Introduction
Complete activation of T cells in response to donor
alloantigen presentation requires the delivery of two separate, but
complimentary signals. One is delivered via the antigen-specific
T-cell receptor, following recognition of an alloantigen in the
context of a major histocompatibility complex molecule expressed on
an antigen-presenting cell. The other is introduced by the
costimulatory molecules, which result from the ligation of the
costimulatory receptor-ligand pair to deliver a positive or
negative activation signal (1).
The T-cell costimulatory molecules are predominantly comprised of
the B7/CD28 superfamily and the tumor necrosis factor (TNF) family.
In previous years, numerous members of the CD28 and B7 families
have been identified, including programmed death-1 (PD-1), B and T
lymphocyte attenuator, and the PD-L1, PD-L2, B7-H2, B7-H3 and B7-H4
ligands, which share similar protein domain structures consisting
of one Ig-V-like domain and one Ig-C-like domain, which reside in
the extracellular portion of the molecule (2-7).
B7-H3 is a novel member of the human B7 family,
which shares 20–27% amino acids with other B7 family members
(5). Differential splicing of
human B7-H3 leads to a 4Ig domain (VCVC) transcript (4IgB7-H3) and
a 2Ig domain (containing V1 and C2) transcript (2IgB7-H3) (8). The 4Ig transcript is the dominant
form in human tissues. By contrast, the mouse B7-H3 gene encodes
only a single VC domain form of B7-H3. Human and mouse B7-H3 mRNAs
are expressed broadly in lymphoid and non-lymphoid organs (5,9).
Soluble B7-H3-Ig binds to activated T cells, costimulating their
proliferation and the production of interferon (IFN)-γ (5). However, several studies have
indicated that B7-H3 has an inhibitory function. 2IgB7-H3 and
4IgB7-H3 have similar functions and can downregulate T cell
responses (10,11). Thus, further investigations are
required to elucidate the contradictory results regarding the
functions of B7-H3.
In order to analyze the expression pattern of B7-H3
at the protein level, and compare the biological function of the
two B7-H3 isoforms, the present study generated one B7-H3
monoclonal antibody (mAb; clone 11F4), which was able to recognize
2IgB7-H3 and 4IgB7-H3. The 11F4 mAb was used to detect the
expression of B7-H3 in tumor cell lines. The B7-H3 protein was
expressed broadly on the surface of the tumor cells, with the
exception of certain hematopoietic cell lines. Subsequently, the
2IgB7-H3 and 4IgB7-H3-Fc fusion protein was obtained using
NIH3T3/2IgB7-H3, and the NIH3T3/4IgB-H3 stable expressing system
was constructed. Notably, 2IgB7-H3 and 4IgB7-H3 exhibited a similar
costimulatory function in promoting T cell proliferation. The 11F4
mAb inhibited the positive effect activated by the B7-H3 fusion
proteins.
Materials and methods
Preparation and culture of cells
The SP2/0 mouse myeloma cell line, NIH/3T3 mouse
embryo fibroblast cell line, HEK293 human embryonic kidney cell
line, EAhy926 endothelial cell line, the human tumor cell lines
(HO8910, H460, SKOV3, SW-1990, Patu8988, HepG2, MDA-MB-231, HeLa
and MGC-803), the human malignant hematopoietic cell lines (Raji,
Daudi, Jurkat, U266, U937, K562 and HL-60) and RPMI-8226) were
obtained from American Type Culture Collection (Manassas, CA, USA).
All the cell lines were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) or standard Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal calf serum (FCS; GE Healthcare
Life Sciences, Logan, UT, USA), 100 U/ml penicillin and 100
μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and 2
mM L-glutamine (Sigma-Aldrich). The cells were cultured in a 5%
CO2, 37°C incubator.
Animals and reagents
The present study was approved by the ethics
committee of the Affiliated Hospital of Nantong University
(Nantong, China). Female BALB/c mice (age, six weeks) were
purchased from the Department of Experimental Animal (Shanghai
Institute of Biological Products, Ministry of Health of China,
Shanghai China). The three mice were housed together in an
environment of 25°C with a natural light/dark cycle and food and
water provided every day. A series of anti-human mAbs, including
mouse monoclonal CD3 (clone UCHT1; 1:3,000; cat. no. ab119110) and
mouse monoclonal IgG1 (1:3,000; cat. no. ab91353) were purchased
from Abcam (Cambridge, MA, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, Texas, USA; cat. no. sc-2031;
1:3,000). Functional CD28 monoclonal mouse antibody (1:1,000; cat.
no. MAB342), and anti-His Tag mouse monoclonal antibody (1:1,000;
cat. no. MAB050) were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA).
Preparation of T cells and DCs
Peripheral blood mononuclear cells (PBMCs) were
separated from 50 ml healthy human peripheral blood (Nantong
Central Blood Bank, Nantong, China) using Ficoll-Hypaque (TBD
science, Tianjin, China) gradient centrifugation at 800 x g for 20
min at room temperature. Monocytes were prepared from the PBMCs by
removing non-adherent cells to deplete the T cells, B cells and
natural killer cells following 2 h incubation in 6-well culture
plates at 37°C. The T lymphocytes were isolated by classic
erythrocyte-rosetting due to binding between sheep red blood cells
and T lymphocytes (12,13). The purity of the T lymphocytes was
>90%. The adherent monocytes were cultured in RPMI-1640 medium
containing 10% FCS with human recombinant GM-CSF (500 U/ml; R&D
Systems, Inc.) and human recombinant IL-4 (200 U/ml; R&D
Systems, Inc.) to obtain monocyte-derived dendritic cells (mdDCs).
Maturation of the mdDCs was achieved by incubating the cells with
TNF-α (100 U/ml; R&D Systems, Inc.) for 48 h at 37°C.
Production of B7-H3 cDNA
Total RNA was prepared from the mdDCs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and was used
for complementary DNA (cDNA) synthesis with a Superscript
First-Strand Synthesis kit (Takara Bio, Inc., Dalian, China),
according to the manufacturer's instructions. The genes encoding
the extracellular domain of the two B7-H3 isoforms (accession
number: NM_001024736 and accession number: NM_025240.2) were
amplified from the pMD19-T/4IgB7-H3 and pMD19-T/2IgB7-H3 vectors
using polymerase chain reaction (PCR) amplification with the
following primers: B73b-A
5′-GCTAAGCTTCTGCGTCGGCGGGGCAGCCCT-3′ (Hind III site
shown in italics) and B73b-B
5′-CCTGGATCCTCAGGCTATTTCTTGTCCA-3′ (BamH I site shown
in italics) using Premix Taq™ (Takara Bio, Inc.). The thermocycling
conditions were as follows: 3 min at 95°C for polymerase
activation; followed by 35 cycles of denaturation at 95°C for 40
sec, annealing at 57°C for 45 sec, and extension at 72°C for 1
min.
Expression of the 4IgB7-H3 and 2IgB7-H3
recombinant proteins
The 4IgB7-H3 and 2IgB7-H3 cDNA were subcloned into
the pSectag2B mammalian expression vector (Invitrogen; Thermo
Fisher Scientific, Inc.), which carries the C-terminal His-tag for
purification and detection. The two types of cDNA were respectively
introduced into NIH3T3 cells, which contain endogenous furin to
cleave propeptide and release the mature metalloprotease (14), using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The transfected cells
were maintained in DMEM supplemented with 10% FCS, 100 U/ml
penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine at
37°C, with 95% air and 5% CO2. Cells expressing the
recombinant protein were selected in medium containing 400
μg/ml hygromycin-B (Invitrogen; Thermo Fisher Scientific,
Inc.), in which cells expressing high levels of 4IgB7-H3 and
2IgB7-H3 were selected by single-cell cloning. The recombinant
4IgB7-H3 and 2IgB7-H3 was collected from the supernatant of the
confluent cells grown in serum-free medium, and purified through
the C-terminal His-tag using Protino Ni-NTA Agarose
(Macherey-Nagel, Düren, Germany).
Generation and characterization of mouse
anti-human B7-H3 mAbs
The six-week-old BALB/c mice were separately
immunized with four injections of purified recombinant 4IgB7-H3 at
21-day intervals. The first three injections were 0.1 mg
subcutaneous injections and the fourth was a 0.05 mg caudal vein
injection. Subsequently, 4 days after the final injection, the
immunized mice were sacrificed by cervical dislocation. The spleen
cells were separated using a mesh screen. The splenocytes and
murine myeloma SP2/0 cells were fused at a ratio of 10:1 by PEG4000
(Invitrogen; Thermo Fisher Scientific, Inc.) within 2 min. The
cells were subsequently cultured at 37°C in 5% CO2. The
procedure was conducted according to the method described by Groth
and Scheidegger (15). Following 2
weeks of hypoxanthine-aminopterin-thymidine selection
(Sigma-Aldrich), one hybridoma cell line secreting mouse anti-human
B7-H3 mAb (11F4) was obtained, exhibiting high reactivity with
recombinant 4IgB7-H3 using an enzyme linked immunosorbent assay
(ELISA). The mAbs were purified on Protein G-Sepharose CL4B
(Pharmacia, Uppsala, Sweden) affinity columns, and the Ig isotypes
were identified using rapid test paper (Roche Diagnostics,
Varilhes, France).
Binding assay
A 96-well plate (Corning Incorporated, Corning, NY,
USA) was coated with recombinant 4IgB7-H3 or 2IgB7-H3 protein (10
μg/ml; 100 μl/well) in 0.05 M carbonate/bicarbonate
buffer (pH 9.6) overnight at 4°C. Recombinant human
lipopolysaccharide-binding protein (LBP) with a C-terminal 6-His
tag (R&D Systems, Inc.) was used as a negative control protein.
The wells were washed three times with 0.1% Tween
20-phosphate-buffered saline (PBS; PBST; Sigma-Aldrich) and blocked
with 2% (w/v) bovine serum albumin (BSA; Sigma-Aldrich) in PBS for
2 h at 37°C. Following incubation, the plate was incubated with PBS
containing the 11F4 B7-H3 mAb (1 μg/μl, between
1:80,000 and 1:625) for 1 h at 37°C. Following washing six times
with PBST, the plate was incubated with HRP-conjugated goat
anti-mouse IgG in 0.1% PBST-BSA buffer at 37°C for another 1 h,
followed by incubation with tetramethylbenzidine detection reagent
(Thermo Fisher Scientific, Inc.). The reactions were terminated
with 3 M sulfuric acid (Sigma-Aldrich) and the absorbance of each
sample was read at 450 nm on a microplate reader (Varioskan™ Flash;
Thermo Fisher Scientific, Inc.). All the groups were performed in
triplicate, respectively.
Flow cytometric analysis
For flow cytometric analysis, the target cells
(1×106) were incubated with mouse anti-human B7-H3 11F4
mAbs for 30 min at 37°C and washed with PBS. Fluorescein
isothiocyanate (FITC)-labeled goat anti-mouse IgG (BD Biosciences,
San Jose, CA, USA), as a secondary antibody, was added for another
30 min at 37°C at a concentration of 1 mg/L. Following washing with
PBS, the cells were analyzed using a flow cytometer (Cytomics FC
500, Beckman Coulter, Brea, CA, USA) and with Expo32 MultiCOMP
software (Beckman Coulter).
Western blot analysis
Western blot analysis was performed to analyze the
binding capacity of the 11F4 mAb to the 4IgB7-H3 and 2IgB7-H3
protein on NIH3T3/4IgB7-H3 or NIH3T3/2IgB7-H3 cells, respectively.
NIH3T3/mock cells were used as a negative control. The cell lysate
was separated using 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, transferred onto nitrocellulose filters and
stained with 11F4 mAb. The protein bands were visualized using ECL
Western Blotting Substrate (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
T cell proliferation
For the T cell proliferation assay, a 96-well
flat-bottom plate was precoated with anti-CD3 mAb (0.3
μg/ml) at 4°C overnight. The purified T cells were then
seeded into the wells (1×105/well) to co-culture with
recombinant human IgG (hIgG), 2IgB7-H3 and 4IgB7-H3 (0–8
μg/ml), respectively. The 11F4 mAb (10 μg/ml) was
added to the culture system, with or without anti-CD28 mAb (1
μg/ml). Cell Counting Kit-8 (CCK-8) solution (16) was added to each well, and the cells
were incubated at 37°C for another 5 h. The absorbance was measured
at 450 nm using a microplate reader (Thermo Fisher Scientific,
Inc.). All experiments were performed in triplicate.
Results
Purified human 4IgB7-H3 and 2IgB7-H3
fusion protein
The transfected NIH3T3 cell lines secreting
recombinant the 4IgB7-H3 and 2IgB7-H3 protein were successfully
constructed. The supernatant was collected and purified by the
C-terminal His-tag using Protino Ni-NTA Agarose. The recombinant
protein was well identified by the mouse monoclonal anti-His
antibody (Santa Cruz Biotechnology, Inc.; cat. no. sc-8036;
Fig. 1).
Establishment of 11F4, a novel mouse
anti-human B7-H3 mAb
The Balb/c mice were immunized with purified
recombinant 4IgB7-H3 protein and the splenocytes were fused with
murine myeloma SP2/0 cells. Following repeated screening using
recombinant 4IgB7-H3 protein and multiple subcloning using ELISA,
one hybridoma, termed 11F4, was obtained. The isotope of 11F4 was
mouse IgG1 with a κ light chain, and the hybridoma contained
>100 chromosomes, indicating it was fusant (data not shown).
Notably, 11F4 was observed to bind specifically to both the
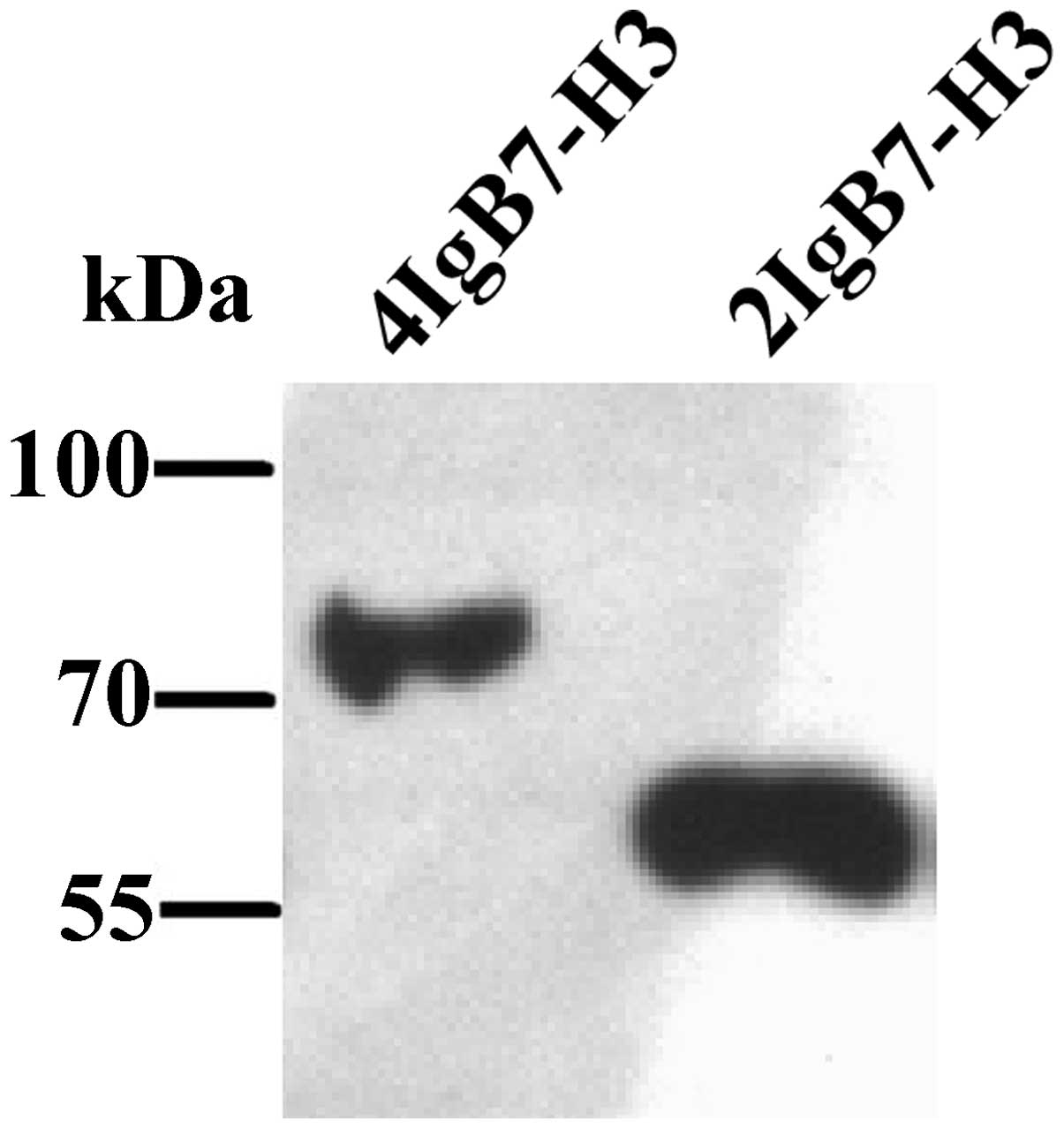
recombinant 4IgB7-H3 and 2IgB7-H3 protein (Fig. 2). In addition, the NIH3T3/4IgB7-H3
and NIH3T3/2IgB7-H3 transfectants were stained by 11F4 using flow
cytometry (Fig. 3A) and western
blotting (Fig. 3B).
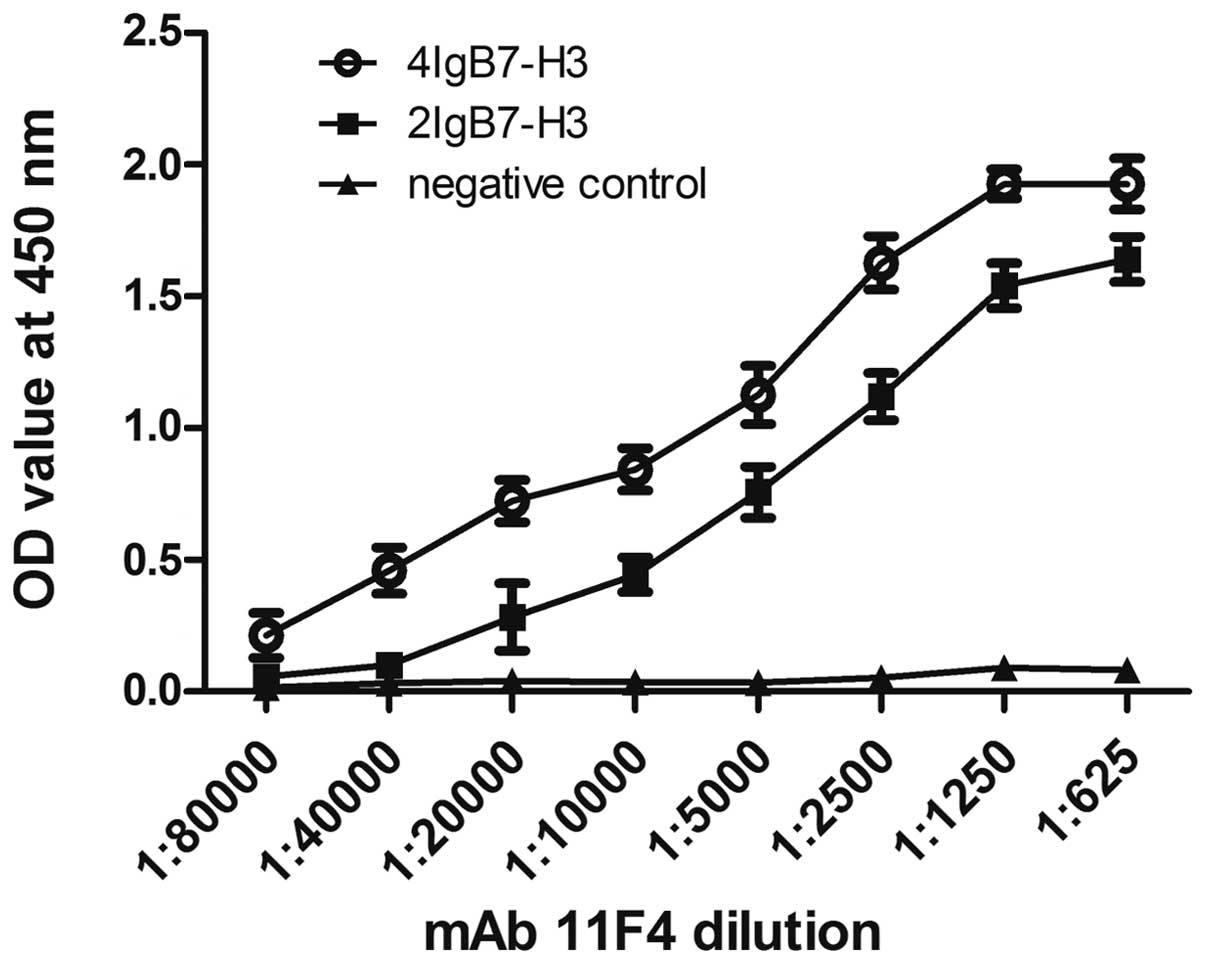 | Figure 2Comparison of the binding activity of
the 11F4 anti-B7-H3 mAb to purified human 4IgB7-H3 and 2IgB7-H3
proteins. Purified 4IgB7-H3 and 2IgB7-H3 proteins (10 μg/ml;
100 μl/well) were coated on a 96-well plate overnight at
4°C, respectively. Recombinant human lipopolysaccharide-binding
protein with a C-terminal 6-His tag was used as a negative control
protein. Following blocking with 2% bovine serum albumin in PBS,
the plate was incubated with PBS containing the 11F4 B7-H3 mAb (1
μg/μl, 1:80,000-1:625) for 1 h at 37°C. Horseradish
peroxidase-conjugated goat anti-mouse IgG was used as the second
antibody. Finally, the plate was incubated with
tetramethylbenzidine and terminated with 3 M sulfuric acid. The
absorbance of each sample was read at 450 nm. All the experiments
were performed in triplicate. Incubation with 11F4 B7-H3 mAb at
1:5,000 and 1:625 was statistically compared with incubation at
1:80,000. Values are expressed as the mean ± standard error of the
mean (n=3). *P<0.001. NS, not significant; PBS,
phosphate-buffered saline; mAB, monoclonal antibody; OD, optical
density. |
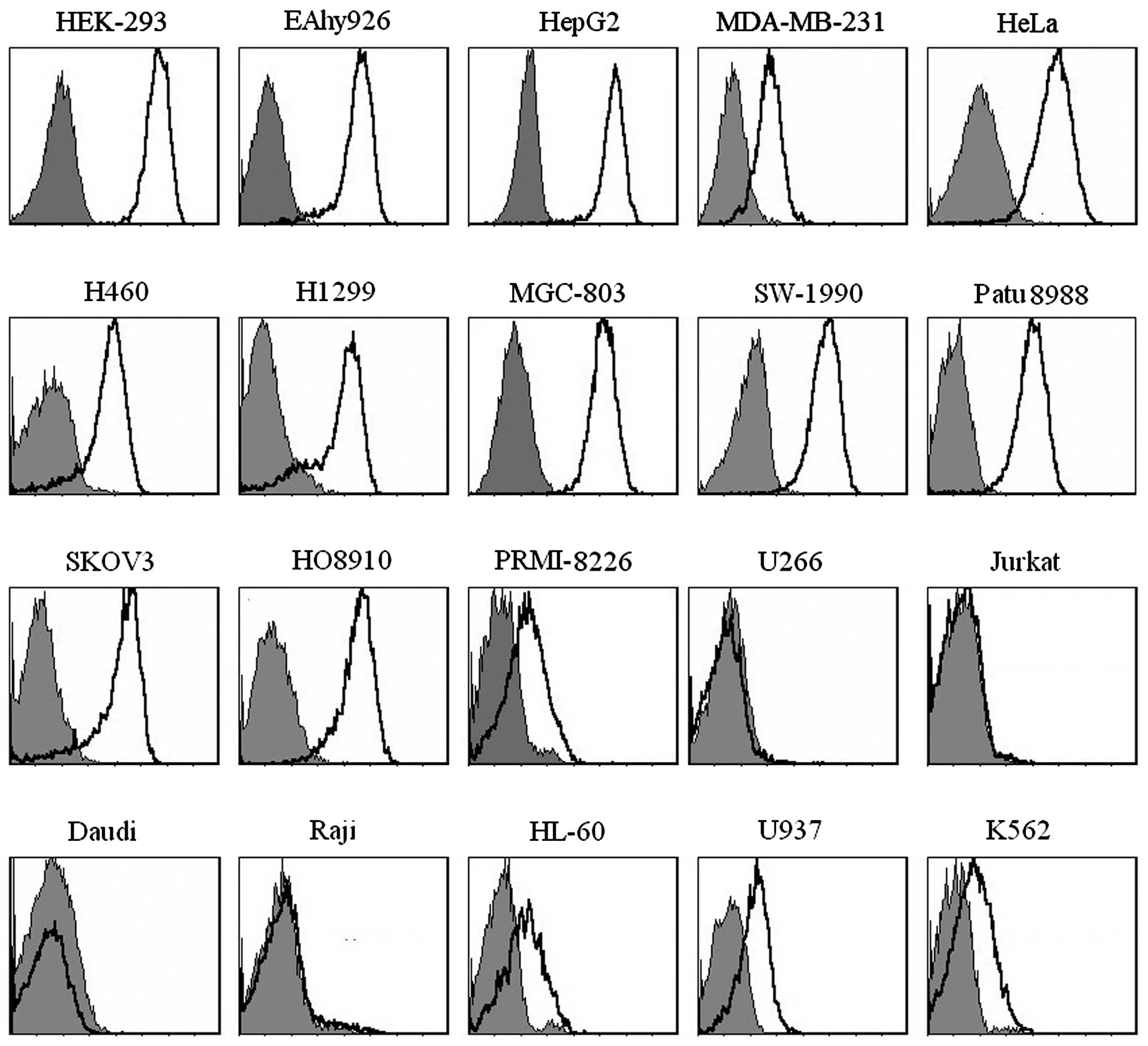
B7-H3 protein is broadly expressed on the
human tumor cell lines
The expression patterns of B7-H3 on human tumor cell
lines were detected by 11F4 using flow cytometry. The results
demonstrated that B7-H3 was broadly and highly expressed on tumor
cell lines, whereas no B7-H3 was detected was detected at had low
levels on human malignant hematopoietic cell lines (Fig. 4).
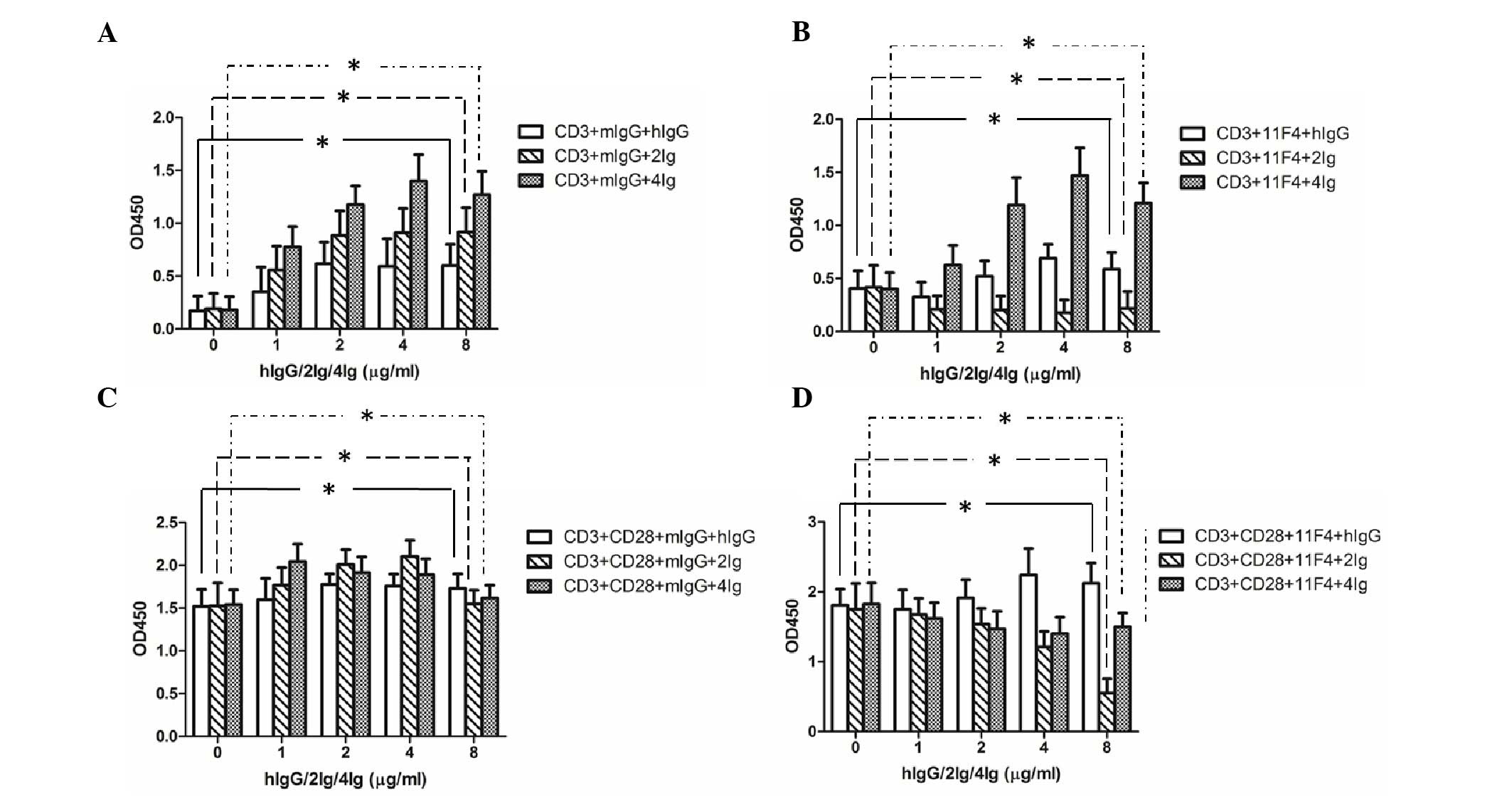
2IgB7-H3 and 4IgB7-H3 signaling enhance
T-lymphocyte proliferation and 11F4 inhibits this enhancement
effect
The purified T cells were stimulated with the
anti-CD3 mAb and co-cultured with recombinant hIgG, 2IgB7-H3 and
4IgB7-H3, respectively. The results revealed that 2IgB7-H3 and
4IgB7-H3 were able to promote T-cell proliferation, and the 11F4
antibody exhibited an inhibitory effect, to a certain extent. In
addition, the inhibitory effect on the 2IgB7-H3 group was more
marked, compared with the effect on the 4IgB7-H3 group (Fig. 5A and B). In the presence of
anti-human CD28 mAb, the effect on proliferation was enhanced.
Notably, the inhibitory effect of 11F4 was markedly increased
following co-incubation with anti-CD3 mAb, anti-CD28 mAb and
recombinant B7-H3 (Fig. 5C and
D).
Discussion
B7-H3 is a type I transmembrane protein belonging to
the B7 superfamily (5,9). B7 superfamily proteins provide
stimulatory or inhibitory accessory signals for T cell responses.
Due to exon duplication, the extracellular architecture of B7-H3 is
characterized by a single IgV-IgC-like (2IgB7-H3) or
IgV-IgC-IgV-IgC-like (4IgB7-H3) domain (8,9). The
different roles of 2IgB7-H3 and 4IgB7-H3 in the immune system
remain to be fully elucidated. In the present study,
NIH3T3/4IgB7-H3 and NIH3T3/2IgB7-H3 transfectants were successfully
constructed, and the two isoforms of recombinant protein were
obtained (Fig. 1). In addition, a
novel mAb, termed, 11F4 against human 2IgB7-H3 and 4IgB7-H3 was
established (Figs. 2 and 3).
B7-H3 are ubiquitously expressed in a wide spectrum
of tissues, however, its protein expression is relatively limited
and maintained at low levels. The mRNA transcription of B7-H3 is
inconsistent with its protein expression, suggesting the existence
of a complex post-transcriptional regulatory mechanism. In
addition, the expression of B7-H3 has been described in
malignancies, including glioma, and lung, pancreatic, ovarian,
breast, gastric and colon cancer (17-22).
However, a correlation between the expression of B7-H3 on cancer
cells and pathological results remains to be fully elucidated. In
the present study, using the 11F4 mAb, high expression levels of
B7-H3 were broadly expressed on tumor cell lines at the protein
level. Normal cell lines, including HEK-293 and EAhy926 cell lines
also exhibited high expression levels of B7-H3. Notably, B7-H3 was
not be detected on several human malignant hematopoietic cell
lines, including U266, Jutkat, Daudi and Raji cells, while
PRMI-8226, HL-60, U937 and K562 cells were observed to exhibit low
or moderate expression levels of B7-H3 (Fig. 4), similar to previously reported
observations (23,24). It has been reported that the
majority of these tumor cell lines express 4IgB7-H3, rather than
2IgB7-H3 (24).
As with other members of the B7 family, B7-H3 is
important in costimulatory pathways in T cells. However, the
function of B7-H3 remains controversial. Chapoval et al
reported that B7-H3 costimulates the proliferation of
CD4+ and CD8+ T cells, enhances the induction
of cytotoxic T cells and selectively stimulates IFN-γ production in
the presence of T cell receptor signaling (5). Other reports have indicated that
B7-H3, with its two isoforms of 2IgB7-H3 and 4IgB7-H3, may have a
similar inhibitory function (10,11).
In the T cell proliferation assay performed in the present study,
the biological functions of these two isoforms were found to be
similar, positive regulating the T cells. The 11F4 mAb effectively
inhibited the B7-H3 signal, resulting in inhibition of T cell
proliferation.
In conclusion, the present study successfully
generated a novel specific mouse anti-human B7-H3 mAb (11F4), with
the ability to recognize 4IgB7-H3 and 2IgB7-H3. Using this mAb,
high expression levels of B7-H3 were observed in tumor cells, as in
previous reports. The recombinant 4IgB7-H3 and 2Ig-B7-H3 proteins
enhanced T cell proliferation, and the effect of B7-H3 on the
promotion of T cell proliferation was further verified using 11F4
in an inhibitory assay. The role of 4IgB7-H3 and 2Ig-B7-H3 on
accessory signals in T cell functions, and whether the two isoforms
have different receptors, requires further investigation.
References
|
1
|
Chen W, Hou Z, Li C, Xiong S and Liu H:
Cloning and characterization of porcine 4Ig-B7-H3: a potent
inhibitor of porcine T-cell activation. PLoS One. 6:e213412011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selenko-Gebauer N, Majdic O, Szekeres A,
Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl
WF, Stockinger H, et al: B7-H1 (programmed death-1 ligand) on
dendritic cells is involved in the induction and maintenance of T
cell anergy. J Immunol. 170:3637–3644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong C, Juedes AE, Temann UA, Shresta S,
Allison JP, Ruddle NH and Flavell RA: ICOS co-stimulatory receptor
is essential for T-cell activation and function. Nature.
409:97–101. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K and Chen L:
B7-H3: A costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tafuri A, Shahinian A, Bladt F, Yoshinaga
SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan
G, et al: ICOS is essential for effective T-helper-cell responses.
Nature. 409:105–109. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steinberger P, Majdic O, Derdak SV,
Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF,
Stöckl J and Knapp W: Molecular characterization of human
4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J
Immunol. 172:2352–2359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun M, Richards S, Prasad DV, Mai XM,
Rudensky A and Dong C: Characterization of mouse and human B7-H3
genes. J Immunol. 168:6294–6297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling V, Wu PW, Spaulding V, Kieleczawa J,
Luxenberg D, Carreno BM and Collins M: Duplication of primate and
rodent B7-H3 immunoglobulin V- and C-like domains: Divergent
history of functional redundancy and exon loss. Genomics.
82:365–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suh WK, Gajewska BU, Okada H, Gronski MA,
Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et
al: The B7 family member B7-H3 preferentially down-regulates T
helper type 1-mediated immune responses. Nat Immunol. 4:899–906.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Indiveri F, Huddlestone J, Pellegrino MA
and Ferrone S: Isolation of human T lymphocytes: Comparison between
nylon wool filtration and rosetting with neuraminidase (VCN) and
2-aminoethylisothiouronium bromide (AET)-treated sheep red blood
cells (SRBC). J Immunol Methods. 34:107–115. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madsen M, Johnsen HE, Hansen PW and
Christiansen SE: Isolation of human T and B lymphocytes by
E-rosette gradient centrifugation. Characterization of the isolated
subpopulations. J Immunol Methods. 33:323–336. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shapiro J, Sciaky N, Lee J, Bosshart H,
Angeletti RH and Bonifacino JS: Localization of endogenous furin in
cultured cell lines. J Histochem Cytochem. 45:3–12. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de StGroth SF and Scheidegger D:
Production of monoclonal antibodies: Strategy and tactics. J
Immunol Methods. 35:1–21. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishiyama M, Miyazono Y, Sasamoto K, Ohkura
Y and Ueno K: A highly water-soluble disulfonated tetrazolium salt
as a chromogenic indicator for NADH as well as cell viability.
Talanta. 44:1299–1305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Z, Luther N, Ibrahim GM, Hawkins C,
Vibhakar R, Handler MH and Souweidane MM: B7-H3, a potential
therapeutic target, is expressed in diffuse intrinsic pontine
glioma. J Neurooncol. 111:257–264. 2013. View Article : Google Scholar
|
|
18
|
Boland JM, Kwon ED, Harrington SM,
Wampfler JA, Tang H, Yang P and Aubry MC: Tumor B7-H1 and B7-H3
expression in squamous cell carcinoma of the lung. Clin Lung
Cancer. 14:157–163. 2013. View Article : Google Scholar
|
|
19
|
Fauci JM, Sabbatino F, Wang Y,
Londoño-Joshi AI, Straughn JM Jr, Landen CN, Ferrone S and
Buchsbaum DJ: Monoclonal antibody-based immunotherapy of ovarian
cancer: Targeting ovarian cancer cells with the B7-H3-specific mAb
376.96. Gynecol Oncol. 132:203–210. 2014. View Article : Google Scholar
|
|
20
|
Arigami T, Narita N, Mizuno R, Nguyen L,
Ye X, Chung A, Giuliano AE and Hoon DS: B7-h3 ligand expression by
primary breast cancer and associated with regional nodal
metastasis. Ann Surg. 252:1044–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu
KF, Zhao JM, Zhang GB and Zhang XG: Relationship between
co-stimulatory molecule B7-H3 expression and gastric carcinoma
histology and prognosis. World J Gastroenterol. 12:457–459. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M,
Tan Y, Wang HT, Lu BF and Zhang XG: Clinical significance and
regulation of the costimulatory molecule B7-H3 in human colorectal
carcinoma. Cancer Immunol Immunother. 59:1163–1171. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castriconi R, Dondero A, Augugliaro R,
Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV,
Moretta L, et al: Identification of 4Ig-B7-H3 as a
neuroblastoma-associated molecule that exerts a protective role
from an NK cell-mediated lysis. Proc Natl Acad Sci U S A.
101:12640–12645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou YH, Chen YJ, Ma ZY, Xu L, Wang Q,
Zhang GB, Xie F, Ge Y, Wang XF and Zhang XG: 4IgB7-H3 is the major
isoform expressed on immunocytes as well as malignant cells. Tissue
Antigens. 70:96–104. 2007. View Article : Google Scholar : PubMed/NCBI
|