreased in the hippocampi of mice
and rats from the aged group compared with that in the young and
adult groups, and suggests that the decreased CAT expression may be
closely associated with age-related changes in the aged
hippocampus.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(grant no. NO2011-0022812), by Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Science, ICT and future Planning (grant no.
NRF-2014R1A2A2A01005307), and by the 2014 Research Grant from
Kangwon National University.
References
|
1
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
2
|
Berlett BS and Stadtman ER: Protein
oxidation in aging, disease, and oxidative stress. J Biol Chem.
272:20313–20316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui K, Luo X, Xu K and Ven Murthy MR: Role
of oxidative stress in neurodegeneration: Recent developments in
assay methods for oxidative stress and nutraceutical antioxidants.
Prog Neuropsychopharmacol Biol Psychiatry. 28:771–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants, and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beckman KB and Ames BN: The free radical
theory of aging matures. Physiol Rev. 78:547–581. 1998.PubMed/NCBI
|
|
6
|
Genovese T, Mazzon E, Di Paola R,
Crisafulli C, Muià C, Bramanti P and Cuzzocrea S: Increased
oxidative-related mechanisms in the spinal cord injury in old rats.
Neurosci Lett. 393:141–146. 2006. View Article : Google Scholar
|
|
7
|
Du Z, Yang Q, Zhou T, Liu L, Li S, Chen S
and Gao C: D-galactose-induced mitochondrial DNA oxidative damage
in the auditory cortex of rats. Mol Med Rep. 10:2861–2867.
2014.PubMed/NCBI
|
|
8
|
Gao Y, Yan Y and Huang T: Human
age-related cataracts: Epigenetic suppression of the nuclear factor
erythroid 2-related factor 2-mediated antioxidant system. Mol Med
Rep. 11:1442–1447. 2015.
|
|
9
|
Reiter RJ: Oxidative processes and
antioxidative defense mechanisms in the aging brain. FASEB J.
9:526–533. 1995.PubMed/NCBI
|
|
10
|
Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ,
Chi ZF and Wu W: Sirtuin 1 activation enhances the
PGC-1α/mitochondrial antioxidant system pathway in status
epilepticus. Mol Med Rep. 11:521–526. 2015.
|
|
11
|
Kang SW, Rhee SG, Chang TS, Jeong W and
Choi MH: 2-Cys peroxiredoxin function in intracellular signal
transduction: Therapeutic implications. Trends Mol Med. 11:571–578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matés JM, Pérez-Gómez C and Núñez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999. View Article : Google Scholar
|
|
13
|
Chen X, Stern D and Yan SD: Mitochondrial
dysfunction and Alzheimer's disease. Curr Alzheimer Res. 3:515–520.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hedden T and Gabrieli JD: Insights into
the ageing mind: A view from cognitive neuroscience. Nat Rev
Neurosci. 5:87–96. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirino T and Sano K: Selective
vulnerability in the gerbil hippocampus following transient
ischemia. Acta Neuropathol. 62:201–208. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kril J, Patel S, Harding A and Halliday G:
Patients with vascular dementia due to microvascular pathology have
significant hippocampal neuronal loss. J Neurol Neurosurg
Psychiatry. 72:747–751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morrison JH and Hof PR: Selective
vulnerability of cortico-cortical and hippocampal circuits in aging
and Alzheimer's disease. Prog Brain Res. 136:467–486. 2002.
View Article : Google Scholar
|
|
18
|
Hussain S, Slikker W Jr and Ali SF:
Age-related changes in antioxidant enzymes, superoxide dismutase,
catalase, glutathione peroxidase and glutathione in different
regions of mouse brain. Int J Dev Neurosci. 13:811–817. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carrillo MC, Kanai S, Sato Y and Kitani K:
Age-related changes in antioxidant enzyme activities are region and
organ, as well as sex, selective in the rat. Mech Ageing Dev.
65:187–198. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ciriolo M, Fiskin K, De Martino A,
Corasaniti M, Nistico G and Rotilio G: Age-releated changes in Cu,
Zn superoxide dismutase, Se-dependent and -independent glutathione
peroxidase and catalase activities in specific areas of rat brain.
Mech Ageing Dev. 61:287–297. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorbunova V, Bozzella MJ and Seluanov A:
Rodents for comparative aging studies: From mice to beavers. Age
(Dordr). 30:111–119. 2008. View Article : Google Scholar
|
|
22
|
Vanhooren V and Libert C: The mouse as a
model organism in aging research: Usefulness, pitfalls and
possibilities. Ageing Res Rev. 12:8–21. 2013. View Article : Google Scholar
|
|
23
|
Quinn R: Comparing rat's to human's age:
How old is my rat in people years? Nutrition. 21:775–777. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demetrius L: Aging in mouse and human
systems. Ann N Y Acad Sci. 1067:66–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gorbunova V, Bozzella MJ and Seluanov A:
Rodents for comparative aging studies: From mice to beavers. Age
(Dordr). 30:111–119. 2008. View Article : Google Scholar
|
|
26
|
Institute of laboratory animal research,
committee for the update of the guide for the care and use of
laboratory animals, national research council: Guide for the care
and use of laboratory animals. 8th edition. Washington, (DC):
National Academies Press; pp. 2202011
|
|
27
|
Lee CH, Ahn JH, Park JH, Yan BC, Kim IH,
Lee DH, Cho JH, Chen BH, Lee JC, Cho JH, et al: Decreased
insulin-like growth factor-I and its receptor expression in the
hippocampus and somatosensory cortex of the aged mouse. Neurochem
Res. 39:770–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohk TG, Yoo KY, Park SM, Shin BN, Kim IH,
Park JH, Ahn HC, Lee YJ, Kim MJ, Kim TY, et al: Neuronal damage
using fluoro-jade B histofluorescence and gliosis in the striatum
after various durations of transient cerebral ischemia in gerbils.
Neurochem Res. 37:826–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang IK, Yoo KY, Jung BK, Cho JH, Kim DH,
Kang TC, Kwon YG, Kim YS and Won MH: Correlations between neuronal
loss, decrease of memory, and decrease expression of brain-derived
neurotrophic factor in the gerbil hippocampus during normal aging.
Exp Neurol. 201:75–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flood DG and Coleman PD: Neuron numbers
and sizes in aging brain: Comparisons of human, monkey and rodent
data. Neurobiol Aging. 9:453–463. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Serrano F and Klann E: Reactive oxygen
species and synaptic plasticity in the aging hippocampus. Ageing
Res Rev. 3:431–443. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sohal RS, Ku HH, Agarwal S, Forster MJ and
Lal H: Oxidative damage, mitochondrial oxidant generation and
antioxidant defenses during aging and in response to food
restriction in the mouse. Mech Ageing Dev. 74:121–133. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai J, Rodriguez AM, Melendez JA and
Cederbaum AI: Overexpression of catalase in cytosolic or
mitochondrial compartment protects HepG2 cells against oxidative
injury. J Biol Chem. 274:26217–26224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rong Y, Doctrow SR, Tocco G and Baudry M:
EUK-134, a synthetic superoxide dismutase and catalase mimetic,
prevents oxidative stress and attenuates kainate-induced
neuropathology. Proc Natl Acad Sci USA. 96:9897–9902. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee WH, Kumar A, Rani A, Herrera J, Xu J,
Someya S and Foster TC: Influence of viral vector-mediated delivery
of superoxide dismutase and catalase to the hippocampus on spatial
learning and memory during aging. Antioxid Redox Signal.
16:339–350. 2012. View Article : Google Scholar :
|
|
36
|
Olsen RH, Johnson LA, Zuloaga DG, Limoli
CL and Raber J: Enhanced hippocampus-dependent memory and reduced
anxiety in mice over-expressing human catalase in mitochondria. J
Neurochem. 125:303–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clausen A, Doctrow S and Baudry M:
Prevention of cognitive deficits and brain oxidative stress with
superoxide dismutase/catalase mimetics in aged mice. Neurobiol
Aging. 31:425–433. 2010. View Article : Google Scholar :
|