Introduction
Perimenopause, or menopause transition, is the stage
of women's reproductive life which begins several years prior to
menopause, and one of the characteristics of this is ovarian aging
(OA). In this period, women's ovarian function and estrogen
secretion declines, and the body enters the menopausal transition,
during which a number of clinical symptoms appear (1–3).
Increasing evidence indicates that a number of environmental and
dietary factors may contribute to OA through oxidative
stress-related mechanisms (4–6).
Oxidative stress has been implicated to serve an
important role in the perimenopause. Women who are undergoing
perimenopause have a reduced ability to repair DNA (2). Oxidative damage may also be
responsible for the declines in ovarian function and oocyte quality
with age (7,8). Previous studies have proposed that
reduced ovarian antioxidant gene expression is a factor in the
oxidative damage to ovarian lipids, proteins and DNA (9,10).
p53 is vital in regulating the
p19ARF-p53-p21-retinoblastoma (Rb) pathway, which serves
a critical role in the regulation of cell proliferation,
differentiation and aging (11,12).
When p53 is activated, the pathway becomes activated, inhibiting
cell proliferation and thus promoting cellular aging. The response
of p53 to oxidative stress depends on the type and extent of stress
signals that activate p53, which may promote or prevent aging
(13). High level of oxidative
stress can activate p53 and lead to apoptosis and senescence
(14,15).
Millions of women suffer from the discomforts of the
perimenopause which is characterized by ovarian aging. The search
for effective anti-aging medicines is may be key to avoiding
numerous diseases associated with aging. Previous studies indicated
that certain native medicines may alter ovarian follicular
development and affect ovarian follicle reserves, thereby affecting
female reproductive aging (16–19).
Yifuning (YFN), a traditional Chinese medicine recipe, is composed
of Ranae Oviductus (Rana temporaria chensinensis David) and
Zedoary Turmeric Oil (oil from Curcuma wenuyjin Y. H. Chen
et C. Ling). They are common ingredients in prescriptions of
anti-aging medication and for treating climacteric syndrome in
women, as shown in Chinese Pharmacopoeia (2010 edition) (20) and some modern pharmacological
studies (21). In a previous
study, it was reported that Yifuning was able to improve
perimenopausal symptoms in women (22–24),
and YFN was indicated to improve symptoms in ovariectomized rats
(25). Ovarian aging is closely
associated with the perimenopause, however, the ability of YFN to
affect ovarian function remains unclear. In the present study, the
effect of YFN on OA in aging female rats and the underlying
molecular mechanisms were investigated.
Materials and methods
Reagents
YFN was obtained from the College of Traditional
Chinese Medicine of the Southern Medical University (Guangzhou,
China) and was prepared in vegetable oil at different
concentrations. Diethylstilbestrol (lot no. 20100829) was obtained
from Hefei Jiulian Pharmaceutical Company Ltd., (Hefei, China). The
rabbit monoclonal p21 (cat. no. 9313) and Rb (cat. no. 9313)
antibodies were purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA). The rabbit polyclonal p19 (cat. no. sc-1066)
and the 8-hydroxydeoxyguanosine (8-OHDG; cat. no. sc-139586)
antibodies were purchased from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA). The mouse monoclonal p53 antibody (cat. no.
PB0076, 2524) was purchased from Bioworld Technology, Inc.,
(Shanghai, China) for the immunohistochemical analyses or from Cell
Signaling Technology, Inc., for western blotting. Malondialdehyde
(MDA), glutathione peroxidase (GSH-Px), catalase (CAT), hydrogen
peroxide (H2O2) and superoxide dismutase
(SOD) assay kits were obtained from the Nanjing Jiancheng
Bioengineering Institute (Nanjing, China).
Animals and treatment
Female Sprague Dawley rats were purchased from the
Southern Medical University Animal Center (Guangzhou, China) at 3
(n=6) and 16–18 months of age (n=35). Animal care and use was
performed in accordance with the Animal Research Institute
Committee guidelines of Southern Medical University Animal Center.
The study was approved by the Southern Medical University Animal
Care and Use Committee. Estrous cycling in adult female rats was
evaluated every morning for a minimum of 14 days using vaginal
cytology (9). Following the
evaluation of estrous cycling, the selected aging rats (n=30) with
irregular cycles were divided into five experimental groups (n=6
per group): O-C group (Old control group); DT group (rats were
treated with diethylstilbestrol, 0.05 mg/kg); YFN-H group (rats
were treated with the high dose of YFN, 2.0 g/kg); YFN-L group
(rats were treated with the low dose of YFN, 1.0 g/kg); Y-C group
(the 3 months old rats were kept as the young control group). The
animals were maintained under controlled light (12 h light/dark
cycle) and temperature (20–24°C) throughout the study, including
during the treatment period, and received a standard diet and water
ad libitum. The rats were treated with DT and YFN for 6
weeks. All non-treated animals received corresponding doses of the
vehicle (vegetable oil).
All animals received humane care according to the
Guidelines for the Ethical Care of Experimental Animals of the
European Union (26). Following
treatment, the rats were sacrificed under 10% chloral hydrate
anesthesia, followed by abdominal aortic blood collection, and the
ovarian tissues were collected and immediately frozen in either
liquid nitrogen (for western blot determinations),
RNAlater® (for PCR determinations), or 4% formaldehyde
(for the immunohistochemical analyses).
Determination of biochemical
parameters
Blood samples were collected and centrifuged at 3000
× g for 15 min at 4°C, and serum estradiol (E2),
testosterone (Te) and progesterone levels were measured using
electrochemiluminescence immunoassays (Roche Diagnostics GmbH,
Mannheim, Germany). Oxidative stress (OS) indicators GSH-Px, MDA,
SOD, H2O2, and CAT activity were measured
according to the manufacturer's recommended instructions (Nanjing
Jiancheng Bioengineering Institute).
Immunohistochemical staining
Immunohistochemistry was performed as previously
described (27). Following
dewaxing, antigens were retrieved in 0.01 M sodium citrate buffer
for 5 min by pressure cooking. Sections were blocked for 30 min in
normal 16.6% swine serum, which was diluted 1:5 in Tris-buffered
saline (pH=7.4), supplemented with 5% bovine serum albumin, then
further blocking with a streptavidin/biotin blocking kit (Vector
Laboratories, Ltd., Peterborough, UK). The sections were incubated
overnight with 8-OHDG and p53 primary antibodies at 4°C (diluted
1:100 in blocking solution). At room temperature the primary
antibodies were detected following incubation with
biotinylated-swine anti-rabbit secondary antibody and
streptavidin-horseradish peroxidase (cat. nos. MP-7451 and SA-5014;
Vector Laboratories, Ltd.) mixtures for 30 min. Using a
3,3′-diaminobenzidine staining kit (Gene Tech, Ltd., Shanghai,
China) the bound antibodies were visualized. The slides were
counterstained with hematoxylin, dehydrated and coverslipped. They
were photographed using a Nikon Eclipse light microscope (Nikon
Corporation, Tokyo, Japan). Similar areas of staining between
groups were compared to assess the effect.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the ovarian tissues
using RNA TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). A total of 5 μg of total RNA for
each sample was reverse-transcribed into cDNA using a cDNA
synthetisis kit (Takara Bio, Inc., Otsu, Japan). The cDNA was
diluted 1/100, then 5 μl was used as a template for each PCR
reaction. The PCR reaction parameters were as follows: 95°C for 10
min, 35 cycles of denaturing at 94°C for 30 sec, annealing at 60°C
for 30 sec, extension at 72°C for 60 sec and 7 min at 72°C. qPCR
was performed on an ABI PRISM 7300 PCR System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using SYBRGreen Taq qPCR
Mastermix (Takara Bio, Inc.). The expression levels of the target
genes (p19, p21, p53 and Rb) were used to generate standard curves,
which were normalized against an endogenous reference gene,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequences of
the primers used in qPCR were as follows: GAPDH forward,
5′-ATTGTCAGCAATGCATCCTG-3′ and reverse, 3′-ATGGACTGTGGTCATGAGCC-5′;
p19 forward, 5′-GGTCACCGACAGGCA TAACT-3′ and reverse,
3′-CCAGAAGTGAAGCCAAGGAG-5′; p53 forward, 5′-GTTCCGAGAGCTGAATGAGG-3′
and reverse, 3′-AGGATGCAGAGGCTGTCAGT-5′; p21 forward,
5′-TGCAATGAGGGACCAGTACA-3′ and reverse, 3′-CCTGAGCCTGTTTCGTGTCT-5′;
and Rb forward, 5′-TTGGCTAACGTGGGAGAAAG-3′ and reverse,
3′-AATGGCATCTCATCCAGGTC-5′. The mRNA sequences of GAPDH, p19, p53,
p21 and Rb were obtained from the UCSC Genome Bioinformatics
website (https://genome.ucsc.edu/), and the
primers were designed by Invitrogen (Thermo Fisher Scientific,
Inc.). GAPDH was used as a housekeeping gene to compare the
samples. PCR was performed three times for each sample and each
gene. Expression levels were calculated using the 2−ΔΔCq
method (28).
Western blotting analyses
Ovarian tissues were powdered with liquid nitrogen
and lysed in lysis buffer (140 mM NaCl, 10 mM EDTA, 10% glycerol,
1% NP40, 20 mM Tris base, pH 7.5) containing protease-inhibitor (1
mM of phenylmethane sulfonyl fluoride). Then, the homogenized
tissues were centrifuged at 4°C 14,000 × g for 30 min and
supernatants were subjected to western blot analysis. Protein was
quantified using a Bicinchoninic Acid Protein Assay kit (Pierce
Biotechnology; Thermo Fisher Scientific, Inc.). Denatured samples
(20 μg) were resolved on 5–12% SDS-PAGE gels and 5% stacking
gel. The proteins were then transferred nitrocellulose membranes.
Non-specific binding of the membranes was blocked with
Tris-buffered saline (TBS) containing 5% (w/v) non-fat dry milk and
0.1% (v/v) Tween-20 (TBS-T) for 1 h at room temperature. Membranes
were washed 3 times with TBS-T for 10 min and incubated with a
specific primary antibodies (p19, p21, p53 and Rb; dilution
1:1,000) overnight at 4°C. The membranes were then washed with
TBS-T and incubated with horseradish-peroxidase conjugated goat
anti-rabbit (dilution 1:10,000; cat. no. 7074) and goat anti mouse
(dilution 1:10,000; cat. no. 7077) secondary antibody (Cell
Signaling Technology, Inc.) for 1 h at room temperature. Using an
enhanced chemiluminescence (ECL) detection system the immune
complexes were visualized. Protein expression levels were
determined using an image analyzer (LAS-3000; Fujifilm, Tokyo,
Japan).
Statistical analyses
Statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Differences
in estrous cycles, sex hormones and OS indicators were evaluated
using one-way analysis of variance. The transformed data and
ovarian mRNA expression levels were analyzed using general linear
regression procedures. The data are presented as the mean ±
standard deviation.
Results
Estrous cycling and reproductive organ
weights
As shown in Fig.
1A, the results indicated that the estrous cycles of rats in
the O-C group were prolonged compared with rats in the Y-C group
(P<0.001). The estrous cycles of rats in the DT- and YFN-treated
groups were markedly shorter compared with the O-C group (P=0.047).
The weights of the rats in the Y-C group significantly differed
from the weights in the O-C group (P=0.025), however, the body
weights of the rats in the DT, YFN-L and YFN-H-treated groups did
not differ from the weights in the O-C group (P=0.063), as shown in
Fig. 1B.
 | Figure 1Body weights, organ indices, and
estrous cycle data. (A) Following 6 weeks of administration, the
DT, YFN-L and YFN-H-treated groups had 3–5 day regular estrous
cycles, which significantly differs from the cycles in the O-C
group. (B) The body weights were not significantly different
between the DT-treated, O-C, YFN-L and YFN-H groups, and only the
body weights in the Y-C group differed significantly from those of
the O-C group (P=0.025). (C) Ovarian weights, adjusted for body
weight, in the YFN-H group increased significantly from those in
the O-C group (P=0.001). (D) The uterine indices in the DT-treated
and YFN-H groups differed significantly compared with those of the
O-C group (P=0.045 and 0.001, respectively). The YFN-H group
displayed larger improvements in the uterine index, with values
that were similar to those of the Y-C group. Comparison of serum
(E) estradiol, (F) testosterone and (G) progesterone levels in the
rats. (H) SOD, (I) GSH-Px and (J) CAT activities and (K) MDA and
(L) H2O2 levels in the sera of the rats. The
SOD, GSH-Px, and CAT activities and the MDA and
H2O2 levels differed significantly among the
groups (F(4,25)=73.300, P<0.001; F(4,25)=9.133, P<0.001; F(4,25)=29.787, P<0.001; F(4,25)=22.593, P<0.001; and F(4,25)=71.087, P<0.001, respectively).
Values are presented as the mean ± standard deviation, n=6 rats per
group. **P<0.01 vs. the Y-C group, and
#P<0.05 and ##P<0.01 vs. the O-C group.
DT, diethylstilbestrol; YFN-L, yifuning-low dose; YFN-H, YFN-high
dose; O-C, old control; Y-C, young control; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; MDA,
malondialdehyde. |
The ovarian index is equal to the weight of the
ovaries divided by the body weight of the rat multiplied by 100,
while the uterine index is equal to the uterine quality divided by
the body weight of the rat multiplied by 100. As shown in Fig. 1C and D, following 6 weeks of
lavage, the ovarian index of the O-C group did not differ from that
of the Y-C group (P=0.053). The ovarian index of the YFN-H group
was significantly improved compared with that of the O-C group
(P=0.001). The uterine index of the O-C group was significantly
reduced compared with that of the Y-C group (P<0.001). The DT
and YFN-H-treated groups displayed marked improvements in uterine
index compared with the O-C group (P=0.045 and P=0.001,
respectively), and the uterine index of the YFN-H group was greater
than that of the DT-treated group (similar to that of the Y-C
group).
Effects of YFN treatment on serum
E2, Te and progesterone levels
As shown in Fig.
1E–G, it was observed that YFN treatment increased the levels
of sex hormones in the natural aging model rats. The levels of
E2 and Te in the O-C group were significantly reduced
compared with those in the Y-C group (P<0.001). The levels of
progesterone were reduced in the O-C group compared with the Y-C
group but did not differ significantly (P=0.055). DT and YFN
treatment altered the levels of sex hormones in the rats,
increasing the serum E2 and Te levels. The serum levels
of E2 and Te in the DT-treated, YFN-H, and YFN-L groups
were significantly increased compared with the O-C group (P=0.035,
P=0.001 and P=0.005, respectively).
Effects of YFN treatment on GSH-Px, SOD
and CAT activity and MDA and H2O2 levels
As shown in Fig.
1H–J, SOD, GSH-Px, and CAT activity in the serum of O-C group
were significantly reduced compared with those of the Y-C group
(P<0.001, P=0.003 and P<0.001, respectively). In addition,
the MDA and H2O2 concentrations in the serum
of the O-C group were significantly increased compared with those
of the Y-C group (P=0.001 and 0.014, respectively). Treatment with
different doses of YFN markedly increased SOD activity compared
with the O-C group (P<0.001). Similarly, SOD activity levels
were significantly increased compared with the O-C group
(P<0.01). However, GSH-Px activity was not significantly
upregulated in the DT, YFN-H and YFN-L-treated groups compared with
the O-C group (P=0.055). CAT activity in the YFN-H group was
markedly increased compared with the O-C group (P=0.007) and was
similar to the activity in the Y-C group. In addition, compared
with the O-C group, MDA and H2O2 expression
levels in the YFN-H (P=0.025 and P=0.001, respectively) and YFN-L
groups (P=0.015 and P<0.001, respectively) were significantly
reduced. The DT-treated group also displayed significantly lower
levels of H2O2 than the O-C group
(P<0.001), as shown in Fig. 1K and
L.
Effects of YFN on oxidative DNA damage
and p53 protein expression
Compared with the Y-C group, it was observed that
oxidative damage in the O-C group was significantly increased as
shown in Fig. 2A–E. The number of
8-OHDG-positive cells in the ovaries of rats in the O-C group was
significantly higher than in the Y-C group (P<0.001). In
addition, a marked reduction in 8-OHDG expression was observed
following treatment with the different doses of YFN and in the
DT-treated group compared with the O-C group (P=0.045). Fewer
8-OHDG-positive cells were observed in the ovaries from the YFN-H
group compared with the O-C group, resembling the results that were
observed in the Y-C group.
 | Figure 2Effects of YFN on 8-OHDG and p53
protein expression in the ovaries. Immunostaining was used to
detect the protein expression of the oxidative damage marker
8-OHDG. Representative immunostaining images show DNA damage
(8-OHDG) in the (A) DT-treated, (B) Y-C, (C) O-C, (D) YFN-L and (E)
YFN-H groups and p53 expression in the (F) DT-treated, (G) Y-C, (H)
O-C, (I) YFN-L and (J) YFN-H groups. (K) Graph showing the number
of 8-OHDG and p53 positive cells in the ovarian tissues from each
group. The data are presented as the mean ± standard deviation
(n=3) of the percentage of ovarian cells that stained positive.
Positive 8-OHDG staining differed significantly among the groups
(F(4,10)=14.478, P<0.001). Positive p53
staining differed significantly among the groups (F(4,10)=45.033, P<0.001).
**P<0.01 vs. the Y-C group. #P<0.05 and
##P<0.01 vs. the O-C group. Magnification, ×200. YFN,
yifuning; 8-OHDG, ; DT, diethylstilbestrol; Y-C, young control;
O-C, old control; YFN-L, YFN-low dose; YFN-H, YFN-high dose; IHC,
immunohistochemistry. |
To observe ovarian oxidative stress in the O-C group
and to further elucidate the mechanisms of action underlying the
antioxidant activity of YFN, p53 protein expression was evaluated
using immunohistochemistry, as presented in Fig. 2F–J. This indicated that p53 was
predominantly localized to the nuclei of cells in the follicles,
oocytes, corpus luteum and mesenchyme. p53 was expressed in a
higher percentage of cells in luteal cells than in follicular
cells. The number of cells that stained positive for p53 protein
expression in the ovarian tissue in the O-C group was significantly
higher than the number in the Y-C group (P<0.001). Positive p53
staining in the ovaries of the YFN-H and YFN-L-treated groups was
reduced compared with the O-C group, and these differences were
significant (P<0.001). Similarly, positive p53 staining in the
DT-treated group was significantly reduced compared with the O-C
group (P<0.001). p53 expression in both YFN-treated groups was
significantly lower than in the DT-treated group and was similar to
the Y-C group.
p19, p53, p21, and Rb mRNA
expression
p19, p53, p21, and Rb are involved in age-associated
signal transduction pathway arrest. As shown in Fig. 3A–D, p19, p53, p21 and Rb mRNA
levels were significantly reduced in the rats in the O-C group
(P=0.011, P=0.007, P<0.001 and P=0.008) compared with the Y-C
group. However, significant elevations were observed in the YFN-L
(P=0.012) and YFN-H (P=0.008) groups compared with the O-C group,
and a partial, but significant, elevation was observed in the
DT-treated group (P=0.003) compared with the O-C group.
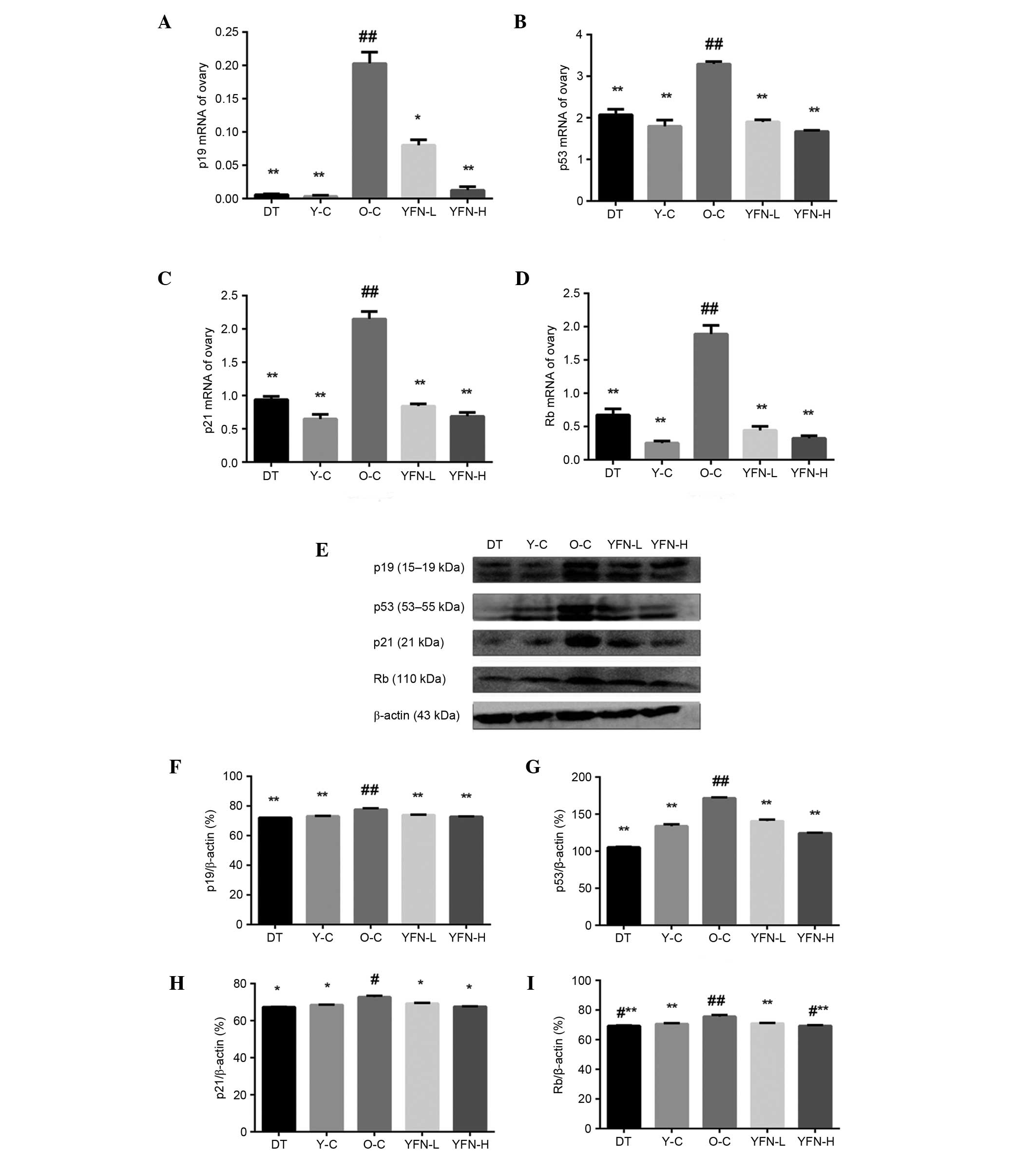 | Figure 3mRNA expression levels of (A) p19,
(B) p53, (C) p21 and (D) Rb mRNA expression in the ovaries. The
values were normalized against GAPDH as a reference gene. p19, p53,
p21 and Rb mRNA expression differed significantly among the groups
(F(4,10)=234.014, P<0.001; F(4,10)=158.515, P<0.001; F(4,10)=283.181, P<0.001; and F(4,10)=224.121, P<0.001). (E) Image of
western blotting for p19, p53, p21, and Rb protein expression in
the ovaries. (F) p19, (G) p53, (H) p21 and (I) Rb protein
expression significantly differed among the groups (F=98.099,
P<0.001, F=956.654, P<0.001; F=80.415, P<0.001; and
F=20.563, P<0.001, respectively). Values are presented as the
mean ± standard deviation, n=3. *P<0.05 and
**P<0.01 vs. the Y-C group. #P<0.05 and
##P<0.01 vs. the O-C group. GAPDH, glyceraldehyde
3-phosphate dehydrogenase; DT, diethylstilbestrol; Y-C, young
control; O-C, old control; YFN-L, yifuning-low dose; YFN-H,
YFN-high dose. |
Effects of YFN on p19, p53, p21, and Rb
protein expression
The protein expression levels of p19, p53, p21, and
Rb were also investigated. As shown in Fig. 3E–I, the levels of p19, p53, p21 and
Rb increased as a result of aging (the O-C group vs. the Y-C group;
P<0.001, P<0.001, P=0.046 and P<0.001). However, treatment
with YFN significantly reduced the age-induced p19, p53, p21 and Rb
activity (all vs. the O-C group; P<0.001, P<0.001, P=0.031
and P<0.001). In addition, p19, p53, p21 and Rb expression
levels were significantly increased compared with the O-C and DT
group.
Discussion
Ovarian aging is one of the characteristics of
perimenopausal symptoms. Regular estrous cycles represent ovarian
function. Alterations in estrous cycles are a convenient and
effective index which can be used to monitor reproductive
conditions. Estrus in rats typically occurs in regular 4 to 5 day
cycles, and continues to occur until the rats reach the age of
10–12 months. During aging, the cycle grows longer or becomes
irregular, following which estrus eventually stops. In the present
study, the estrous cycles of rats in the YFN-treated groups had
markedly shortened and YFN groups displayed marked improvements in
ovarian index and uterine index compared with the O-C group, which
suggests that YFN may promote cell recovery and delay ovarian aging
to a certain extent.
At present, estrogen replacement therapy is widely
used to treat perimenopausal symptoms in which a number of
deficiencies and side effects exist. In a previous study, it was
reported that the influence of YFN on E2 was similar to
that of DT, therefore, DT was selected as a control in the present
study, in which it exhibited the same effect. Reproductive
endocrine hormone levels and the ovarian and uterine indices in the
aging rats were observed to be improved following treatment with
YFN. In addition, YFN-L treatment was superior to YFN-H in
restoring reproductive endocrine hormone activity. The effects of
YFN treatment on progesterone differed from the effects of DT
treatment. It has been suggested that the common symptoms of the
menopause are caused by dramatic declines and fluctuations in the
levels of estrogen and, to some extent, P (29). Approaches that are aimed at
alleviating symptoms generally focus on restoring these hormones in
the body. It has been suggested that Asian women experience fewer
menopausal symptoms because their diets are rich in soy proteins
and phytoestrogens, and because they experience less stress and
lead healthier lifestyles (30–32).
The influence of YFN on hormones was similar to that of DT,
however, YFN contains lower natural estrogens, with estrogen levels
ten times lower than in DT. This indicates that the formation of
reproductive endocrine hormones was not only affected by estrogens
in YFN. YFN may affect the feedback that controls pituitary hormone
synthesis and release. YFN may affect the level of hormones such as
DT, but YFN contains lower levels of estrogen, suggesting that YFN
treatment may reduce the need for hormone replacement therapy and
reduce its associated side effects. We will further study these
indications.
YFN is rich in nutritional antioxidant ingredients.
In the present study, YFN significantly increased the levels of
enzymatic antioxidants and reduced the accumulation of lipid
peroxidation. The O-C group exhibited higher MDA and
H2O2 levels and lower GSH-Px, SOD and CAT
activity compared with Y-C group. Certain previous studies have
indicated that oxidative stress is higher in women who are
experiencing perimenopausal symptoms than those who are not
experiencing these symptoms (3,33).
The levels of lipoperoxides in perimenopausal women have been
reported to be increased and the activities of antioxidant enzymes
which can protect the body against oxidation damage reduced
(2,34).
The present study also examined an indicator of
oxidative DNA damage, 8-OHDG. Sai et al (35) found that during aging the process
of the accumulation of oxidative DNA damage varies among organs.
Kaneko et al (36) reported
that 8-OHDG begins accumulating in the DNA of rat organs at 24
months of age or older. However, certain other studies have
reported no significant alterations in oxidative DNA damage with
age (37–39). A previous study reported that
8-OHDG is expressed in ovarian interstitial cells and follicles
with increasing age (10). The
data from the current study indicates an increase in 8-OHDG
expression levels in the ovaries of the O-C group. YFN treatment
protected against increased oxidative stress in the aging rats,
reducing 8-OHDG expression during age-related ovarian oxidative DNA
damage. We speculate that elevated repair activity may contribute
to the protective effects of YFN. Therefore, one of the mechanisms
underlying the ability of YFN to delay ovarian aging may be defense
against oxidative stress.
As a tumor suppressor protein, p53 is also a
redox-active transcription factor that organizes and directs
cellular responses, in the face of a variety of stresses that lead
to genomic instability. Oxidative factors are activated in response
to stress signals. p53 regulates its target genes and initiates
stress responses, which include cell cycle arrest, apoptosis and/or
senescence (40–43). In unstressed mammalian cells, p53
has a short half-life and is normally maintained at low levels
(44), while it is activated upon
oxidative stress or DNA damage. Studies have indicated that
cellular reactive oxygen species (ROS) can be modulated by p53 in a
number of ways. Multiple pathways exist that integrate redox and
p53 signaling, in which various redox signals converge on p53
target genes to determine cell fate, such as apoptosis, cell cycle
arrest and DNA repair. For example, excess ROS production in the
mitochondria resulting from treatments with chemotherapeutic agents
has been demonstrated to lead to apoptosis (45,46),
whereas oxidative stress that occurs in the nucleus stimulates
p53-dependent DNA repair (47). As
a redox-sensitive protein, p53 is also under redox regulation that
determines cell fate via the selection of target genes. Other
parameters, including cell type, source of stress and the intensity
of stimuli, also determine the outcomes of the interaction between
ROS and p53, which may limit any generalized mechanistic
explanation of interaction between p53 and ROS. High levels of ROS
activate p53 under severe oxidative stress conditions or
irreparable damage, then in turn leads to p53-mediated apoptosis
and senescence (14,15). Furthermore, activated p53 protein
induces the expression of a set of pro-oxidant genes, which include
p53-inducible gene 3 (PIG3), PIG6, ferredoxin reductase, Bax and
p53 upregulated modulator of apoptosis (15,48).
The induction of these genes can increase intracellular ROS levels
and further sensitize cells to oxidative stress to eliminate
damaged cells through apoptosis and p53-mediated senescence
(49). In the present study, the
number of p53-positive cells in the ovaries of rats in the O-C
group increased, while YFN treatment resulted in a significant
reduction. We speculate that p53 was activated by oxidative stress
and irreparable DNA damage in aged rats and that YFN improves the
ability to repair DNA and alter redox regulation.
In addition, p53 is involved in the G1/S
checkpoint of the p19ARF-p53-p21-pRb cell cycle pathway,
which ensures that eukaryotic cells proliferate and divide in an
orderly and programmed manner. Alterations in p19ARF
expression which leads to the activation of p53 is a critical step
in the senescence response (50).
In response to DNA damage, the tumor suppressor gene p21 is
activated by p53 and inhibits the activation of cyclin/Cdk
complexes, then blocking cell cycle progression from G1
to S phase (51). Rb regulates the
transition between the G1 and S phases of the cell cycle
(52). Dephosphorylation of Rb
during G1 progression results in cell cycle arrest. This
dephosphorylation may be caused by the overexpression of p21, which
inhibits Cdk (53). The
p19ARF-p53-p21-pRb pathway is activated in a variety of
cell types in response to a number of cellular stresses, including
senescence. In the present study, it was demonstrated that the
p19ARF-p53-p21-pRb pathway was activated in the model
rats, and that YFN was able to reduce the expression of p19, p53,
p21 and Rb. We speculate that another regulatory effect that is
mediated by YFN to defer aging in the ovaries may be the blockade
of the p19ARF-p53-p21-pRb pathway to promote cell
differentiation and proliferation, thereby slowing the aging
process in the ovaries.
The results of the present study suggest that YFN
defers ovarian aging by upregulating antioxidants and
downregulating negative regulators of proliferation, including p19,
p53, p21 and Rb, in ovarian tissues. In addition, YFN reduces
ovarian damage, promotes cell proliferation and restores the
ability of the ovaries to produce sex hormones, thereby relieving
the symptoms of the menopausal transition. To conclude, the
anti-aging effects of YFN in the ovaries may be mediated by
promoting antioxidant activity and proliferation. However, the
possibility cannot be excluded that natural ovarian aging mediates
endogenous oxidative stress that blocks cell cycle progression,
thus leading to senescence. Further investigation is required to
clarify the association between oxidative stress and the cell
cycle. At present, the ability of p53 to function as an inducer of
antioxidant genes, which may suppress p53-activated reductions in
oxidative stress and enhance ovarian function, remains to be
elucidated.
Acknowledgments
The present study was supported by grants from
Natural Science Foundation of China (grant no. 81001701), Pearl
River S&T Nova Program of Guangzhou Science & Technology
Project (grant no. 2013J2200030), China Postdoctoral Science
Foundation Funded Project (grant no. 2013T60965) to Dr Lei Liang
and grants from Guangdong Provincial Administration of Traditional
Chinese Medicine (grant no. 2010230 to Professor Zhi-Jian Guo).
References
|
1
|
Harman D: Aging: A theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tatone C, Carbone MC, Falone S, Aimola P,
Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM and
Amicarelli F: Age-dependent changes in the expression of superoxide
dismutases and catalase are associated with ultra structural
modifications in human granulosa cells. Mol Hum Reprod. 12:655–660.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zitňanová I, Rakovan M, Paduchová Z,
Dvořáková M, Andrezálová L, Muchová J, Simko M, Waczulíková I and
Duračková Z: Oxidative stress in women with perimenopausal
symptoms. Menopause. 11:1249–1255. 2011. View Article : Google Scholar
|
|
4
|
Banu SK, Samuel JB, Arosh JA, Burghardt RC
and Aruldhas MM: Lactational exposure to hexavalent chromium delays
puberty by impairing ovarian development, steroidogenesis and
pituitary hormone synthesis in developing Wistar rats. Toxicol Appl
Pharmacol. 232:180–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banu SK, Stanley JA, Lee J, Stephen SD,
Arosh JA, Hoyer PB and Burghardt RC: Hexavalent chromium-induced
apoptosis of granulose cells involves selective sub-cellular
translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl
Pharmacol. 251:253–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devine PJ, Perreault SD and Luderer U:
Roles of reactive oxygen species and antioxidants in ovarian
toxicity. Biol Reprod. 86:272012. View Article : Google Scholar :
|
|
7
|
Das S, Chattopadhyay R, Ghosh S, Ghosh S,
Goswami SK, Chakravarty BN and Chaudhury K: Reactive oxygen species
level in follicular fluid-embryo quality marker in IVF. Hum Reprod.
21:2403–2407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiener-Megnazi Z, Vardi L, Lissak A,
Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M
and Dirnfeld M: Oxidative stress indices in follicular fluid as
measured by the thermo chemiluminescence assay correlate with
outcome parameters in vitro fertilization. Fertil Steril. 82(Suppl
3): S1171–S1176. 2004. View Article : Google Scholar
|
|
9
|
Goldman JM, Murr AS and Cooper RL: The
rodent estrous cycle: Characterization of vaginal cytology and its
utility in toxicological studies. Birth Defects Res B Dev Reprod
Toxicol. 80:84–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim J and Luderer U: Oxidative damage
increases and antioxidant gene expression decreases with aging in
the mouse ovary. Biol Reprod. 84:775–782. 2011. View Article : Google Scholar :
|
|
11
|
Finlay CA, Hinds PW and Levine AJ: The p53
proto-oncogene can act as a suppressor of transformation. Cell.
57:1083–1093. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simpson JF and Page DL: The p53 tumor
suppressor gene in ductal carcinoma in situ of the breast. Am J
Pathol. 156:5–6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Z, Lin M and Wu R: The regulation of
aging and longevity: A new and complex role of p53. Genes Cancer.
2:443–452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bensaad K and Vousden KH: p53: New roles
in metabolism. Trends Cell Biol. 17:286–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang L, Zhang XH, Zhou Y, Huang YJ and
Deng HZ: Protective effect of Oviductus Ranae capsules on the
reproductive organs of aged mice. Nan Fang Yi Ke Da Xue Xue Bao.
28:982–985. 2008.In Chinese. PubMed/NCBI
|
|
17
|
Pend J, Deng HZ, Ma DD, Wei LC, Zheng YX
and Liang L: The effects of Oviductus Ranae on the proliferation
and secretion of ovarian granulosa cells in rats. Shi Zhen Guo Yi
Guo Yao. 24:532–535. 2013.In Chinese.
|
|
18
|
Xu LW, Kluwe L, Zhang TT, Li SN, Mou YY,
Sang Z, Ma J, Lu X and Sun ZJ: Chinese herb mix Tiáo-Gēng-Tāng
possesses antiaging and antioxidative effects and upregulates
expression of estrogen receptors alpha and beta in ovariectomized
rats. BMC Complement Altern Med. 11:1372011. View Article : Google Scholar
|
|
19
|
Li WY, Wu KL, Yao JC, Du LY and Liu YH:
Effects of Zishen Yangyin Decoction on sex hormones levels and
antioxidant capacity of ovariectomized rats. Zhong Cheng Yao.
35:673–677. 2013.In Chinese.
|
|
20
|
Chinese Pharmacopoeia Commission: Chinese
Pharmacopoeia: Part 1. China Medical Science Press; Beijing: pp.
239–257. 2010
|
|
21
|
Zhao WY and Sun GC: Research progress of
Ranae Oviductus. J Shenyang Pharmaceut Univ. 1:68–72. 1996.
|
|
22
|
Yun M and Wei X: Pharmacodynamics of
Yifuning soft capsule. Zhe Jiang Da Xue Xue Bao. 29:62–65. 2005.In
Chinese.
|
|
23
|
Wu ZX, Wang XH, Liu H and Deng HZ: Effects
of the mixture of Rhizoma Curcumae and Oviducts Ranae on estrogen
and its receptor expressions in ovariectomized rats. Nan Fang Yi Ke
Da Xue Xue Bao. 28:746–749. 2008.In Chinese. PubMed/NCBI
|
|
24
|
Liu XW and Deng HZ: Clinical observation
of Yifuning's treatment of menopause syndrome. J Tradit Med Sci
Tech. 6:3532002.In Chinese.
|
|
25
|
Xiao W, Deng HZ, Ma Y and Chen YY:
Laboratory study of the yi-fu-ning soft gelatin capsules in
treating climacteric syndrome. Zhongguo Zhong Yao Za Zhi.
28:253–257. 2003.In Chinese.
|
|
26
|
Council of Europe: Directive 2010/63/EU of
the European Parliament and of the Council of 22 September 2010 on
the protection of animals used for scientific purposes. J Eur.
276:82–128. 2010.
|
|
27
|
Singavarapu R, Buchinsky N, Cheon DJ and
Orsulic S: Whole ovary immunohistochemistry for monitoring cell
proliferation and ovulatory wound repair in the mouse. Reprod Biol
Endocrinol. 8:982010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
29
|
Archer DF and Oger E: Estrogen and
progestogen effect on venous thromboembolism in menopausal women.
Climacteric. 3:235–240. 2012. View Article : Google Scholar
|
|
30
|
Cheng G, Wilczek B, Warner M, Gustafsson
JA and Landgren BM: Isoflavone treatment for acute menopausal
symptoms. Menopause. 14:468–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiechi LM, Putignano G, Guerra V,
Schiavelli MP, Cisternino AM and Carriero C: The effect of a soy
rich diet on the vaginal epithelium in postmenopause: A randomized
double blind trial. Maturitas. 45:241–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uesuqi T, Fukui Y and Yamori Y: Beneficial
effects of soybean isoflavone supplementation on bone metabolism
and serum lipids in postmenopausal Japanese women: A four-week
study. J Am Coll Nutr. 21:97–102. 2002. View Article : Google Scholar
|
|
33
|
Sánchez Rodríguez MA, Zacarías Flores M,
Arronte Rosales A and Mendoza Núñez VM: Effect of hormone therapy
with estrogens on oxidative stress and quality of life in
postmenopausal women. Ginecol Obstet Mex. 81:11–22. 2013.In
Spanish.
|
|
34
|
Mesalić L, Tupković E, Kendić S and Balić
D: Correlation between hormonal and lipid status in women in
menopause. Bosn J Basic Med Sci. 8:188–192. 2008.
|
|
35
|
Sai K, Takagi A, Umemura T, Hasegawa R and
Kurokawa Y: Changes of 8-hydroxydeoxyguanosine levels in rat organ
DNA during the aging process. J Environ Pathol Toxicol Oncol.
11:139–143. 1992.PubMed/NCBI
|
|
36
|
Kaneko T, Tahara SM and Matsuo M:
Non-linear accumulation of 8-hydroxy-2-deoxyguanosine, a marker of
oxidized DNA damage, during aging. Mutat Res. 316:277–285. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anson RM, Sentürker S, Dizdaroglu M and
Bohr VA: Measurement of oxidatively induced base lesions in liver
from Wistar rats of different ages. Free Radic Biol Med.
27:456–462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fraga CG, Shigenaga MK, Park JW, Degan P
and Ames BN: Oxidative damage to DNA during aging:
8-hydroxy-2-deoxyguanosine in rat organ DNA and urine. Proc Natl
Acad Sci USA. 87:4533–4537. 1990. View Article : Google Scholar
|
|
39
|
Wang YJ, Ho YS, Lo MJ and Lin JK:
Oxidative modification of DNA bases in rat liver and lung during
chemical carcinogenesis and aging. Chem Biol Interact. 94:135–145.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levine AJ, Hu W and Feng Z: The P53
pathway: What questions remain to be explored? Cell Death Differ.
13:1027–1036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng Z and Levine AJ: The regulation of
energy metabolism and the IGF-1/mTOR pathways by the p53 protein.
Trends Cell Biol. 20:427–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y, Jungsuwadee P, Vore M, Butterfield
DA and St Clair DK: Collateral damage in cancer chemotherapy:
Oxidative stress in nontargeted tissues. Mol Interv. 7:147–156.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hwang PM, Bunz F, Yu J, Rago C, Chan TA,
Murphy MP, Kelso GF, Smith RA, Kinzler KW and Vogelstein B:
Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced
apoptosis in colorectal cancer cells. Nat Med. 7:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ueno M, Masutani H, Arai RJ, Yamauchi A,
Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J and Nikaido T:
Thioredoxin-dependent redox regulation of p53-mediated p21
activation. J Biol Chem. 274:35809–35815. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rivera A and Maxwell SA: The p53-induced
gene-6 (proline oxidase) mediates apoptosis through a
calcineurin-dependent pathway. J Biol Chem. 280:29346–29354. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lyakhov IG, Krishnamachari A and Schneider
TD: Discovery of novel tumor suppressor p53 response elements using
information theory. Nucleic Acids Res. 36:3828–3833. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu G and Chen X: The ferredoxin reductase
gene is regulated by the p53 family and sensitizes cells to
oxidative stress-induced apoptosis. Oncogene. 21:7195–7204. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu B, Chen Y and St Clair DK: ROS and
p53: A versatile partnership. Free Radic Bio Med. 44:1529–1535.
2008. View Article : Google Scholar
|
|
52
|
Kamijo T, Zindy F, Roussel MF, Quelle DE,
Downing JR, Ashmun RA, Grosveld G and Sherr CJ: Tumor suppression
at the mouse INK4a locus mediated by the alternative reading frame
product p19ARF. Cell. 91:649–659. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Waldman T, Kinzler KW and Vogelstein B:
p21 is necessary for the p53-mediated G1 arrest in human cancer
cells. Cancer Res. 55:5187–5190. 1995.PubMed/NCBI
|

















