Introduction
Cardiovascular disease is a major cause of mortality
worldwide and has markedly increased in prevalence. It is
characterized by high disability and mortality rates. In a previous
study, rats that received an acute isoproterenol overdose (ISO-OV)
suffered cardiac apex ischemia-reperfusion damage and arrhythmia,
and subsequently underwent cardiac remodeling and dysfunction. At
two weeks, myocytes exhibited systolic and diastolic
Ca2+ mishandling, thus, post-ISO-OV mitochondrial
dysfunction may underlie decreased cardiac contractility, ATP
depletion and exacerbated oxidative stress (1). S100 Ca2+-binding protein
A1 (S100A1) is an important regulator of cardiac function and
vascular biology. S100A1 is a Ca2+ sensor protein
involved in Ca2+ signal transduction pathways, and is
important for gene expression, secretion, apoptosis, cell
differentiation and muscle contraction. S100A1 interacts with the
sarcoplasmic reticulum ATPase (SERCA2a) and ryanodine receptor 2
(RyR2), which primarily results in improved Ca2+
handling and contractile function (2–5).
S100A1 may enhance transient Ca2+ amplitudes and
decrease diastolic Ca2+ overload via increased
Ca2+-induced Ca2+ release (CICR) from the
sarcoplasmic reticulum (SR) and decreased diastolic SR
Ca2+ leak to improve cardiomyocyte function (2–8).
Previously, a mouse model demonstrated that a stable increase in
the expression level of Sl00A1 protein significantly enhanced
myocardial contractility (9).
S100A1 gene knockout mice exhibit acute contractile dysfunction,
myocardial apoptosis, early myocardial remodeling, a severely
damaged adrenergic signaling system and rapid heart failure
(4). In other animal studies,
S100A1 gene-targeted therapy reversed experimental heart failure
and improved cardiac performance (9,10).
However, the understanding how S100A1 effects modifications to
cardiac energy metabolism, Ca2+-binding proteins and
cytoskeletal proteins following acute myocardial infarction (AMI)
remains relatively limited. LTQ OrbiTrap mass spectrometry is a
protein quantification strategy that provides relative and absolute
measurements of proteins in complex mixtures (11). The present study used LTQ OrbiTrap
mass spectrometry to analyze the expression profiles of energy
metabolism-associated proteins, Ca2+-binding proteins
and cytoskeletal proteins when S100A1 was overexpressed by an
adenovirus following AMI. The current study aims to identify the
target proteins that are associated with Ad-S100A1-EGFP following
AMI, and finding alternative therapies to reconstitute the
energetic state.
Materials and methods
Ethics statement
The current study was conducted with approval from
the Ethics Committee of Shandong Provincial Hospital Affiliated to
Shandong University (Jinan, Shandong, China).
Adenovirus constructs
The construction of recombinant adenoviruses that
carry S100A1 and enhanced green fluorescent protein (EGFP;
Ad-S100A1-EGFP), and only EGFP (Ad-EGFP) was performed by Shanghai
Jikai Gene Chemical Technology Co., Ltd. (Shanghai, China)
Acute heart failure model
All animal procedures and experiments were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (12). A total of 20 male Wistar rats
(12–14 weeks old; 250–300 g) were provided by the Experimental
Animal Center of Shandong University (Shan-dong, China). The rats
were housed at a constant temperature of 22°C and a 12-h dark:light
cycle. Rats were fed standard laboratory chow and watered ad
libitum. The animals were randomly divided into 4 groups (3
rats used/time-point) as follows: i) Ad-S100A1-EGFP group,
following ligation of the coronary artery for 20 min,
1×1010 plaque-forming units (pfu) of Ad-S100A1-EGFP in
20 µl were injected into the anterolateral wall of the left
ventricle (LV) using a 30-gauge needle; ii) control group, only the
coronary artery was ligated without any treatment; iii) Ad-EGFP
group, following ligation of the coronary artery for 20 min,
1×1010 pfu of Ad-EGFP in 20 µl were injected into
the anterolateral wall of the LV; and iv) physiological saline
group, following ligation of the coronary artery for 20 min, 20
µl physiological saline was injected into the anterolateral
wall of the LV.
The rats were anesthetized with an intraperitoneal
injection of 30 mg/100 g chloral hydrate and endotracheal
intubation was performed. A breathing machine was used to
facilitate breathing. The animals were ventilated using an
SAR-830/AP small animal ventilator (CWE, Inc., Ardmore, PA, USA) at
a rate of 70 breaths per minute. A left thoracotomy was performed
between the third-fourth intercostal space and the pericardium was
opened. The proximal left anterior descending coronary artery,
which is in the left atrium, and the pulmonary arterial cone
boundary, 2–3 mm below the left auricle, were encircled and ligated
using a 6-0 silk suture. The chest was subsequently closed. A left
anterior descending artery ligation in a rat model produces a large
area of lateral wall infarction, which may induce acute heart
failure.
The rats were euthanized on postoperative days 1, 7
and 14 with an intraperitoneal injection of 40 mg/100 g chloral
hydrate. On day 14, acquired echocardiography was performed to
obtain the LV end-diastolic diameter (LVEDD), LV end-systolic
diameter (LVESD), LV systolic and diastolic posterior (LVPWs and
LVPWd, respectively), LV ejection fraction (LVEF) and fractional
shortening (FS).
Protein extraction
LV samples (50 mg) were obtained then immediately
snap-frozen and stored in liquid nitrogen. The samples were
pulverized under liquid nitrogen into a fine powder, which was
homogenized in a lysate buffer containing 8 mol/l urea, 1 mol/l
dithiothreitol (13) (Roche
Diagnostics GmbH, Mannheim, Germany), leupeptinand, aprotinin,
pepstatin (all 1 mg/ml), radioimmunoprecipitation assay buffer and
0.1% phenylmethanesulfonyl fluoride (w/v; Sigma-Aldrich, St. Louis,
MO, USA) and then lysed on ice for 30 min. The samples were lysed
further by sonication for 2 min. The total lysate was centrifuged
for 15 min at 4°C and 10,000–14,000 × g, and the final supernatant
was collected.
Sample processing
The protein concentrations of the cleared lysates
were then determined using sample processing. Protein extracts (30
µl) were mixed with 200 µl 8 M urea in 0.1 mol/l
Tris/HCl (pH 8.5) in a filter unit and centrifuged at 14,000 × g
for 15 min, following which the flow-through was discarded from the
collection tube. Iodoacetamide solution (0.05 mol/l in urea) was
added, then mixed at 600 rpm in a thermo-mixer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 1 min and incubated without
mixing for 20 min. The filter units were centrifuged at 14,000 × g
for 10 min. Urea (100 µl) was added to the filter unit and
centrifuged at 14,000 × g for 15 min. This step was repeated twice.
NH4HCO3 (100 µl 0.05 M in water) was
added to the filter unit and centrifuged at 14,000 x g for 10 min.
This step was repeated twice. The filter units were transferred to
new centrifuge tubes. NH4HCO3 (40 µl)
and trypsin were added (enzyme-to-protein ratio, 1:100) and mixed
at 600 rpm in a thermo-mixer for 1 min. The units were incubated in
a wet chamber at 37°C for 20 h to achieve complete digestion.
Subsequently, the filter units were centrifuged again at 14,000 × g
for 10 min, and NH4HCO3 (40 µl) was
added and centrifuged at 14,000 x g for 10 min. The final solution
was dried in a vacuum and the samples were stored at −80°C.
Samples were purified with a C18 column
(ReproSil-Pur, Dr. Maisch GmbH, Entringen, Germany). The samples
were mixed with 40 µl 0.1% trifluoroacetic acid (TFA) to
achieve a pH<4. Acetonitrile (100%, 200 µl) was added to
a wet ZipTip and centrifuged at 800 × g for 2 min. This step was
repeated twice. A total of 200 µl 0.1% TFA was added to the
wet ZipTip and centrifuged at 800 x g for 2 min. This step was
repeated twice. The samples were repeatedly drawn 8 times through
the ZipTip. The ZipTip was washed twice with 0.1% TFA and
centrifuged at 800 x g for 2 min. The peptides were eluted with 40
µl formic acid, dried under a vacuum and stored at
−80°C.
LTQ OrbiTrap
Four injections were made into a NanoLC 1000 (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) interfaced to the LTQ
OrbiTrap elite mass spectrometer (Thermo Fisher Scientific, Inc.)
via a nanosource. The samples were loaded onto a 150 µm × 2
cm peptrap 300 A C18 pre-column (ReproSil-Pur, Dr. Maisch GmbH) in
solvent A (99.9% water/0.1% formic acid) and desalted for 10 min.
The peptides were eluted into a 75 µm × 25 cm 100 A C18
analytical column (self-packed; ReproSil-Pur, Dr. Maisch GmbH) and
separated with a linear gradient of 5-30% solvent B (99.9%
acetonitrile/0.1% formic acid) in 5 min, and then 69% solvent B for
115 min. The flow rate used was 500 nl/min. The survey scans were
acquired in the OrbiTrap with a resolving power of 60,000 m/z 400
and an automated gain control target level of 1×106. The
25 most abundant ions were selected for fragmentation using
collision-induced dissociation in the linear ion trap. The
precursor ions were fragmented with He gas for 30 ms with a
normalized collision energy of 35. The dynamic exclusion parameters
were set to exclude ions previously selected for fragmentation for
1 min. All data were acquired in reduced profile mode to
accommodate further downstream processing.
Protein identification and
quantification
Protein identification was accomplished via Proteome
Discoverer version 1.4 (Thermo Fisher Scientific, Inc.) and Mascot
Server version 2.4 (www.matrixscience.com/server.html). The Mascot search
engine was used to identify consolidated data in the Uniprot rat
protein database (www.uniprot.org), with carbamidomethylation + 57,005
selected as the fixed modification and oxidation of methionine +
15,995, light-marked dimethylation + 28,0313 (C- and N-terminal)
and heavy-marked dimethylation + 32,0564 (C- and N-terminal) set as
the variable modifications. The mass tolerance was set to 10 ppm,
and the MS/MS tolerance was set to 0.8 Da (14). The trypsin enzymolysis maximum
leakage cut-off value was set to 2, and the important threshold
value was set to 0.01 to ensure a false discovery rate of <1%.
The protein quantification was obtained via unique peptides.
P<0.05 was considered to indicate a statistically significant
difference for protein quantification. To designate significant
changes in protein expression, fold changes <2.0 were set as the
cut-offs. The analysis was performed twice.
Bioinformatic analyses
Database analyses were performed with Protein
Analysis Through Evolutionary Relationships (PANTHERU; www.pantherdb.org) tools. The PANTHER Classification
System was designed to classify proteins (and their genes) to
facilitate high-throughput analysis.
Western blot analysis
The LTQ OrbiTrap protein expression results were
validated using western blot analysis. The total protein extracts
used for western blotting were obtained from the described
experiments. Protein concentrations were quantified using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China). The samples of 50 µg total proteins were separated
using 12% and 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (ZSGB-Bio, Beijing, China) and transferred onto
polyvinylidene fluoride membranes (Merck Millipore, Darmstadt,
Germany) via electro-blotting. The membranes were incubated in TBST
containing 5% non-fat dried milk for 1 h at 25°C. The membranes
were then probed with primary mouse monoclonal anti-myosin light
chain 3 (MLC3; ab680; Abcam, Cambridge, UK), rabbit polyclonal
anti-cardiac troponin I (cTnI; ab47003; Abcam) and rabbit
polyclonal anti-heat shock protein 70 (HSP70; 4872S; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibodies at 1:1,000 dilution
overnight at 4°C. Horseradish peroxidase-conjugated goat anti-mouse
and anti-rabbit antibodies (cat no. SA00001-2; Proteintech Group,
Inc., Chicago, IL, USA) were used as the secondary antibodies at a
dilution of 1:2,000. Results were visualized with an enhanced
chemiluminescence assay (Thermo Fisher Scientific, Inc.). The bands
were evaluated by Image J software (version 2.0; National
Institutes of Health, Bethesda, MD, USA). Experiments were repeated
3 times)
Immunohistochemical (IHC) staining
The myocardial tissue was fixed in formalin,
embedded in conventional paraffin, sectioned (5 µm
thickness) and stained using an SP-9001 IHC staining kit (ZSGB-Bio)
in accordance with the manufacturer's instructions. The anti-MLC 3
(1:200) and anti-cTnI (1:100) antibodies were incubated with the
sections overnight at 4°C, then incubated at 37°C for 30 min with
biotin-labeled IgG and streptavidin-biotin complex solution. The
specimens were stained with 3,3′-diaminobenzidine and
counter-stained with hematoxylin. Phosphate-buffered saline was
used for the negative control specimens. The specimens were
observed and images were captured using a light microscope (Leica
DM4000B, Leica Microsystems, Germany, magnification, x400). Brown
reaction granules observed in the cells indicated positive
staining.
Statistical analysis
One-way analysis of variance was used to determine
significant differences between Ad-S100A1-EGFP group and control
group, followed by Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Proteomic results
The data from the LTQ OrbiTrap demonstrated that
cardiac tissue samples from the Ad-S100A1-EGFP group (day 14)
contained 1,507 different proteins. These proteins included
well-known markers associated with the cytoskeleton, energy
metabolism and actin. In the day 14 control group cardiac tissues,
1,711 proteins were detected, and 1,573 proteins were
differentially expressed when the two groups were compared. A
2.0-fold difference in expression was used as the cut-off. Of the
differentially expressed proteins, 208 were associated with
high-level processes and their biological functions were
identified. Metabolism-associated and Ca2+-binding
proteins were present in the LV tissue of the Ad-S100A1-EGFP group.
Furthermore, searching the protein database revealed that 134
differentially expressed proteins were closely associated with
energy metabolism. The majority of proteins were important enzymes
involved in energy metabolism. The present study focused on the
proteins that were differentially expressed in the Ad-S100A1-EGFP
group tissue.
Energy metabolism-associated
proteins
Between the Ad-S100A1-EGFP and control groups, 20
distinct carbohydrate metabolism-associated proteins were
differentially expressed in the myocardial tissues. There were 6
proteins significantly downregulated in the Ad-S100A1-EGFP group,
whereas 14 proteins were upregulated in the Ad-S100A1-EGFP group.
The majority of the upregulated proteins were enzymes involved in
glycolysis and the tricarboxylic acid cycle, including citrate
synthase, succinate dehydrogenase (ubiquinone) iron-sulfur subunit,
fumarate hydratase and malate dehydrogenase. These important
enzymes limit the catabolism of carbohydrate and energy support
(Table I).
 | Table ICarbohydrate metabolism-associated
proteins differentially expressed in the Ad-S100A1-EGFP group. |
Table I
Carbohydrate metabolism-associated
proteins differentially expressed in the Ad-S100A1-EGFP group.
| Accession | Unique peptide
no. | P-value | Fold | Description | Average normalized
abundance
| Panther protein
class |
|---|
| S100A1 | Control |
|---|
| MDHM_RAT | 2 | 2.00e-002 | 178.94 | Malate
dehydrogenase | 4.09e+005 | 2.29e+003 | Dehydrogenase |
| CISY_RAT | 2 | 9.52e-004 | 4.62 | Citrate
synthase | 1.28e+005 | 2.77e+004 | Transferase
lyase |
| DHSB_RAT | 1 | 3.00e-002 | 2.58 | Succinate
dehydrogenase [ubiquinone] iron-sulfur subunit | 6.30e+004 | 2.44e+004 | Dehydrogenase |
| HXK1_RAT | 3 | 1.38e-004 | 2.22 | Hexokinase-1 | 5.97e+005 | 2.68e+005 | Carbohydrate
kinase |
| KPB1_RAT | 2 | 1.00e-002 | 2.02 | Phosphorylase b
kinase regulatory subunit alpha, skeletal muscle isoform | 4.99e+004 | 2.46e+004 | Kinase
activator |
| MCCA_RAT | 2 | 3.07e-003 | 2.55 | Methylcrotonoyl-CoA
carboxylase subunit alpha | 3.23e+006 | 1.26e+006 | Ligase |
| HCDH_RAT | 2 | 2.00e-002 | 20.50 |
Hydroxyacyl-coenzyme A dehydrogenase, | 2.88e+005 | 1.40e+004 | Dehydrogenase
hydratase epimerase/racemase |
| PYC_RAT | 2 | 2.00e-002 | 2.04 | Pyruvate
carboxylase | 6.61e+004 | 3.24e+004 | Ligase |
| FUMH_RAT | 4 | 3.66e-003 | 5.18 | Fumarate
hydratase | 6.42e+004 | 1.24e+004 | Hydratase |
| UD2B2_RAT | 1 | 6.18e-004 | 2.69 | UDP-glucuronos
yltransferase 2B2 | 2.82e+004 | 1.05e+004 |
Glycosyltransferase |
| G3P_RAT | 5 | 1.02e-006 | −4.80 |
Glyceraldehyde-3-phosphate
dehydrogenase | 3.10e+005 | 1.49e+006 | Dehydrogenase |
| PCCA_RAT | 5 | 5.17e-003 | 2.20 | Propionyl-CoA
carboxylase alpha chain | 5.43e+005 | 2.46e+005 | Ligase |
| K6PF_RAT | 3 | 2.81e-004 | 3.01 |
6-phosphofructokinase, muscle type | 1.48e+005 | 4.92e+004 | Carbohydrate
kinase |
| PCKGC_RAT | 4 | 2.78e-003 | 3.09 | Phosphoenolpyruvate
carboxykinase, cytosolic | 1.49e+006 | 4.83e+005 | Decarboxylase |
| HCDH_RAT | 2 | 2.00e-002 | 20.50 |
Hydroxyacyl-coenzyme A dehydrogenase | 2.88e+005 | 1.40e+004 | Dehydrogenase |
| DO2_RAT | 2 | 1.16e-003 | −5.27 |
Dihydrolipoyllysine-residue
succinyltransferase component of 2-oxoglutarate dehydrogenase
complex | 5.42e+003 | 2.86e+004 |
Acetyltransferase |
| ECHM_RAT | 1 | 3.08e-004 | −20.17 | Enoyl-CoA
hydratase, | 3971.92 | 8.01e+004 |
Acetyltransferase |
| NSDHL_RAT | 2 | 5.65e-005 | −41.27 |
Sterol-4-alpha-carboxylate 3-
dehydrogenase, decarboxylating | 2306.09 | 9.52e+004 | Oxidoreductase |
| SCOT1_RAT | 3 | 6.50e-004 | −2.41 |
Succinyl-CoA:3-ketoacid-coenzyme A
transferase 1 | 2.42e+005 | 5.83e+005 | Epimerase/racemase
transferase |
| ECHA_RAT | 3 | 3.67e-004 | −4.18 | Trifunctional
enzyme subunit alpha | 2.97e+004 | 1.24e+005 | Dehydrogenase |
Regarding the 27 proteins involved in lipid
metabolism, 16 distinct proteins in the myocardial tissue were
significantly upregulated in the Ad-S100A1-EGFP group. The
upregulated proteins were associated with the following PANTHER
classes: reductase, synthesis, transferase, ligase and lyase
ribosomal protein (Table II).
 | Table IILipid metabolism-associated proteins
differentially expressed in the Ad-S100A1-EGFP group. |
Table II
Lipid metabolism-associated proteins
differentially expressed in the Ad-S100A1-EGFP group.
| Accession | Unique
peptides | P-value | Fold | Description | Average normalized
abundance
| Panther protein
class |
|---|
| S100A1 | Control |
|---|
| DHRS4_RAT | 3 | 9.90e-004 | 2.42 |
Dehydrogenase/reductase SDR family member
4 | 4.64e+005 | 1.92e+005 | Dehydrogenase |
| ACSL5_RAT | 3 | 2.00e-002 | 2.04 |
Long-chain-fatty-acid-CoA ligase 5 | 7.54e+005 | 3.71e+005 | Ligase |
| HMCS1_RAT | 2 | 2.00e-002 | 4.60 |
Hydroxymethylglutaryl-CoA synthase | 7.58e+005 | 1.65e+005 | Transferase |
| ACADM_RAT | 2 | 1.64e-003 | 2.93 | Medium-chain
specific acyl-CoA dehydrogenase | 1.20e+005 | 4.10e+004 | Transferase |
| MCCA_RAT | 2 | 3.07e-003 | 2.55 | Methylcrotonoyl-CoA
carboxylase subunit alpha | 3.23e+006 | 1.26e+006 | Ligase |
| CAV3_RAT | 1 | 2.34e-004 | 3.44 | Caveolin-3 | 7.31e+005 | 2.13e+005 | Transmembrane
receptor regulatory/adaptor protein |
| HCDH_RAT | 2 | 2.00e-002 | 20.50 |
Hydroxyacyl-coenzyme A dehydrogenase, | 2.88e+005 | 1.40e+004 | Dehydrogenase
hydratase epimerase/racemase |
| MCAT_RAT | 2 | 6.06e-003 | 2.05 | Mitochondrial
carnitine/acylcarnitine carrier protein | 2.61e+005 | 1.28e+005 | Amino acid
transporter |
| ACSF2_RAT | 4 | 4.81e-004 | 2.84 | Acyl-CoA synthetase
family member2 | 2.69e+005 | 9.47e+004 | Dehydrogenase |
| CAV1_RAT | 2 | 2.00e-002 | 2.12 | Caveolin-1 | 2.96e+005 | 1.39e+005 | Transmembrane
receptor regulatory/adaptor protein |
| ECHP_RAT | 3 | 4.82e-004 | 2.35 | Peroxisomal
bifunctional enzyme | 2.25e+005 | 9.55e+004 | Dehydrogenase |
| RL8_RAT | 1 | 8.22e-003 | 3.07 | 60S ribosomal
protein L8 | 9.43e+004 | 3.07e+004 | Ribosomal
protein |
| PYC_RAT | 2 | 2.00e-002 | 2.04 | Pyruvate
carboxylase, mitochondrial | 6.61e+004 | 3.24e+004 | Ligase |
| UD2B2_RAT | 1 | 6.18e-004 | 2.69 | UDP-glucuronos
yltransferase 2B2 | 2.82e+004 | 1.05e+004 |
glycosyltransferase |
| PCCA_RAT | 1 | 5.17e-003 | 2.20 | Propionyl-CoA
carboxylase alpha chain, mitochondrial | 5.43e+005 | 2.46e+005 | Ligase |
| DECR_RAT | 7 | 3.29e-004 | 2.92 | 2,4-dienoyl-CoA
reductase | 1.16e+006 | 3.98e+005 | Dehydrogenase |
| LBR_RAT | 1 | 2.80e-003 | −468.97 | Lamin-B
receptor | 4.45 | 2.09e+003 | Receptor |
| ADT1_RAT | 1 | 9.97e-006 | −11.39 | ADP/ATP translocase
1 | 6.63e+004 | 7.55e+005 | Amino acid
transporter |
| ECHM_RAT | 1 | 3.08e-004 | −20.17 | Enoyl-CoA
hydratase, mitochondrial | 3971.92 | 8.01e+004 |
Acetyltransferase |
| NSDHL_RAT | 2 | 5.65e-005 | −41.27 |
Sterol-4-alpha-carboxylate
3-dehydrogenase, decarboxylating | 2306.09 | 9.52e+004 | Oxidoreductase |
| ECHA_RAT | 3 | 3.67e-004 | −4.18 | Trifunctional
enzyme subunit alpha | 2.97e+004 | 1.24e+005 | Dehydrogenase |
| ETFA_RAT | 3 | 2.00e-002 | −3.17 | Electron transfer
flavoprotein subunit alpha | 1.14e+004 | 3.63e+004 | Epimerase/racemase
transferase |
| FAS_RAT | 1 | 3.80e-005 | −∞ | Fatty acid
synthase | 0.00 | 602.35 |
Acyltransferase |
| SCOT1_RAT | 3 | 6.50e-004 | −2.41 |
Succinyl-CoA:3-ketoacid-coenzyme A
transferase 1 | 2.42e+005 | 5.83e+005 | Epimerase/racemase
transferase |
| CDS2_RAT | 1 | 7.24e-007 | −∞ | Phosphatidate
cytidylyltransferase 2 | 0.00 | 2749.92 |
Nucleotidytranferase |
| ACADL_RAT | 4 | 8.86e-004 | −2.06 | Long-chain specific
acyl-CoA dehydrogenase | 5.98e+004 | 1.23e+005 | Transferase |
| MPCP_RAT | 6 | 6.45e-008 | −2.36 | Phosphate carrier
protein | 4.93e+005 | 1.17e+006 | Amino acid
transporter |
The current study identified 14 stimuli response
proteins, and 12 differentially expressed proteins that were
upregulated in the Ad-S100A1-EGFP group compared with the control
group. The majority of the upregulated proteins were important for
ATP binding, ATPase activity, the stress response, the immune
response and potassium channel activity. Although downregulated
proteins were observed, the number was low compared with the
upregulated proteins. There were 2 proteins significantly
downregulated in the Ad-S100A1-EGFP group, whereas 12 proteins were
upregulated (Table III).
 | Table IIIResponse to stimulus proteins
differentially expressed in the Ad-S100A1-EGFP group. |
Table III
Response to stimulus proteins
differentially expressed in the Ad-S100A1-EGFP group.
| Accession | Unique peptide
no. | P-value | Fold | Description | Average normalized
abundance
| Panther protein
class |
|---|
| S100A1 | Control |
|---|
| CTP5B_RAT | 1 | 2.00e-002 | 367.49 |
Contactin-associated protein like 5-2 | 4.28e+005 | 1.17e+003 | Transporter |
| ABCC9_RAT | 1 | 2.64e-006 | 5.97 | ATP-binding
cassette sub-family C member 9 | 7.53e+004 | 1.26e+004 | ATP-binding
cassette (ABC) transporter |
| MSH2_RAT | 4 | 2.68e-003 | 3.62 | DNA mismatch repair
protein Msh2 | 2.72e+005 | 7.53e+004 | DNA binding
protein |
| HRH4_RAT | 1 | 4.85e-005 | 3.51 | Histamine H4
receptor | 1.59e+005 | 4.51e+004 | G-protein coupled
receptor |
| GPR37_RAT | 3 | 2.10e-003 | 2.24 | Probable G-protein
coupled receptor 37 | 1.12e+005 | 4.99e+004 | G-protein coupled
receptor |
| CO1A2_RAT | 4 | 2.33e-005 | 2.27 | Collagen alpha-2(I)
chain | 6.43e+005 | 2.84e+005 | Transporter |
| CO8B_RAT | 2 | 5.12e-003 | 2.13 | Complement
component C8 beta chain | 2.07e+005 | 9.75e+004 | Apolipoprotein |
| COBA1_RAT | 3 | 1.00e-002 | 2.24 | Collagen
alpha-1(XI) chain | 6.35e+004 | 2.83e+004 | Transporter |
| PLAK_RAT | 2 | 5.00e-002 | 10.52 | Junction
plakoglobin | 8.56e+004 | 8.14e+003 | Signaling
molecule |
| RBM43_RAT | 1 | 1.00e-002 | 2.27 | RNA-binding protein
43 | 1.24e+005 | 5.46e+004 | Transcription
cofactor |
| CSF1_RAT | 1 | 1.05e-003 | 3.81 | Macrophage
colony-stimulating factor 1 | 2.69e+004 | 7.054e+003 | Cytokine |
| HSP74_RAT | 2 | 1.95e-003 | 2.27 | Heat shock 70 kDa
protein 4 | 9.09e+004 | 4.01e+004 | Hsp70 family
chaperone |
| APOH_RAT | 1 | 7.98e-003 | −15.96 | Beta-2-glycoprotein
1 | 2.38e+003 | 3.80e+004 | Apolipoprotein |
| MK13_RAT | 3 | 2.00e-002 | −4.46 | Mitogen-activated
protein kinase 13 | 8.47e+004 | 3.78e+005 | No-receptor
serine/threonine protein kinase |
The present study identified 12
Ca2+-binding proteins; 7 were upregulated and 5
downregulated in the Ad-S100A1 EGFP group compared with the control
group. The majority of the upregulated proteins were important for
cardiac muscle contraction, muscle filament sliding and vascular
tone modulation. The present study identified 12 Ca2+
binding proteins; 7 were upregulated and 5 were downregulated in
the Ad-S100A1 EGFP group compared with the control group (Table IV). The majority of the
upregulated proteins were important for cardiac muscle contraction,
muscle filament sliding and vascular tone modulation.
 | Table IVCalcium-binding proteins
differentially expressed in the Ad-S100A1-EGFP group. |
Table IV
Calcium-binding proteins
differentially expressed in the Ad-S100A1-EGFP group.
| Accession | Unique peptide
no. | P-value | Fold | Description | Average normalized
abundance
| Panther protein
class |
|---|
| S100A1 | Control |
|---|
| EFHD2_RAT | 1 | 5.69e-003 | 2.49 | EF-hand
domain-containing protein D2 | 2.68e+005 | 1.07e+005 | Calcium-binding
protein |
| MCAT_RAT | 2 | 6.06e-003 | 2.05 | Mitochondrial
carnitine/acylcarnitine carrier protein | 2.61e+005 | 1.28e+005 | Mitochondrial
carrier protein transfer/carrier protein calmodulin |
| NELL1_RAT | 1 | 5.57e-003 | 3.95 | Protein kinase
C-binding protein NELL1 | 6.33e+005 | 1.60e+005 | Calmodulin |
| SPT21_RAT | 2 | 2.00e-002 | 2.40 |
Spermatogenesis-associated protein 21 | 3.50e+005 | 1.46e+005 | Calmodulin |
| GELS_RAT | 3 | 9.93e-005 | 4.15 | Gelsolin | 6.52e+005 | 1.57e+005 | Calcium-binding
protein |
| EHD1_RAT | 1 | 4.66e-004 | 2.13 | EH
domain-containing protein 1 | 2.08e+004 | 9.78e+003 | Calcium-binding
protein |
| GPAT3_RAT | 1 | 2.00e-002 | 5.12 |
Glycerol-3-phosphate acyltransferase
3 | 1.12e+004 | 2.18e+003 | Calmodulin |
| CALL3_RAT | 1 | 9.19e-005 | −4.37 | Calmodulin-like
protein 3 | 4.11e+004 | 1.80e+005 | Calmodulin |
| MYL3_RAT | 4 | 1.07e-004 | −4.43 | Myosin light chain
3 | 3.23e+005 | 1.43e+006 | Calmodulin |
| CALX_RAT | 3 | 8.97e-003 | −7.48 | Calnexin | 1.11e+004 | 8.31e+004 | Calcium-binding
protein |
| ADT1_RAT | 1 | 9.97e-006 | −11.39 | ADP/ATP translocase
1 | 6.63e+004 | 7.55e+005 | Amino acid
transporter |
| MPCP_RAT | 6 | 6.45e-008 | −2.36 | Phosphate caaarrier
protein | 4.93e+005 | 1.17e+006 | Mitochondrial
carrier protein transfer/carrier protein calmodulin |
The current study identified 22 cytoskeletal
proteins, 16 of which were upregulated in the Ad-S100A1-EGFP group
compared with the control group. The majority of the upregulated
proteins were involved in electron transport during muscle
contraction, cellular Ca2+ ion homeostasis, actin
filament capping, actin filament severing and actin filament
polymerization. Although downregulated proteins were identified,
there were fewer compared with the upregulated proteins. Compared
with the control group, 6 proteins were downregulated in the
Ad-S100A1-EGFP group, whereas 16 proteins were upregulated
(Table V).
 | Table VCytoskeletal proteins differentially
expressed in the Ad-S100A1-EGFP group. |
Table V
Cytoskeletal proteins differentially
expressed in the Ad-S100A1-EGFP group.
| Accession | Unique peptide
no. | P-value | Fold | Description | Average normalized
abundance
| Panther protein
class |
|---|
| S100A1 | Control |
|---|
| MARE3_RAT | 1 | 3.36e-003 | 2.32 |
Microtubule-associated protein RP/EB
family member 3 | 2.97e+005 | 1.28e+005 | Non-motor
microtubule binding protein |
| TNNI3_RAT | 2 | 1.01e-003 | 2.95 | Troponin I, cardiac
muscle | 9.94e+005 | 3.37e+005 | Non-motor actin
binding protein |
| CALD1_RAT | 4 | 6.72e-004 | 2.07 | Non-muscle
caldesmon | 2.96e+005 | 1.43e+005 | Non-motor actin
binding protein |
| MYBPH_RAT | 1 | 3.00e-002 | 2.28 | Myosin-binding
protein H | 1.98e+005 | 8.68e+004 | Non-reporter
serine/threonine protein kinase |
| MYPC_RAT | 1 | 9.94e-003 | 2.22 | Myosin-binding
protein C, cardiac-type | 2.73e+006 | 1.23e+006 | Non-reporter
serine/threonine protein kinase |
| KIF1C_RAT | 4 | 8.37e-003 | 2.14 | Kinesin-like
protein KIF1C | 1.37e+005 | 6.40e+004 | Microtubule binding
motor protein |
| KIF22_RAT | 2 | 9.43e-003 | 2.24 | Kinesin-like
protein KIF22 | 1.64e+004 | 7287.25 | Microtubule binding
motor protein |
| TBA1A_RAT | 1 | 9.20e-004 | 2.91 | Tubulin alpha-1A
chain | 1.98e+005 | 6.81e+004 | tubulin |
| ABLM2_RAT | 2 | 6.67e-003 | 3.53 | Actin-binding LIM
protein 2 | 6.01e+004 | 1.70e+004 | Structural
protein |
| MYH3_RAT | 5 | 3.06e-003 | 2.35 | Myosin-3 | 1.93e+005 | 8.21e+004 | Actin binding motor
protein |
| PLAK_RAT | 2 | 5.00e-002 | 10.52 | Junction
plakoglobin | 8.56e+004 | 8141.00 | Cytoskeletal
protein |
| ARP2_RAT | 2 | 3.37e-003 | 2.11 | Actin-related
protein 2 | 6.08e+004 | 2.88e+004 | Actin and actin
related protein |
| CAPZB_RAT | 1 | 1.78e-004 | 3.58 | F-actin-capping
protein subunit beta | 4.29e+004 | 1.20e+004 | Non-motor actin
binding protein |
| GELS_RAT | 3 | 9.93e-005 | 4.15 | Gelsolin | 6.52e+005 | 1.57e+005 | Calcium-binding
protein |
| LMNA_RAT | 3 | 4.88e-003 | 2.60 | Prelamin-A/C | 1.28e+005 | 4.94e+004 | Structural
protein |
| MYH11_RAT | 2 | 1.94e-003 | 2.86 | Myosin-11
(Fragments) | 5.37e+005 | 1.88e+005 | Actin binding motor
protein |
| MYL3_RAT | 4 | 1.07e-004 | −4.43 | Myosin light chain
3 | 3.23e+005 | 1.43e+006 | calmodulin |
| TPM1_RAT | 3 | 5.62e-006 | −3.46 | Tropomyosin alpha-1
chain | 7.44e+005 | 2.57e+006 | Actin binding motor
protein |
| ACTB_RAT | 2 | 9.32e-005 | −18.34 | Actin, cytoplasmic
1 | 1955.75 | 3.59e+004 | Actin and actin
related protein |
| GFAP_RAT | 2 | 6.29e-003 | −7.03 | Glial fibrillary
acidic protein | 2342.24 | 1.65e+004 | Structural
protein |
| MACF1_RAT | 4 | 1.69e-003 | −2.39 | Microtubule-actin
cross-linking factor 1 | 2.28e+004 | 5.45e+004 | Non-motor actin
binding protein |
| MARK2_RAT | 1 | 8.36e-003 | −2.77 |
Serine/threonine-protein kinase MARK2 | 1.07e+005 | 2.98e+005 | No-receptor
serine/threonine protein kinase |
MLC 3, cTnI and HSP70
The 218 identified proteins were associated with the
cytoskeleton, Ca2+-binding, cellular protein
modifications, translation, catalytic activity, apoptosis,
biological regulation and energy metabolism. The identified
proteins included cTnI, MLC 3 and HSP70. Western blot analysis was
performed to further validate the distribution of these proteins in
the myocardial tissues of the Ad-S100A1-EGFP and control groups.
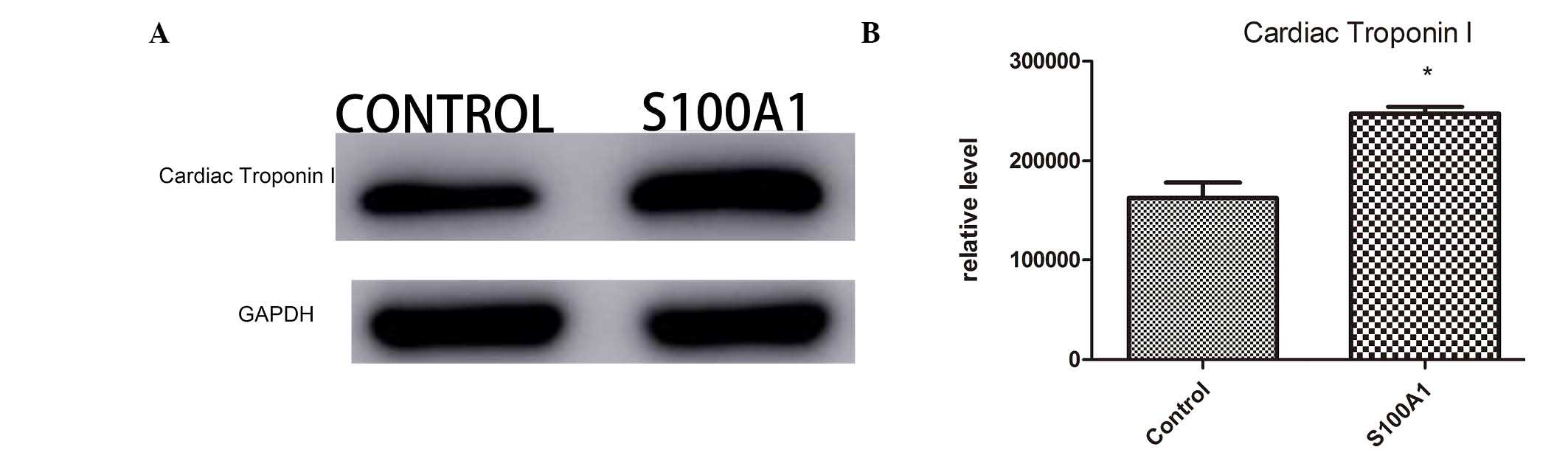
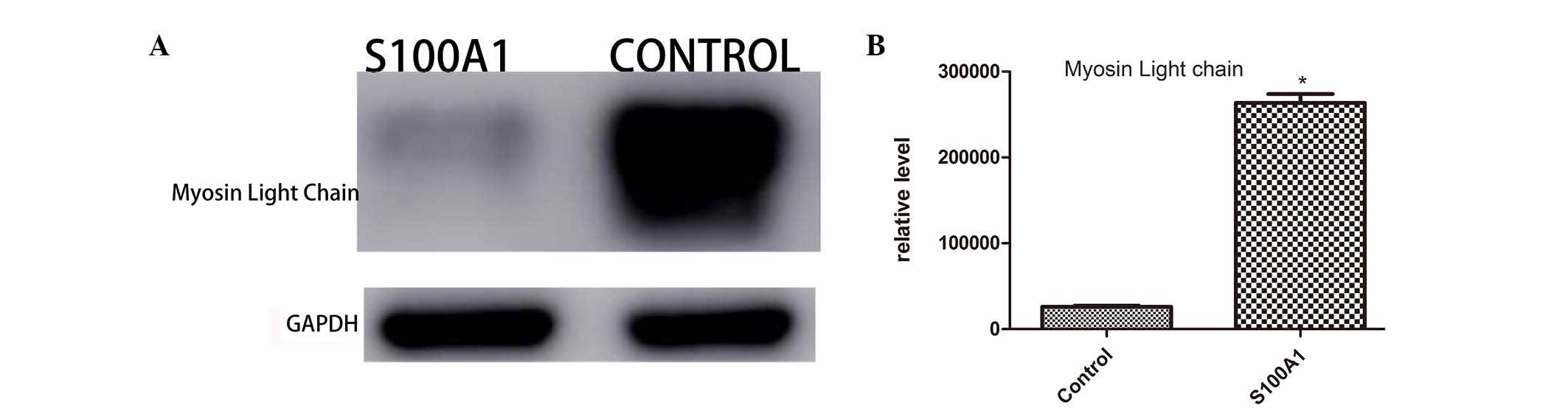
The changes in the protein levels of cTnI (Fig. 1), MLC 3 (Fig. 2) and HSP70 (Fig. 3) were determined using western blot
analysis. The western blot results were generally consistent with
the changes detected by LTQ OrbiTrap. All proteomic experiments
were performed at least twice to validate the reliability of the
LTQ OrbiTrap results. Thus, the present proteomics data are
reliable.
IHC analysis
Fig. 4 demonstrates
positive immunoreactions for the different markers (cTnI, MLC 3 and
HSP70) analyzed in the Ad-S100A1-EGFP and control myocardial
tissues. The IHC results demonstrated that the proteins were
localized to the cytoplasm and nucleus of myocardial cells.
Compared with the control group, expression of cTnI and HSP70 was
increased in the cytoplasm of the Ad-S100A1-EGFP group. By
contrast, cytoplasmic MLC 3 expression was higher in the control
group compared with the Ad-S100A1-EGFP group.
Discussion
Main finding
The main finding of the current proteomics study is
that the majority of the differentially expressed proteins
identified were upregulated in the Ad-S100A1-EGFP group compared
with the control group. Additionally, the majority of proteins were
important enzymes involved in energy metabolism. In the
Ad-S100A1-EGFP group, various Ca2+-binding and
cytoskeletal proteins were upregulated. These findings suggest that
energy production status and cardiac muscle contraction are
important for the recovery of cardiac function.
The present study successfully identified and
analyzed the protein profile of Ad-S100A1-EGFP group rats. To the
best of our knowledge, this is the first study to analyze the
protein profile of rats administered with Ad-S100A1-EGFP adenovirus
using LTQ OrbiTrap.
Proteomics and energy metabolism status
in the Ad-S100A1-EGFP group
According to the PANTHER classification used in the
present study, energy metabolism status and cardiac muscle
contraction are important factors in during cardiac function
recovery following acute heart failure. Furthermore, previous
experimental data suggest a close association between LV
remodeling, energy metabolism status and acute heart failure
(15). Compared with the control
group, 14 carbohydrate metabolism-associated proteins were
upregulated in the Ad-S100A1-EGFP group, including malate
dehydrogenase, citrate synthase, succinate dehydrogenase
(ubiquinone) iron-sulfur subunit, fumarate hydratase hexokinase-1,
6-phosphofructokinase, phosphorylase b kinase regulatory subunit
alpha, phosphoenolpyruvate carboxykinase and UDP-glucuronosyl
transferase 2B2. Additionally, a number of the significantly
upregulated proteins were important enzymes involved in glycolysis
and the tricarboxylic acid cycle. These findings indicate that
carbohydrate anabolism was upregulated in the Ad-S100A1-EGFP group.
The heart is an absolute aerobic organ, thus the majority of the
energy required for its activity is obtained from the aerobic
oxidation of fatty acids and competing energy substrates (including
glucose, ketones, lactate and amino acids). It is understood that
there is a clear decrease in fatty acid oxidation capacity during
advanced heart failure and glucose uptake is doubled compared with
controls. Given that optimal cardiac function under normal and
pathological conditions is dependent on glycolysis and pyruvate
oxidation (13), the increase in
carbohydrate metabolism-associated proteins may also contribute to
the increased ventricular contractility in the Ad-S100A1-EGFP
group. Of the differentially expressed proteins involved in lipid
metabolism, 16 proteins were upregulated, which suggests lipid
metabolism was increased in the Ad-S100A1-EGFP group. Furthermore,
these upregulated proteins had closely associated functions,
particularly long-chain-fatty-acid-CoA ligase 5, which is crucial
for the regulation of lipid metabolism. In addition, the functions
of these proteins in fatty acid oxidative metabolism may exert an
important effect on cardiomyocyte energy supply. Fatty acid
β-oxidation involves four enzymes (acyl CoA dehydrogenase, 2,3
enoyl CoA hydratase, 3-OH acyl CoA dehydrogenase and 3-keotacyl CoA
thiolase) (15). The proteomic
screening in the present study detected the expression of acyl-CoA
dehydrogenase (medium-chain and long-chain), enoyl CoA hydratase
and hydroxyacyl CoA dehydrogenase. Among these proteins, acyl-CoA
dehydrogenase (medium-chain) and hydroxyacyl CoA dehydrogenase were
significantly upregulated in the Ad-S100A1-EGFP group compared with
the control group. 2,4-dienoyl CoA reductase is the auxiliary
enzyme for fatty acid β-oxidation and acyl-CoA dehydrogenase
catalyzes the initial rate-limiting step in mitochondrial fatty
acid β-oxidation (16).
Hydroxyacyl-coenzyme A dehydrogenase is essential for the
mitochondrial β-oxidation of short chain fatty acids and it exerts
its highest activity toward 3-hydroxybutyryl-CoA. The upregulation
of acyl-CoA dehydrogenase and hydroxyacyl-coenzyme A dehydrogenase
in the ventricular tissue of Ad-S100A1-EGFP group rats may be
beneficial for myocardial function. Oxidative phosphorylation is
the principal process by which ATP is formed (17). Changes in the expression of enzymes
involved in respiratory chain complexes may impair energy
production in myocytes. Various modifications to energy metabolism
were demonstrated in the Ad-S100A1-EGFP group. The current study
demonstrated that many proteins were upregulated in the
Ad-S100A1-EGFP group. Impaired energy production or consumption
during acute heart failure tended to reflect chronic heart failure.
The application of quantitative proteomics based on LTQ OrbiTrap
technology effectively evaluated the protein expression in
myocardial tissues. In addition to carbohydrates and lipids, other
metabolites, including certain amino acids and aldehydes, may also
influence energy status.
S100A1 proteins and Ca2+
The present study detected the expression of 12
Ca2+-binding proteins in the myocardial tissues. Of
these proteins, 7 were upregulated and 5 downregulated in the
Ad-S100A1 EGFP group compared with the control group. The majority
of the upregulated proteins, including EF hand domain-containing
protein and gelsolin, are important for cardiac muscle contraction,
muscle filament sliding and vascular tone modulation. The current
study also detected 22 cytoskeletal proteins, 16 of which were
upregulated in the Ad-S100A1 EGFP group compared with the control
group. These cytoskeletal proteins, including myosin binding
protein, myosin 11, myosin 3, and troponin I, are closely
associated with Ca2+. The majority of the upregulated
proteins are important for electron transport in muscle
contraction, cellular Ca2+ ion homeostasis, actin
filament capping, actin filament severing and actin filament
polymerization. Ca2+ acts as a global second messenger
involved in numerous cellular processes. Ca2+ is a vital
regulator of muscle contraction and energy production, and
modulates various other cellular functions, including secretion and
transcription coupling, synaptic transmission, hormonal regulation,
cell cycle control, fertilization and vision (18). In the heart, Ca2+ is
released from the cytoplasmic Ca2+ transients to control
important Ca2+-dependent enzymes of the tricarboxylic
acid cycle and ATP synthase (complex V of the respiratory chain).
Many Ca2+-sensor proteins contain a specific
Ca2+ binding motif (helix-loop-helix, referred to as the
EF hand). Calmodulin (CAM), regarded as the prototypical
Ca2+-sensor, has four EF hands, and its associated
family members include troponin-C (TnC) and MLCs. The S100 proteins
contain two Ca2+-binding motifs, a classical C-terminal
EF hand and an S100-specific N-terminal EF hand. S100A1 protein has
high tissue and cell specificity, and is preferentially abundant in
the heart. It regulates Ca2+ homeostasis, contractile
inotropy and energy production. At the molecular level, S100A1
interacts with the cardiac isoforms of RyR2, SERCA2A, phospholamban
(PLN), titin and mitochondrial F1-ATP synthase (19,20)
in a Ca2+-dependent manner. S100A1 has previously been
demonstrated to exert a dual effect via its interaction with RyR2.
During systole, the opening of the L-type Ca2+ channel
(LTCC) triggers SR Ca2+ release via RyR2 and SR
Ca2+ reuptake is conducted by SERCA, whereas the
Na+-Ca2+-exchanger (NCX) extrudes
Ca2+ from the cardiomyocyte to maintain steady-state
conditions. It stimulates RyR2 to increase systolic function via
the enhancement of CICR from the SR (21). S100A1 interacts with RyR2 and the
SERCA-phospholamban-complex. Increasing S100A1 protein levels
increases systolic SR Ca2+ release via RyR2 without an
effect on LTCC activity. Furthermore, inducing closure of RyR2
channels during diastole, S100A1 decreases the Ca2+
spark frequency to reduce the leakage of Ca2+ from the
SR (22). Thus, S100A1 stimulates
Ca2+ uptake into the SR by directly interacting and
stimulating the SERCA2A Ca2+-pump. It also interacts
with PLN to repress its inhibitory effect on SERCA2A (20). Both effects significantly increase
muscle relaxation via the rapid removal of cytoplasmic
Ca2+ following contraction.
S100A1 directly interacts with the α- and β-chains
of F1-ATPase at the inner mitochondrial membrane to stimulate ATP
production in a Ca2+-dependent manner. S100A1 couples
cardiac Ca2+ cycling with Ca2+-dependent
mitochondrial energy production (20). The S100A1/F1-ATPase interference in
the mitochondria enhances the generation of cytoplasmic ATP in
cardiomyocytes (5). The current
study demonstrated that 14 carbohydrate metabolism-associated
proteins were upregulated in the Ad-S100A1-EGFP group, including
malate dehydrogenase, citrate synthase, succinate dehydrogenase
[ubiquinone] iron-sulfur subunit, hexokinase-1, pyruvatecarboxylase
and 6-phosphofructokinase. The increase in carbohydrate
metabolism-associated proteins may also contribute to the recovery
of ventricular contraction function in the Ad-S100A1-EGFP group.
S100A1 controls contraction and relaxation via interactions at
different target sites. S100A1 functions independently of
β-adrenergic receptor effects without increasing heart rate,
cardiac hypertrophy, cardiac arrhythmias, myocardial fibrosis or
other adverse reactions. The effects of S100A1 in the heart involve
cAMP, and the protein kinase A and CAM-dependent kinase-II
phosphorylation systems (21),
which depend on trans-sarcolemmal Ca2+ fluxes. S100A1
does not affect Ca2+ entry via dihydropyridine
receptors, or efflux via the NCX in forward or reverse mode
(22).
cTnI
Tn consists of three subunits whose names indicate
their roles: Ca2+-binding TnC; inhibitory TnI; and
tropomyosin-binding TnT. cTnI is specifically expressed in the
myocardium. cTnI is an essential regulator of sarcomere contraction
and relaxation. Together with cTnC and cTnT, cTnI binds different
tropomyosin sites on the thin actin filament in response to
Ca2+ (23). In
diastole, when intracellular [Ca2+] is low, cTnI binds
at the outer domain of actin to maintain tropomyosin, which blocks
myosin-binding sites on the thin filament and prevents force
development. In systole, when intracellular [Ca2+] is
high, Ca2+ binds to cTnC and induces a conformational
change. This change results in cTnI release from actin and moves
tropomyosin closer to the groove of the actin filament, thus,
enabling actin-myosin interactions and cardiomyocyte force
development (24). cTnI may be a
potential treatment target for heart failure because of its
important role in the regulation of heart contraction and
relaxation. A previous study examined cTnI phosphorylation in
healthy and diseased hearts via the activation of the β-adrenergic
receptor pathway. cTnI is a regulator of the Frank-Starling
mechanism, which regulates heart function by increasing stroke
volume with increased in ventricular filling (end-diastolic volume)
(22).
MLC
Essential and regulatory MLCs, which have
Ca2+-binding EF hand motifs, bind to the neck region of
myosin heavy chains. MLCs modulate the Ca2+ sensitivity
of cross-bridge cycling to control concerted cardiac contractility.
MLCs are important for cardiac and skeletal muscle function. When
phosphorylated at a specific serine at position 19 by MLC kinase,
MLCs induce conformational changes, stimulate myosin-actin
interactions and improve cardiomyocyte contraction (25–27).
Furthermore, a unique N-terminal domain of essential MLCs directly
binds actin to modulate actin-myosin cross-bridge cycling (28–30).
Notably, cardiomyocyte-specific overexpression of the N-terminus of
human essential MLC (ELC) in rats leads to enhanced cardiac
contractility (30,31). In hypertropy, human ELC partially
replaced expression of the MLC 3. The MLC 3-to-ELC isoform shift
induced a positive inotropic effect (32). It was previously identified that
ventricular ELC is cleaved by caspase-3 at a noncanonical cleavage
site (33).
HSP70
HSPs comprise a family of intracellular proteins
with cytoprotective function. These proteins are induced by
stresses and acute conditions, and have previously been
demonstrated as essential molecular chaperones involved in cell
survival following stress (34,35).
Animal models indicate that HSP70 overexpression protects
myocardial tissues against ischemia (36). HSP70 has a protective function
during acute stress and in patients with chronic stress (36). It has also been previously
demonstrated that HSP70 is associated with ischemia and reperfusion
following coronary bypass grafting (37). In an acute coronary infarction
animal model, Zhang et al (38) transplanted bone marrow cells into
the ischemic area. Reverse transcription-polymerase chain reaction
demonstrated that following bone marrow transplantation, HSP70
expression was upregulated in cardiomyocytes from the infarction
and peri-infarction areas, and acute ischemic cardiac function was
improved. Furthermore, in acute myocardial infarction or other
stress conditions, the body provides protection to the ischemic
myocardium via the increased production of HSP. The level of HSP
expression is associated with the degree of myocardial protection
following AMI. In the present study, the protein expression of
HSP70 was significantly increased in the Ad-S100A1-EGFP group.
The majority of the differentially expressed energy
metabolism-associated and Ca2+-binding proteins in the
Ad-S100A1-EGFP group following AMI were upregulated. This indicates
that energy production and contractile ability were enhanced in the
ventricular myocardium of the Ad-S100A1-EGFP group. Ca2+
is crucial for the recovery of myocardial function in S100A1
transgenic rats (2). To the best
of our knowledge, the current study is the first proteomic analysis
of S100A1-adenoviral overexpression using LTQ OrbiTrap, and the
results of the study may provide comprehensive insights into the
mechanisms of S100A1 in the recovery of heart function following
AMI.
Abbreviations:
|
S100A1
|
S100 calcium binding protein A1
ISO-OV, isoproterenol overdose
|
|
AMI
|
acute myocardial infarction
|
|
PANTHER
|
Protein Analysis Through Evolutionary
Relationships
|
|
SERCA2a
|
sarcoplasmic reticulum ATPase
|
|
RyR2
|
ryanodine receptor 2
|
|
EGFP
|
enhanced green fluorescent protein
|
|
LVEDD
|
left ventricular end-diastolic
diameter
|
|
LVESD
|
left ventricular end-systolic
diameter
|
|
LVPWs
|
LVPWd, left ventricular systolic and
diastolic posterior
|
|
LVEF
|
left ventricular ejection fraction
|
|
FS
|
fractional shortening
|
|
cTnI
|
cardiac troponin I
|
|
MLC
|
myosin light chain
|
|
ELC
|
essential myosin light chain
|
|
HSP70
|
heat shock protein 70
|
Acknowledgments
The current study was supported by the Medical
Science and Technology Development Program of Shandong Province
(grant no. 2014WSA01015).
References
|
1
|
Willis BC, Salazar-Cantú A, Silva-Platas
C, Fernández-Sada E, Villegas CA, Rios-Argaiz E, González-Serrano
P, Sánchez LA, Guerrero-Beltrán CE, García N, et al: Impaired
oxidative metabolism and calcium mishandling underlie cardiac
dysfunction in a rat model of post-acute isoproterenol-induced
cardiomyopathy. Am J Physiol Heart Circ Physiol. 308:H467–wH477.
2015. View Article : Google Scholar
|
|
2
|
Most P, Pleger ST, Völkers M, Heidt B,
Boerries M, Weichenhan D, Löffler E, Janssen PM, Eckhart AD,
Martini J, et al: Cardiac adenoviral S100A1 gene delivery rescues
failing myocardium. J Clin Invest. 114:1550–1563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Most P, Remppis A, Pleger ST, Löffler E,
Ehlermann P, Bernotat J, Kleuss C, Heierhorst J, Ruiz P, Witt H, et
al: Transgenic overexpression of the Ca2+-binding protein S100A1 in
the heart leads to increased in vivo myocardial contractile
performance. J Biol Chem. 278:33809–33817. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boerries M, Most P, Gledhill JR, Walker
JE, Katus HA, Koch WJ, Aebi U and Schoenenberger CA: Ca2+
-dependent interaction of S100A1 with F1-ATPase leads to an
increased ATP content in cardiomyocytes. Mol Cell Biol.
27:4365–4373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamasaki R, Berri M, Wu Y, Trombitás K,
McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M and
Granzier H: Titin-actin interaction in mouse myocardium: Passive
tension modulation and its regulation by calcium/S100A1. Biophys J.
81:2297–2313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Most P, Bernotat J, Ehlermann P, Pleger
ST, Reppel M, Börries M, Niroomand F, Pieske B, Janssen PM,
Eschenhagen T, et al: S100A1: A regulator of myocardial
contractility. Proc Natl Acad Sci USA. 98:13889–13894. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Most P, Boerries M, Eicher C, Schweda C,
Völkers M, Wedel T, Söllner S, Katus HA, Remppis A, Aebi U, et al:
Distinct subcellular location of the Ca2+-binding protein S100A1
differentially modulates Ca2+-cycling in ventricular rat
cardiomyocytes. J Cell Sci. 118:421–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Most P, Seifert H, Gao E, Funakoshi H,
Völkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR Jr,
Eckhart AD, et al: Cardiac S100A1 protein levels determine
contractile performance and propensity toward heart failure after
myocardial infarction. Circulation. 114:1258–1268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pleger ST, Shan C, Ksienzyk J, Bekeredjian
R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G,
et al: Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart
failure in a preclinical large animal model. Sci Transl Med.
3:92ra642011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pleger ST, Most P, Boucher M, Soltys S,
Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, et al:
Stable myocardial-specific AAV6-S100A1 gene therapy results in
chronic functional heart failure rescue. Circulation.
115:2506–2515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ross PL, Huang YN, Marchese JN, Williamson
B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, et
al: Multiplexed protein quantitation in Saccharomyces cerevisiae
using amine-reactive isobaric tagging reagents. Mol Cell
Proteomics. 3:1154–1169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Institute of Laboratory Animal Resources
(US); Committee on Care, Use of Laboratory Animals National
Institutes of Health (US); Division of Research Resources: Guide
for the care and use of laboratory animals. 8th edition. National
Academies Press; Washington, DC: 2011
|
|
13
|
Stanley WC, Lopaschuk GD, Hall JL and
McCormack JG: Regulation of myocardial carbohydrate metabolism
under normal and ischaemic conditions. Potential for
pharmacological interventions. Cardiovasc Res. 33:243–257. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang B, Li Y, Zhao L, Yuan S, Wang Z, Li B
and Chen Q: Stable isotope dimethyl labeling combined with LTQ mass
spectrometric detection, a quantitative proteomics technology used
in liver cancer research. Biomed Rep. 1:549–554. 2013.
|
|
15
|
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal
JS and Stanley WC: Myocardial fatty acid metabolism in health and
disease. Physiol Rev. 90:207–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andresen BS, Olpin S, Poorthuis BJ,
Scholte HR, Vianey-Saban C, Wanders R, Ijlst L, Morris A,
Pourfarzam M, Bartlett K, et al: Clear correlation of genotype with
disease phenotype in very-long-chain acyl-CoA dehydrogenase
deficiency. Am J Hum Genet. 64:479–494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raha S and Robinson BH: Mitochondria,
oxygen free radicals, disease and ageing. Trends Biochem Sci.
25:502–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carafoli E: Calcium-a universal carrier of
biological signals. Delivered on 3 July 2003 at the special FEBS
meeting in Brussels. FEBS J. 272:1073–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heizmann CW, Ackermann GE and Galichet A:
Pathologies involving the S100 proteins and rage. Subcell Biochem.
45:93–138. 2007. View Article : Google Scholar
|
|
20
|
Most P, Remppis A, Pleger ST, Katus HA and
Koch WJ: S100A1: A novel inotropic regulator of cardiac
performance. Transition from molecular physiology to
pathophysiological relevance. Am J Physiol Regul Integr Comp
Physiol. 293:R568–R577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Völkers M, Loughrey CM, Macquaide N,
Remppis A, DeGeorge BR Jr, Wegner FV, Friedrich O, Fink RH, Koch
WJ, Smith GL and Most P: S100A1 decreases calcium spark frequency
and alters their spatial characteristics in permeabilized adult
ventricular cardiomyocytes. Cell Calcium. 41:135–143. 2007.
View Article : Google Scholar
|
|
22
|
Kettlewell S, Most P, Currie S, Koch WJ
and Smith GL: S100A1 increases the gain of excitation-contraction
coupling in isolated rabbit ventricular cardiomyocytes. J Mol Cell
Cardiol. 39:900–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi T and Solaro RJ: Calcium, thin
filaments and the integrative biology of cardiac contractility.
Annu Rev Physiol. 67:39–67. 2005. View Article : Google Scholar
|
|
24
|
Lehman W and Craig R: Tropomyosin and the
steric mechanism of muscle regulation. Adv Exp Med Biol.
644:95–109. 2008. View Article : Google Scholar
|
|
25
|
Zhi G, Herring BP and Stull JT: Structural
requirements for phosphorylation of myosin regulatory light chain
from smooth muscle. J Biol Chem. 269:24723–24727. 1994.PubMed/NCBI
|
|
26
|
Chan JY, Takeda M, Briggs LE, Graham ML,
Lu JT, Horikoshi N, Weinberg EO, Aoki H, Sato N, Chien KR and
Kasahara H: Identification of cardiac-specific myosin light chain
kinase. Circ Res. 102:571–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andreev OA, Saraswat LD, Lowey S,
Slaughter C and Borejdo J: Interaction of the N-terminus of chicken
skeletal essential light chain 1 with F-actin. Biochemistry.
38:2480–2485. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Timson DJ, Trayer HR and Trayer IP: The
N-terminus of A1-type myosin essential light chains binds actin and
modulates myosin motor function. Eur J Biochem. 255:654–662. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Timson DJ, Trayer HR, Smith KJ and Trayer
IP: Size and charge requirements for kinetic modulation and actin
binding by alkali 1-type myosin essential light chains. J Biol
Chem. 274:18271–18277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdelaziz AI, Segaric J, Bartsch H,
Petzhold D, Schlegel WP, Kott M, Seefeldt I, Klose J, Bader M,
Haase H and Morano I: Functional characterization of the human
atrial essential myosin light chain (hALC–1) in a transgenic rat
model. J Mol Med (Berl). 82:265–274. 2004. View Article : Google Scholar
|
|
31
|
Fewell JG, Hewett TE, Sanbe A, Klevitsky
R, Hayes E, Warshaw D, Maughan D and Robbins J: Functional
significance of cardiac myosin essential light chain isoform
switching in transgenic mice. J Clin Invest. 101:2630–2639. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morano M, Zacharzowski U, Maier M, Lange
PE, Alexi-Meskishvili V, Haase H and Morano I: Regulation of human
heart contractility by essential myosin light chain isoforms. J
Clin Invest. 98:467–473. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moretti A, Weig HJ, Ott T, Seyfarth M,
Holthoff HP, Grewe D, Gillitzer A, Bott-Flügel L, Schömig A,
Ungerer M and Laugwitz KL: Essential myosin light chain as a target
for caspase-3 in failing myocardium. Proc Natl Acad Sci USA.
99:11860–11865. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hightower LE and Guidon PT Jr: Selective
release from cultured mammalian cells of heat-shock (stress)
proteins that resemble glia-axon transfer proteins. J Cell Physiol.
138:257–266. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Knowlton AA, Eberli FR, Brecher P, Romo
GM, Owen A and Apstein CS: A single myocardial stretch or decreased
systolic fiber shortening stimulates the expression of heat shock
protein 70 in the isolated, erythrocyte-perfused rabbit heart. J
Clin Invest. 88:2018–2025. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trost SU, Omens JH, Karlon WJ, Meyer M,
Mestril R, Covell JW and Dillmann WH: Protection against myocardial
dysfunction after a brief ischemic period in transgenic mice
expressing inducible heat shock protein 70. J Clin Invest.
101:855–862. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dybdahl B, Wahba A, Lien E, Flo TH, Waage
A, Qureshi N, Sellevold OF, Espevik T and Sundan A: Inflammatory
response after open heart surgery: Release of heat-shock protein 70
and signaling through toll-like receptor-4. Circulation.
105:685–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Guo J, Zhang P, Liu Y, Jia Z,
Feng X, Li Z, Li W, Ma K, Zhou C and Li L: Transplantation of bone
marrow cells up-regulated the expressions of HSP32 and HSP70 in the
acute ischemic myocardium. Beijing Da Xue Xue Bao. 35:476–480.
2003.In Chinese. PubMed/NCBI
|