Introduction
Sepsis, characterized by whole-body inflammation, is
caused by a severe systemic infection (1). Sepsis is a leading cause of mortality
in critically ill patients and the predominant cause of mortality
in non-coronary intensive care units (2–4). The
overproduction of cytokines that are induced by an infectious
stimulus is the hallmark of sepsis, and leads to multiple organ
dysfunction and consequently to a high mortality (5). Myocardial dysfunction is a recognized
manifestation of this lethal condition (6–8).
Resveratrol (RESV) is a polyphenolic phytoalexin
that has previously been suggested to exert cardioprotective
effects (9,10). It has been suggested to cause the
‘French Paradox’; the low incidence of cardiovascular diseases in
the French population despite a high consumption of wine and
saturated fat (11). In addition,
RESV is reported to be beneficial for sepsis-induced myocardial
dysfunction (12). However, the
underlying mechanism remains unclear.
Sirtuin 1 (Sirt1), a member of the silent mating
type information regulator family of proteins, has multiple
protective effects in various diseases (13–15).
Sirt1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy
(16) and is reported to suppress
lung inflammasome activation in a murine model of sepsis (17). However, whether Sirt1 activation is
involved in the protective effect of RESV against sepsis-induced
myocardial dysfunction remains unclear.
The current study used a rat model of sepsis
involving cecal ligation and puncture (CLP) to investigate whether
RESV was protective against sepsis-induced myocardial injury. In
addition, the role of Sirt1 was evaluated in this model of
CLP-induced sepsis.
Materials and methods
Animals and reagents
The present study was approved by the Committee for
Animal Research of the Fourth Military Medical University (Xi'an,
China). All animal experiments were performed in accordance with
the National Institutes of Health Guidelines on the Use of
Laboratory Animals (Bethesda, MD, USA). A total of 40 healthy,
specific pathogen-free, male Sprague-Dawley rats (age, 3 months;
weight, 200–250 g), were obtained from the Animal Center of the
Fourth Military Medical University (Xi'an, China). Rats were housed
in constant humidity (50%) and temperature (25±2°C) animal
facilities with free access to standard laboratory food and water
with a 12 h light/dark cycle.
RESV was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). The Sirt1 inhibitor, EX527, was
purchased from Tocris Bioscience (Bristol, UK). Rabbit antibodies
against Sirt1 (cat. no. 9475), acetylated-Forkhead box O1
(Ac-FoxO1; cat. no. 2880), B cell lymphoma 2 apoptosis regulator
(Bcl-2; cat. no. 2870), and Bcl-2 associated protein X apoptosis
regulator (Bax; cat. no. 2772) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Antibodies against
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat. no. BM1623),
and goat anti-rabbit (for Sirt1, Ac-FoxO1, Bcl-2 and Bax; cat. no.
BA1039) and goat anti-mouse (for GAPDH; cat. no. BA1089) secondary
antibodies were purchased from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China).
CLP surgery
To establish sepsis, rats were subjected to a CLP
procedure. Animals were generally anesthetized with chloral hydrate
(Sigma-Aldrich; Merck Millipore) (350 mg/kg) via intraperitoneal
injection, and an abdominal midline incision (2–3 cm) was created
to expose the cecum. The cecum was exteriorized and isolated, and a
midpiece ligation of the cecum was made using 4–0 silk. The cecum
was then perforated below the ligation twice with an 18-gauge
needle, and a small amount of stool was extruded through the
puncture holes to ensure patency. The cecum was then relocated to
its normal intra-abdominal position and the abdomen was closed by
suturing the muscle and skin. For the sham-operated animals, the
cecum was isolated without ligation and puncturing. All animals
received 0.9% saline solution (40 ml/kg of body weight)
subcutaneously immediately following the surgery and every
subsequent 24 h.
Experimental protocol
A total of 40 rats were randomly divided into 5
groups of 8 animals: Sham operation without CLP; CLP; CLP + RESV;
CLP + RESV + EX527; and CLP + EX527. RESV was administered
intraperitoneally at 60 mg/kg per rat, at 3, 12 and 24 h
post-surgery. The Sirt1 inhibitor, EX527, was dissolved in dimethyl
sulfoxide and diluted to the final concentration with normal
saline. EX527, or the same volume of vehicle, was intraperitoneally
injected at a dose of 5 mg/kg every two days, beginning 8 days
prior to CLP surgery. A CLP + EX527 group was included to assess
any contribution of EX527 to CLP-induced sepsis.
Hemodynamic assessment
Rats were anesthetized with chloral hydrate at 48 h
post-CLP surgery, and pressure tracings of cardiac function were
analyzed using an RM-6280 Multi-channel Physiological Signal
Recording system (Chengdu Science Instrument Factory, Chengdu,
China) for the assessment of cardiac function. A high-fidelity,
pressure-transducing catheter, filled with heparinized saline, was
inserted via the right carotid artery into the left ventricle. When
the rats returned to a stable condition, hemodynamic changes,
including left ventricular systolic pressure (LVSP) and left
ventricular end-diastolic pressure (LVEDP), and their first
derivative with respect to time (LV±dP/dtmax) were
measured continuously (over 3 different periods, 10 cardiac cycles
for each).
Myocardial apoptosis
Rats were anesthetized with chloral hydrate and
sacrificed by thoracotomy. Hearts were harvested at 48 h post-CLP
surgery and fixed in 4% paraformaldehyde for 48 h. Following
embedding in paraffin, 5 µm thick sections of whole hearts were
obtained. Terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL staining) was used to assess the number of
apoptotic myocardial cells. TUNEL kits were purchased from Roche
Diagnostics GmbH (Mannheim, Germany). TUNEL reaction mixture (50
µl) was added to each sample and the slides incubated in a
humidified atmosphere for 60 min at 37°C in the dark. Slides were
rinsed with phosphate-buffered saline (PBS; pH 7.4) three times,
for 5 min each time. To detect the nuclei, slides were incubated
with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room
temperature in the dark, rinsed with PBS three times, for 5 min
each time, and observed under a fluorescence microscope.
TUNEL-positive nuclei were green and DAPI-positive nuclei were
blue. The percentage of apoptotic nuclei (apoptotic nuclei/total
nuclei ×100) was calculated in 5 randomly chosen fields per slide
(3 slides per section and 3 sections per rat, 26 rats in
total).
Detection of tumor necrosis factor
(TNF)-α in the serum and myocardial tissue
Blood samples were collected from the abdominal
aorta at the time of sacrifice 48 h post-CLP surgery and
centrifuged (1,000 × g for 15 min) to obtain serum that was
stored at −80°C prior to further analysis. Serum and heart samples,
harvested and stored at −80°C until analysis, were processed to
measure TNF-α levels with an enzyme-linked immunosorbent assay
(ELISA) kit (cat. no. JER-06; Joyee Biotechnics Co., Ltd.,
Shanghai, China), used according to the manufacturer's
protocol.
Evaluation of myeloperoxidase (MPO)
levels
Myocardial tissues were collected 48 h after CLP
surgery. An MPO Activity assay kit (cat. no. A044; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) was employed to
detect the level of MPO in the myocardial tissue.
Western blotting
Left ventricular myocardial tissue was lysed in
sample buffer (Pulse Yuan Biological Technology Co., Ltd., Xi'an,
China), homogenized, and centrifuged at 10,000 × g for 15
min. A bicinchoninic acid assay was performed for protein
quantification. Equal amounts of total protein (40 µg) were
separated on 10–15% denaturing gels by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The proteins were
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA) and incubated in 10% skimmed milk in
Tris-buffered saline and 0.1% Tween-20 (TBST) for 2 h. The membrane
was then incubated with primary antibodies against Sirt1, Ac-FoxO1,
Bcl-2, or Bax, diluted 1:1,000, at 4°C overnight. GAPDH was
selected as the loading control. The blots were washed with TBST
and incubated with the appropriate secondary antibodies conjugated
to horseradish peroxidase diluted 1:5,000, for 1 h at room
temperature, and then washed with TBST. Immunoreactive bands were
detected using an enhanced chemiluminescence detection reagent
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) using a Bio-Rad imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and quantified using Quantity One software
version 4.62 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error.
Significant differences among groups were evaluated by Student's
t-test for unpaired data, or one-way analysis of variance
followed by Dunnett's t-test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RESV attenuates sepsis-induced
myocardial injury in rats
CLP-induced sepsis resulted in a severe impairment
in cardiovascular performance at 48 h post-CLP surgery. In the CLP
group, the LVSP was significantly decreased (P<0.001; Fig. 1A), the LVEDP was significantly
elevated (P<0.001; Fig. 1B),
and LV±dP/dtmax were significantly decreased compared
with the sham group (P<0.001 and P<0.001, respectively;
Fig. 1C and D). Treatment with
RESV significantly prevented all of these detrimental effects
induced by CLP (P<0.05 CLP + RESV vs. CLP group; Fig. 1). EX527, a selective Sirt1
inhibitor, significantly attenuated the effects induced by RESV on
LVSP, LVEDP and LV±dP/dtmax (P<0.05, CLP + RESV +
EX527 group vs. CLP + RESV group; Fig.
1). No significant difference was detected between the CLP and
CLP+EX527 groups.
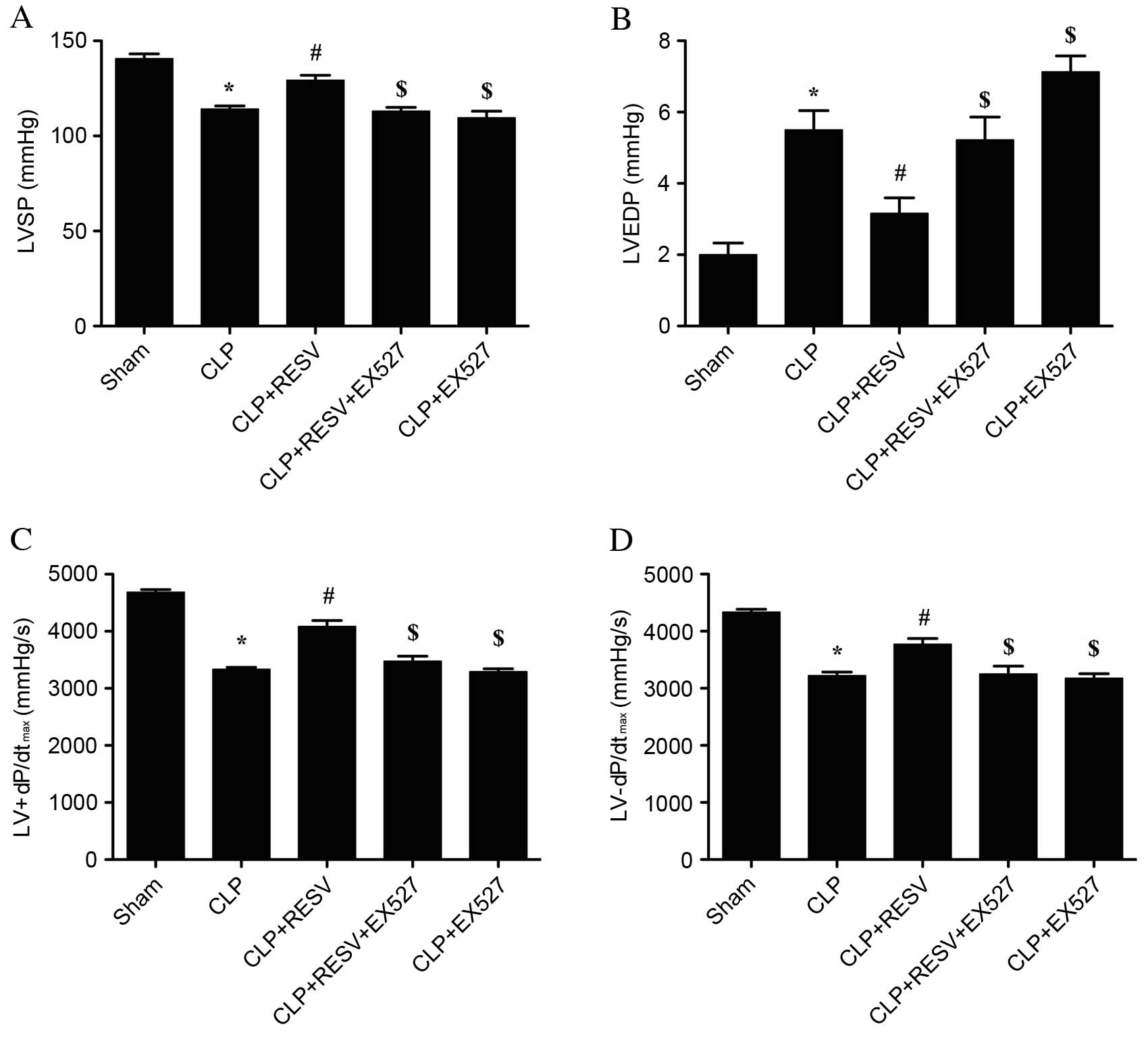 | Figure 1.Invasive hemodynamic evaluation
suggests that RESV improves cardiac function. (A) LVSP, (B) LVEDP,
(C) LV+dP/dtmax and (D) LV-dP/dtmax were
measured in rats following CLP and treatment with RESV, EX527 or
both. Data are presented as the mean ± standard error, n=8 per
group. *P<0.05 vs. sham group, #P<0.05 vs. CLP
group, $P<0.05 vs. CLP + RESV group. LVSP, left
ventricular systolic pressure; CLP, cecal ligation and puncture;
RESV, resveratrol; LVEDP, left ventricular end diastolic pressure;
LV±dP/dtmax, instantaneous first derivation of left
ventricle pressure. |
RESV reduces myocardial apoptosis in
septic rats
The number of TUNEL-positive cells detected in
myocardial tissue was increased in the CLP group compared with the
sham group (P<0.001; Fig. 2),
indicating a significantly higher degree of apoptosis. There was a
significant reduction in TUNEL-positive staining in the
RESV-treated group compared with the CLP group (P=0.0014; Fig. 2), indicating an anti-apoptotic
effect of RESV. EX527 suppressed the protective effect of RESV;
increasing the apoptotic index in the CLP + RESV + EX527 group
compared with the CLP + RESV group (P=0.0038; Fig. 2). No significant difference was
detected between the CLP and CLP+EX527 groups (P=0.9909).
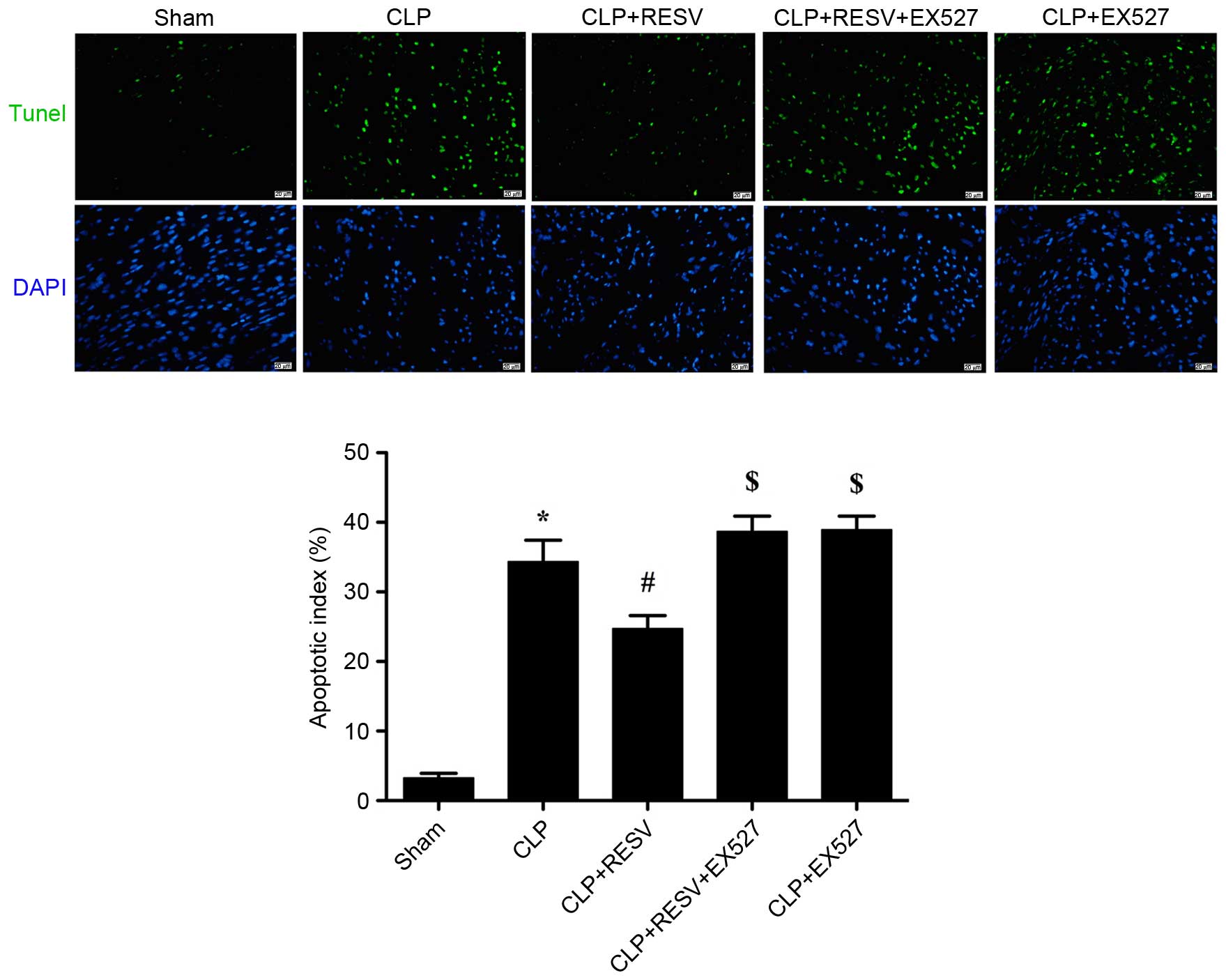 | Figure 2.RESV suppression of sepsis-induced
myocardial apoptosis is abolished by EX527. Apoptotic cells were
detected by TUNEL (green), and the nuclei were detected by DAPI
(blue). Representative images are shown. Magnification, ×400. Scale
bars=20 µm. Data are presented as the mean ± standard error, n=8
per group. *P<0.05 vs. sham group, #P<0.05 vs. CLP
group, $P<0.05 vs. CLP + RESV group. CLP, cecal
ligation and puncture; RESV, resveratrol; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick-end labeling; DAPI,
4′,6-diamidino-2-phenylindole. |
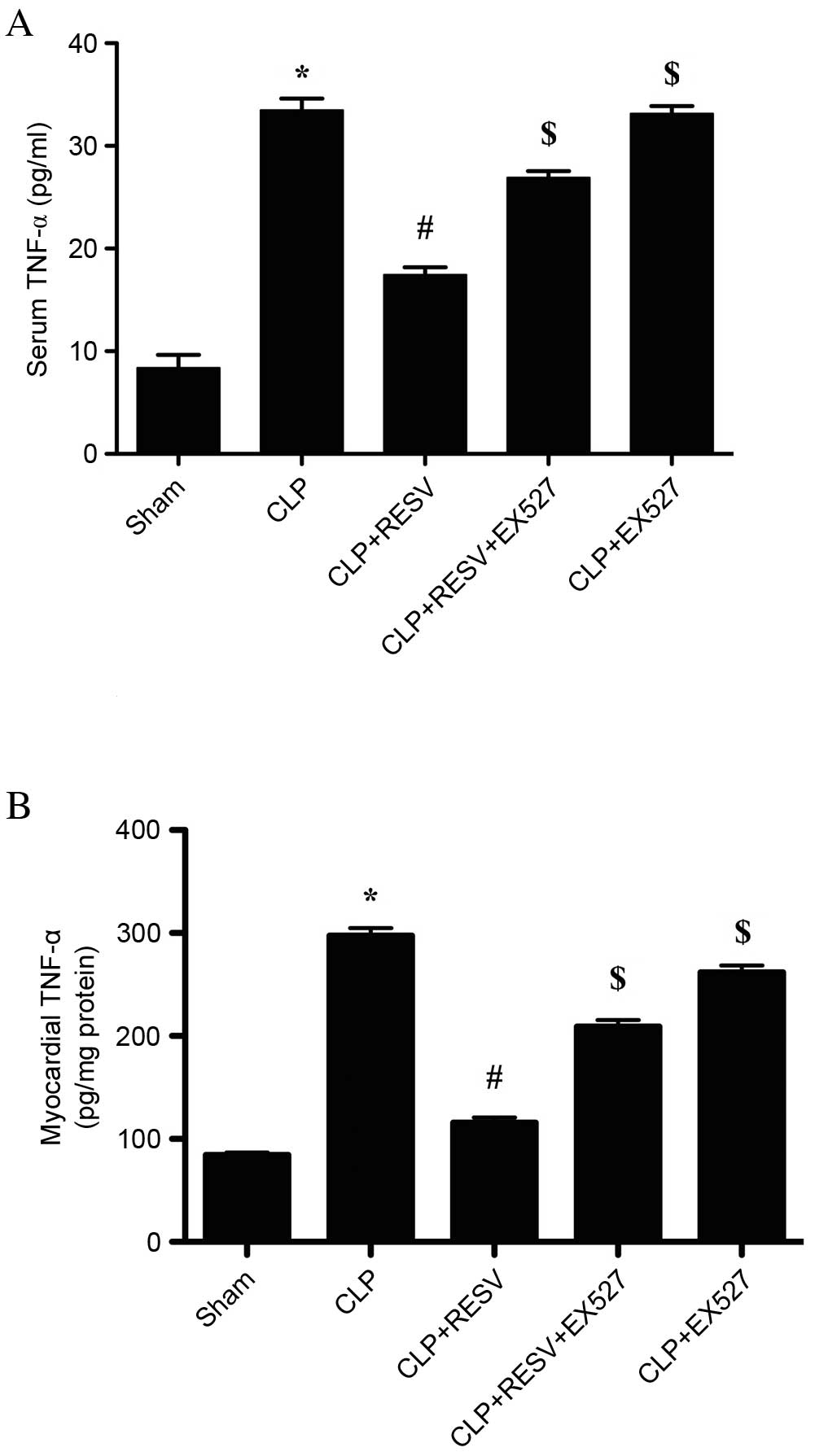
RESV suppresses the sepsis-induced
production of TNF-α
An inflammatory response accompanied the CLP-induced
sepsis. In the CLP group, the level of TNF-α was significantly
increased in serum (P<0.001; Fig.
3A) and myocardial tissue compared with the sham group
(P<0.001; Fig. 3B). Treatment
with RESV significantly decreased the levels of TNF-α in the CLP +
RESV group compared with the CLP group in serum (P<0.001;
Fig. 3A) and myocardial tissue
(P<0.001; Fig. 3B). EX527
virtually eliminated the protective effect of RESV treatment in the
CLP + RESV + EX527 group compared with the CLP + RESV group in
serum (P<0.001; Fig. 3A) and
myocardial tissue (P<0.001; Fig.
3B). No significant difference was detected between the CLP and
CLP+EX527 groups (P=0.9992) in serum; however, in the myocardial
tissue, EX527 significantly decreased the level of TNF-α compared
with the CLP group (P=0.0008).
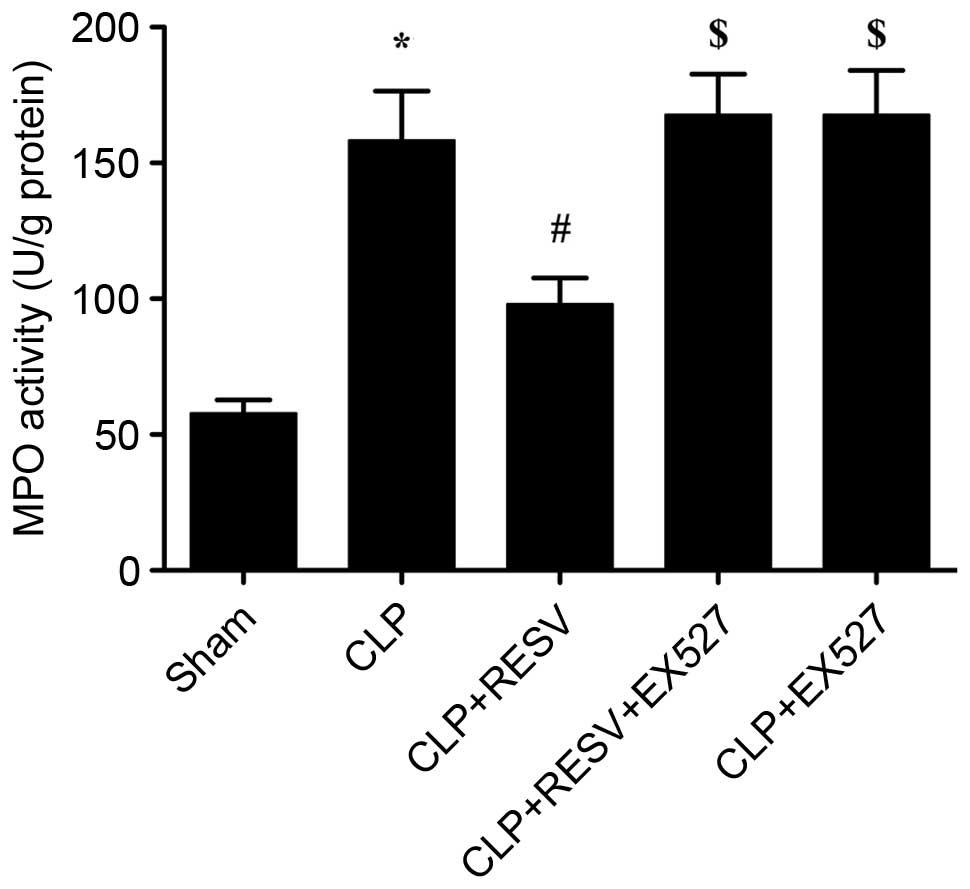
RESV inhibits neutrophil infiltration
of myocardial tissue in septic rats
MPO, a heme protein predominantly expressed in
neutrophils, is synthesized and stored in azurophilic granules of
granulocytes and monocytes, and accounts for 5% of dry cell weight
(18–20). The extent of neutrophil
infiltration is partially reflected by the activity of MPO within a
tissue. As demonstrated in Fig. 4,
the activity of MPO in hearts from the CLP group was significantly
increased compared with sham group (P=0.0004). By contrast, the
activity of MPO was significantly reduced in the CLP + RESV group
compared with the CLP group (P=0.0372). Furthermore, this effect
was largely abolished by EX527 in the CLP + RESV + EX527 group
compared with the CLP + RESV group (P=0.0130), indicating that the
activation of Sirt1 may have an important role in the protective
effect of RESV. No significant difference was detected between the
CLP and CLP+EX527 groups (P=0.9878).
Expression and effect of Sirt1 in
septic rats treated with RESV
To investigate the role of Sirt1 in injured
myocardial tissue of rats with CLP-induced sepsis, the protein
expression levels of Sirt1, Ac-FoxO1, Bcl-2 and Bax were assessed
by western blot analysis (Fig. 5).
The CLP group displayed significantly lower Sirt1 expression
(P<0.001; Fig. 5A), higher
Ac-FoxO1 expression (P<0.001; Fig.
5B), lower Bcl-2 expression (P<0.001; Fig. 5C) and higher Bax expression
(P<0.001; Fig. 5D) than the
sham group. RESV prevented the CLP-induced downregulation of Sirt1
and Bcl-2 expression in the CLP + RESV group compared with the CLP
group (P<0.001 and P<0.001, respectively; Fig. 5A and C), and, similarly, prevented
CLP-induced upregulation of Ac-FoxO1 and Bax (P<0.001 and
P<0.001, respectively; Fig. 5B and
D). In the CLP + RESV+ EX527 group, all protective effects of
RESV were prevented compared with the CLP + RESV group (P<0.05;
Fig. 5). No significant
differences in Sirt1, Ac-FoxO1 and Bcl-2 were detected between the
CLP and CLP+EX527 groups (P=0.9969, P=0.5170 and P=0.9936,
respectively); however, Bax was significantly decreased with the
application of EX527 (P<0.001). This suggested that Sirt1 is
important for the protective effect of RESV against CLP-induced
myocardial injury.
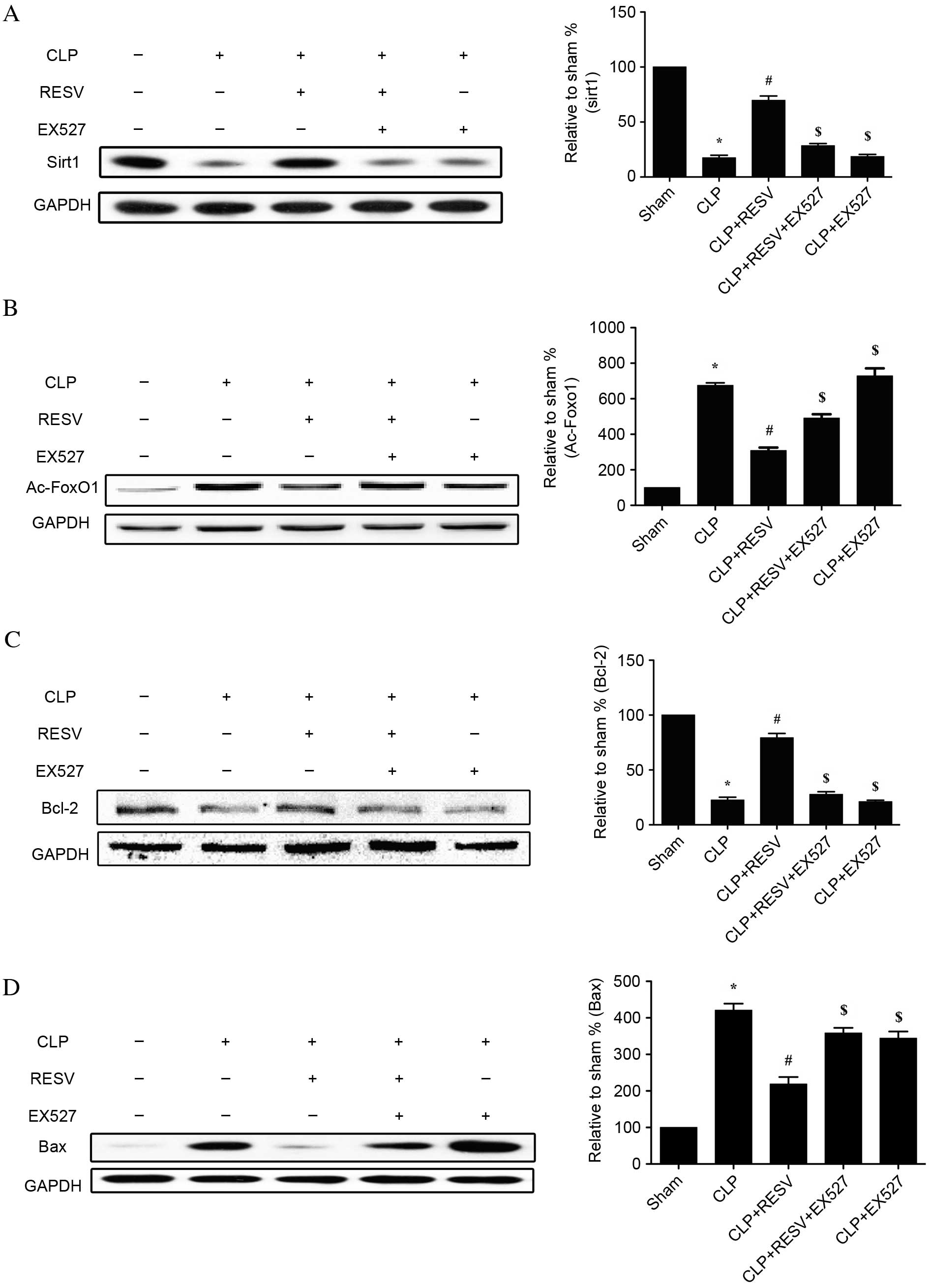 | Figure 5.Effect of RESV on the protein
expression levels of Sirt1, Ac-FoxO1, Bcl-2 and Bax following
sepsis. Western blot analysis and quantification of the protein
expression levels of (A) Sirt1, (B) Ac-FoxO1, (C) Bcl-2 and (D)
Bax. Representative images of western blot results are shown.
Quantification was relative to GAPDH. Data are presented as the
mean ± standard error, n=8 per group. *P<0.05 vs. sham group,
#P<0.05 vs. CLP group, and $P<0.05 vs.
CLP + RESV group. CLP, cecal ligation and puncture; RESV,
resveratrol; Sirt1, sirtuin 1; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; Ac-FoxO1, acetylated-Forkhead box O1, Bcl-2, B cell
lymphoma 2 apoptosis regulator; Bax, Bcl-2 associated protein X
apoptosis regulator. |
Discussion
In the present study, a CLP-induced rat model of
sepsis was established to mimic human sepsis, and used to
investigate sepsis-induced cardiac dysfunction and the protective
effect of RESV. Treatment with RESV was demonstrated to
significantly suppress myocardial apoptosis, neutrophil
infiltration and TNF-α production during sepsis. Additionally,
Sirt1 was demonstrated to be involved in the cardioprotective
effect of RESV.
Sepsis is a lethal condition characterized by a
systemic inflammatory response syndrome, induced by a severe
infection leading to multiple organ dysfunctions (21–23).
The cardiovascular system is frequently affected by sepsis, and
this effect has been studied for nearly six decades (23). Patients with sepsis and myocardial
depression are at a 50–70% greater risk of death than patients
without cardiovascular complications (23), indicating that myocardial injury is
an urgent problem to resolve. The underlying mechanisms of
sepsis-induced myocardial dysfunction are thought to include
metabolic changes (24), autonomic
dysregulation (25), mitochondrial
dysfunction (26), cell apoptosis
(27), and inflammation (28). TNF-α is important during
sepsis-induced inflammation (29).
It has previously been reported that the application of murine
monoclonal anti-TNF antibodies induced a transient improvement in
ventricular function in patients with sepsis, suggesting that TNF
may be involved in sepsis (30).
Furthermore, TNF-α exerts a negative inotropic effect on the heart,
leading to a decrease in blood pressure and cardiac output
(29). TNF-α can also activate
other inflammatory cells, such as neutrophils, triggering an
inflammatory cascade (31). Thus,
neutrophil infiltration is another factor associated with
myocardial dysfunction induced by sepsis (32).
RESV, a natural phenolic anti-oxidant, has
therapeutic benefits in sepsis (12,33–35).
Recently, RESV was reported to attenuate microvascular inflammation
in sepsis (36). Additionally,
RESV was suggested to suppress high-mobility group protein box 1
nucleocytoplasmic translocation in sepsis-induced liver injury
(13) and to alleviate
sepsis-induced myocardial dysfunction via the Nrf2 transcription
factor (35). In the present
study, RESV was suggested to ameliorate myocardial dysfunction,
suppress TNF-α activity in the serum and myocardium, and inhibit
neutrophil accumulation in the myocardial tissue.
EX527 abolished the protective effect of RESV,
indicating Sirt1 activation is closely associated with the RESV
mechanism of action. Sirt1, a nicotinamide adenine
dinucleotide+-dependent class III histone deacetylase,
is involved in numerous pathophysiological processes. Sirt1 is
essential for protein deacetylation and the regulation of
pro-inflammatory cytokine release, apoptosis, stress resistance,
metabolism, mitochondrial biogenesis, autophagy, senescence,
differentiation and aging (9,37–39).
The activation of Sirt1 leads to the deacetylation and activation
of FoxO, which promotes the synthesis of superoxide dismutase and
catalase (40), therefore
protecting the cell against oxidative stress. In addition, Sirt1
upregulates Bcl-2 expression and downregulates Bax expression,
leading to an anti-apoptotic effect (41). In accordance with previous studies
(42–44), the results of the present study
suggest that RESV suppresses myocardial apoptosis by upregulating
Bcl-2 expression and downregulating Bax expression. In addition,
RESV promoted the deacetylation and activation of FoxO1, as
evidenced by the decrease in Ac-FoxO1 detected by western blotting.
However, the selective Sirt1 inhibitor, EX527, abolished the
protective effects of RESV, indicating the involvement of Sirt1 in
this protective effect. The inclusion of the control group, CLP +
EX527, throughout the study additionally excluded any contribution
of EX527 itself to either the CLP-induced sepsis or the
RESV-induced protections.
In conclusion, the results of the present study
demonstrate that RESV ameliorates cardiac dysfunction and apoptosis
induced by sepsis, and suppresses TNF-α production and neutrophil
accumulation. Additionally, activation of Sirt1 signaling is
involved in the protective effects of RESV. The present study,
therefore, provides evidence supporting further investigations into
the clinical use of RESV in the treatment of sepsis-induced
myocardial injury.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81170185).
References
|
1
|
Annane D, Bellissant E and Cavaillon JM:
Septic shock. Lancet. 365:63–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frolkis I, Klein Y, Locker C, Adi N, Dahan
E, Uretzsky G, Shapira I and Sorkine P: Vipera aspis venom reduces
lethality and down-regulates tumor necrosis factor-alpha in a rat
model of LPS-induced sepsis. Cytokine. 49:319–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock, 2012. Intensive Care
Med. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An R, Zhao L, Xi C, Li H, Shen G, Liu H,
Zhang S and Sun L: Melatonin attenuates sepsis-induced cardiac
dysfunction via a PI3K/Akt-dependent mechanism. Basic Res Cardiol.
111:82016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Court O and Kumar A, Parrillo JE and Kumar
A: Clinical review: Myocardial depression in sepsis and septic
shock. Crit Care. 6:500–508. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Poll T and van Deventer SJ:
Cytokines and anticytokines in the pathogenesis of sepsis. Infect
Dis Clin North Am. 13:413–426, ix. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borra MT, Smith BC and Denu JM: Mechanism
of human SIRT1 activation by resveratrol. J Biol Chem.
280:17187–17195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YG, Zhu W, Tao JP, Xin P, Liu MY, Li JB
and Wei M: Resveratrol protects cardiomyocytes from oxidative
stress through SIRT1 and mitochondrial biogenesis signaling
pathways. Biochem Biophys Res Commun. 438:270–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu BL, Zhang X, Zhang W and Zhen HN: New
enlightenment of French Paradox: Resveratrol's potential for cancer
chemoprevention and anti-cancer therapy. Cancer Biol Ther.
6:1833–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smeding L, Leong-Poi H, Hu P, Shan Y,
Haitsma JJ, Horvath E, Furmli S, Masoom H, Kuiper JW, Slutsky AS,
et al: Salutary effect of resveratrol on sepsis-induced myocardial
depression. Crit Care Med. 40:1896–1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Lu Y, Yao J, Li Z, Chen Z, Wang G,
Jing H, Zhang X, Li M, Peng J and Tian X: Novel role of
resveratrol: Suppression of high-mobility group protein box 1
nucleocytoplasmic translocation by the upregulation of sirtuin 1 in
sepsis-induced liver injury. Shock. 42:440–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu F, Burk D, Gao Z, Yin J, Zhang X, Weng
J and Ye J: Angiogenic deficiency and adipose tissue dysfunction
are associated with macrophage malfunction in SIRT1-/− mice.
Endocrinology. 153:1706–1716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motta MC, Divecha N, Lemieux M, Kamel C,
Chen D, Gu W, Bultsma Y, McBurney M and Guarente L: Mammalian SIRT1
represses forkhead transcription factors. Cell. 116:551–563. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao R, Ma Z, Hu Y, Chen J, Shetty S and Fu
J: Sirt1 restrains lung inflammasome activation in a murine model
of sepsis. Am J Physiol Lung Cell Mol Physiol. 308:L847–L853. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arnhold J: Properties, functions, and
secretion of human myeloperoxidase. Biochemistry (Mosc). 69:4–9.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thom SR, Milovanova TN, Bogush M, Yang M,
Bhopale VM, Pollock NW, Ljubkovic M, Denoble P, Madden D, Lozo M
and Dujic Z: Bubbles, microparticles, and neutrophil activation:
Changes with exercise level and breathing gas during open-water
SCUBA diving. J Appl Physiol (1985). 114:1396–1405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mallat Z, Hugel B, Ohan J, Lesèche G,
Freyssinet JM and Tedgui A: Shed membrane microparticles with
procoagulant potential in human atherosclerotic plaques: A role for
apoptosis in plaque thrombogenicity. Circulation. 99:348–353. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calis J, van Woensel J and Lemson J:
Severe sepsis and septic shock. N Engl J Med. 369:20622013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Merx MW and Weber C: Sepsis and the heart.
Circulation. 116:793–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stanley WC, Recchia FA and Lopaschuk GD:
Myocardial substrate metabolism in the normal and failing heart.
Physiol Rev. 85:1093–1129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharshar T, Gray F, de la Grandmaison G
Lorin, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC,
Gajdos P and Annane D: Apoptosis of neurons in cardiovascular
autonomic centres triggered by inducible nitric oxide synthase
after death from septic shock. Lancet. 362:1799–1805. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Qin W, Qiu X, Cao J, Liu D and Sun
B: A novel role of exogenous carbon monoxide on protecting cardiac
function and improving survival against sepsis via mitochondrial
energetic metabolism pathway. Int J Biol Sci. 10:777–788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou X, Xu J, Yao S, Li J, Yang Y and Yang
L: Endoplasmic reticulum stress-mediated autophagy protects against
lipopolysaccharide-induced apoptosis in HL-1 cardiomyocytes. Exp
Physiol. 99:1348–1358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang T, Yan T, Du J, Wang S and Yang H:
Apigenin attenuates heart injury in lipopolysaccharide-induced
endotoxemic model by suppressing sphingosine kinase 1/sphingosine
1-phosphate signaling pathway. Chem Biol Interact. 233:46–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antonucci E, Fiaccadori E, Donadello K,
Taccone FS, Franchi F and Scolletta S: Myocardial depression in
sepsis: From pathogenesis to clinical manifestations and treatment.
J Crit Care. 29:500–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vincent JL, Bakker J, Marécaux G,
Schandene L, Kahn RJ and Dupont E: Administration of anti-TNF
antibody improves left ventricular function in septic shock
patients. Results of a pilot study. Chest. 101:810–815. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu X, Zhang B, Fan R, Zhao L, Wang Y,
Zhang S, Kaye AD, Huang L and Pei J: U50, 488H inhibits neutrophil
accumulation and TNF-α induction induced by ischemia-reperfusion in
rat heart. Cytokine. 56:503–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Unnewehr H, Rittirsch D, Sarma JV, Zetoune
F, Flierl MA, Perl M, Denk S, Weiss M, Schneider ME, Monk PN, et
al: Changes and regulation of the C5a receptor on neutrophils
during septic shock in humans. J Immunol. 190:4215–4225. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kolgazi M, Sener G, Cetinel S, Gedik N and
Alican I: Resveratrol reduces renal and lung injury caused by
sepsis in rats. J Surg Res. 134:315–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holthoff JH, Wang Z, Seely KA, Gokden N
and Mayeux PR: Resveratrol improves renal microcirculation,
protects the tubular epithelium, and prolongs survival in a mouse
model of sepsis-induced acute kidney injury. Kidney Int.
81:370–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hao E, Lang F, Chen Y, Zhang H, Cong X,
Shen X and Su G: Resveratrol alleviates endotoxin-induced
myocardial toxicity via the Nrf2 transcription factor. PLoS One.
8:e694522013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Buechler NL, Yoza BK, McCall CE
and Vachharajani VT: Resveratrol attenuates microvascular
inflammation in sepsis via SIRT-1-Induced modulation of adhesion
molecules in ob/ob mice. Obesity (Silver Spring). 23:1209–1217.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bishayee A, Waghray A, Barnes KF, Mbimba
T, Bhatia D, Chatterjee M and Darvesh AS: Suppression of the
inflammatory cascade is implicated in resveratrol chemoprevention
of experimental hepatocarcinogenesis. Pharm Res. 27:1080–1091.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun W, Wang W, Kim J, Keng P, Yang S,
Zhang H, Liu C, Okunieff P and Zhang L: Anti-cancer effect of
resveratrol is associated with induction of apoptosis via a
mitochondrial pathway alignment. Adv Exp Med Biol. 614:179–186.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Daitoku H, Hatta M, Matsuzaki H, Aratani
S, Ohshima T, Miyagishi M, Nakajima T and Fukamizu A: Silent
information regulator 2 potentiates Foxo1-mediated transcription
through its deacetylase activity. Proc Natl Acad Sci USA.
101:10042–10047. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kalle AM, Mallika A, Badiger J, Alinakhi
Talukdar P and Sachchidanand: Inhibition of SIRT1 by a small
molecule induces apoptosis in breast cancer cells. Biochem Biophys
Res Commun. 401:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo S, Yao Q, Ke Z, Chen H, Wu J and Liu
C: Resveratrol attenuates high glucose-induced oxidative stress and
cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol.
412:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang R, Liu YY, Liu XY, Jia SW, Zhao J,
Cui D and Wang L: Resveratrol protects neurons and the myocardium
by reducing oxidative stress and ameliorating mitochondria damage
in a cerebral ischemia rat model. Cell Physiol Biochem. 34:854–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin Y, Zhu J, Zhang X, Wang J, Xiao W, Li
B, Jin L, Lian J, Zhou L and Liu J: Inhibition of cardiomyocytes
hypertrophy by resveratrol is Associated with Amelioration of
endoplasmic reticulum stress. Cell Physiol Biochem. 39:780–789.
2016. View Article : Google Scholar : PubMed/NCBI
|