Introduction
Lung cancer is a malignant neoplasm associated with
high morbidity and is the leading cause of cancer-associated
mortality worldwide (1). Non-small
cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases
(1–3). The majority of patients with NSCLC
are elderly at the time of diagnosis (2). The five-year survival rate for NSCLC
is 15%, which is reduced to 4% following the development of
metastases (1), therefore research
to prolong survival times for such patients is of vital
importance.
Personalized therapy, based on the genetic
characteristics of patients, is the most effective method for the
treatment of lung cancer (2).
Anaplastic lymphoma kinase (ALK) inhibitors such as crizotinib can
be used to treat NSCLC successfully, greatly improving the
prognosis of patients with lung cancer that have ALK gene mutations
(3). Crizotinib is a tyrosine
kinase inhibitor with activity against ALK, MET proto-oncogene
receptor tyrosine kinase and ROS proto-oncogene 1 receptor tyrosine
kinase (4). It was the first
molecular targeted drug to undergo clinical development for the
treatment of patients with ALK-positive advanced NSCLC, where it
exhibited an objective response rate (ORR) of ~60% (5). Crizotinib has been approved for the
treatment of patients with ALK-positive lung cancer by the Food and
Drug Administration (6).
Recently, a clinical trial evaluated the effects of
first-line crizotinib vs. pemetrexed-cisplatin in patients with
advanced ALK-positive NSCLC and demonstrated that crizotinib
resulted in increased progression-free survival and ORR, with fewer
side effects (7). Crizotinib is,
therefore, an attractive therapeutic option for patients with
ALK-positive lung cancer (7,8).
However, in virtually all patients receiving
crizotinib therapy, drug resistance occurs within approximately one
year, via complex resistance mechanisms (9). New approaches for solving drug
resistance and delaying its occurrence are therefore required,
including investigating methods to increase the sensitivity of
conventional treatments, including chemotherapy, radiation therapy
and molecular targeted therapy.
An important member of the microRNA (miR)-200
family, miR-200c, exhibits low-expression in numerous tumor tissues
and can regulate epithelial-mesenchymal transition (EMT), tumor
invasion and metastasis (10–12).
The overexpression of miR-200c in mesenchymal cells can promote
mesenchymal-epithelial transition (MET) (11), and enhance the sensitivity of lung
cancer cells to various chemotherapy drugs, epidermal growth factor
receptor tyrosine kinase inhibitors and radiotherapy (13–15).
In addition, previous reports have demonstrated that miR-200c
regulates EMT by targeting ZEB1 and ZEB2 (11,16).
However, whether it can improve the sensitivity of ALK-positive
NCI-2228 lung cancer cells to crizotinib is unknown. In the present
study, the regulation mechanism of miR-200c in ALK-positive lung
cancer cells to crizotinib was investigated.
Materials and methods
Cell culture and treatment
NCI-2228 cells, echinoderm microtubule-associated
protein-like 4-ALK positive lung cancer cells, were purchased
from the American Type Culture Collection (Manassas, VA, USA). A549
cells and H460 cells were obtained from Basic Medical Research
Institute, Chinese Academy of Medical Sciences (Beijing, China).
Cells were cultured in RPMI1640/Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Shanghai Luo
Biological Technology Co., Ltd., Shanghai, China) without
antibiotics, and maintained in a humidified 5% CO2 atmosphere at
37°C.
To establish the crizotinib-resistant cell line,
NCI-2228/CRI, 5 ml NCI-2228 cell suspension (1×106
cells/ml) was seeded in cell culture plates prior to treatment with
80 nM crizotinib (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) until >70% confluence was reached. The concentration of
crizotinib was then increased to 160, 200, 300, 400, 500, 600, 700
and 800 nM on a bi-weekly basis. Following approximately six months
of treatment, NCI-2228/CRI cells were resistant to 800 nM
crizotinib.
Cell transfection
Transient transfection was performed using
Lipofectamine 2000 Transfection Reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocols. Briefly, ~1×104 cells were first seeded in cell culture
plates. At 50% confluence, cells were transfected with miR-200c
mimic or miR-200c inhibitor (cat. nos. 4464066 and 4464084,
respectively; Invitrogen; Thermo Fisher Scientific, Inc.) using
Lipofectamine 2000 transfection reagent in RPMI1640/DMEM without
serum. Control reactions were simultaneously performed using the
miR-200c mimic negative control (NC) or miR-200c inhibitor NC (cat.
nos. 4464058 and 4464076, respectively; Invitrogen; Thermo Fisher
Scientific, Inc.). At 24 h following transfection, cells were
collected for subsequent experiments.
Cell viability and invasion
assays
Cell viability was determined using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells in RPMI1640 culture medium were first seeded onto
96-well plates at a density of 5×103 cells/well and cultured for 24
h. The medium was then replaced with serum-free fresh medium plus
crizotinib. NCI-2228 cells were treated with 0, 12.5, 25, 50, 100
and 200 nM crizotinib, whereas the drug-resistant NCI-2228/CRI
cells were treated with 0, 800, 1,600, 2,000, 4,000 and 8,000 nM
crizotinib. Following 48 h incubation, 100 µl MTT was added to each
well and the cells were incubated for a further 4 h. The medium was
subsequently discarded before 100 µl of 10% sodium dodecyl sulfate
(SDS) was added into each well, and the absorbance was read at 492
nm using the Multiskan FC Microplate Photometer (Thermo Fisher
Scientific, Inc.). The half maximal inhibitory concentration (IC50)
was calculated using SPSS (SPSS, Inc., Chicago, IL, USA). All
experiments were performed in triplicate.
The invasion assay was performed using Transwell
inserts in 24-well dishes as described previously (17). For each Transwell, the number of
migrated cells in five random fields of view were counted using a
light microscope.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of cells was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. Total RNA (1 µg) was used to generate
the first strand cDNA using RevertAid™ First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
for 1 h at 42°C. qPCR reactions were performed using Go
Taq® Green Master Mix (Promega Corporation, Madison, WI,
USA) according to the manufacturer's protocol. Thermal cycling
conditions were as follows: Denaturation for 2 min at 95°C followed
by 40 cycles of 95°C for 15 sec, 60°C for 1 min and 72°C for 1–2
min. The mRNA expression levels of GAPDH were used as the internal
control. The relative expression levels of each target gene were
calculated using the 2-ΔΔCq method (18). Sequences for the PCR primers used
are presented in Table I.
 | Table I.Primer pairs used for amplification
reactions. |
Table I.
Primer pairs used for amplification
reactions.
| Gene | Oligonucleotide
sequence (5′-3′) |
|---|
| GAPDH | Forward:
CGGATTTGGTCGTATTGGG |
|
| Reverse:
TGCTGGAAGATGGTGATGGGATT |
| E-cadherin | Forward:
TGGACAGGGAGGATTTTGAG |
|
| Reverse:
ACCTGAGGCTTTGGATTCCT |
| N-cadherin | Forward:
CCACAGCTCCACCATATGACT |
|
| Reverse:
CCCCAGTCGTTCAGGTAATC |
| Vimentin | Forward:
AGTGCCTGGAACGTCAGATG |
|
| Reverse:
CAGCAGCTTCCTGTAGGTGG |
| CD24 | Forward:
AGAGATAACCCTGCCCGAGG |
|
| Reverse:
GTCTAGCAGGATGCTGGGTG |
| ZEB1 3′UTR | Forward:
GACTAGTCATTTCAGACATGGAC |
|
| Reverse:
CCCAAGCTTGGGAGTGAATTTCAAAATTTA |
| ZEB1 | Forward:
CTCGAGCATTTAGACACAAGCG |
|
| Reverse:
TTGCCCTTCCTTTCCTGTGT |
| ZEB2 | Forward:
ACGGTATTGCCAACCCTCTG |
|
| Reverse:
TGCATTCTTCACTGGACCATCT |
| miR-200c | Forward:
GGCCTAATACTGCCGGGTAAT |
|
| Reverse:
CAGTGCGTGTCGTGGAGT |
| miR-200c RT |
GTCGTATCCAGTGCGTGTCGTGGAGTCG |
|
|
GCAATTGCACTGGATACGATCCATC |
Luciferase reporter assay
The 3′-untranslated region (UTR) for zinc finger
E-box binding homeobox 1 (ZEB1) was PCR-amplified from
genomic DNA as described previously (19), and inserted downstream of the
firefly luciferase gene in the pmiR-REPORT or the pmiR control
plasmid (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
PCR primers used to amplify the ZEB1 3′-UTR are presented in
Table I. NCI-2228 cells were
co-transfected with 2 µg of the firefly luciferase reporter vector
and 0.5 µg of the control pRL-TK vector containing Renilla
luciferase (Promega Corporation) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. For each group, 20 nM of the miR-200c
mimic or miR-200c mimic negative control was used. Following 48 h
transfection, cells were lysed by incubating with 500 µl lysis
buffer (Promega Corporation) for 15 min. The firefly and
Renilla luciferase activities were then measured using the
GloMax 96 Microplate Luminometer (Promega Corporation) and the
Dual-Luciferase Reporter assay system (Promega Corporation)
according to the manufacturer's protocols.
Western blot analysis
Transfected cells were harvested for immunoblot
analysis following 72 h incubation. Cells were lysed in lysis
buffer (Beyotime Institute of Biotechnology, Jiangsu, China). The
protein concentration of cell lysates was determined by using
Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) according to the manufacturer's protocols. Proteins (15 µg)
were separated by SDS-polyacrylamide gel electrophoresis on 10–15%
gels and transferred to polyvinylidene fluoride membranes.
Membranes were blocked with 8% non-fat dry milk for 1 h at room
temperature before they were immunoblotted overnight at 4°C with
the following primary antibodies: Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; dilution, 1:1,000; cat. no. 10494-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA), E-cadherin (dilution,
1:1,000; cat. no. 20874-1-AP; ProteinTech Group, Inc.), N-cadherin
(dilution, 1:1,000; cat. no. 13769-1-AP; ProteinTech Group, Inc.),
Vimentin (dilution, 1:1,000; cat. no. 10366-1-AP; ProteinTech Group
Inc.) and CD24 (dilution, 1:1,000; cat. no. 18330-1-AP; ProteinTech
Group, Inc.), ZEB1 (dilution, 1:2,000; cat. no. sc-25388; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and ZEB2 (dilution,
1:2,000; cat. no. sc-48789; Santa Cruz Biotechnology, Inc.). After
washing three times with phosphate-buffered saline plus 0.05%
Tween-20, the membrane was incubated with the horseradish
peroxidase-conjugated IgG secondary antibody (dilution, 1:1,000;
cat. no. RPN4301; GE Healthcare Life Sciences, Shanghai, China) for
2 h at room temperature. Signals were detected with UltraECL
Western Blot Detection reagent (Beyotime Institute of
Biotechnology) and images visualized on Kodak film (Kodak,
Rochester, NY, USA) and quantified with Quantity One Software
version 4.4 (Bio-Rad Laboratories Inc.). All experiments were
performed in triplicate.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All analysis was performed using SPSS version 13.5
(SPSS, Inc.). Comparisons of continuous measurement data were
conducted with Student's t-test. Comparisons among treatment
groups and controls were conducted using one-way analysis of
variance with a post-hoc Tukey test. P<0.05 was considered to
indicate a statistically significant difference.
Results
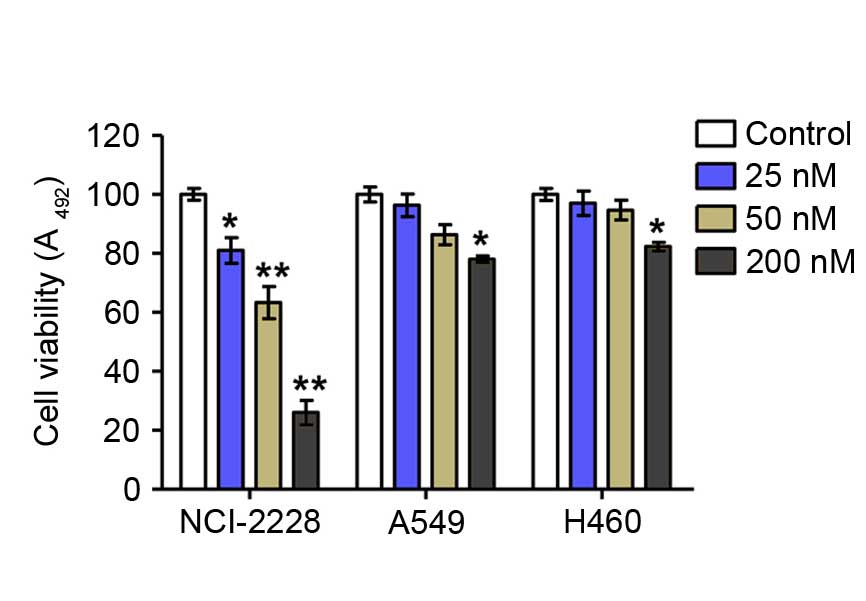
Crizotinib reduces lung cancer cell
viability of NCI-2228
Assessment of cell viability by MTT assay revealed
that crizotinib significantly inhibited the proliferation of
NCI-2228 cells in vitro in a dose-dependent manner compared
with untreated control cells (5 nM, P=0.016; 10 nM, P=0.001; 20 nM,
P=0.002), but had only a weak inhibitory effect on the viability of
A549 and H640 cells compared with untreated control (Fig. 1).
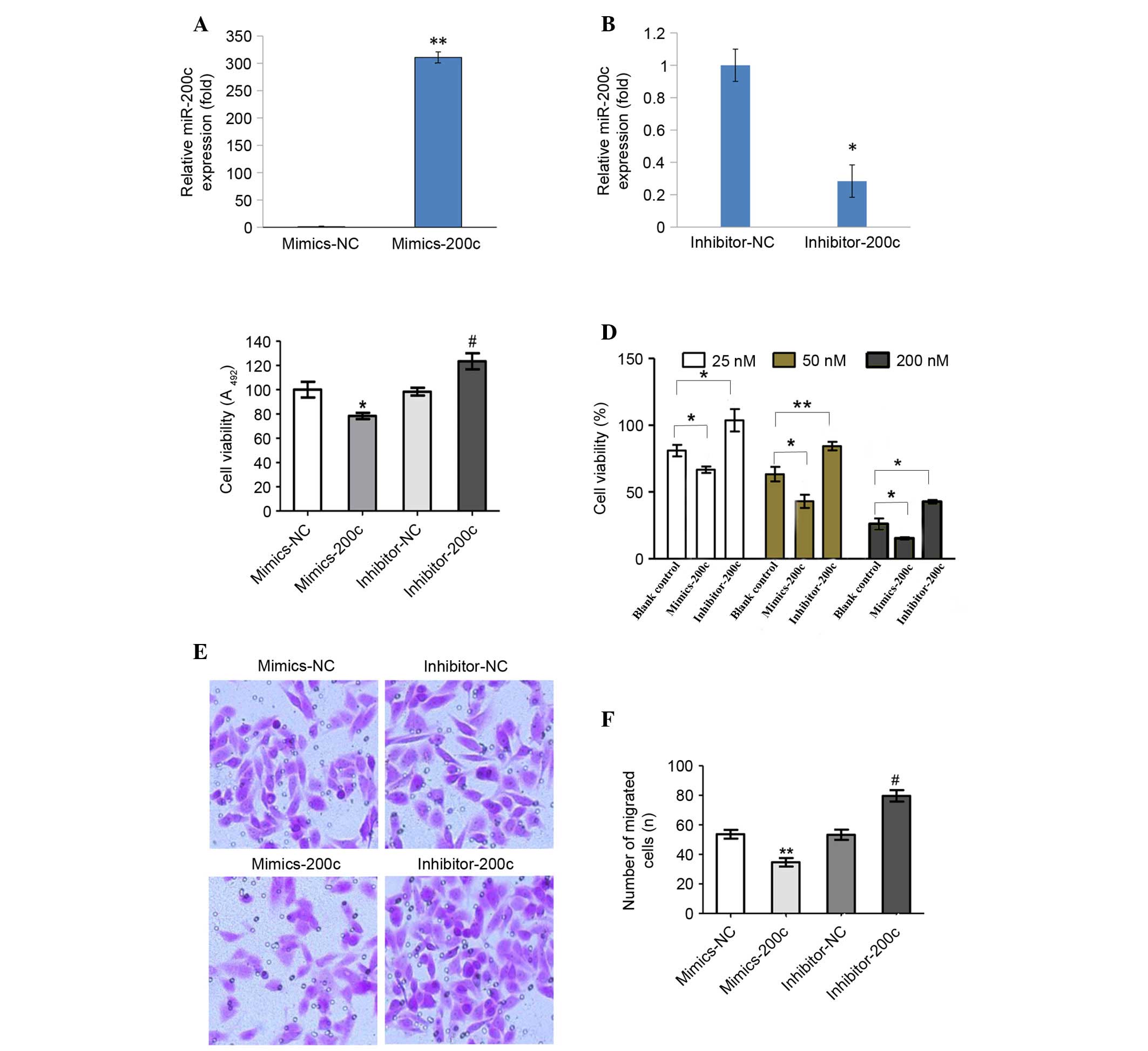
miR-200c inhibits proliferation,
migration and invasion of NCI-2228 cells
NCI-2228 cells were utilized to elucidate the
regulatory mechanism of miR-200c expression in NSCLC. NCI-2228
cells were treated with miR-200c mimic, miR-200c inhibitor,
miR-200c mimic NC or miR-200c inhibitor NC, with expression of
miR-200c subsequently analyzed by RT-qPCR (Fig. 2A and B). Compared with the mimic
NC, miR-200c expression levels increased 310-fold following
transfection with miR-200c mimic (P=0.001; Fig. 2A), while miR-200c expression levels
decreased 3.5-fold following transfection with miR-200c inhibitor,
compared with the inhibitor NC (P=0.033; Fig. 2B).
Cell viability (Fig. 2C
and D) and invasion assays (Fig.
2E and F) demonstrated that transfection with miR-200c mimic
significantly inhibited cell viability (P=0.036; Fig. 2C). With increasing concentrations
of miR-200c mimic, a significant decrease in the viability of cells
exposed to 25, 50 and 200 nM crizotinib was observed (P=0.011,
P=0.038 and P=0.025, respectively; Fig. 2D). In addition, the migration and
invasion capabilities of NCI-2228 cells were inhibited when
compared with mimic NC (P=0.009; Fig.
2F). By contrast, transfection with miR-200c inhibitors
significantly enhanced cell viability (P=0.027) and invasion
(P=0.017) compared with the inhibitor NC.
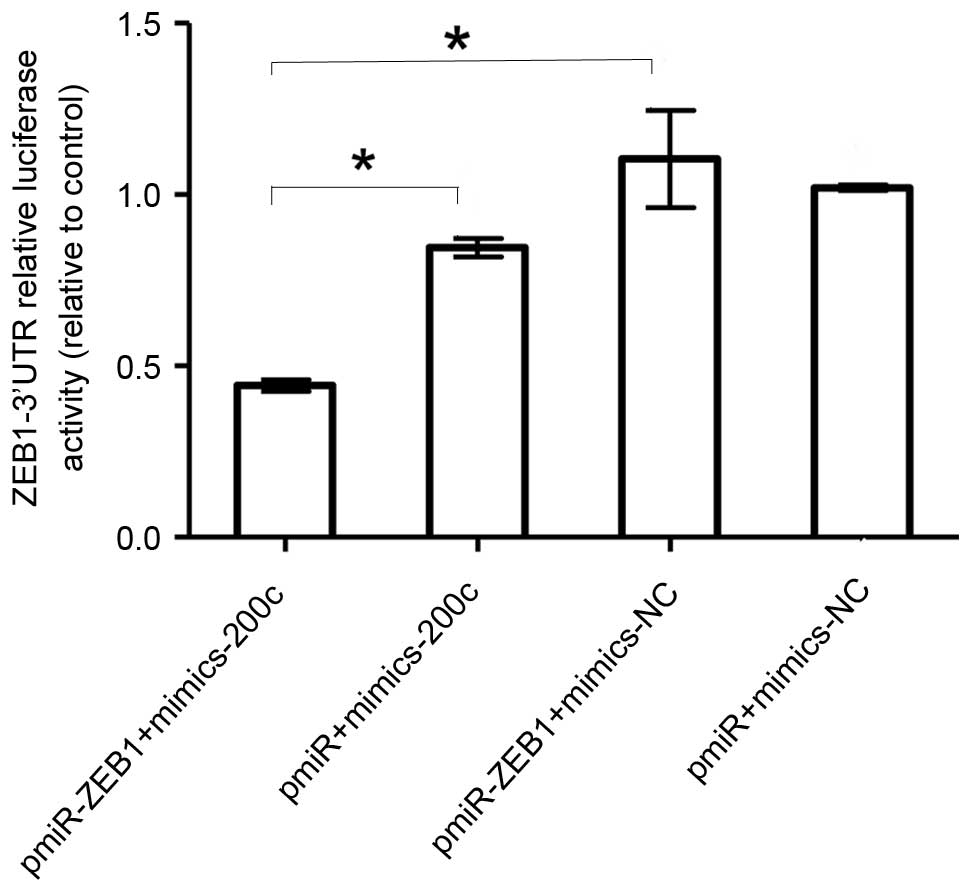
miR-200c targets ZEB1 in NCI-2228
cells directly
To understand how miR-200c suppresses lung cancer
migration and invasion, the coaction of miR-200c and miR-200c
target of E-cadherin transcriptional repressor, ZEB1, were analyzed
by luciferase reporter assay. The miR-200c was transfected with the
ZEB1 3′-UTR luciferase reporter gene into NCI-2228 cells. The
results demonstrated that the luciferase activity of pmiR-ZEB1 was
significantly decreased following transfection of miR-200c mimics
when compared with cells transfected with pmiR-ZEB1+mimics-NC
(P=0.01; Fig. 3).
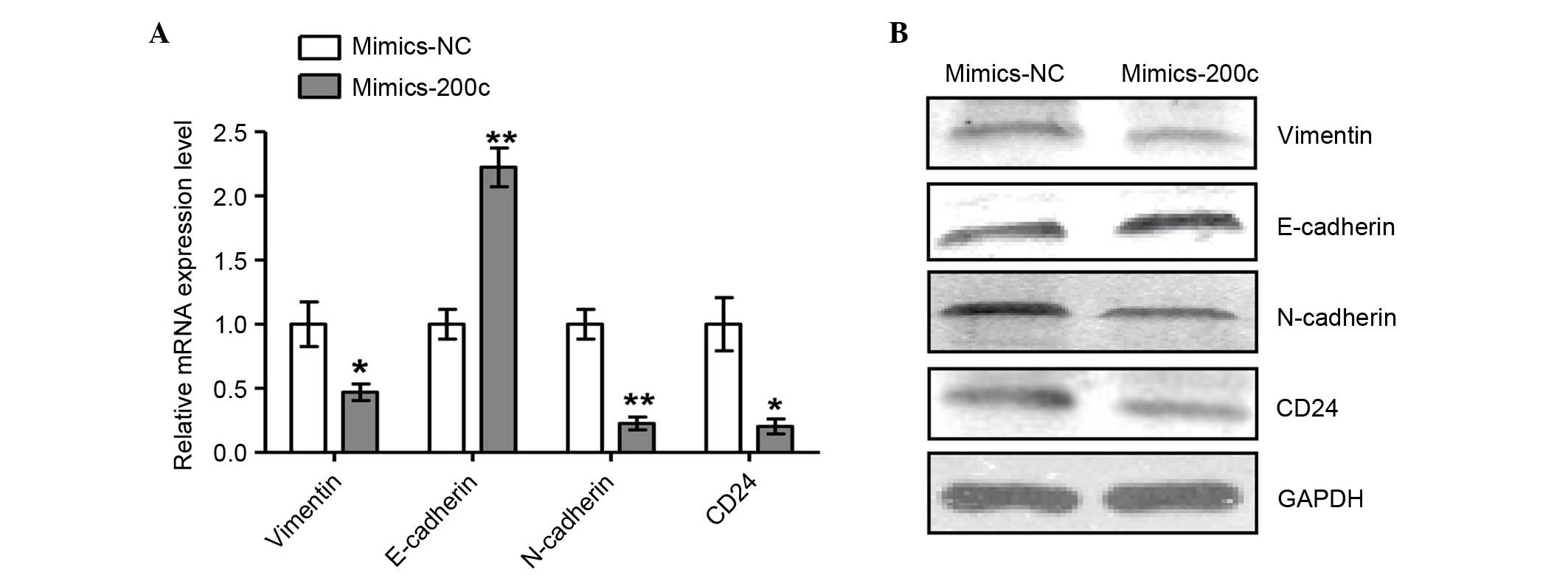
Increased miR-200c reverses EMT in
NCI-2228 cells
To further examine the regulation of miR-200c
expression in NCI-2228 cells, the miR-200c was overexpressed
through transfection with miR-200c mimic, and the mRNA (Fig. 4A) and protein (Fig. 4B) expression levels of epithelial
and mesenchymal cells markers analyzed. Expression levels of
mesenchymal markers N-cadherin, Vimentin and CD24 were
significantly decreased when compared with NC (P=0.004, P=0.045 and
P=0.021, respectively), while expression of the epithelial marker
E-cadherin was increased following transfection with miR-200c mimic
(P=0.003) compared with mimics NC, suggesting that EMT was reversed
in NCI-2228 cells upon overexpression of miR-200c.
NCI-2228/CRI cells exhibit higher
proliferation, migration and invasion rates
The crizotinib-resistant cell line, NCI-2228/CRI,
was successfully established in approximately six months, with
NCI-2228/CRI cells displaying viable growth in high concentrations
of crizotinib compared with the parental cell line, NCI-2228
(Fig. 5A and B). Furthermore, the
NCI-2228/CRI cells displayed a certain degree of resistance to the
ALK inhibitor LDK378 and chemotherapeutic drugs, paclitaxel and
cisplatin (data not shown). NCI-2228/CRI cells exhibited increased
invasion rates compared with NCI-2228 (P=0.035; Fig. 5C) and appeared spindle-shaped
compared to the oval shape of NCI-2228 cells (Fig. 5D). In addition, the relative
expression of miR-200c in NCI-2228/CRI cells was also demonstrated
to be reduced by 24.93-fold when compared with NCI-2228 cells
(P=0.014; Fig. 5E).
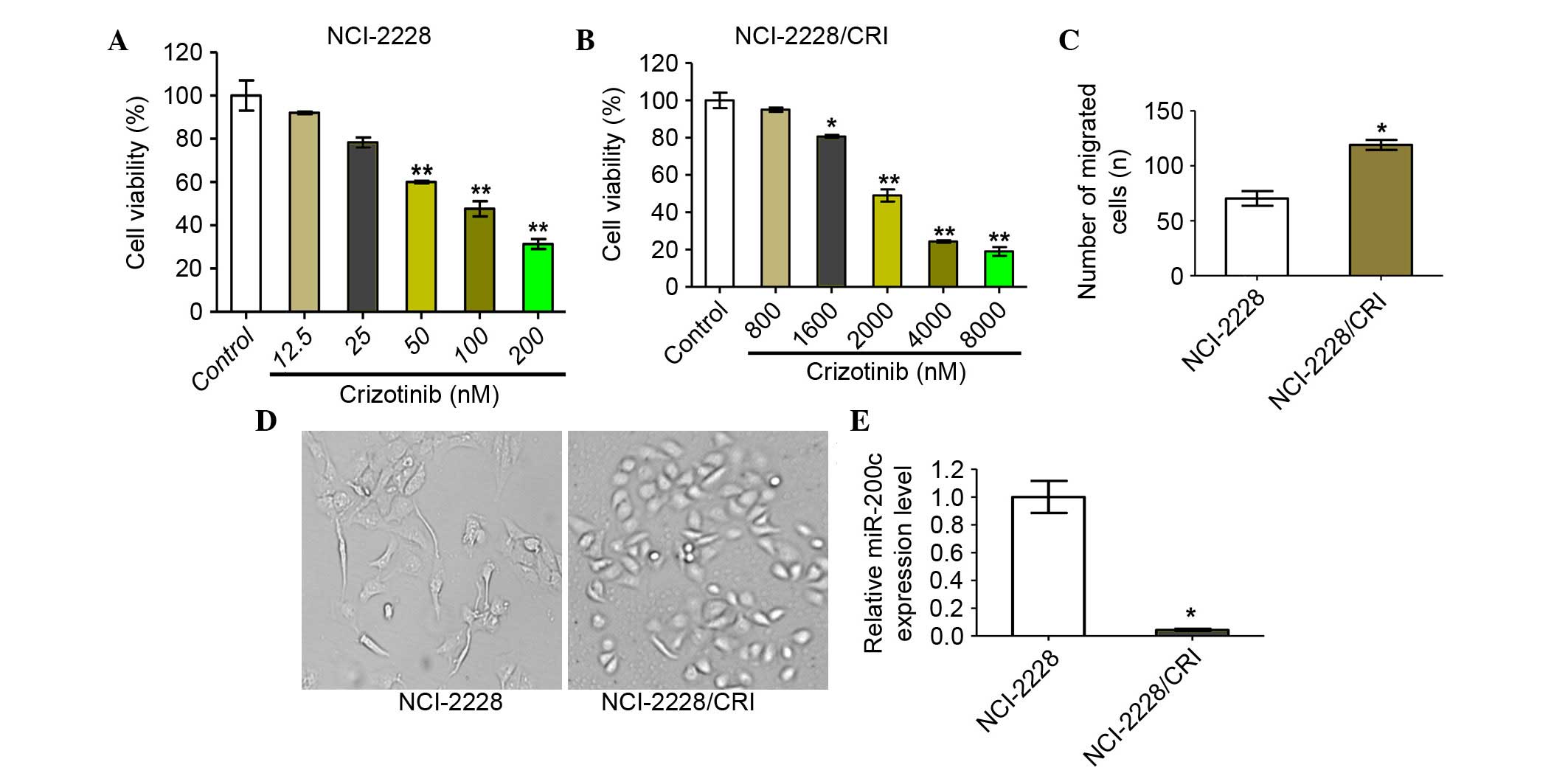 | Figure 5.NCI-2228/CRI cells exhibit higher
proliferation, migration and invasion rates than NCI-2228. Cell
viability was determined by MTT assay following 24 h treatment of
(A) NCI-2228 cells with 0, 12.5, 25, 50, 100 and 200 nM crizotinib
and (B) NCI-2228/CRI cells with 0, 800, 1,600, 2,000, 4,000 and
8,000 nM crizotinib. Transwell assays were used to analyze the
invasive properties of NCI-2228 and NCI-2228/CRI cells, with (C)
migrated cells plotted as the average number of cells per field of
view (original magnification, ×200) and (D) representative images
of the assays are presented. (E) Total RNA was extracted from
NCI-2228 or NCI-2228/CRI cells and subjected to reverse
transcription-quantitative polymerase chain reaction to measure
miR-200c. Values are presented as the mean ± standard deviation of
three independent experiments. *P<0.05 and **P<0.01 vs.
control. |
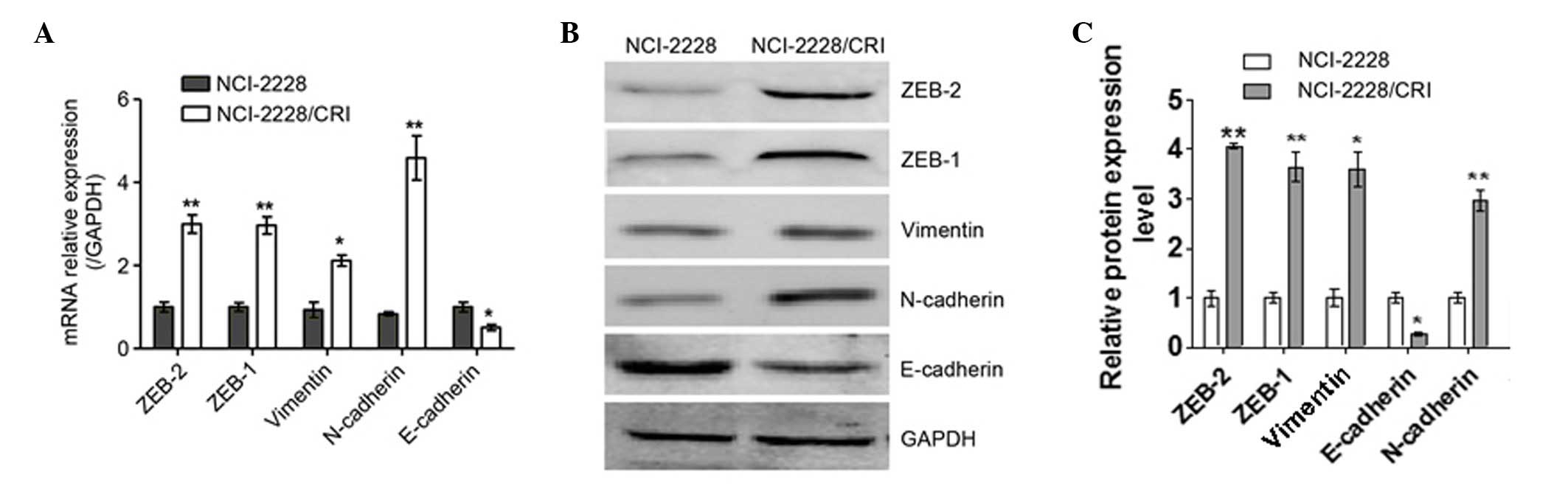
EMT occurs in NCI-2228/CRI cells
To elucidate the resistance mechanism of
NCI-2228/CRI cells to crizotinib, the mRNA (Fig. 6A) and protein (Fig. 6B) expression levels of epithelial
and mesenchymal cell markers were analyzed. The mRNA expression
levels of N-cadherin and Vimentin were increased in NCI-2228/CRI
cells compared with the parental cell line (P=0.004 and P=0.013,
respectively; Fig. 5A), while
E-cadherin was decreased in NCI-2228/CRI cells (P=0.017; Fig. 5A). The same trends in protein
expression were also observed (Fig.
6B). In addition, the mRNA levels of ZEB1 and ZEB2
(transcription factors involved in EMT) were increased in
NCI-2228/CRI cells compared with NCI-2228 (P=0.009 and P=0.003,
respectively; Fig. 6A), with
increased protein expression similarly observed by western blotting
(Fig. 6B). The present study,
therefore, suggests that EMT occurs in NCI-2228/CRI cells.
Increased miR-200c improves the
sensitivity of NCI-2228/CRI to crizotinib
The function of miR-200c in NCI-2228/CRI cells was
assessed by introducing miR-200c mimics to the cell line, resulting
in significantly increased miR-200c mRNA levels (P=0.001; Fig. 7A) and significantly reduced
viability of NCI-2228/CRI cells (P=0.008; Fig. 7B) compared with mimics NC. In
addition, miR-200c inhibited the migration and invasion of
NCI-2228/CRI cells compared with cells transfected with mimics NC
(P=0.038; Fig. 7C and D). Analysis
of mRNA (Fig. 7E) and protein
(Fig. 7F and G) expression levels
of epithelial and mesenchymal cell markers revealed that
overexpression of miR-200c in NCI-2228/CRI cells resulted in
decreased expression of N-cadherin (mRNA, P=0.032; protein,
P=0.048), Vimentin (mRNA, P=0.035; protein, P=0.018) and CD24
(mRNA, P=0.030; protein, P=0.004) compared with NC, and increased
expression of E-cadherin compared with NC (mRNA, P=0.017; protein,
P=0.03). Overexpression of miR-200c therefore appears to reverse
EMT to MET in NCI-2228/CRI cells, which may result in improved
sensitivity of NCI-2228/CRI cells to crizotinib.
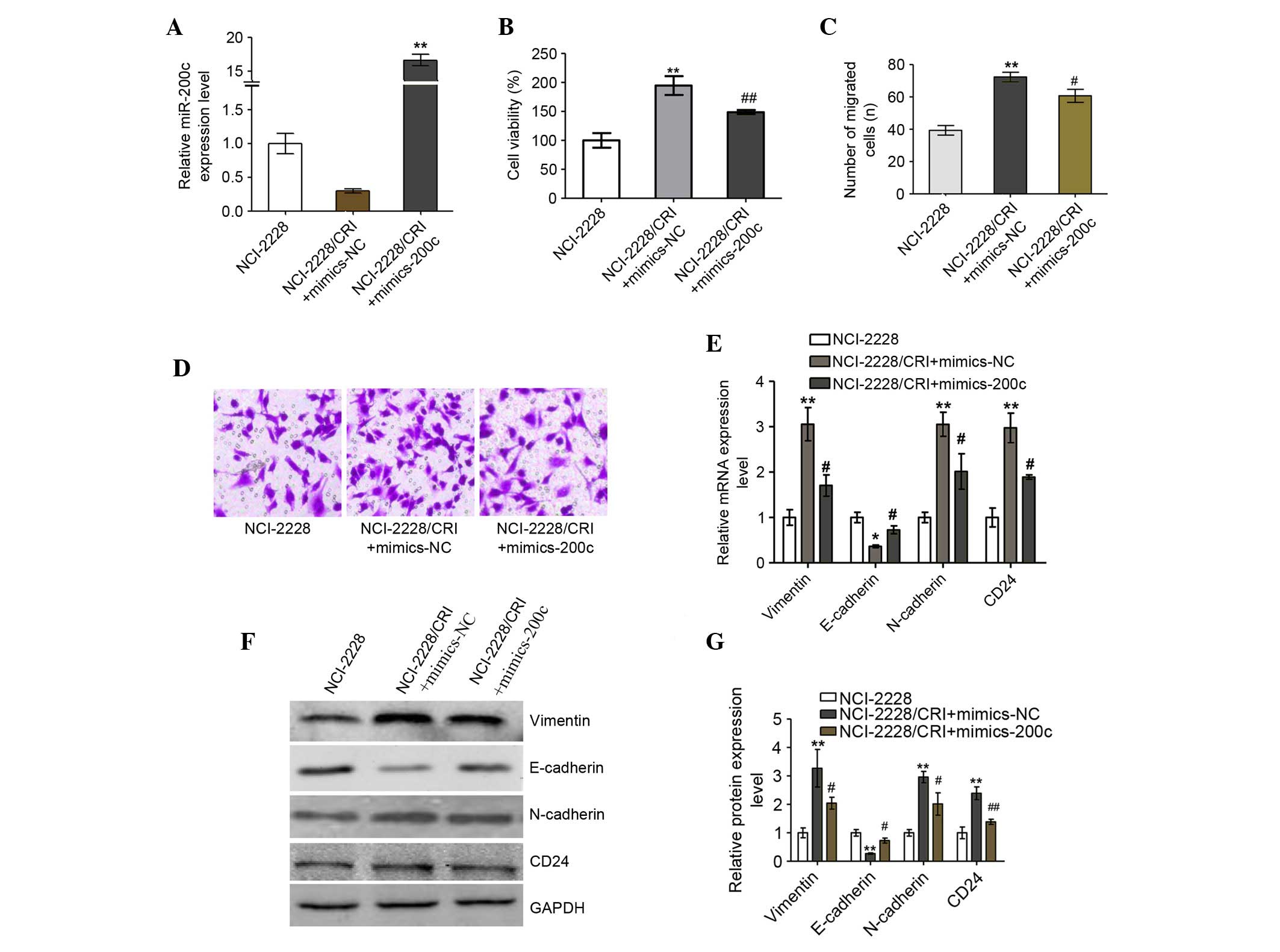 | Figure 7.Increased miR-200c reverses the
epithelial-mesenchymal transition to mesenchymal-epithelial
transition in NCI-2228/CRI cells. (A) NCI-2228/CRI cells were
treated with mimic-200c or mimic-NC and levels of miR expression
were analyzed by RT-qPCR. (B) Cell viability was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Transwell assays using a Matrigel-coated chamber were used
to analyze the invasive properties of the transfected NCI-2228/CRI
cells, with (C) migrated cells plotted as the average number of
cells per field of view (magnification, ×200) and (D)
representative images of the assays presented. (E) Total RNA was
extracted from NCI-2228 and transfected NCI-2228/CRI cells, and
subjected to RT-qPCR. GAPDH was used as an internal control. (F)
Cell lysates from NCI-2228 or transfected NCI-2228/CRI were
subjected to western blot analysis, and (G) the relative protein
levels of N-cadherin, Vimentin, E-cadherin and CD24 were analyzed
using GAPDH as the loading control. Values are presented as the
mean ± standard deviation of three independent experiments.
**P<0.01 vs. NCI-2228 group; #P<0.05 and
##P<0.01 vs. NCI-2228/CRI + mimics-NC group. miR,
microRNA; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; NC, negative control; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
It is widely accepted that the miR-200 family are
involved in the proliferation, invasion, metastasis and drug
resistance of various types of tumor (19–26).
As an important member of the miR-200 family, miR-200c displays low
expression in numerous tumor tissues, and can regulate EMT, tumor
invasion and metastasis (10–12).
However, few studies examining the effect of miR-200c in the
resistance of crizotinib have been conducted. The present study
reported that miR-200c expression was significantly lower in
NCI2228/CRI cells compared with NCI2228 cells, suggesting that
miR-200c may be associated with resistance to crizotinib.
EMT is associated with acquired crizotinib
resistance (27,28). In the present study, overexpression
of miR-200c was demonstrated to increase the expression of
E-cadherin, and decrease the expression of Vimentin and N-cadherin.
The results indicated that EMT was reversed in NCI-2228. In
addition, morphological changes to the spindle-cell shape of
NCI-2228 were observed in NCI-2228/CRI cells compared with parental
cells, and were accompanied by a decrease in E-cadherin, and
increase in N-cadherin and Vimentin expression, indicating the
occurrence of EMT in NCI-2228/CRI cells. Furthermore, invasion and
migration capabilities were significantly increased in NCI-2228/CRI
cells compared with the parental NCI-2228 cell line. The authors
speculate that overexpression of miR-200c in NCI-2228/CRI cells may
serve a role in improving the sensitivity of cells to crizotinib.
Moreover, NCI-2228/CRI cells exhibited resistance to conventional
chemotherapy drugs, such as paclitaxel, cisplatin and the
next-generation ALK tyrosine kinase inhibitor LDK378, which is
similar to the previous report (data not shown) (28). The changes to EMT marker expression
levels also suggested that EMT is involved in resistance to drugs,
including chemotherapeutics and molecular targeted drugs (28,29).
Drug resistance mechanisms are divided into
pharmacological and biological mechanisms (9). Regulation of EMT by miR-200c has been
previously demonstrated to be mediated by a negative feedback loop
targeting ZEB1 or ZEB2 (11,30).
ZEB1 and ZEB2 mediate EMT by repressing the transcription of
E-cadherin in order to enhance cell migration and invasion
(31–33). The present study examined the
mechanism by which miR-200c regulates migration and invasion in
NCI-2228 cells and demonstrated that miR-200c directly targets
ZEB1, thereby increasing E-cadherin expression in NCI-2228 cells.
This suggests that miR-200c inhibits migration and invasion of
NCI-2228 cells indirectly by upregulating E-cadherin through direct
targeting of ZEB1. Expression of ZEB1 and ZEB2 was also observed to
be higher in NCI-2228/CRI cells compared with NCI-2228 cells, and
was accompanied by decreased expression levels of E-cadherin. The
evidence, therefore, suggests that miR-200c is involved in
preventing EMT in NCI-2228 cells through targeting of ZEB1.
In summary, EMT may represent an acquired resistance
mechanism associated with crizotinib treatment. Migration and
invasion of NCI-2228 cells can be inhibited by miR-200c through
direct targeting of ZEB1 and indirect upregulation of E-cadherin.
The results of the present study suggest that overexpression of
miR-200c may improve crizotinib sensitivity by reversing EMT. The
miR-200c may, therefore, serve as a potent target for therapy,
which warrants further investigation using clinical samples.
Acknowledgements
The present study was supported by the Medical
Research Key Project Plan of Hebei Province (grant no. 20160065).
The authors thank the Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences (Beijing, China) for providing the A549
and H460 cell lines.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blanco R, Maestu I, de la Torre MG,
Cassinello A and Nuñez I: A review of the management of elderly
patients with non-small-cell lung cancer. Ann Oncol. 26:451–463.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zimmermann S, Dziadziuszko R and Peters S:
Indications and limitations of chemotherapy and targeted agents in
non-small cell lung cancer brain metastases. Cancer Treat Rev.
40:716–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rothschild SI and Gautschi O: Crizotinib
in the treatment of non-small-cell lung cancer. Clin Lung Cancer.
14:473–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malik SM, Maher VE, Bijwaard KE, Becker
RL, Zhang L, Tang SW, Song P, Liu Q, Marathe A, Gehrke B, et al: US
Food and Drug Administration approval: Crizotinib for treatment of
advanced or metastatic non-small cell lung cancer that is
anaplastic lymphoma kinase positive. Clin Cancer Res. 20:2029–2034.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS,
Squire J, et al: Molecular testing guideline for selection of lung
cancer patients for EGFR and ALK tyrosine kinase inhibitors:
Guideline from the college of American pathologists, international
association for the study of lung cancer and association for
molecular pathology. Arch Pathol Lab Med. 137:828–860. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iacono D, Chiari R, Metro G, Bennati C,
Bellezza G, Cenci M, Ricciuti B, Sidoni A, Baglivo S, Minotti V and
Crinò L: Future options for ALK-positive non-small cell lung
cancer. Lung Cancer. 87:211–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bracken CP, Gregory PA, Kolesnikoff N,
Bert AG, Wang J, Shannon MF and Goodall GJ: A double-negative
feedback loop between ZEB1-SIP1 and the microRNA-200 family
regulates epithelial-mesenchymal transition. Cancer Res.
68:7846–7854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cochrane DR, Spoelstra NS, Howe EN,
Nordeen SK and Richer JK: MicroRNA-200c mitigates invasiveness and
restores sensitivity to microtubule-targeting chemotherapeutic
agents. Mol Cancer Ther. 8:1055–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bryant JL, Britson J, Balko JM, Willian M,
Timmons R, Frolov A and Black EP: A microRNA gene expression
signature predicts response to erlotinib in epithelial cancer cell
lines and targets EMT. Br J Cancer. 106:148–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi L, Zhang S, Wu H, Zhang L, Dai X, Hu
J, Xue J, Liu T, Liang Y and Wu G: MiR-200c increases the
radiosensitivity of non-small-cell lung cancer cell line A549 by
targeting VEGF-VEGFR2 pathway. PloS One. 8:e783442013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang H, Deng M, Tang Y and Xie X, Guo J,
Kong Y, Ye F, Su Q and Xie X: miR-200b and miR-200c as prognostic
factors and mediators of gastric cancer cell progression. Clin
Cancer Res. 19:5602–5612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Wang Y, Shan B, Han J, Zhu H, Lv
Y, Fan X, Sang M, Liu XD and Liu W: The downregulation of
miR-200c/141 promotes ZEB1/2 expression and gastric cancer
progression. Med Oncol. 32:4282015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paterson EL, Kazenwadel J, Bert AG,
Khew-Goodall Y, Ruszkiewicz A and Goodall GJ: Down-regulation of
the miRNA-200 family at the invasive front of colorectal cancers
with degraded basement membrane indicates EMT is involved in cancer
progression. Neoplasia. 15:180–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tamagawa S, Beder LB, Hotomi M, Gunduz M,
Yata K, Grenman R and Yamanaka N: Role of miR-200c/miR-141 in the
regulation of epithelial-mesenchymal transition and migration in
head and neck squamous cell carcinoma. Int J Mol Med. 33:879–886.
2014.PubMed/NCBI
|
|
22
|
van Jaarsveld MT, Helleman J, Boersma AW,
van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH,
Berns EM, Verweij J, et al: miR-141 regulates KEAP1 and modulates
cisplatin sensitivity in ovarian cancer cells. Oncogene.
32:4284–4293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregory PA, Bracken CP, Smith E, Bert AG,
Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, et al:
An autocrine TGF-beta/ZEB/miR-200 signaling network regulates
establishment and maintenance of epithelial-mesenchymal transition.
Mol Biol Cell. 22:1686–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vrba L, Jensen TJ, Garbe JC, Heimark RL,
Cress AE, Dickinson S, Stampfer MR and Futscher BW: Role for DNA
methylation in the regulation of miR-200c and miR-141 expression in
normal and cancer cells. PloS One. 5:e86972010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Brenn T, Brown ER, Doherty V and
Melton DW: Differential expression of microRNAs during melanoma
progression: miR-200c, miR-205 and miR-211 are downregulated in
melanoma and act as tumour suppressors. Br J Cancer. 106:553–561.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt
L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS,
Borre M, et al: Coordinated epigenetic repression of the miR-200
family and miR-205 in invasive bladder cancer. Int J Cancer.
128:1327–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HR, Kim WS, Choi YJ, Choi CM, Rho JK
and Lee JC: Epithelial-mesenchymal transition leads to crizotinib
resistance in H2228 lung cancer cells with EML4-ALK translocation.
Mol Oncol. 7:1093–1102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kogita A, Togashi Y, Hayashi H, Sogabe S,
Terashima M, De Velasco MA, Sakai K, Fujita Y, Tomida S, Takeyama
Y, et al: Hypoxia induces resistance to ALK inhibitors in the H3122
non-small cell lung cancer cell line with an ALK rearrangement via
epithelial-mesenchymal transition. Int J Oncol. 45:1430–1436.
2014.PubMed/NCBI
|
|
29
|
Shen W, Pang H, Liu J, Zhou J, Zhang F and
Liu L, Ma N, Zhang N, Zhang H and Liu L: Epithelial-mesenchymal
transition contributes to docetaxel resistance in human non-small
cell lung cancer. Oncol Res. 22:47–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Li X, Ren S, Chen X, Zhang Y, Zhou
F, Zhao M, Zhao C, Chen X, Cheng N, et al: miR-200c overexpression
is associated with better efficacy of EGFR-TKIs in non-small cell
lung cancer patients with EGFR wild-type. Oncotarget. 5:7902–7916.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Gao D, Wang H, Li X, Yang J, Yan X,
Liu Z and Ma Z: Negative feedback loop between p66Shc and ZEB1
regulates fibrotic EMT response in lung cancer cells. Cell Death
Dis. 6:e17082015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh AB, Sharma A, Smith JJ, Krishnan M,
Chen X, Eschrich S, Washington MK, Yeatman TJ, Beauchamp RD and
Dhawan P: Claudin-1 up-regulates the repressor ZEB-1 to inhibit
E-cadherin expression in colon cancer cells. Gastroenterology.
141:2140–2153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar : PubMed/NCBI
|