Introduction
Crohn's disease (CD) is a chronic inflammatory
disorder of the gastrointestinal tract. The precise cause remains
unknown; however, genetic, immunological, infective and
environmental factors have been proposed to contribute to its
pathogenesis (1,2).
Advances in the understanding of the pathogenesis of
CD over the last decade have enabled the development of agents
directed at rational therapeutic targets. Selective blocking of
inflammatory cytokines through the introduction of novel
biologicals, including anti-tumor necrosis factor-α agents, has
yielded considerable clinical benefit (3). This approach validated the targeting
of inflammatory cytokines as a strategy for treating ongoing
disease; however, the long-term safety and efficacy of such
therapeutic agents remains uncertain. Additionally, certain
patients may not respond to this treatment, exhibit intolerance, or
both. Thus, result in the requirement of an alternative therapeutic
approach. The other treatments currently available include total
parenteral nutrition and elemental diets, restriction of oral food
intake and in severe cases, hospitalization. Therefore, a safer and
more effective treatment method for CD is desirable.
Daikenchuto (TU-100) is a traditional Japanese
herbal medicine (Kampo), which consists of a mixture of powdered
extracts of dried Japanese pepper, processed ginger and ginseng
radix, and maltose powder (4).
TU-100 is a frequently prescribed Kampo medicine in Japan,
particularly for the treatment of postoperative paralytic and
adhesive ileus and ischemic intestinal disorders (5–8).
Previous studies have demonstrated the effects of TU-100 on
intestinal motility, adhesion, vasodilatation, inflammation and
bacterial translocation (9–11). A
previous study reported that the beneficial effect of TU-100 is
primarily mediated by the increased release of adrenomedullin (ADM)
from intestinal epithelial cells (12).
ADM, is a 52-amino-acid peptide, which was
originally identified in human pheochromocytoma tissue. Subsequent
studies have demonstrated that the ADM protein and mRNA are widely
distributed in various tissues and organs (13–15).
ADM promotes vasodilation, tissue repair and anti–inflammatory
actions in the intestine. Exogenous administration of ADM has been
demonstrated to exhibit therapeutic potential in various models of
inflammatory disease, including colitis (16–18)
and arthritis (19). In addition,
it has been reported that TU-100 exerted beneficial effects in a
mouse model of colitis through the induction of ADM release
(20,21).
On the basis of these previous studies, double-blind
placebo controlled randomized clinical trials using TU-100 in
patients with CD, postoperative paralytic ileus, functional
constipation and irritable bowel syndrome have been conducted in
Japan (funded by the Japanese Foundation For Multidisciplinary
Treatment of Cancer: Identifier nos. JFMC39-0902, JFMC40-1001 and
JFMC42-1002) and the United States (identifier nos. NCT00871325,
NCT01139216, NCT01388933, and NCT01348152). However, to the best of
our knowledge, the effects of TU-100 on the blood plasma levels of
ADM in patients with CD have not been described previously.
The primary aim of the current study was to evaluate
the efficacy of TU-100 treatment on the circulating ADM levels in
patients with active CD. Additional objectives included the
assessment of the effect of the drug on the disease activity and
its safety.
Materials and methods
Ethical approval
This project was performed in accordance with the
principles laid out in the Declaration of Helsinki and with the
approval of the Medical Ethics Committee of Kurume University
Hospital (Kurume, Japan). Written informed consent was obtained
from the participants.
TU-100 preparation
TU-100 was prepared as a dried powdered extract of
Panacis Ginseng Radix (3.0 g), Zanthoxyli Fructus (2.0 g) and
Zingiberis Siccatum Rhizoma (5.0 g), purchased from Tsumura &
Co., Ltd. (Tokyo, Japan).
Detection of ADM blood plasma levels
ADM occurs in two molecular forms, the active and
mature ADM (m-ADM) form and the inactive and glycine-extended ADM
(gly-ADM) form (22). Total ADM
(t-ADM) is the sum of the levels of m-ADM and gly-ADM. The present
study determined the plasma levels of m-ADM and t-ADM by
enzyme-linked immunosorbent assays (ELISA; Shionogi & Co.,
Ltd., Tokyo, Japan). These assay systems use two monoclonal
antibodies against human ADM, one that recognizes the ring
structure of human ADM in each kit and another that recognizes the
C-terminal sequence in the m-ADM kit or ADM-(25–36) in the t-AM
kit. The assay determined human m-ADM or t-ADM by placing it
between the two antibodies without plasma extraction. The detection
limit was 2 fmol/ml for t-ADM and 1 fmol/ml for m-ADM. The plasma
levels of calcitonin gene-related peptide (CGRP) were also
determined by ELISA; the detection limit of this assay was 7.8
pg/ml (Bertin Pharma, Montigny le Bretonneux, France).
Patient selection and study design
An open-label, preliminary trial was conducted at
Kurume University School of Medicine between September 2007 and
June 2008. A total of 10 patients with active CD (3 men, 7 women;
mean age, 37.3 years; mean duration of CD, 11.7 years) participated
in this study (Table I). The
diagnosis of CD was based on characteristic clinical, endoscopic,
radiological and histological features. The patients had been
unresponsive or intolerant to the standard treatment methods for CD
for at least 4 weeks. All baseline anti-inflammatory therapies were
continued throughout the current study (Table I). No dietary alterations were made
after the patients entered the study.
 | Table ICharacteristics of 10 patients with
active CD who completed 8 weeks of treatment with TU-100. |
Table I
Characteristics of 10 patients with
active CD who completed 8 weeks of treatment with TU-100.
| Total number of
patients | 10 |
| Mean age ± SE
(years) | 37.3±15.5 |
| Number of males | 3 |
| Mean duration of
disease since diagnosis ± SE (years) | 11.7±13.2 |
| Mean baseline IOIBD ±
SE | 3.9±0.55 |
| Mean baseline CRP ±
SE (mg/dl) | 1.2±0.3 |
| Location of disease,
n | |
| Ilial | 2 |
| Colonic | 1 |
| Ileocolonic | 7 |
| Disease behavior,
n |
| Inflammatory | 1 |
| Stricturing | 6 |
| Penetrating | 3 |
| Previous intestinal
resection, n | 6 |
| Concomitant
medication, n (%) |
| Mesalamine | 10 (100) |
| Corticosteroids | 4 (40) |
| Antibiotics | 0 (0) |
| Azathioprine | 1 (10) |
| Anti-tumor necrosis
factor | 2 (20) |
| Nutritional
therapy | 7 (70) |
Patients received 15 g TU-100 (5 g three times a day
administered orally) per day for 8 consecutive weeks. Plasma levels
of m-ADM, t-ADM and CGRP were determined before and after the
8-week administration.
The response to the treatment was evaluated biweekly
using the routine laboratory variables, including serum hemoglobin
and C-reactive protein (CRP) and the International Organization for
the Study of Inflammatory Bowel Disease (IOIBD) score (23). The IOIBD score is based on 10
components, specifically the presence/absence of: i) Abdominal
pain, ii) bowel evacuation 6 times or more per day, iii) anal
lesion, iv) fistula, v) complication, vi) abdominal tumor, vii)
body weight decrease, viii) fever of 38°C or higher, ix) abdominal
tenderness and x) hemoglobin level of 10 g/dl or lower. Presence of
each of these components is assigned 1 point, and the total number
of points is expressed as the IOIBD score of the patients.
Statistical analysis
Results are expressed as the mean ± standard error.
All the statistical analyses were performed using SPSS version
12.0.2 J (SPSS Inc., Chicago, IL, USA). Data were compared using
Wilcoxon signed-rank tests or paired Student's t tests. Spearman's
rank correlation test was used where appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of TU-100 on the circulating ADM
levels
The t-ADM and m-ADM levels in blood plasma of
patients at the beginning and the end of treatment with TU-100 are
presented in Fig. 1. Detectable
levels of ADM were identified in all of the plasma samples.
Following 8 weeks of treatment with TU-100, the patients had
significantly elevated levels of plasma t-ADM (20.2±1.7 fmol/ml)
compared with levels prior to treatment (16.4±1.1 fmol/ml;
P=0.0218) and plasma m-ADM (2.2±0.1 vs. 1.7±0.1 fmol/ml; P=0.0284).
Simultaneous determinations revealed the absence of detectable CGRP
in the plasma in the majority of subjects.
Effect of TU-100 on the disease activity
of CD
The changes in the activity of the disease during
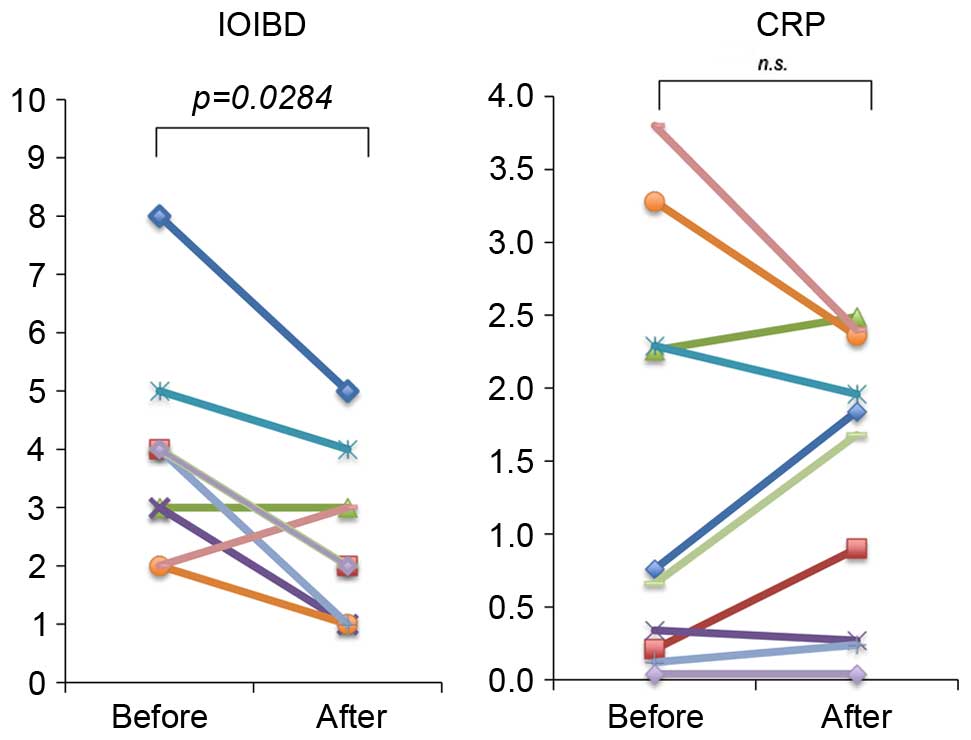
TU-100 treatment are presented in Fig.
2. The IOIBD score significantly improved over time, with a
decrease in the score from 3.9±0.5 to 2.4±0.4 (P=0.0284). A
favorable trend was observed in the serum CRP concentration;
however, no significant difference was identified. The effect of
TU-100 treatment on the components of the IOIBD scoring system was
also examined (Fig. 3). Out of the
10 components, the scores for abdominal pain and tenderness were
significantly decreased after 8 weeks when compared with the scores
at the start of the study (P=0.014 and P=0.046, respectively). No
significant differences were identified for the remaining
components.
Correlation between the circulating
levels of ADM and the disease activity
As presented in Fig.
4, no significant association was observed between the plasma
concentrations of ADM and the IOIBD scores or serum CRP levels.
Side effects
TU-100 was well tolerated in all 10 patients
enrolled in the present study. The patients felt well and did not
experience any side effects. No febrile or allergic reactions were
observed. No changes were encountered in the routine blood
biochemical parameters, including liver and renal function tests,
during/after TU-100 treatment. Additionally, no adverse events
related to TU-100 were observed.
Discussion
To the best of our knowledge, the present study was
the first to evaluate the efficacy, circulating levels of ADM,
disease activity and safety of TU-100 in patients with CD.
Due to the heterogeneous nature of TU-100, the
underlying mechanism of its therapeutic effect has not been fully
elucidated. However, observations in animal models indicate that
the effect of TU-100 may be attributed to the induction of ADM
release. TU-100 also improves colonic injury by enhancing
endogenous ADM release (20).
Additionally, the beneficial effect of TU-100 was reduced by an ADM
inhibitor (11). Systemically
administered ADM had a similar beneficial effect on colonic injury
(16–19).
Therefore, there was considerable interest in
determining whether TU-100 promoted ADM release in patients with
CD. The present study determined that TU-100 treatment induced the
release of the active form of ADM, which possibly resulted in a
significant improvement of the disease activity. These findings
suggest that the efficacy of TU-100 against CD may be contributed
to the induction of ADM release. To the best of our knowledge, the
present study was the first to determine the circulating levels of
ADM in blood plasma during TU-100 treatment in humans. At present,
the current findings on the clinical response of patients with CD
to TU-100 are preliminary. However, as TU-100 has been observed to
promote ADM release in human subjects is encouraging and further
clinical investigations should be performed.
The mechanisms underlying the effect of TU-100 on
the release of ADM remain to be fully elucidated. TU-100
components, including 6-shogaol and hydroxy-α-sanshool, contain an
activator of the Transient Receptor Potential (TRP)-channels
(4). Kono et al (24) demonstrated that TU-100 and its
component 6-shogaol increase the intestinal blood flow via
enhancement of ADM release mediated by the stimulation of TRP
ankyrin 1 in intestinal epithelial cells (24). It possible that a component of
TU-100, such as 6-shogaol, is responsible for ADM release through
TRP channel stimulation. Determination of the signaling pathways
involved in the TRP channel-ADM axis is an important area for
further investigation.
No direct correlation was observed between the
circulating levels of ADM and the IOIBD scores during TU-100
treatment. Therefore, an additional unrecognized peptide may be
involved in the response to TU-100 treatment, in addition to ADM.
Further investigation is required to elucidate the precise
mechanisms underlying the actions of TU-100 action in human
patients with CD.
The primary advantage of TU-100 is that it appears
to lack any side effects or toxicity (4). In particular, TU-100 led to no
allergic reactions or any significant changes in blood biochemical
parameters, including liver and renal function tests, during the
study period. Standard therapy for CD consists of drugs that
primarily act on the intestinal immune-component cells, including
lymphocytes and macrophages to reduce the release of various
inflammatory mediators (3).
However, the preventive action of TU-100 differs from these
anti-inflammatory approaches. Combined therapy of TU-100 and
standard therapeutic agents is of interest and warrants
evaluation.
The findings of the current study suggest that
treating CD using ADM may be a novel approach to treat CD (16–19).
In addition, ADM, particularly its active form, are rapidly cleared
from the blood, which decreases the systemic effects (25). By contrast, TU-100 appears to
affect the endogenous ADM system not systemically, but locally and
in a sustained manner. Such sustained and localized increase of ADM
induced by TU-100 may be advantageous, as it may lead to the
biological effect of ADM being primarily confined to the disease
sites (12).
A potential limitation of the current study is that
it included a small number of patients. The patients were monitored
for 8 weeks subsequent to the administration of the drug; however,
a trial with a greater number of patients and longer-term follow-up
period is required.
In conclusion, the present study provides novel
evidence to suggest that TU-100 may be an effective treatment for
CD, and exerts this effect at least in part through inducing the
release of ADM. Additionally, no significant side effects or
toxicity were observed during this trial. Based on these
encouraging preliminary results, a larger, multicenter randomized
controlled clinical trial of this therapeutic agent should be
conducted for patients with CD.
Acknowledgments
The present study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Health and Welfare. The
abstract was presented at the UEG Week 2013 Poster Presentations
October 14 2013, and published in United European Gastroenterol J
1, A135–A587 (1 Suppl): 2013.
References
|
1
|
Neurath MF and Finotto S: The many roads
to inflammatory bowel diseases. Immunity. 25:189–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strober W, Fuss I and Mannon P: The
fundamental basis of inflammatory bowel disease. J Clin Invest.
117:514–521. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermeire S, Ferrante M and Rutgeerts P:
Recent advances: Personalised use of current Crohn's disease
therapeutic options. Gut. 62:1511–1515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kono T, Kanematsu T and Kitajima M: Exodus
of Kampo, traditional Japanese medicine, from the complementary and
alternative medicines: Is it time yet? Surgery. 146:837–840. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Endo S, Nishida T, Nishikawa K, Nakajima
K, Hasegawa J, Kitagawa T, Ito T and Matsuda H: Dai-kenchu-to, a
Chinese herbal medicine, improves stasis of patients with total
gastrectomy and jejunal pouch interposition. Am J Surg. 192:9–13.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Itoh T, Yamakawa J, Mai M, Yamaguchi N and
Kanda T: The effect of the herbal medicine dai-kenchu-to on
post-operative ileus. J Int Med Res. 30:428–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwai N, Kume Y, Kimura O, Ono S, Aoi S and
Tsuda T: Effects of herbal medicine Dai-Kenchu-to on anorectal
function in children with severe constipation. Eur J Pediatr Surg.
17:115–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suehiro T, Matsumata T, Shikada Y and
Sugimachi K: The effect of the herbal medicines dai-kenchu-to and
keishi-bukuryo-gan on bowel movement after colorectal surgery.
Hepatogastroenterology. 52:97–100. 2005.PubMed/NCBI
|
|
9
|
Tokita Y, Satoh K, Sakaguchi M, Endoh Y,
Mori I, Yuzurihara M, Sakakibara I, Kase Y, Takeda S and Sasaki H:
The preventive effect of Daikenchuto on postoperative
adhesion-induced intestinal obstruction in rats.
Inflammopharmacology. 15:65–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murata P, Kase Y, Ishige A, Sasaki H,
Kurosawa S and Nakamura T: The herbal medicine Dai-kenchu-to and
one of its active components [6]-shogaol increase intestinal blood
flow in rats. Life Sci. 70:2061–2070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kono T, Koseki T, Chiba S, Ebisawa Y,
Chisato N, Iwamoto J and Kasai S: Colonic vascular conductance
increased by Daikenchuto via calcitonin gene-related peptide and
receptor-activity modifying protein 1. J Surg Res. 150:78–84. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kono T, Kaneko A, Hira Y, Suzuki T,
Chisato N, Ohtake N, Miura N and Watanabe T: Anti-colitis and
-adhesion effects of daikenchuto via endogenous adrenomedullin
enhancement in Crohn's disease mouse model. J Crohns Colitis.
4:161–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eto T: A review of the biological
properties and clinical implications of adrenomedullin and
proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and
vasodilating peptides. Peptides. 22:1693–1711. 2001. View Article : Google Scholar
|
|
14
|
Julián M, Cacho M, García MA,
Martín-Santamaría S, de Pascual-Teresa B, Ramos A, Martínez A and
Cuttitta F: Adrenomedullin: A new target for the design of small
molecule modulators with promising pharmacological activities. Eur
J Med Chem. 40:737–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marutsuka K, Nawa Y, Asada Y, Hara S,
Kitamura K, Eto T and Sumiyoshi A: Adrenomedullin and
proadrenomudullin N-terminal 20 peptide (PAMP) are present in human
colonic epithelia and exert an antimicrobial effect. Exp Physiol.
86:543–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonzalez-Rey E, Fernandez-Martin A, Chorny
A and Delgado M: Therapeutic effect of urocortin and adrenomedullin
in a murine model of Crohn's disease. Gut. 55:824–832. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talero E, Sánchez-Fidalgo S, de la Lastra
CA, Illanes M, Calvo JR and Motilva V: Acute and chronic responses
associated with adrenomedullin administration in experimental
colitis. Peptides. 29:2001–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashizuka S, Ishikawa N, Kato J, Yamaga J,
Inatsu H, Eto T and Kitamura K: Effect of adrenomedullin
administration on acetic acid-induced colitis in rats. Peptides.
26:2610–2615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez-Rey E, Chorny A, O'Valle F and
Delgado M: Adrenomedullin protects from experimental arthritis by
down-regulating inflammation and Th1 response and inducing
regulatory T cells. Am J Pathol. 170:263–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kono T, Omiya Y, Hira Y, Kaneko A, Chiba
S, Suzuki T, Noguchi M and Watanabe T: Daikenchuto (TU-100)
ameliorates colon microvascular dysfunction via endogenous
adrenomedullin in Crohn's disease rat model. J Gastroenterol.
46:1187–1196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaneko A, Kono T, Miura N, Tsuchiya N and
Yamamoto M: Preventive effect of TU-100 on a Type-2 model of
colitis in mice: Possible involvement of enhancing adrenomedullin
in intestinal epithelial cells. Gastroenterol Res Pract.
2013:3840572013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishikimi T, Shibasaki I, Iida H, Asakawa
H, Matsushita Y, Mori H, Mochizuki Y, Okamura Y, Horinaka S,
Kangawa K, et al: Molecular forms of adrenomedullin in pericardial
fluid and plasma in patients with ischaemic heart disease. Clin Sci
(Lond). 102:669–677. 2002. View Article : Google Scholar
|
|
23
|
Myren J, Bouchier IA, Watkinson G, Softley
A, Clamp SE and de Dombal FT: The O.M.G.E. multinational
inflammatory bowel disease survey 1976–1982. A further report on
2,657 cases. Scand J Gastroenterol Suppl. 95:1–27. 1984.
|
|
24
|
Kono T, Kaneko A, Omiya Y, Ohbuchi K, Ohno
N and Yamamoto M: Epithelial transient receptor potential ankyrin 1
(TRPA1)-dependent adrenomedullin upregulates blood flow in rat
small intestine. Am J Physiol Gastrointest Liver Physiol.
304:G428–G436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikimi T, Matsuoka H, Shimada K, Matsuo
H and Kangawa K: Production and clearance sites of two molecular
forms of adrenomedullin in human plasma. Am J Hypertens.
13:1032–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|