Introduction
Breast cancer remains a major health problem for
women worldwide; accounting for one in three newly diagnosed cases
of cancer in women. Targeting the estrogen receptor (ER) has
achieved marked progress in the treatment of patients with
ER-positive breast cancer. Although the ER is critical for breast
cancer carcinogenesis, the most commonly expressed hormone receptor
in invasive breast cancer is the androgen receptor (AR), which is
expressed in 72.9% of primary breast cancer cases (1). Furthermore, the AR has been
characterized in terms of its role as a predictive factor, and the
clinical significance of its expression in breast cancer. The AR is
inhibitory in breast cancer, counteracting the oncogenic effect of
the ER. Due to this crosstalk, AR expression is associated with
improved prognosis in ER-positive breast cancer (2,3).
However, the prognostic value of AR expression in triple negative
breast cancer (TNBC) remains unclear, with certain studies
suggesting reduced mortality (4),
and others indicating poor prognosis (5). A recent systematic review of 19
separate studies investigated the association between AR
expression, overall survival and disease free survival, and
revealed that the expression of AR is a marker of good prognosis,
with an approximate doubling of overall survival at 3 or 5 years
(6). Therefore, further
examination of the role of the AR in breast cancer is essential,
and may contribute to the development of novel therapies for
AR-positive tumors, particularly ER−, progesterone
receptor (PR)−, AR+ breast cancer patients
who derive limited benefit from current chemotherapeutic
strategies.
MicroRNAs (miRNAs) are small non-coding RNAs, ~22
nucleotides in length, which regulate the expression of multiple
genes by degrading messenger RNA (mRNA) or interrupting the
translation process (7). Aberrant
expression of miRNAs contributes to cancer pathogenesis by inducing
oncogenes, inhibiting tumor suppressor genes or disrupting
important signaling pathways. Dysregulation of miRNAs occurs in
breast cancer pathogenesis. Sets of genes have been identified to
be dysregulated in breast cancer using miRNA expression profiling
studies (8,9). Furthermore, functional studies have
confirmed miRNAs as tumor suppressors and oncogenes in breast
cancer (10).
miRNAs impact the pathobiology of hormone-regulated
cancers. The potential association between AR expression and miRNA
signature in prostate cancer has been examined. A miRNA profiling
study of 6 prostate cancer (PCa) cell lines, 9 prostate cancer
xenograft models and 9 prostate carcinoma samples identified a
significant link between miRNAs and AR expression (11), and revealed a potential correlation
for further investigation. Similarly, in 2007, Shi et al
(12) observed differential
expression of miR-125b in androgen-dependent and -independent PCa
cells, as well as in benign and malignant prostate tissues. This
study suggested that androgen-AR signaling may regulate the
differential expression of miR-125b. Furthermore, a study in 2011
described 71 miRNAs that influenced the expression levels of AR in
human PCa cells, and 13 miRNAs were validated to regulate the long
AR 3′untranslated region (UTR) (13). Taken together, these findings
indicate a potential link between AR expression and miRNAs. In
breast cancer, miRNAs have been studied primarily with regard to ER
and human epidermal growth receptor 2. AR-associated miRNAs in
breast cancer have been less well investigated.
To reveal the association between miRNAs and AR in
breast cancer, miRNA expression profiling was performed in breast
cancer cell lines representative of various AR expressions. Further
target prediction was conducted on the significantly dysregulated
miRNAs. Target genes were classified into different pathways
according to their biological functions, as determined by the Gene
Ontology (GO) system. Vascular endothelial growth factor (VEGF) and
mammalian target of rapamycin (mTOR) signaling pathways were
demonstrated to correlate with the significantly dysregulated
miRNAs. The results of the present study revealed a correlation
between differential miRNA expression and AR expression levels in
breast cancer, and described a putative interaction between the AR,
VEGF and mTOR signaling pathways. These results may improve
understanding of the role of the AR in breast cancer.
Materials and methods
Cell culture
The Hs578T, MDA-MB-231, MCF-7 and SK-BR-3 human
breast cancer cell lines were purchased from the American Type
Culture Collection (Manassas, VA, USA). Hs578T and MCF-7 cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin/streptomycin and 4 mg/ml insulin, whereas MDA-MB-231 and
SK-BR-3 cells were cultured in RPMI 1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS and 1%
penicillin/streptomycin. All cells were cultured at 37°C, in an
atmosphere of 5% CO2.
RNA preparation
Samples were harvested from Hs578T, MDA-MB-231,
MCF-7 and SK-BR-3 human breast cancer cells. Total RNA was prepared
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.), and the quality and quantity of the RNA were assessed using
a NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.). Optical
density (OD) 260/280 ≥1.6 and OD 260/230 ≥1 indicated acceptable
RNA purity, whereas acceptable RNA integrity (RNA integrity number
≥5) was determined using an Agilent RNA 6000 Nano assay (Agilent
Technologies, Inc., Santa Clara, CA, USA). Gel electrophoresis was
used to evaluate genomic DNA contamination.
miRNAs and gene expression
analysis
RNA samples were subjected to Human
OneArray® version 6 (Phalanx Biotech Group, Hsinchu,
Taiwan). Data were analyzed with Rosetta Resolver System software
(Rosetta Biosoftware, Seattle, WA, USA). Standard selection
criteria were used to identify differentially expressed genes: i)
Absolute log2 fold change ≥0.585; absolute fold change
≥1.5 and ii) false discovery rate <0.05, which were subsequently
categorized into up and downregulated genes for AR-positive vs.
-negative breast cancer cells.
miRNA target prediction and miRNA-gene
interaction analysis
The miRNAs whose expression was significantly
dysregulated between AR-positive and -negative breast cancer cells
were selected for target prediction. Predicted target genes were
identified by at least three of the seven well-established
databases: DIANA
(diana.imis.athena-innovation.gr/DianaTools/index.php), miRanda
(www.microrna.org/microrna/home.do), miBridge
(sitemaker.umich.edu/mibridge/home), PicTar
(pictar.mdc-berlin.de/), PITA (genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNA22 (cm.jefferson.edu/rna22/) and
TargetScan (www.targetscan.org/vert_71/). Pathway enrichment
analysis was performed using the GO system to gain insight into the
molecular networks and canonical pathways associated with
differentially expressed miRNA. Of the enriched pathways with a
P-value of <0.05, the associations between the differentially
expressed miRNAs and their candidate target genes were visualized
with an interaction network, for the VEGF and mTOR signaling
pathways.
Results
miRNAs are differentially expressed in
AR-positive and -negative breast cancer cell lines
Previous reports have indicated that AR protein
expression levels may be affected by a set of miRNAs in PCa cells
(13); another 25 miRNAs have been
reported to be differentially expressed in PCa compared with
matched benign tissues (14). This
evidence suggests that there is an association between miRNA
expression and the AR signaling pathway. However, the mechanism
underlying AR-mediated regulation of miRNAs in PCa and TNBC remains
unclear. To further identify differentially expressed miRNAs in
breast cancer cell lines with varying AR expression, miRNA
expression profiling was performed in breast cancer cell lines
representative of AR-negative (Hs578T:
ER−/PR−/HER2−/AR−) and
AR-positive (MDA-MB-231:
ER−/PR−/HER2−/AR+;
MCF-7:
ER+/PR+/HER2−/AR+;
SK-BR-3:
ER−/PR−/HER2+/AR+)
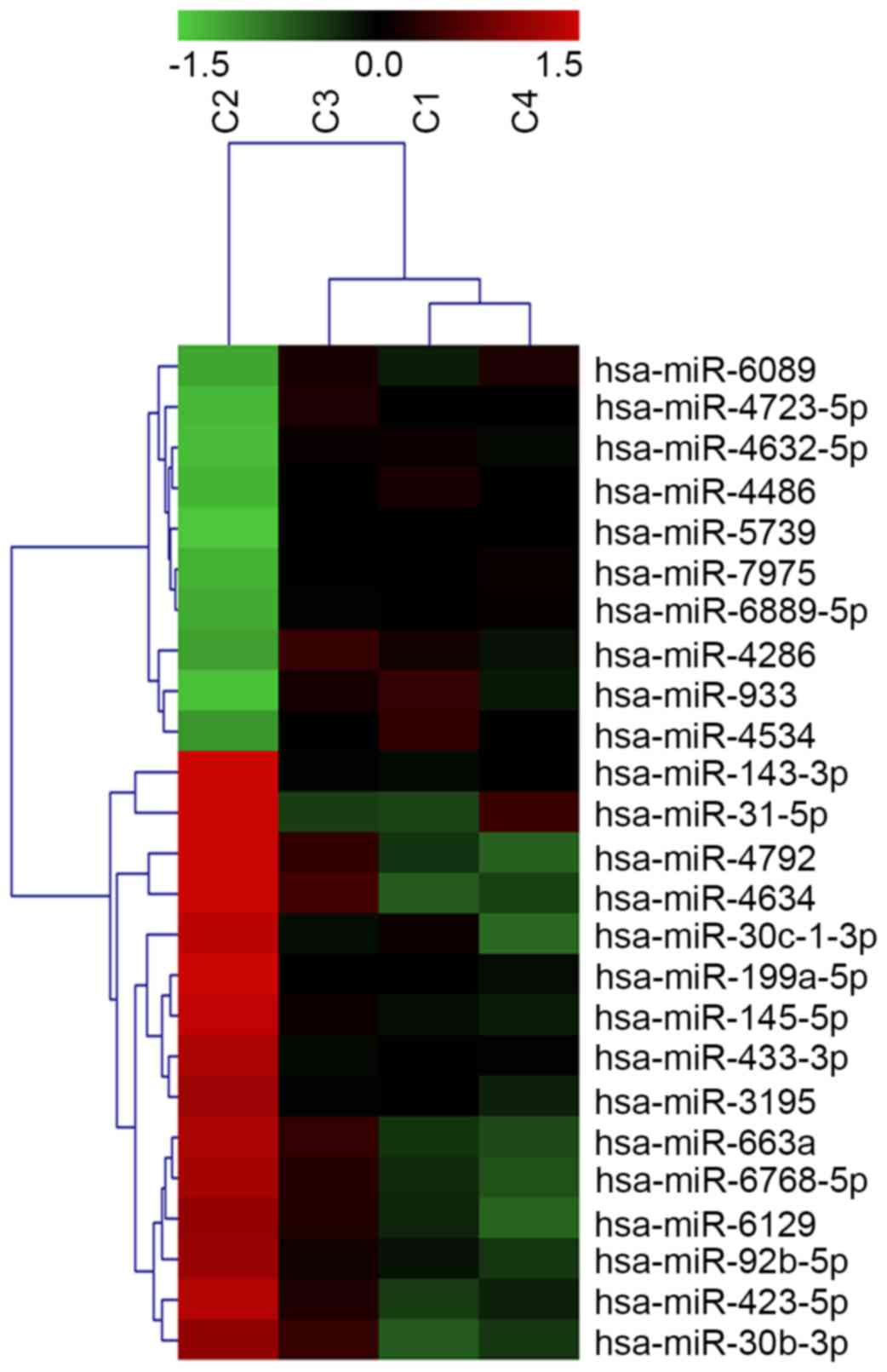
molecular subtypes. A total of 153 miRNAs were identified as
significantly altered in AR-positive vs. AR-negative cell lines,
with at least a threefold change in expression (P<0.05; Fig. 1). Of the 52 upregulated miRNAs,
miR-933 and miR-5793 had the most significant increases, with
miR-933 expression undergoing the greatest upregulation (2.83-fold;
P=0.026). Of the 101 downregulated miRNAs, miR-4792 expression
underwent the greatest decrease (5.93-fold; P=0.002).
Predicted targets of the
differentially expressed miRNAs and their involvement in signaling
pathways
As a single miRNA may target multiple mRNA
transcripts, the dysregulation of sets of miRNAs may impact
significantly on cancerogenesis via the regulation of multiple
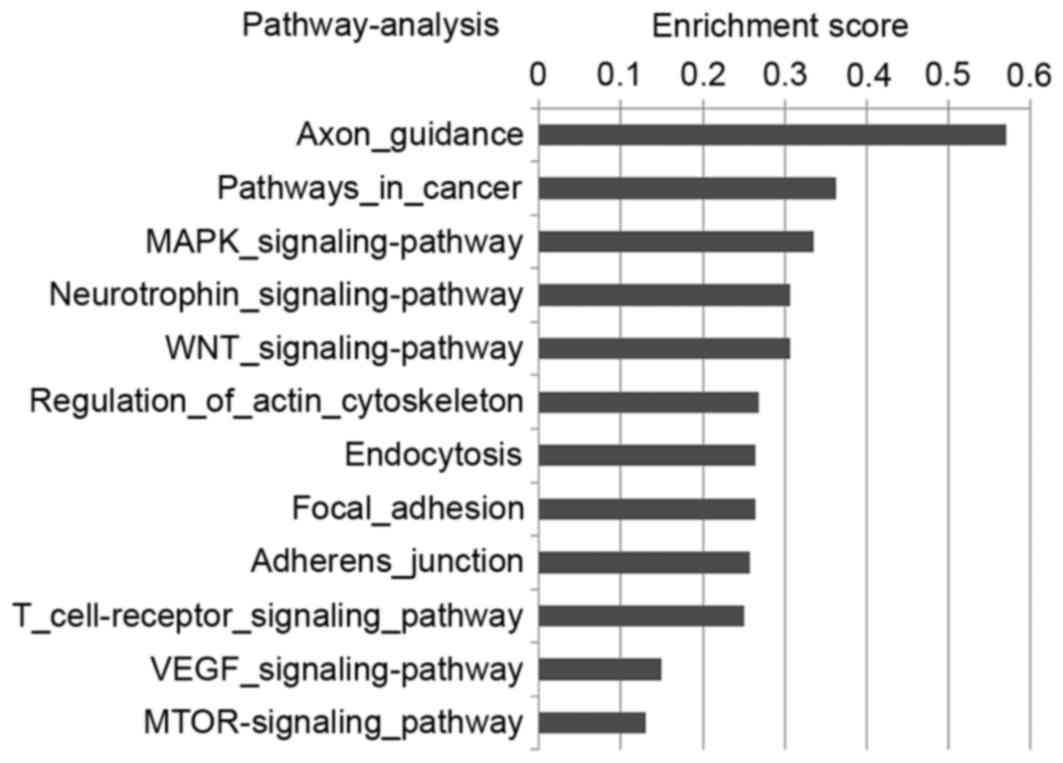
signaling pathways. To determine the probable biological functions
of the differentially expressed miRNAs, the potential targets of
the 153 differently expressed miRNAs were predicted in seven online
databases: DIANA, miRanda, miRBridge, PicTar, PITA, RNA22 and
TargetScan. A total of 5,576 target genes were predicted, and
classified into different pathways according to their basic
biological functions by the GO system. Multiple effectors of
pathways involved in tumor cell proliferation and invasion were
characterized, for example in the VEGF and mTOR signaling pathways
(Fig. 2). Due to the importance of
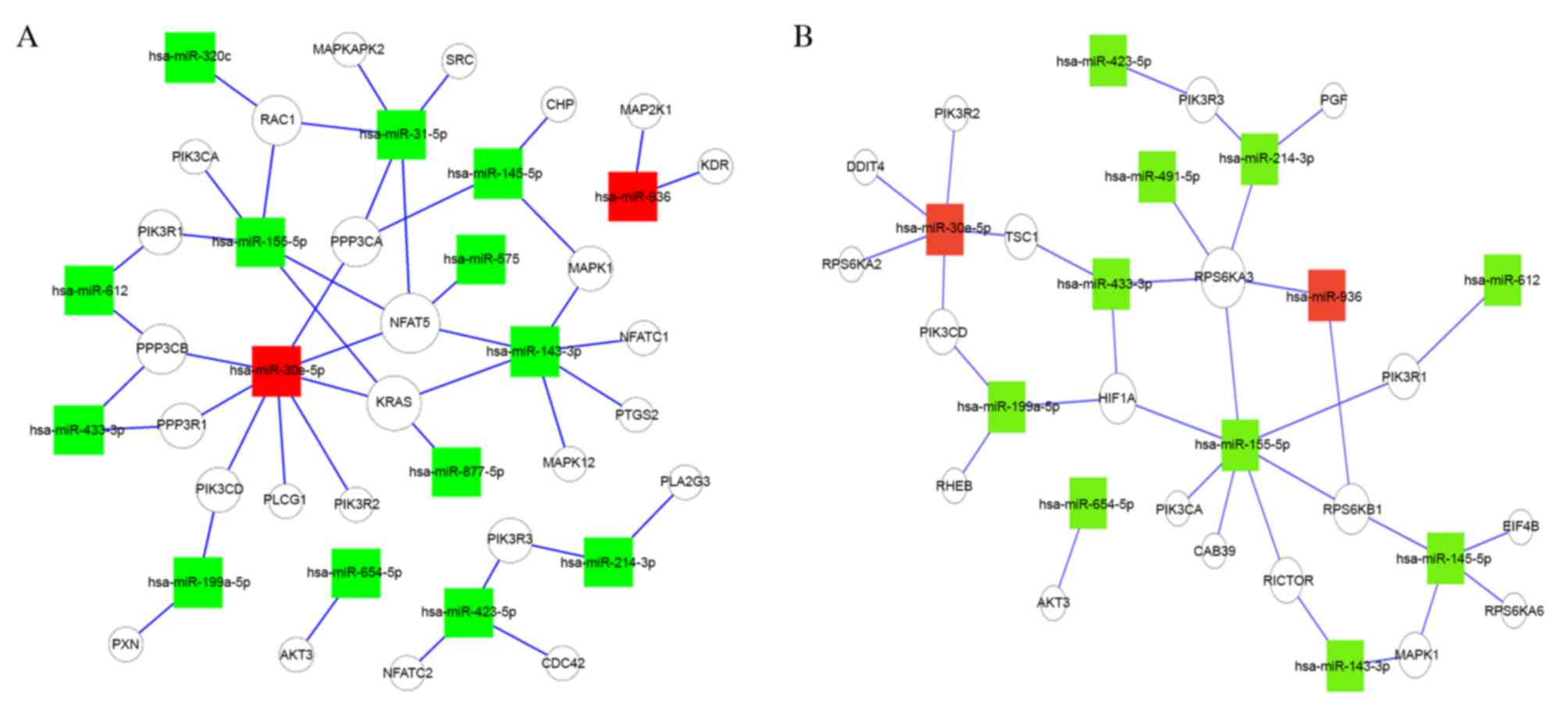
the VEGF and mTOR signaling pathways in breast cancer, the
VEGF-miRNA-target gene network (Fig.
3A) and mTOR-miRNA-target gene network (Fig. 3B) were constructed to reveal the
association between AR-associated miRNAs and their target effectors
in the two signaling pathways. The present study revealed a
putative role of AR in the VEGF and mTOR signaling pathways,
suggesting that AR may regulate the pathways through up or
downregulating miRNAs. Further work is required to improve the
understanding of the role of AR in these signaling pathways.
Discussion
The present study performed a screening to identify
AR-associated miRNAs in four breast cancer cell lines. By focusing
on miRNAs that were associated with AR in three separate cell
lines, cell-dependent bias was reduced. A total of 153 miRNAs were
identified as differentially expressed. Of these, 52 were
upregulated and 101 were downregulated. By predicting the targets
of differentially expressed miRNAs, interactions between AR, mTOR
and VEGF signaling pathways were indicated, suggesting that the AR
may interact with miRNAs and contribute to breast cancer
carcinogenesis via VEGF or mTOR signaling pathways. To date,
studies validating the association between the AR and miRNA
expression have primarily been conducted in PCa. Few studies have
reported the role of AR-associated miRNAs in breast cancer. Thus,
the present study increases the understanding of the role of AR in
breast cancer.
miRNAs have previously been implicated in the
regulation of AR signaling in prostate cancer. Chromatin
immunoprecipitation analysis confirmed AR binding to putative
androgen responsive elements (AREs) within the promoter regions of
these miRNAs, thereby regulating miRNA expression (15). Porkka et al (11) in 2007 were the first to reveal an
association between the AR and miRNAs. Following miRNA expression
profiling of PCa cell lines and carcinoma samples, hierarchical
clustering of the aberrantly expressed miRNAs accurately separated
the carcinomas according to AR status, indicating that the AR may
regulate the expression of miRNAs. Furthermore, a number of studies
have described the roles of miRNAs as regulators of AR activity.
Östling et al (13)
demonstrated that the majority of the miRNAs regulating the AR
targeted an extended 6 kb 3′UTR. A total of 71 unique miRNAs were
identified to influence the level of AR expression in PCa cell
lines. Therefore, there exists a potential link between AR
signaling and miRNAs, with implications for the development of
strategies to inhibit AR function via miRNAs-associated
pathways.
A differential miRNA expression pattern, involving
153 miRNAs, was detected in the present study, 52 upregulated and
101 downregulated, thus identifying those miRNAs that may be
significant in breast cancer development. Certain significantly
dysregulated miRNAs in the upregulated and downregulated groups
have been previously associated with breast cancer progression.
Notably, certain of the significantly downregulated miRNAs have
been associated with breast cancer cell functions, including
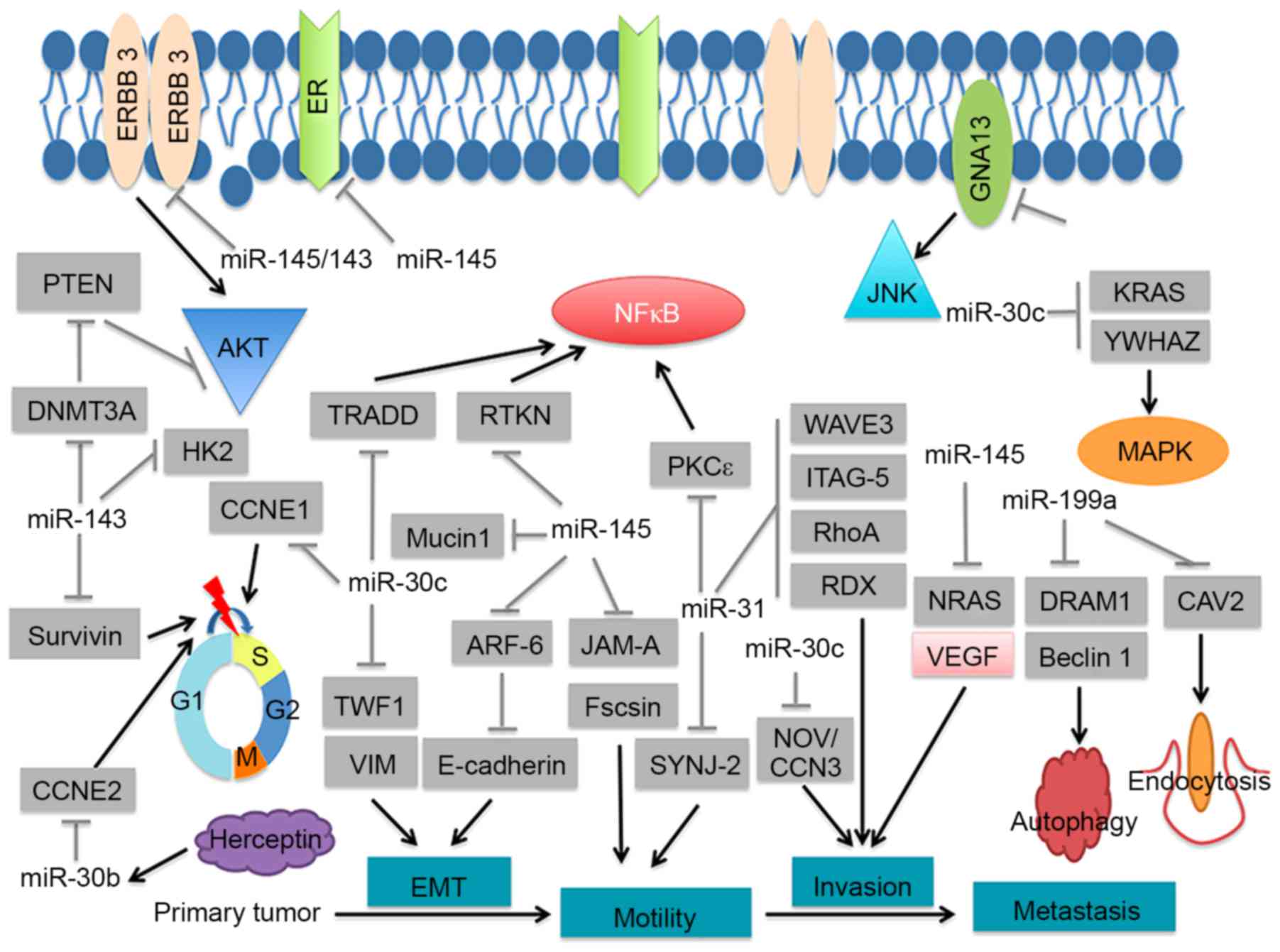
proliferation, invasion and drug-resistance (Table I; Fig.
4). For example, miR-143 and miR-145, components of the
miR-143/145 cluster, were significantly downregulated. miR-143 and
miR-145 have been demonstrated to possess anti-tumorigenic activity
(Table I). Furthermore, expression
of the miR-143/145 cluster was revealed to function as a tumor
suppressor in breast cancer via the inhibition of ERBB3 (17). Stimulation of EGF pathways,
including ERBB3, leads to enhanced AR stability and promotes AR
binding to AREs. Therefore, the miR-143/145 cluster may be involved
in the underlying mechanism of the AR in breast cancer.
Furthermore, miR-31, an miRNA that was significantly downregulated
in the present study, has been identified to directly target and
destabilize the AR through its coding sequence. The mutual
regulation between miR-31 and AR maintains prostate cellular
homeostasis, and the loss of miR-31 contributes to PCa progression
through unregulated AR expression. In breast cancer, miR-31 serves
as a tumor suppressor via inhibition of guanine nucleotide binding
protein alpha 13, WAS protein family member 3, β1-Integrin and
protein kinase C ε (Table I).
miR-181, another dysregulated miRNA in the present study, has been
demonstrated to indirectly target AR to function as a growth
suppressive miRNA in prostate cancer (40). Taken together, the dysregulated
miRNAs identified by the present study may regulate the expression
of AR in breast cancer. It may be useful to determine whether the
dysregulated pattern is implicated in the role of the AR in breast
cancer. The majority of the upregulated miRNAs identified in the
present study have not been widely reported as aberrantly expressed
in other cancers, which suggests that AR-associated breast cancer
may have a specific signature. Further studies are required to
confirm the expression of the dysregulated miRNAs by polymerase
chain reaction anaylsis.
 | Table I.Significantly downregulated miRNAs in
the present study are associated with breast cancer. |
Table I.
Significantly downregulated miRNAs in
the present study are associated with breast cancer.
| Author, year | miRNA | Cancer | Target gene | Clinical
specimen/animal model/cell line | Function | Ref. |
|---|
| Ng, 2014 | miR-143 | Breast | DNMT3A | MDA-MB-231,
T47D | Inhibit cell
proliferation | 16 |
| Yan, 2014 |
|
| ERBB3 | Breast cancer
tissues, MDA-MB-231, MCF-7, xenograft mouse model | Inhibit cell
proliferation and invasion | 17 |
| Jiang, 2012 |
|
| HK2 | MDA-MB-231,
ZR-75-30, xenograft mouse model | Inhibit cell
proliferation and invasion | 18 |
| Yu, 2012 |
|
| Survivin | MCF-7 | Inhibit cell
proliferation | 19 |
| Rasheed, 2015 | miR-31 | Breast | GNA13 | Breast cancer
tissues, MDA-MB-231, MCF-10a | Inhibit cell
invasion | 20 |
| Augoff, 2011 |
|
| β1-Integrin | MDA-MB-231 | Inhibit cell
invasion | 21 |
| Sossey-Alaoui,
2011 |
|
| Wave3 | Breast cancer
tissues, MDA-MB-231 | Inhibit cell
invasion | 22 |
| Körner, 2013 |
|
| PKCε | MDA-MB-231,
MCF-10A | Inhibit cell
invasion | 23 |
| Valastyan,
2010 |
|
| RDX ITGA5 RhoA | MDA-MB-231,
xenograft mouse model | Inhibit cell
invasion | 24 |
| Ben-Chetrit,
2015 |
|
| SYNJ2 | Breast cancer
tissues, MD-MB-231, MCF-10a, xenograft mouse model | Inhibit cell
invasion | 25 |
| Dobson, 2014 | miR-30c | Breast | NOV/CCN3 | MDA-MB-231 | Promote cell
invasion | 26 |
| Shukla, 2015 |
|
| TRADD CCNE1 | Breast cancer
tissues, MDA-MB-231 | Inhibit cell
proliferation and invasion | 27 |
| Tanic, 2012 |
|
| KRAS | MDA-MB-436 | Inhibit cell
growth | 28 |
| Bockhorn, 2013 |
|
| TWF1 VIM | MDA-MB-231,
xenograft mouse model | Inhibit cell
invasion | 10 |
| Bockhorn, 2013 |
|
| TWF1 | Breast cancer
tissues, MDA-MB-231, xenograft mouse model | Inhibit
chemo-resistance | 29 |
| Fang, 2014 |
|
| YWHAZ | MCF-7, xenograft
mouse model | Inhibit
chemo-resistance | 30 |
| Yi, 2013 | miR-199a | Breast | DRAM1 Beclin 1 | MDA-MB-231,
MCF-7 | Regulate
apoptosis | 31 |
| Shatseva, 2011 |
|
| CAV-2 | MDA-MB-231 | Inhibit cell
proliferation | 32 |
| Yan, 2014 | miR-145 | Breast | ERBB3 | Breast cancer
tissues, MDA-MB-231, MCF-7, xenograft mouse model | Inhibit cell
proliferation and invasion | 17 |
| Eades, 2015 |
|
| ARF6 | Breast cancer
tissues, MDA-MB-231 | Inhibit cell
invasion | 33 |
| Zou, 2012 |
|
| N-RAS | Breast cancer
tissues, MDA-MB-231, MCF-7, | Inhibit cell
growth, Invasion, and | 34 |
|
|
|
| VEGF-A | xenograft mouse
model | angiogenesis |
| Wang, 2009 |
|
| RTKN | MFC-7 | Inhibit cell
growth | 35 |
| Spizzo, 2010 |
|
| ER-α | MDA-MB-231,
MCF-7 | Inhibit cell
growth | 36 |
| Gotte, 2010 |
|
| JAM-A Fascin | MB-MDA-231, MCF-7,
MDA-MB-468, SK-BR-3 | Inhibit cell
invasion and motility | 37 |
| Liu, 2014 |
|
| Muc-1 | Breast cancer
tissues, MDA-MB-231, LM2-4142 | Inhibit cell growth
and invasion | 38 |
| Ichikawa, 2012 | miR-30b-3p | Breast | CCNE2 | SKBR3; BT474 | Inhibit cell
growth | 39 |
However, studies on AR-associated miRNAs in breast
cancer are limited. To the best of our knowledge, only two studies
have been performed to date. Nakano et al (41) identified miRNAs induced by
dihydrotestosterone (DHT) in an AR-positive breast cancer cell
line. A total of 13 miRNAs were upregulated and 28 miRNAs were
downregulated significantly. Of the aberrantly expressed miRNAs,
miR-363, with the fold-change (8.15-fold) targets IQ motif- and WD
repeats-containing 1 to influence the mechanism underlying the
action of androgens. Furthermore, to identify critical miRNAs
associated the androgen-induced AR signaling pathway in breast
cancer, Lyu et al (42)
examined global miRNA expression in DHT-treated MCF-7 and
MDA-MB-453 cells. A total of 10 differentially expressed miRNAs
were identified in MDA-MB-453 cells. Of these, 4 upregulated miRNAs
(let-7a, b, c and d) were detected. Androgen-induced AR activating
signal was revealed to directly upregulate let-7a expression,
downregulate CMYC and KRAS expression and inhibit cell
proliferation in ER−, PR−, AR+
breast cancer cells. miR-30b, another critical miRNA associated
with the androgen-induced AR activating signal in breast cancer
(42), was aberrantly expressed in
the present study, which screened the AR 3′UTR for potential miRNA
target sites. miR-30b was predicted by miRanda to target the 3′UTR
of the AR. Taken together, these data suggested a potential link
between AR signaling and miRNAs in breast cancer. To investigate
the role of AR-associated miRNAs in breast cancer, further
investigations are required. The present study has identified a
distinct miRNA expression pattern in AR-positive breast cancer cell
lines, which indicates a critical role for miRNAs in AR-positive
and -negative breast cancer.
Pathway enrichment analysis suggested that the
differentially expressed miRNAs collectively targeted various
signaling pathways associated with cell proliferation and invasion.
Notably, VEGF and mTOR pathways, the key pathways of breast
tumorigenesis, were identified as associated with the deregulated
miRNAs of the present study (43).
Previous studies have revealed that the involvement of miRNAs was
critical for VEGF-induced angiogenesis and mTOR-associated tumor
proliferation (44,45). In the present study, a putative
correlation was revealed between AR, VEGF and mTOR signaling
pathways. The prognostic value of AR expression in TNBC remains
elusive, with certain studies suggesting reduced mortality
(4), and others indicating poor
prognosis (5). Furthermore, our
previous study identified a role for AR in tumorigenesis role and
the inhibitory effect of AR antagonist in AR-positive mesenchymal
stem-like TNBC in vitro and in vivo, suggesting that
AR inhibition may be a potential therapeutic approach for
AR-positive TNBC patients (46).
AR may therefore be a tumorigenic factor in breast cancer.
AR-associated miRNAs may be involved in the VEGF and mTOR signaling
pathways to promote tumor cell proliferation.
In conclusion, the results of the present study
indicated that there is an imbalance of miRNAs levels in
AR-positive breast cancer cells compared with AR-negative cells,
suggesting an important role for miRNAs in the function of the AR
in breast cancer. Following classification of the miRNAs targets
into different pathways, a potential interaction between AR, VEGF
and mTOR signaling pathways was revealed. These results suggested a
potential underlying mechanism of AR in breast cancer via the
dysregulation of the expression pattern of miRNAs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272252) and the
Foundation for Clinical Medicine, Science and Technology Special
Project of Jiangsu Province (grant no. BL2014071) to X. G.
References
|
1
|
Park S, Koo J, Park HS, Kim JH, Choi SY,
Lee JH, Park BW and Lee KS: Expression of androgen receptors in
primary breast cancer. Ann Oncol. 21:488–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsang JY, Ni YB, Chan SK, Shao MM, Law BK,
Tan PH and Tse GM: Androgen receptor expression shows distinctive
significance in ER positive and negative breast cancers. Ann Surg
Oncol. 21:2218–2228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loibl S, Müller BM, von Minckwitz G,
Schwabe M, Roller M, Darb-Esfahani S, Ataseven B, du Bois A,
Fissler-Eckhoff A, Gerber B, et al: Androgen receptor expression in
primary breast cancer and its predictive and prognostic value in
patients treated with neoadjuvant chemotherapy. Breast Cancer Res
Treat. 130:477–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He J, Peng R, Yuan Z, Wang S, Peng J, Lin
G, Jiang X and Qin T: Prognostic value of androgen receptor
expression in operable triple-negative breast cancer: A
retrospective analysis based on a tissue microarray. Med Oncol.
29:406–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu R, Dawood S, Holmes MD, Collins LC,
Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA and Tamimi
RM: Androgen receptor expression and breast cancer survival in
postmenopausal women. Clin Cancer Res. 17:1867–1874. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vera-Badillo FE, Templeton AJ, de Gouveia
P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF,
Ocana A and Amir E: Androgen receptor expression and outcomes in
early breast cancer: A systematic review and meta-analysis. J Natl
Cancer Inst. 106:djt3192014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dvinge H, Git A, Gräf S, Salmon-Divon M,
Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA, et al:
The shaping and functional consequences of the microRNA landscape
in breast cancer. Nature. 497:378–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mulrane L, McGee SF, Gallagher WM and
O'Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bockhorn J, Yee K, Chang YF, Prat A, Huo
D, Nwachukwu C, Dalton R, Huang S, Swanson KE, Perou CM, et al:
MicroRNA-30c targets cytoskeleton genes involved in breast cancer
cell invasion. Breast Cancer Res Treat. 137:373–382. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu
M, Tepper CG, Evans CP, Kung HJ and de Vere White RW: An
androgen-regulated miRNA suppresses Bak1 expression and induces
androgen-independent growth of prostate cancer cells. Proc Natl
Acad Sci USA. 104:19983–19988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Östling P, Leivonen SK, Aakula A, Kohonen
P, Mäkelä R, Hagman Z, Edsjö A, Kangaspeska S, Edgren H, Nicorici
D, et al: Systematic analysis of microRNAs targeting the androgen
receptor in prostate cancer cells. Cancer Res. 71:1956–1967. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin PC, Chiu YL, Banerjee S, Park K,
Mosquera JM, Giannopoulou E, Alves P, Tewari AK, Gerstein MB,
Beltran H, et al: Epigenetic repression of miR-31 disrupts androgen
receptor homeostasis and contributes to prostate cancer
progression. Cancer Res. 73:1232–1244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribas J, Ni X, Haffner M, Wentzel EA,
Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J,
Rodriguez R, et al: miR-21: An androgen receptor-regulated microRNA
that promotes hormone-dependent and hormone-independent prostate
cancer growth. Cancer Res. 69:7165–7169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ng EK, Li R, Shin VY, Siu JM, Ma ES and
Kwong A: MicroRNA-143 is downregulated in breast cancer and
regulates DNA methyltransferases 3A in breast cancer cells. Tumor
Biol. 35:2591–2598. 2014. View Article : Google Scholar
|
|
17
|
Yan X, Chen X, Liang H, Deng T, Chen W,
Zhang S, Liu M, Gao X, Liu Y, Zhao C, et al: miR-143 and miR-145
synergistically regulate ERBB3 to suppress cell proliferation and
invasion in breast cancer. Mol Cancer. 13:2202014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH,
Liang S, Li B, Li Y, Li D, Wang ED and Liu MF: A novel
miR-155/miR-143 cascade controls glycolysis by regulating
hexokinase 2 in breast cancer cells. EMBO J. 31:1985–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu X, Zhang X, Dhakal IB, Beggs M,
Kadlubar S and Luo D: Induction of cell proliferation and survival
genes by estradiol-repressed microRNAs in breast cancer cells. BMC
Cancer. 12:292012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve
PM, Zhou W, Ghosh S and Casey PJ: MicroRNA-31 controls G protein
alpha-13 (GNA13) expression and cell invasion in breast cancer
cells. Mol Cancer. 14:672015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Augoff K, Das M, Bialkowska K, McCue B,
Plow EF and Sossey-Alaoui K: miR-31 is a broad regulator of
β1-integrin expression and function in cancer cells. Mol Cancer
Res. 9:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sossey-Alaoui K, Downs-Kelly E, Das M,
Izem L, Tubbs R and Plow EF: WAVE3, an actin remodeling protein, is
regulated by the metastasis suppressor microRNA, miR-31, during the
invasion-metastasis cascade. Int J Cancer. 129:1331–1343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Körner C, Keklikoglou I, Bender C, Wörner
A, Münstermann E and Wiemann S: MicroRNA-31 sensitizes human breast
cells to apoptosis by direct targeting of protein kinase C epsilon
(PKCepsilon). J Biol Chem. 288:8750–8761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valastyan S, Chang A, Benaich N, Reinhardt
F and Weinberg RA: Concurrent suppression of integrin alpha5,
radixin, and RhoA phenocopies the effects of miR-31 on metastasis.
Cancer Res. 70:5147–5154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ben-Chetrit N, Chetrit D, Russell R,
Körner C, Mancini M, Abdul-Hai A, Itkin T, Carvalho S, Cohen-Dvashi
H, Koestler WJ, et al: Synaptojanin 2 is a druggable mediator of
metastasis and the gene is overexpressed and amplified in breast
cancer. Sci Signal. 8:ra72015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dobson JR, Taipaleenmäki H, Hu YJ, Hong D,
van Wijnen AJ, Stein JL, Stein GS, Lian JB and Pratap J:
hsa-mir-30c promotes the invasive phenotype of metastatic breast
cancer cells by targeting NOV/CCN3. Cancer Cell Int. 14:732014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shukla K, Sharma AK, Ward A, Will R,
Hielscher T, Balwierz A, Breunig C, Münstermann E, König R,
Keklikoglou I and Wiemann S: MicroRNA-30c-2-3p negatively regulates
NF-κB signaling and cell cycle progression through downregulation
of TRADD and CCNE1 in breast cancer. Mol Oncol. 9:1106–1119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanic M, Yanowsky K, Rodriguez-Antona C,
Andrés R, Márquez-Rodas I, Osorio A, Benitez J and Martinez-Delgado
B: Deregulated miRNAs in hereditary breast cancer revealed a role
for miR-30c in regulating KRAS oncogene. PLoS One. 7:e388472012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bockhorn J, Dalton R, Nwachukwu C, Huang
S, Prat A, Yee K, Chang YF, Huo D, Wen Y, Swanson KE, et al:
MicroRNA-30c inhibits human breast tumour chemotherapy resistance
by regulating TWF1 and IL-11. Nat Commun. 4:13932013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang Y, Shen H, Cao Y, Li H, Qin R, Chen
Q, Long L, Zhu XL, Xie CJ and Xu WL: Involvement of miR-30c in
resistance to doxorubicin by regulating YWHAZ in breast cancer
cells. Braz J Med Biol Res. 47:60–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yi H, Liang B, Jia J, Liang N, Xu H, Ju G,
Ma S and Liu X: Differential roles of miR-199a-5p in
radiation-induced autophagy in breast cancer cells. FEBS Lett.
587:436–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shatseva T, Lee DY, Deng Z and Yang BB:
MicroRNA miR-199a-3p regulates cell proliferation and survival by
targeting caveolin-2. J Cell Sci. 124:2826–2836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eades G, Wolfson B, Zhang Y, Li Q, Yao Y
and Zhou Q: lincRNA-RoR and miR-145 regulate invasion in
triple-negative breast cancer via targeting ARF6. Mol Cancer Res.
13:330–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou C, Xu Q, Mao F, Li D, Bian C, Liu LZ,
Jiang Y, Chen X, Qi Y, Zhang X, et al: MiR-145 inhibits tumor
angiogenesis and growth by N-RAS and VEGF. Cell Cycle.
11:2137–2145. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng
L, Zhou H and Zhao RC: miR-145 inhibits breast cancer cell growth
through RTKN. Int J Oncol. 34:1461–1466. 2009.PubMed/NCBI
|
|
36
|
Spizzo R, Nicoloso MS, Lupini L, Lu Y,
Fogarty J, Rossi S, Zagatti B, Fabbri M, Veronese A, Liu X, et al:
miR-145 participates with TP53 in a death-promoting regulatory loop
and targets estrogen receptor-alpha in human breast cancer cells.
Cell Death Differ. 17:246–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Götte M, Mohr C, Koo CY, Stock C, Vaske
AK, Viola M, Ibrahim SA, Peddibhotla S, Teng YH, Low JY, et al:
miR-145-dependent targeting of Junctional adhesion molecule A and
modulation of fascin expression are associated with reduced breast
cancer cell motility and invasiveness. Oncogene. 29:6569–6580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Wang X, Yang X, Liu Y, Shi Y, Ren J
and Guleng B: miRNA423-5p regulates cell proliferation and invasion
by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett.
347:98–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ichikawa T, Sato F, Terasawa K, Tsuchiya
S, Toi M, Tsujimoto G and Shimizu K: Trastuzumab produces
therapeutic actions by upregulating miR-26a and miR-30b in breast
cancer cells. PLoS One. 7:e314222012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tong SJ, Liu J, Wang X and Qu LX:
microRNA-181 promotes prostate cancer cell proliferation by
regulating DAX-1 expression. Exp Ther Med. 8:1296–1300.
2014.PubMed/NCBI
|
|
41
|
Nakano K, Miki Y, Hata S, Ebata A, Takagi
K, McNamara KM, Sakurai M, Masuda M, Hirakawa H, Ishida T, et al:
Identification of androgen-responsive microRNAs and
androgen-related genes in breast cancer. Anticancer Res.
33:4811–4819. 2013.PubMed/NCBI
|
|
42
|
Lyu S, Yu Q, Ying G, Wang S, Wang Y, Zhang
J and Niu Y: Androgen receptor decreases CMYC and KRAS expression
by upregulating let-7a expression in ER−,
PR−, AR+ breast cancer. Int J Oncol.
44:229–237. 2014.PubMed/NCBI
|
|
43
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005.PubMed/NCBI
|
|
44
|
Chang SH, Lu YC, Li X, Hsieh WY, Xiong Y,
Ghosh M, Evans T, Elemento O and Hla T: Antagonistic function of
the RNA-binding protein HuR and miR-200b in post-transcriptional
regulation of vascular endothelial growth factor-A expression and
angiogenesis. J Biol Chem. 288:4908–4921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oneyama C and Okada M: MicroRNAs as the
fine-tuners of Src oncogenic signalling. J Biochem. 157:431–438.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu A, Li Y, Song W, Xu Y, Yang F, Zhang
W, Yin Y and Guan X: Antiproliferative effect of androgen receptor
inhibition in Mesenchymal stem-like triple-negative breast cancer.
Cell Physiol Biochem. 38:1003–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|