Introduction
Due to an aging population, stroke has become the
second leading cause of mortality and adult disability in China.
Stroke-associated neurological deficits exert a negative effect on
the language, cognition and motor function of patients. In
addition, stroke places a heavy emotional burden on caregivers and
a financial burden on health systems. The only Food and Drug
Administration-approved therapeutic agent for the treatment of
ischemic stroke, recombinant tissue plasminogen activator, is
suitable for use only within a narrow time window; as a result only
2% of stroke patients benefit from this therapy. Therefore, the
development of novel agents for the treatment of stroke is
required.
Opioids have been demonstrated to alleviate
ischemia/reperfusion (I/R) injury in a number of organs when
administered prior to ischemia or at the time of reperfusion
(1–3). In addition, certain studies have
demonstrated that opioids may be involved in protecting neurons
from hypoxic and ischemic stress in vivo and in vitro
(4–6). However, the risk of adverse effects,
including respiratory depression, constipation, and physical and
psychological dependence, has made physicians wary of prescribing
these drugs.
Salvinorin A (SA) is a natural hallucinogen from
which herkinorin, the first µ opioid selective agonist, is derived.
Herkinorin does not promote the recruitment of β-arrestin-2 to the
µ-opioid peptide (MOP) receptor, a quality exhibited by the
majority of other opioids, and does not lead to receptor
internalization (7). As certain
studies in mice have revealed that the interaction between
β-arrestin-2 and the opioid receptor is important in the
development of opioid-induced tolerance, constipation and
respiratory depression, herkinorin may provide therapeutic benefits
with limited adverse effects (8,9). It
has been demonstrated that opioid agonists have a protective effect
against ischemic injury (1–3). A
study conducted by Chen et al (10) revealed that administration of SA
improved neurological function and reduced the mortality rate
following hypoxic injury. The present study hypothesized that
herkinorin may have similar positive effects on I/R injury.
Transient middle cerebral artery occlusion (MCAO) in
mice was used to mimic human stroke. Neurobehavioral scores and
sensorimotor functions were examined 24 h and 7 days following
induction of MCAO. Infarct volumes were examined at these time
points using the 2,3,5-triphenyltetrazolium chloride (TTC)
assay.
Conventional protein kinase C γ (cPKCγ) is a member
of the classical PKC subfamily, and its localization is restricted
to brain and spinal cord neurons. It is an important component of
the signal transduction pathways involved in neuroprotection.
Previous studies have demonstrated that ischemic injury protection
is associated with an increase in the membrane translocation of
cPKCγ, from the cytosol to the particulate fraction (11,12).
Therefore, cell fraction and western blot analysis were used to
investigate whether the subcellular localization of cPKCγ is
involved in the protective effects of herkinorin pretreatment
against cerebral I/R injury.
Materials and methods
Animals
A total of 60 adult male C57BL/6 mice (age, 12–14
weeks; weight, 18–22 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice
were housed under conventional conditions (temperature, 21±1°C,
relative humidity 60±10%,) under a 12 h light/dark cycle and were
allowed free access to food and water. Animals were randomly
divided into five groups: i) Naïve, ii) sham, iii) I/R, iv) I/R
with dimethyl sulfoxide (DMSO; I/R+D) and v) I/R with herkinorin
(I/R+H). The experimental procedures were carried out in accordance
with the Animal Protection Law of the People's Republic of China
and were approved by the animal ethics committee of Beijing Tongren
Hospital affiliated to the Capital Medical University.
Animal model
The MCAO model of permanent focal cerebral ischemia
was conducted as previously reported (13). Following anesthesia with an
intraperitoneal injection of 0.06 g/kg pentobarbital sodium
(Ovation Pharmaceuticals, Inc., Deerfield, IL, USA), the left
common carotid artery and ipsilateral external carotid artery were
exposed and ligated via a ventral midline neck incision. An
arteriotomy in the common carotid artery was made, allowing the
introduction of a blunt-tipped 4–0 surgical monofilament (0.23-mm
diameter; Guangzhou Jialing Biotechnology Co., Ltd., Guangzhou,
China) to a point ~12 mm distal to the carotid bifurcation until a
mild resistance was felt, thereby occluding the origin of middle
cerebral artery. The nylon thread was removed to allow reperfusion
1 h following occlusion. In the sham group, the mice underwent
surgical exposure of carotid artery and ligation without insertion
of an intraluminal filament. Body temperature was maintained
throughout the surgery with a heating lamp and thermal blanket.
Mice were placed in a post-operative cage, and kept warm and
undisturbed for a minimum of 2 h for observation.
Drug administration
Mice in the I/R+H group were treated with 10 mg/kg
herkinorin (Abcam, Cambridge, UK) dissolved in 10% DMSO via
intraperitoneal injection 3 h prior to cerebral ischemia induction.
In the I/R+D group, an equal volume of DMSO was administered.
Neurobehavioral analyses
To investigate whether herkinorin promotes
neurologic recovery following I/R, an array of neurobehavioral
analyses were performed at 24 h and 7 days following reperfusion by
an investigator who was blinded to the experimental groups.
Neurological scoring
Neurological scoring was based on that reported by
Rodriguez et al (14) and
was as follows: 0, no neurological dysfunction; 2, slight decrease
in mobility and the presence of passivity; 4, moderate neurological
dysfunction and additional alterations, including moderate
hypomobility, flattened posture, lateralized posture, hunched back,
ataxic gait, decreased body tone and muscular strength, and slight
motor incoordination; 6, disabled but able to walk, with more
marked hypomobility, circling, tremor, jerks and/or convulsions,
forelimb flexion, and moderate motor incoordination; 8, respiratory
distress, total incapacity to move/coordinate; 10, death. If the
criteria for a precise grade given in the scoring list were not
met, the nearest appropriate number was utilized: 1, 3, 5, 7 or.
9.
Pole test
The pole test was performed as described by Matsuura
et al (15), with minor
modifications. The test apparatus consisted of a vertical steel
pole covered with tape, which acted to create a rough surface. The
mice were placed head upward on the top of the pole. The ‘time
turn’ was recorded, which was defined as the time taken by the
mouse to turn completely head downward. In addition, the ‘time
total’ was recorded, defined as the time it took the mouse to
descend down the pole and reach the floor with its front paws. If
the animal was unable to turn completely, the time taken to reach
the floor was also attributed to the time total.
Wire hang test
This test was used to measure neuromuscular strength
as previously described (16).
Mice were placed gently on a wire mesh and the hanging time until
the mice fell onto the soft bedding below was recorded, with a
cut-off time of 300 sec.
Cylinder test
Mice were placed in a clear plexiglass cylinder
(30×20 cm) as previously described (17). Left or right paw vertical
exploration movement was scored for each contact with the glass
wall. Simultaneous contact by the two paws was scored separately. A
total of 20 contacts were recorded for each mouse. The laterality
index was calculated as follows: [Contacts (left)-contacts
(right)]/[contacts (left)+contacts (right)+contacts (both)].
Foot-fault test
This test aimed to measure the accuracy of forepaw
placement on non-equidistant grids, as previously described
(18). The total number of steps
that the mouse used to cross the grid was counted, and the total
number of foot-faults for the left forelimb was recorded. The
results were presented as the percentage of the foot-fault
measurement of the left paw, of the total number of steps.
Measurement of infarct size
Mice were sacrificed at 24 h or 7 days following
reperfusion, and the brain was rapidly removed and cut into 1.5-mm
thick coronal sections (five sections from each brain). The slices
were incubated in a solution of 0.5% TTC (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) dissolved in 10 mM
phosphate-buffered saline at 37°C for 20 min, and subsequently
imaged. The images of stained slices were scanned into a computer
and the infarct volume was determined using Image-Pro®
Plus software version 6.0 (Media Cybernetics Inc., Rockville, MD,
USA), as reported by Wexler et al (19).
Sample preparation and western blot
analysis
Following completion of behavioral testing at 24 h
after reperfusion, animals were deeply anesthetized and
decapitated. Brains were removed and placed into ice-cold (4°C)
artificial cerebral spinal fluid (ACSF: 125.0 mM NaCl, 2.5 mM KCl,
2.0 mM CaCl2, 26.0 mM NaHCO3, 1.25 mM
NaH2PO4, 1.0 mM MgCl2, 10.0 mM
glucose, pH 7.4). Regions from left hemispheres that corresponded
to the ischemic core, peri-infarct region and contralateral
non-ischemic region were dissected, in accordance with a previous
study (20). Briefly, the
left-brain was sectioned (four slices; 2-mm thick) and the tissue
supplied by the anterior cerebral artery was removed through a
longitudinal cut (from top to bottom) ~2 mm from the midline. The
ischemic core and peri-infarct region were separated by a
transverse diagonal cut at approximately the ‘2 o'clock’ position.
A contralateral control consisted of the corresponding regions from
the non-ischemic hemisphere. Samples were frozen in liquid nitrogen
and kept at −80°C until analysis.
Cytosolic and particulate proteins were extracted as
described in a previous study (21). In brief, frozen slices were thawed
and homogenized at −4°C in buffer A [50 mM Tris-Cl, pH 7.5,
containing 2 mM EDTA, 1 mM ethylene glycol-bis (β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid, 100 mM iodoacetamide (SH-group
blocker), 5 g/ml each of leupeptin, aprotinin, pepstatin A and
chymostatin, 50 mM potassium fluoride, 50 nM okadaic acid and 5 mM
sodium pyrophosphate]. Centrifugation of the homogenates was
performed at 30,000 × g for 30 min at 4°C to yield cytosolic
fractions. The pellets were then subsequently resuspended in buffer
B (buffer A containing 0.5% Nonidet P-40) prior to sonication and
centrifugation (5,000 × g, 30 min, 4°C). The resulting
supernatants were particulate fractions. Protein concentrations
were determined using a Bicinchoninic Acid kit (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA); albumin diluted in
buffer A or B served as a standard.
A total of 40 µg protein from each sample was loaded
onto 10% SDS-PAGE gels. Proteins were electrophoresed and
transferred onto polyvinylidene difluoride membranes (GE Healthcare
Life Sciences, Chalfont, UK) at 4°C. Following rinsing with TBS
containing Tween-20 (20 mM Tris-Cl, pH 7.5, 0.15 M NaCl and 0.05%
Tween-20), membranes were blocked with 10% non-fat milk for 1 h at
room temperature. Membranes were subsequently incubated with the
primary antibodies rabbit anti-cPKCγ (1:1,000; catalogue number
SC-98952; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and
mouse anti-β-actin (1:1,000; catalogue number A5441 Sigma-Aldrich;
Merck Millipore) for 3 h at room temperature. Membranes were then
incubated with the horseradish peroxidase-conjugated goat
anti-rabbit or anti-mouse immunoglobulin G secondary antibodies
(1:5,000; catalogue numbers ADI-SAB-300-J and ADI-sab-100-J,
respectively; Stressgen Biotechnologies Corporation Victoria, BC,
Canada) for 1 h at room temperature. Following incubation with
secondary antibodies, an Enhanced Chemiluminescence kit (GE
Healthcare Life Sciences) was used to detect protein bands, which
were quantified with the TINA 2.0 densitometry software (Raytest
GmbH, Straubenhardt, Germany) and normalized to β-actin.
Statistical analysis
Data was analyzed using SPSS software version 13.0
(SPSS, Inc., Chicago, IL, USA). All data are presented as the mean
± standard error. Statistical analysis was performed by one-way
analysis of variance followed by all pairwise multiple comparison
procedures using the Bonferroni test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Herkinorin treatment improves
neurobehavioral and sensorimotor functional recovery from
I/R-induced brain injury
Mice in the sham and naïve groups had a neurological
score of 0 at 24 h and 7 days. Following MCAO, there was a
significant increase in the neurological function scores in the I/R
and I/R+D groups (P<0.001 I/R and I/R+D group vs. naïve and sham
groups at 24 h and 7 days) However, the herkinorin treatment group
demonstrated a significant improvement in neurological deficit at
the two time points (Fig. 1A,
P=0.006 and 0.008, I/R and I/R+D group vs. I/R+H group at 24 h;
P=0.006 and 0.009, I/R and I/R+D group vs. I/R+H group at 7
days).
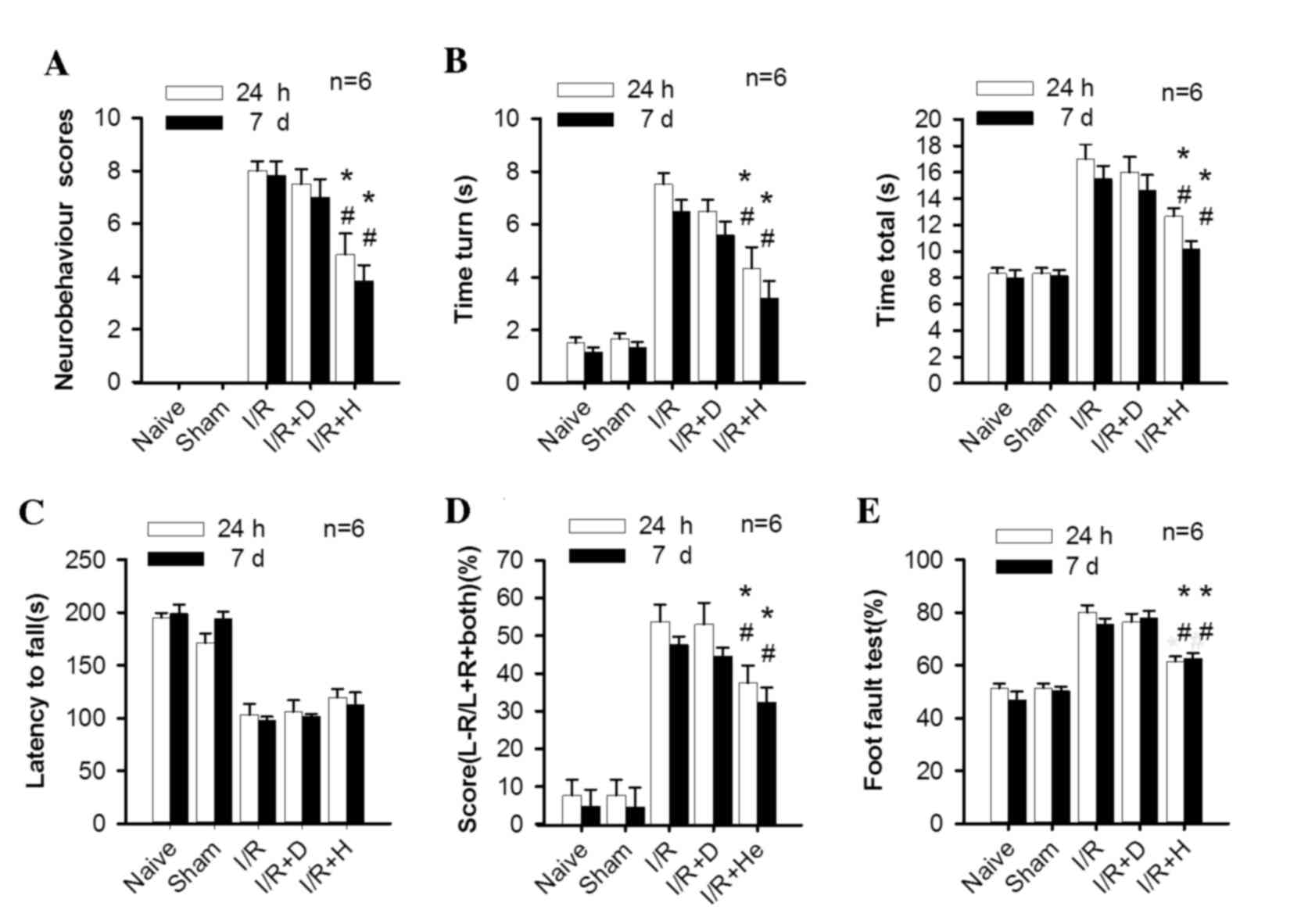 | Figure 1.Herkinorin promotes neurobehavioral
and sensorimotor functional recovery from I/R induced brain injury.
The following assessments were measured at 24 h and 7 days
following reperfusion: (A) Neurological score, (B) time turn and
time total, (C) latency to fall, (D) score (L-R/L+R+both) and (E)
foot-fault test. Following I/R, mice performed worse in all these
tests compared with the naïve and sham groups; herkinorin treatment
significantly attenuated this effect, except in the wire hang test,
where there was no significant improvement. Data are expressed as
the mean ± standard error (n=6) *P<0.05 vs. I/R group;
#P<0.05 vs. I/R+D group. I/R, ischemic/reperfusion;
I/R+D, I/R with dimethyl sulfoxide; I/R+H, I/R with herkinorin;
MCAO, middle cerebral artery occlusion. |
In the pole test, mice from the I/R and I/R+D groups
performed worse compared with those in the sham and naïve groups at
24 h and 7 days. There was a significant decrease of ‘time turn’
and ‘time total’ in the I/R+H group compared with the I/R and I/R+D
groups at the two time points (Fig.
1B, Time turn: P=0.006 and 0.008, I/R and I/R+D group vs. I/R+H
group at 24 h; P=0.007 and 0.008, I/R and I/R+D group vs. I/R+H
group at 7 days. Time total: P=0.005 and 0.007, I/R and I/R+D group
vs. I/R+H group at 24 h; P=0.005 and 0.008, I/R and I/R+D group vs.
I/R+H group at 7 days.).
The wire hanging test revealed a significant
decrease of latency to fall in the I/R and I/R+D groups. However,
there was no statistical difference between the herkinorin-treated
and non-treated groups (Fig.
1C).
In the cylinder test, the mice in the I/R and I/R+D
groups demonstrated significant asymmetrical forelimb use for wall
exploration. By contrast, sham and naïve animals used left and
right paws equally. The forelimb impairment of the contralateral
paw to the lesion site was significantly decreased in animals of
I/R+H group compared with those of the I/R and I/R+D groups at 24 h
and 7 days (Fig. 1D, P=0.004 and
0.006, I/R and I/R+D group vs. I/R+H group at 24 h; P=0.005 and
0.008, I/R and I/R+D group vs. I/R+H group at 7 days).
In the foot fault test, the I/R and I/R+D groups
demonstrated a significant asymmetric impairment in muscle strength
and motor coordination in the limbs of the left side. The
herkinorin treatment group revealed a significant improvement of
motor deficit at the two time points (Fig. 1E, P=0.005 and 0.008, I/R and I/R+D
group vs. I/R+H group at 24 h; P=0.006 and 0.009, I/R and I/R+D
group vs. I/R+H group at 7 days).
Herkinorin reduces infarction
volume
The images of cerebral infarction in the various
groups are presented in Fig. 2,
with the red and white areas indicating non-infarcted and infarcted
brain tissue, respectively. Brain infarct size significantly
increased in the I/R and I/R+D groups at 24 h (Fig. 2A and B) and 7 days (Fig. 2C and D) after reperfusion, compared
with the sham and naïve groups (P<0.001 I/R and I/R+D group vs.
naïve and sham groups at 24 h and 7 days). Herkinorin treatment
reduced I/R-induced cerebral infarction (P=0.006 and 0.009, I/R and
I/R+D group vs. I/R+H group at 24 h; P=0.010 and 0.011, I/R and
I/R+D group vs. I/R+H group at 7 days).
 | Figure 2.Effect of herkinorin on infarct volume
of mice following I/R. (A) Images of TTC-stained brain coronal
sections at 24 h after reperfusion from naïve, sham, I/R, I/R+D and
I/R+H groups, and (B) quantitative analysis of infarct volume in
all groups at this time point. (C) Images and (D) quantification of
brain infarction 7 days after reperfusion. Red and white indicates
non-infarcted and infarcted brain tissue, respectively. Brain
infarct size significantly increased in the I/R and I/R+D groups at
24 h and 7 days after reperfusion, compared with the sham and naïve
groups. Herkinorin treatment reduced I/R-induced infarction. Data
are expressed as the mean ± standard error (n=6) *P<0.05 vs. I/R
group, #P<0.05 vs. I/R+D group. I/R,
ischemic/reperfusion; I/R+D, I/R with dimethyl sulfoxide; I/R+H,
I/R with herkinorin; MCAO, middle cerebral artery occlusion; TTC,
2,3,5-triphenyltetrazolium chloride. |
Herkinorin stabilizes cPKCγ membrane
translocation level in the peri-infarct region of I/R mice
Western blotting was conducted to assess the role of
cPKCγ activation in herkinorin-induced neuroprotection (Fig. 3A). cPKCγ membrane translocation in
the ischemic core and peri-infarct region of the ischemic cortex
demonstrated a significant decrease at 24 h following I/R
(P<0.001 Ic and P of I/R and I/R+D group vs. naïve and sham
groups), and the decrease of cPKCγ membrane translocation in the
peri-infarct region was attenuated by herkinorin pretreatment
(Fig. 3B, P=0.006 and 0.007, P of
I/R and I/R+D group vs. I/R+H group). The results suggested that
herkinorin may protect the mouse brain against ischemic injury via
a stabilization of the cPKCγ membrane translocation level in the
peri-infarct region of I/R mice.
 | Figure 3.Herkinorin alleviates I/R-induced
inhibition of cPKCγ membrane translocation in the ischemic cortex
of mice. (A) Representative results of western blotting
demonstrated the changes in cPKCγ membrane translocation in cortex
from whole brain of naïve and sham groups, and the Ic, P and C of
I/R, I/R+D and I/R+H groups. (B) Quantitative analysis revealed
that cPKCγ membrane translocation levels decreased significantly in
the Ic and P regions of ischemic cortex; however, herkinorin
pretreatment attenuated the I/R-induced inhibition of cPKCγ
membrane translocation in the P region of MCAO mice. White and
horizontally striped bars represent whole brain samples. Data are
expressed as the mean ± standard error (n=6). *P<0.05 vs. sham.
#P<0.05 vs. P of I/R group. I/R,
ischemic/reperfusion; I/R+D, I/R with dimethyl sulfoxide; I/R+H,
I/R with herkinorin; MCAO, middle cerebral artery occlusion; PKC,
protein kinase C; cPKCγ, conventional protein kinase C γ; Ic,
ischemic core; P, peri-infarct region; C, contralateral cortex. |
Discussion
In addition to pain modulation, opioids are involved
in various central processes including motivation, stress, anxiety,
learning and feeding. Opioids mediate neuroprotection via a variety
of mechanisms. Previous studies have reported the neuroprotective
effects of opioids using in vivo mouse and rat models, brain
slices and cultured neurons (5,6).
However, side effects of opioids, including constipation and
respiratory depression limit their use in the clinical setting.
Herkinorin, as a first selective atypical opioid µ agonist derived
from the naturally occurring plant product SA, partially addresses
these problems. An important advantage of this compound is that it
does not promote the recruitment of β-arrestin-2 to the MOP
receptor and does not lead to receptor internalization. As
β-arrestin-2 is important for the development of morphine-induced
tolerance, constipation and respiratory depression, herkinorin may
be a promising therapeutic agent for the treatment of stroke with
fewer adverse effects. Certain studies have investigated the
antinociceptive properties and cerebral vasodilative effects of
herkinorin; however, efforts have seldom been made to evaluate the
neuroprotective action of this compound (22,23).
The present study demonstrated that administration of herkinorin
into I/R mice significantly reduced infarct volume and markedly
improved the recovery of neurological function.
A number of underlying mechanisms have been proposed
to explain how opioid receptor agonists achieve their
neuroprotective effects, the most prominent of which is the
activation and translocation of PKC. PKC is regarded as an
important intermediate in signal transduction and is involved in
the regulation of numerous cellular functions (24). The PKC signaling pathway has been
implicated as a key mediator in the protection against I/R injury.
Furthermore, activation of PKC was proposed to be an important
neuroprotective event by various studies. Wang et al
(25) suggested that the
activation of endogenous PKC is important in the neuroprotective
mechanism underlying electroacupuncture pretreatment following
focal cerebral ischemia. Our previous study revealed that
activation of cPKCγ and novel PKCε may be involved in the
development of cerebral hypoxic preconditioning of mice (21). The majority of studies proposed
pathways of ischemic protection suggesting that endogenous or
exogenous stimuli activated phospholipase C via G-protein, and
diacylglycerol released from phospholipid moieties activated PKC by
translocating it from the cytosol to the membrane.
cPKCγ belongs to the classical subgroup of the PKC
family, and is present only in neurons of the brain and spinal cord
(26). Therefore, cPKCγ as a
central nervous system-specific compound has attracted the
attention of biochemists and neuroscientists investigating
modulation of ischemic injury. Zhang et al (12) suggested that following hypoxic
preconditioning, there is an increased translocation of cPKCγ in
the surviving penumbra of MCAO mice. Hayashi et al (27) suggested that estrogen may induce
translocation of cPKCγ, and exogenous estrogen-induced
neuroprotection was attenuated in cPKCγ-knockout mice. These
studies aimed to explain the role of cPKCγ in neuroprotection via
its translocation from the cytosol to the membrane fraction upon
activation. Certain studies have indicated that translocation of
cPKCγ may be initiated by different types of opioid agonist. Ping
et al (28) revealed a
significant translocation of cPKCγ from the cytosol to the membrane
component of morphine-conditioned rats in a dose-dependent manner.
In addition, repeated intrathecal injection of a selective
µ-receptor agonist, [D-Ala2, N-Me-Phe4,
Gly5-ol]-enkephalin, in the spinal cord of mice results
in a translocation of cPKCγ from the cytosol to the membrane
(29). The present study revealed
that in the group pretreated with herkinorin, the decrease of cPKCγ
membrane translocation in the peri-infarct region induced by I/R
injury was attenuated.
There are numerous molecules and signaling pathways
involved in the neuroprotective effects of herkinorin, and membrane
translocation of cPKCγ only provides a partial explanation of this
phenomenon. The investigation of only the membrane translocation of
cPKCγ is a limitation of the present study. The results of the
present study suggested that herkinorin exerts neuroprotective
effects; a comparison between this atypical opioid agonist and
clinically used morphine-like compounds is required and will be
performed in future studies.
In conclusion, the results of the present study
demonstrated that herkinorin may protect against I/R-induced brain
injury in mice and this may potentially be attributed to the
membrane translocation of cPKCγ upon activation. These results
provide a novel view of the effects and underlying mechanisms of
atypical opioids in neuroprotection, and may indicate a potential
novel therapeutic agent for the treatment of stroke.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301065 to X.C.)
and the Talent Training Plan of Beijing (grant no.
D003034000031).
References
|
1
|
Schultz JE, Hsu AK and Gross GJ: Morphine
mimics the cardioprotective effect of ischemic preconditioning via
a glibenclamide-sensitive mechanism in the rat heart. Circ Res.
78:1100–1104. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato R and Foëx P: Fentanyl reduces
infarction but not stunning via delta-opioid receptors and protein
kinase C in rats. Br J Anaesth. 84:608–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saccani F, Anselmi L, Jaramillo I, Bertoni
S, Barocelli E and Sternini C: Protective role of u opioid receptor
activation in intestinal inflammation induced by mesenteric
ischemia/reperfusion in mice. J Neurosci Res. 90:2146–2153. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SW, Yi JW, Kim YM, Kang JM, Kim DO,
Shin MS, Kim CJ, Lee DI, Kim DH and Lee BJ: Remifentanil alleviates
transient cerebral ischemia-induced memory impairment through
suppression of apoptotic neuronal cell death in gerbils. Korean J
Anesthesiol. 61:63–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fanjun M, Junfa L, Bingxi Z and Fang J:
nPKCepsilon and NMDA receptors participate in neuroprotection
induced by morphine pretreatment. J Neurosurg Anesthesiol.
18:119–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Li J, Yang J, Ji F, Bu X, Zhang N
and Zhang B: Inhibition of PKCgamma membrane translocation mediated
morphine preconditioning-induced neuroprotection against
oxygen-glucose deprivation in the hippocampus slices of mice.
Neurosci Lett. 444:87–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groer CE, Tidgewell K, Moyer RA, Harding
WW, Rothman RB, Prisinzano TE and Bohn LM: An opioid agonist that
does not induce mu-opioid receptor-arrestin interactions or
receptor internalization. Mol Pharmacol. 71:549–557.
2007.PubMed/NCBI
|
|
8
|
Raehal KM, Walker JK and Bohn LM: Morphine
side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp
Ther. 314:1195–1201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang M, Maguma HT, Smith TH, Ross GR,
Dewey WL and Akbarali HI: The role of β-arrestin2 in the mechanism
of morphine tolerance in the mouse and guinea pig gastrointestinal
tract. J Pharmacol Exp Ther. 340:567–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen C, Cui X, Matsunaga F, Ma J, Ma N,
Abel T and Liu R: Salvinorin A decreases mortality and improves
neurological outcome in a neonatal mouse hypoxia model. Transl
Perioper Pain Med. 1:9–13. 2014.
|
|
11
|
Ding J, Ding N, Wang N, Lu Q, Lu N, Yang
D, Bu X, Han S and Li J: Determination of conventional protein
kinase C isoforms involved in high intraocular pressure-induced
retinal ischemic preconditioning of rats. Vision Res. 49:315–321.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang N, Yin Y, Han S, Jiang J, Yang W, Bu
X and Li J: Hypoxic preconditioning induced neuroprotection against
cerebral ischemic injuries and its cPKCγ-mediated molecular
mechanism. Neurochem Int. 58:684–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bu X, Zhang N, Yang X, Liu Y, Du J, Liang
J, Xu Q and Li J: Proteomic analysis of cPKCβII-interacting
proteins involved in HPC-induced neuroprotection against cerebral
ischemia of mice. J Neurochem. 117:346–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodriguez R, Santiago-Mejia J, Gomez C and
San-Juan ER: A simplified procedure for the quantitative
measurement of neurological deficits after forebrain ischemia in
mice. J Neurosci Methods. 147:22–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuura K, Kabuto H, Makino H and Ogawa
N: Pole test is a useful method for evaluating the mouse movement
disorder caused by striatal dopamine depletion. J Neurosci Methods.
73:45–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karl T, Pabst R and von Hörsten S:
Behavioral phenotyping of mice in pharmacological and toxicological
research. Exp Toxicol Pathol. 55:69–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Blizzard KK, Zeng Z, DeVries AC,
Hurn PD and McCullough LD: Chronic behavioral testing after focal
ischemia in the mouse: Functional recovery and the effects of
gender. Exp Neurol. 187:94–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Zhang C, Jiang H, Li Y, Zhang L,
Robin A, Katakowski M, Lu M and Chopp M: Atorvastatin induction of
VEGF and BDNF promotes brain plasticity after stroke in mice. J
Cereb Blood Flow Metab. 25:281–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wexler EJ, Peters EE, Gonzales A, Gonzales
ML, Slee AM and Kerr JS: An objective procedure for ischemic area
evaluation of the stroke intraluminal thread model in the mouse and
rat. J Neurosci Methods. 113:51–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashwal S, Tone B, Tian HR, Cole DJ and
Pearce WJ: Core and penumbral nitric oxide synthase activity during
cerebral ischemia and reperfusion. Stroke. 29:1037–1047. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Niu C, Han S, Zu P, Li H, Xu Q and
Fang L: Identification of protein kinase C isoforms involved in
cerebral hypoxic preconditioning of mice. Brain Res. 1060:62–72.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamb K, Tidgewell K, Simpson DS, Bohn LM
and Prisinzano TE: Antinociceptive effects of herkinorin, a MOP
receptor agonist derived from salvinorin A in the formalin test in
rats: New concepts in mu opioid receptor pharmacology: From a
symposium on new concepts in mu-opioid pharmacology. Drug Alcohol
Depend. 121:181–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji F, Wang Z, Ma N, Riley J, Armstead WM
and Liu R: Herkinorin dilates cerebral vessels via kappa opioid
receptor and cyclic adenosine monophosphate (cAMP) in a piglet
model. Brain Res. 1490:95–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishizuka Y: Protein kinase C and lipid
signaling for sustained cellular responses. The FASEB Journal.
9:484–496. 1995.PubMed/NCBI
|
|
25
|
Wang ZK, Zhang LC, Zhao SH, Zhang JW,
Shang LH, Zhang YL, Lin CR and Guo JK: Effect of electroacupuncture
on cerebral PKC isozyme expression levels in cerebral
ischemia-reperfusion rats. Zhen Ci Yan Jiu. 37:312–317. 2012.(In
Chinese). PubMed/NCBI
|
|
26
|
Saito N and Shirai Y: Protein kinase C
gamma (PKC gamma): Function of neuron specific isotype. J Biochem.
132:683–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi S, Ueyama T, Kajimoto T, Yagi K,
Kohmura E and Saito N: Involvement of gamma protein kinase C in
estrogen-induced neuroprotection against focal brain ischemia
through G protein-coupled estrogen receptor. J Neurochem.
93:883–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ping X, Ma Y, Li Y, Qi C, Sun X, Lv X and
Cui C: Essential role of protein kinase C in morphine-induced
rewarding memory. Neuropharmacology. 62:959–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narita M, Mizoguchi H, Narita M, Nagase H,
Suzuki T and Tseng LF: Involvement of spinal protein kinase Cgamma
in the attenuation of opioid mu-receptor-mediated G-protein
activation after chronic intrathecal administration of [D-Ala2,
N-MePhe4, Gly-Ol(5)]enkephalin. J Neurosci. 21:3715–3720.
2001.PubMed/NCBI
|

















