Introduction
Advanced oxidation protein products (AOPPs) were
initially described by Witko-Sarsat et al in 1996 as a
family of oxidized, dityrosine-containing protein products, which
are formed during oxidative stress by the interaction between
plasma proteins and chlorinated oxidants, and are often carried by
albumin in vivo (1,2). AOPPs are recognized as novel markers
of protein oxidative damage, the intensity of oxidative stress, and
inflammation (3). Significantly
increased concentrations of AOPPs have been detected in several
pathological conditions, including chronic kidney disease, diabetes
mellitus, inflammatory bowel disease and rheumatoid arthritis
(4–6).
Notably, patients with the aforementioned conditions
often exhibit bone loss and have an increased incidence of
fracture, which is defined as secondary osteoporosis. Secondary
osteoporosis is characterized by low bone mass with
micro-architectural alterations in the bone, which can lead to
fragility fractures in the presence of an underlying disease or
medication (7). The exact
underlying mechanisms of this condition remain unclear; however, it
may be hypothesized that AOPPs have a certain role in the
progression of secondary osteoporosis.
In the process of bone remodeling, bone is
constantly renewed by the balance between osteoblastic bone
formation and osteoclastic bone resorption. Previous studies have
demonstrated that AOPPs may inhibit the proliferation and
differentiation of rat osteoblastic cells and rat mesenchymal stem
cells (8,9). As the most abundant cell type in bone
(90–95%), osteocytes function as more than just mechanosensors in
bone homeostasis. It has previously been reported that osteocytes
are a major source of the cytokine receptor activator of nuclear
factor kappa-B ligand (RANKL), which is a ligand for
osteoprotegerin and functions as a key factor for osteoclast
differentiation and activation (10,11).
In addition, osteocytes almost exclusively secrete the protein
sclerostin, which inhibits osteoblast functioning and bone
formation by antagonizing the Wnt signaling pathway (12,13).
Therefore, it has been suggested that osteocytes act as the
commander cells of bone remodeling, since they regulate bone
formation and bone resorption via sclerostin and RANKL. However, it
remains unclear whether AOPPs affect osteocytes or regulate the
production of these factors, thereby causing bone deterioration in
patients with pathological levels of plasma AOPPs.
Oxidative stress induces several signal transduction
pathways, including the mitogen-activated protein kinases (MAPKs)
pathways. MAPKs consist of extracellular signal-regulated kinases
(ERK), c-Jun N-terminal kinases (JNK) and p38 MAPK, and mediate
various cellular activities, including cell growth,
differentiation, survival and death (14,15).
It has previously been reported that JNK/p38 MAPK pathways have a
pivotal role in oxidative stress-induced apoptosis, whereas ERK
exerts effects on cell physiology. However, it remains unknown as
to whether AOPPs activate JNK/p38 MAPK signaling in osteocytes, or
whether these signaling pathways are essential for AOPPs-induced
apoptosis.
The present study aimed to determine the effects of
AOPPs on apoptosis and on the expression of sclerostin and RANKL in
osteocytic MLO-Y4 cells. The results demonstrated that AOPPs
induced apoptosis of MLO-Y4 cells, and increased sclerostin and
RANKL expression in a dose- and time-dependent manner. In addition,
the association between JNK/p38 MAPK signaling and AOPPs-induced
apoptosis was investigated, and it was revealed that sustained
activation of the JNK/p38 MAPK pathways is responsible for
AOPPs-induced apoptosis of osteocytic MLO-Y4 cells.
Materials and methods
Reagents
Mouse serum albumin (MSA), p38 inhibitor SB203580,
JNK inhibitor SP600125, ERK inhibitor PD98059, N-acetylcysteine
(NAC) and apocynin were obtained from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Trypsin-EDTA, fetal bovine serum
(FBS), newborn calf serum, α-minimum essential medium (α-MEM) and
penicillin-streptomycin were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). TRIzol® reagent was
obtained from Invitrogen (Thermo Fisher Scientific, Inc.). The
Prime Script® One Step real time-polymerase chain
reaction (RT-PCR) kit and SYBR Premix Ex Taq were obtained
from Takara Biotechnology Co., Ltd. (Dalian, China).
Radioimmunoprecipitation assay (RIPA) lysis buffer and
phenylmethylsulfonyl fluoride (PMSF) were from Beyotime Institute
of Biotechnology (Shanghai, China). The Detoxi-Gel column was from
Pierce (Thermo Fisher Scientific, Inc.). Cell Death Detection
enzyme-linked immunosorbent assay (ELISA)PLUS kit and Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit were
obtained from Roche Life Science (Indianapolis, IN, USA). Rabbit
anti-sclerostin (cat. no. sc-130258) and anti-RANKL (cat. no.
sc-9073) antibodies were from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA); anti-phosphorylated (P)-p38 (cat. no. #4631S),
anti-p38 (cat. no. #9212S), anti-P-ERK 1/2 (cat. no. #9101S),
anti-ERK 1/2 (cat. no. #9102S), anti-P-JNK (cat. no. #9251S) and
anti-JNK (cat. no. #9252S) antibodies were from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Mouse anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (cat. no. ab8245) antibody and
the Cellular Reactive Species Detection Assay kit were purchased
from Abcam (Cambridge, UK).
AOPPs-MSA preparation and
determination
AOPPs-MSA was prepared in vitro by incubating
20 mg/ml MSA with 200 mM hypochlorous acid (HOCl) for 30 min at
37°C. The mixture was then dialyzed overnight against
phosphate-buffered saline to remove any free HOCl. All prepared
samples were passed through a Detoxi-Gel column to remove
contaminated endotoxin. Endotoxin levels in the preparation were
determined using the Limulus Amebocyte Lysate kit (Sigma-Aldrich;
Merck Millipore) and were shown to be <0.025 EU/ml. AOPP content
in the preparation was determined according to a previously
published method (1). Briefly, 200
µl of the sample or chloramine-T (standard curve; Sigma-Aldrich;
Merck Millipore) were plated in a 96-well plate and mixed with 20
µl acetic acid. The absorbance was read immediately at 340 nm using
a microplate reader (Multiskan MK3; Thermo Fisher Scientific,
Inc.). The content of AOPP in the AOPP-MSA preparation was
68.80±5.35 nmol/mg protein in AOPPs-MSA, and 0.27±0.03 nmol/mg
protein in native MSA.
Cell culture
Mouse osteocyte-like MLO-Y4 cells were kindly
provided by Dr. Ni Guoxin (Southern Medical University, Guangzhou,
China). The cells were cultured on type I collagen-coated plates in
α-MEM at 37°C in a humidified atmosphere containing 5%
CO2. The medium was supplemented with 5% newborn calf
serum (CS), 5% FBS and 1% penicillin-streptomycin.
Protein extraction and western blot
analysis
Cells were lysed with RIPA buffer containing 0.5 mM
PMSF, and the lysates were centrifuged prior to determination of
the protein concentration using a bicinchoninic acid kit
(Sigma-Aldrich; Merck Millipore). A total of 30 µg of protein was
loaded per sample channel, and the proteins were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8%
gels). The separated proteins were then transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
blocking buffer (5% defatted milk) and incubated at room
temperature for 1 h. The membranes were initially probed with
anti-sclerostin (1:500 dilution), anti-RANKL (1:500 dilution),
anti-P-p38, anti-P-ERK 1/2 and anti-P-JNK (all 1:1,000 dilution)
primary antibodies, and incubated at 4°C overnight. Subsequently,
membranes were stripped and reprobed with anti-ERK 1/2, anti-JNK,
anti-p38 (all 1:1,000 dilution) or anti-GAPDH (1:500 dilution) for
normalization and densitometric analysis. Blots were scanned and
visualized using the Odyssey detector, as mentioned below. After
extensive washing with Tris-buffered saline/Tween 20 (TBS-T), the
membranes were incubated for 1 h at room temperature with the
secondary antibody [IRDye® 680LT-conjugated donkey
anti-rabbit immunoglobulin G (IgG) (H + L) antibody; cat. no. P/N
925–68023, 1:20,000 dilution). Images were detected using the
Odyssey detector (LI-COR Biosciences, Lincoln, NE, USA).
RNA extraction and reverse
transcription-quantitative (RT-qPCR)
Total RNA was extracted from the lysed cells using
TRIzol® reagent. Aliquots of each RNA extraction were
then reverse transcribed simultaneously into cDNA using the
PrimeScript® One Step RT-PCR kit, according to the
manufacturer's protocol. RT-qPCR was performed in a total volume of
20 µl in duplicate using the SYBR® Premix Ex Taq
kit and Fast Real-time PCR system 7300 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The PCR reaction mixture contained 2X
SYBR® Premix Ex Taq™ (10 µl), PCR forward primer (10 µM;
0.4 µl), PCR reverse primer (10 µM; 0.4 µl), ROX Reference Dye II
(50x; 0.4 µl), RT reaction solution (cDNA) (2.0 µl) and
double-distilled H2O (6.8 µl). The PCR thermocycling
conditions were as follows: Stage 1: 95°C for 30 sec, repeat one
time; Stage 2: 95°C for 5 sec, 60°C for 34 sec, repeat 40 times;
Stage 3: 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec,
repeat one time. The mRNA value for each gene was normalized
relative to the mouse GAPDH mRNA levels in the same RNA samples,
and the results were quantified using the 2−ΔΔCT method
(16). Primer sequences were as
follows: GAPDH, forward 5′-ATGGCCTTCCGTGTTCCTAC-3′, reverse
5′-CACCTTCTTGATGTCATCATACTTG-3′; sclerostin (gene name, SOST),
forward 5′-GCCTCCTCAGGAACTAGAGAAC-3′, reverse
5′-TACTCGGACACGTCTTTGGTG-3′; and RANKL, forward
5′-TGTACTTTCGAGCGCATGATG-3′ and reverse
5′-AGGCTTGTTTCATCCTCCTG-3′.
Determination of intracellular
formation of reactive oxygen species (ROS)
Intracellular ROS were detected in MLO-Y4 cells
using the Cellular Reactive Species Detection Assay kit (Deep Red
Fluorescence). Briefly, ROS assay solution (100 µl/well) was added
to the treated or untreated cells at a density of 3,000 cells per
well, and the cells were incubated in a 5% CO2
atmosphere at 37°C in an incubator for 1 h. The fluorescence signal
was monitored at an excitation wavelength of 650 nm and an emission
wavelength of 675 nm. Untreated cells were used to determine
background fluorescence. Data were expressed as percentage of
controls.
Measurement of apoptotic cell
death
To detect apoptosis, the Cell Death Detection
ELISAPLUS kit was used. Briefly, MLO-Y4 cells were seeded in
96-well plates at a density of 3,000 cells/well and were incubated
overnight in α-MEM supplemented with 5% FBS and 5% CS at 37°C in a
humidified atmosphere containing 5% CO2. On the
following day, the cells were treated with either MSA (control) or
various concentrations of AOPPs for 60 min, or with 200 µg/ml AOPPs
for the indicated time. The cells were then lysed, and the
supernatant was analyzed using the Cell Death Detection ELISAPLUS
kit, according to the manufacturer's protocol.
Annexin V/propidium iodide (PI)
staining assay
Another apoptosis analysis was conducted using the
Annexin V/PI assay. The Annexin V-FITC Apoptosis Detection kit was
used in accordance with the manufacturer's protocol. Briefly,
MLO-Y4 cells were serum-starved for 24 h, and were pretreated for
30 min at 37°C with JNK inhibitor SP600125 (30 µM), p38 inhibitor
SB203580 (30 µM), ROS scavenger NAC (10 µM) and NADPH oxidase
inhibitor apocynin (100 µM), prior to incubation with the indicated
concentration of AOPPs-MSA for 24 h. Cells were trypsinized and
double-stained with FITC-conjugated Annexin V and PI. Cells were
analyzed using a flow cytometer (Beckman Coulter, Brea, CA,
USA).
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± standard deviation. One-way analysis of
variance was performed, followed by a least significant difference
(LSD) method when P<0.05 for multiple comparisons. Statistical
analyses were conducted using SPSS 19.0 (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
AOPPs induce apoptosis of MLO-Y4
cells
The present study initially investigated whether
AOPPs induced apoptosis of osteocytic MLO-Y4 cells (Fig. 1). As determined by Cell Death
Detection ELISA kit and Annexin V/PI staining, treatment of the
cells with AOPPs significantly induced apoptosis in a dose- and
time-dependent manner (Fig. 1A and
B). Subsequently, the present study examined the effects of JNK
inhibitor SP600125 (30 µM), p38 inhibitor SB203580 (30 µM), ROS
scavenger NAC (10 µM) and NADPH oxidase inhibitor apocynin (100 µM)
on AOPPs-induced apoptosis. Pretreatment of the cells with these
agents significantly reversed AOPPs-induced apoptosis (Fig. 1B).
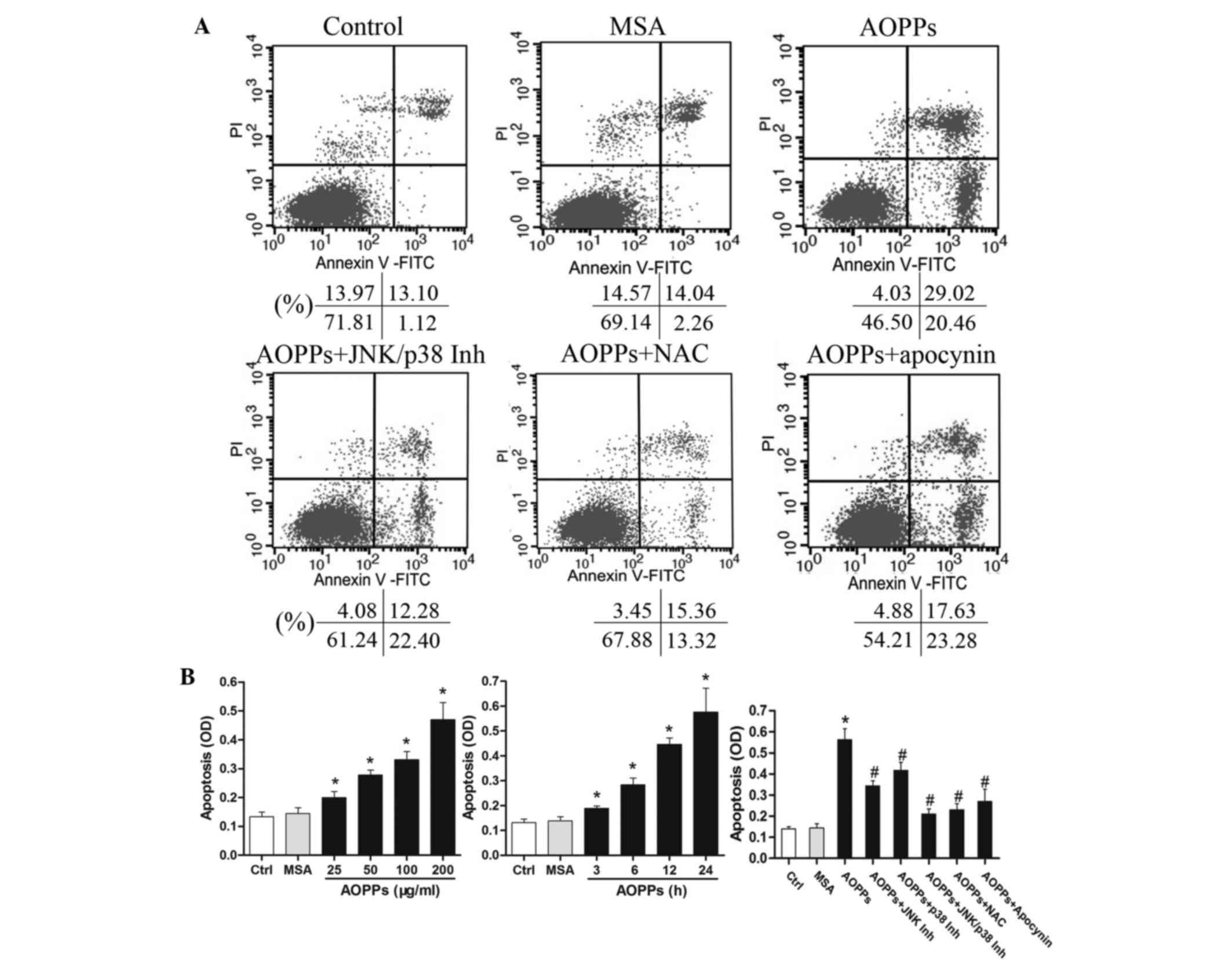 | Figure 1.Treatment with advanced oxidation
protein products (AOPPs) significantly induced apoptosis of
cultured MLO-Y4 cells. (A) Serum-starved MLO-Y4 cells were
pretreated with c-Jun N-terminal kinases (JNK) inhibitor SP600125
(30 µM), p38 inhibitor SB203580 (30 µM), reactive oxygen species
(ROS) scavenger N-acetylcysteine (NAC) (10 µM) and NADPH oxidase
inhibitor apocynin (100 µM), prior to incubation with 200 µg/ml
AOPPs-mouse serum albumin (MSA) for 24 h. The percentage of
apoptotic cells was estimated by Annexin V/propidium iodide (PI)
staining. Dot plot graphs present the percentage of viable cells,
early-phase apoptotic cells, late-phase apoptotic cells and
necrotic cells. (B) Apoptotic cells were also detected using the
Cell Death Detection enzyme-linked immunosorbent assay (ELISA) kit.
MLO-Y4 cells were incubated with the indicated concentrations of
AOPPs for 24 h, or with 200 µg/ml AOPPs for the indicated
durations, and were subjected to ELISA assay. AOPPs induced
apoptosis of MLO-Y4 cells in a dose- and time-dependent manner. To
verify the effects of JNK/p38-mitogen-activated protein kinases
signaling, ROS and NADPH oxidase on AOPPs-induced apoptosis, the
experiments were repeated in the presence of SP600125, SB203580,
NAC and apocynin. Data are presented as the mean ± standard
deviation of three independent experiments. Analysis of variance;
*P<0.05 vs. control; #P<0.05 vs. AOPPs-treated
group. Ctrl, untreated cells; AOPPs, AOPPs-MSA; FITC, fluorescein
isothiocyanate; Inh, inhibitor; OD, optical density. |
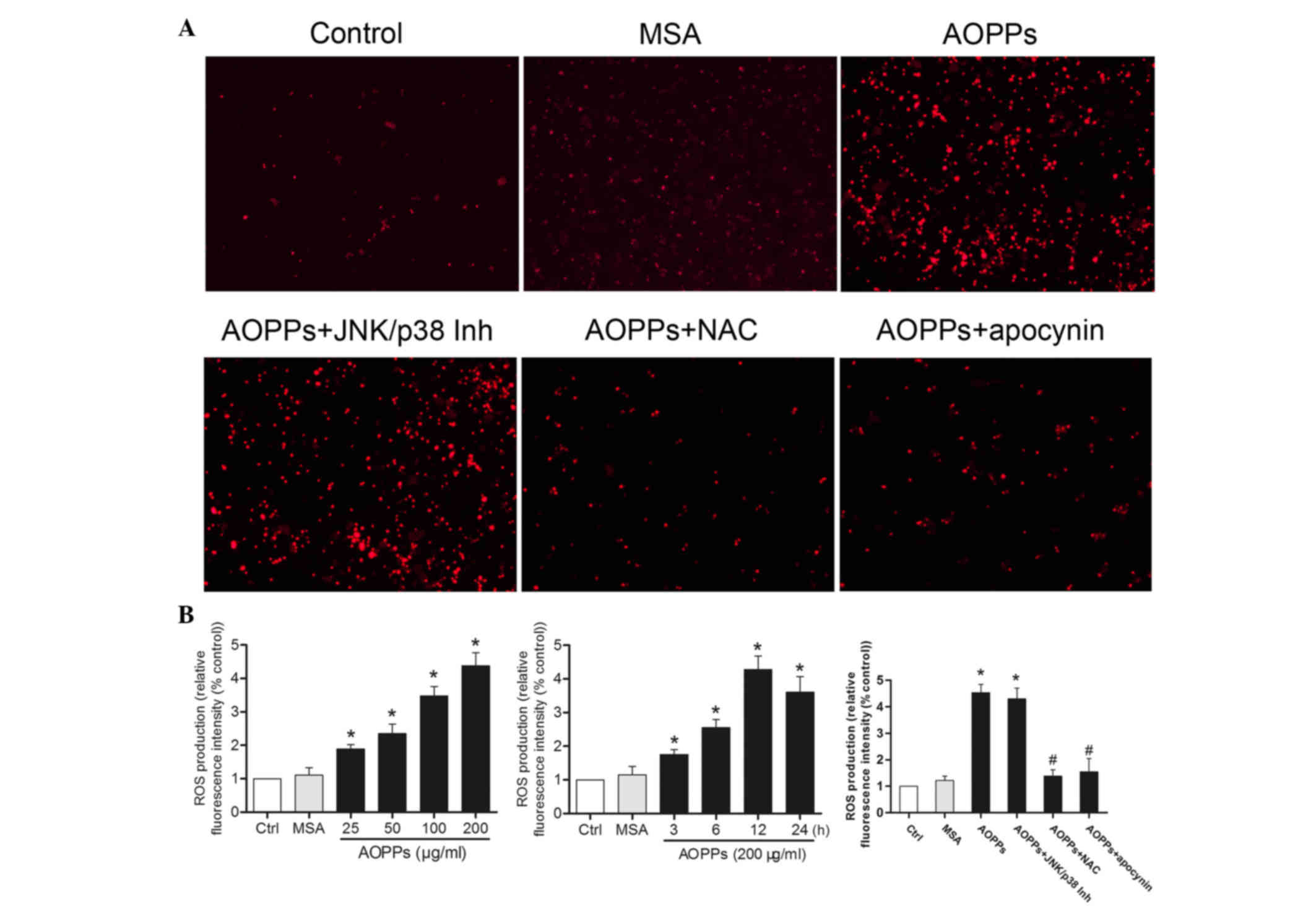
AOPPs enhance intracellular ROS
generation in MLO-Y4 cells
ROS generation was detected using the Cellular
Reactive Species Detection Assay kit (Fig. 2). Treatment of MLO-Y4 cells with
AOPPs induced a significant increase in intracellular ROS
production, in a dose- and time-dependent manner (Fig. 2B). Conversely, AOPPs-induced ROS
generation was suppressed by NAC pretreatment, but not by SP600125
or SB203580 treatment. In addition, the NADPH oxidase inhibitor
apocynin exhibited inhibitory action on ROS generation (Fig. 2A and B).
AOPPs increase the expression levels
of sclerostin and RANKL in MLO-Y4 cells
Sclerostin and RANKL are associated with bone
remodeling by regulating osteoblastogenesis and osteoclastogenesis.
Therefore, the present study examined the effects of AOPPs on the
expression levels of sclerostin and RANKL in MLO-Y4 cells. As shown
in Fig. 3, exposure of MLO-Y4
cells to AOPPs significantly increased the expression of
sclerostin, at the protein and mRNA levels (Fig. 3A-C), in a dose- and time-dependent
manner. In addition, the effects of AOPPs on the expression levels
of RANKL were detected by western blotting and RT-qPCR. Treatment
with AOPPs also increased the expression levels of RANKL (Fig. 3A, B and D). However, treatment of
the cells with medium alone or MSA did not exert any effects on the
expression levels of sclerostin and RANKL.
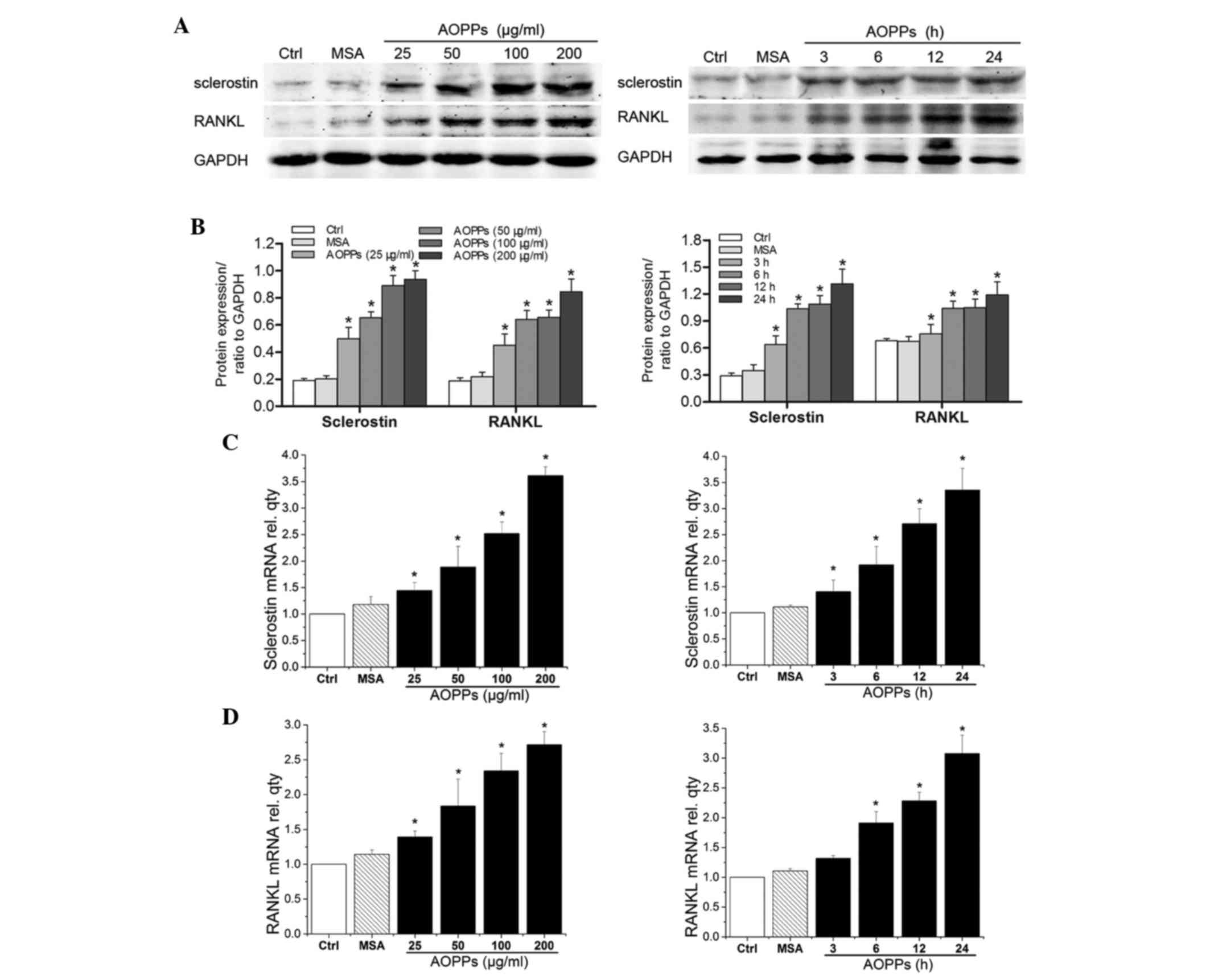 | Figure 3.Treatment with advanced oxidation
protein products (AOPPs) increased the expression levels of
sclerostin and receptor activator of nuclear factor kappa-B ligand
(RANKL) in cultured MLO-Y4 cells. MLO-Y4 cells were incubated with
the indicated concentrations of AOPPs for 24 h, or with 200 µg/ml
AOPPs for the indicated durations, and were subjected to (A and B)
protein and (C and D) mRNA analysis of sclerostin and RANKL.
Treatment with AOPPs increased the expression levels of (A-C)
sclerostin and (A, B and D) RANKL, at the mRNA and protein levels,
in a dose- and time-dependent manner. Data are presented as the
mean ± standard deviation of three independent experiments.
Analysis of variance; *P<0.05 vs. control. Ctrl, control; MSA,
mouse serum albumin; AOPPs, AOPPs-MSA; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; rel. qty, relative quantity. |
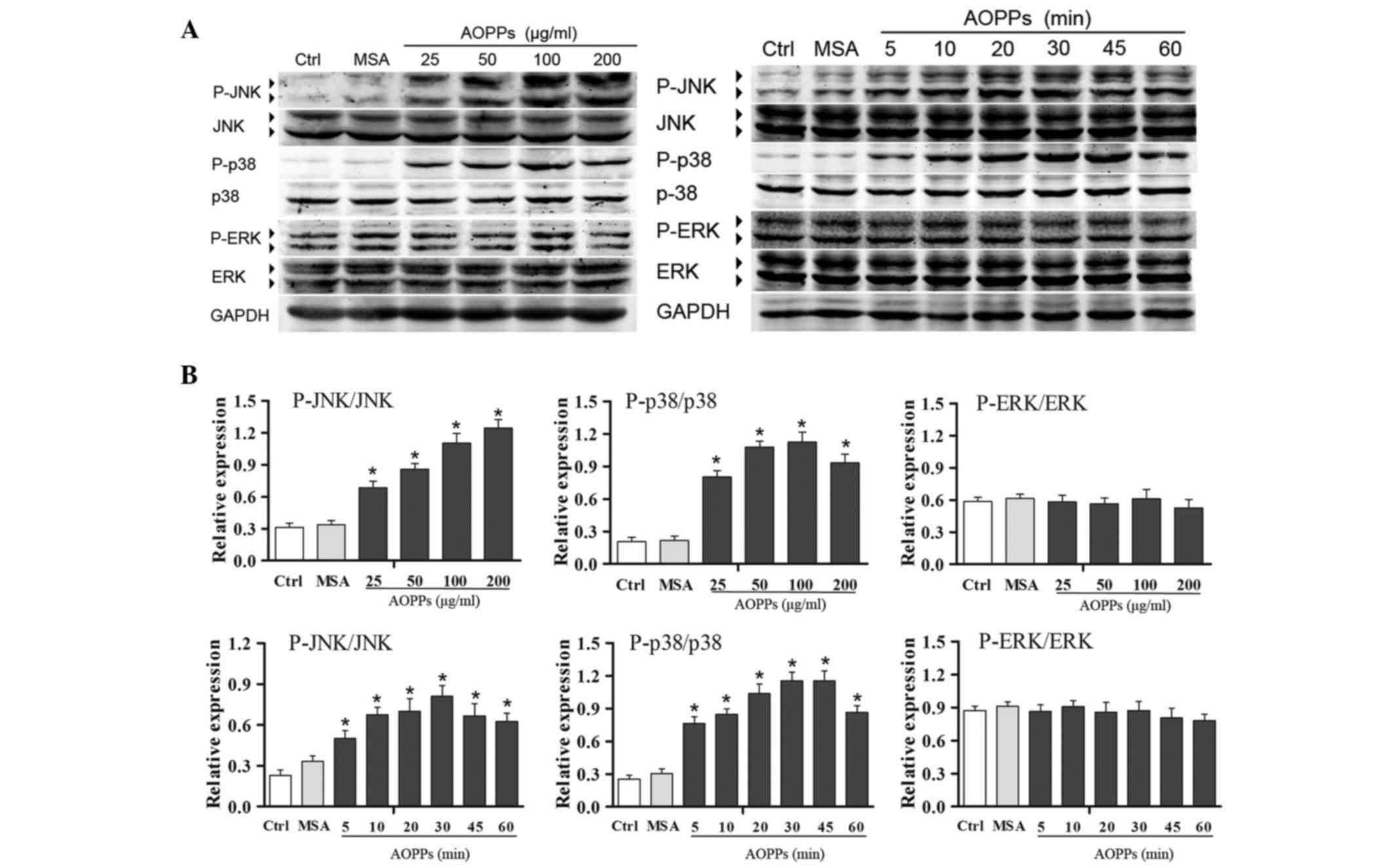
AOPPs induce ROS-dependent activation
of JNK and p38 MAPK
The extent and duration of MAPK activation serves a
key role in regulating cell functions (17,18).
As shown in Fig. 4, activation of
the JNK, p38 and ERK MAPKs was detected by western blotting, using
phosphorylation level as an index of enzyme activity. Treatment of
MLO-Y4 cells with AOPPs markedly induced the activation of JNK and
p38, but not ERK, in a dose- and time-dependent manner. To further
clarify the involvement of the activation of MAPKs in AOPPs-induced
upregulation of sclerostin and RANKL, MLO-Y4 cells were pretreated
with PD98059 (30 µM), SP600125 (30 µM) and SB203580 (30 µM) for 30
min, and were then co-treated with AOPPs (200 µg/ml) for the
indicated durations. As shown in Fig.
5, SP600125 and SB203580, but not PD98059, significantly
inhibited AOPPs-induced upregulation of sclerostin and RANKL, as
confirmed by western blotting. These results indicate that
AOPPs-induced upregulation of sclerostin and RANKL is associated
with activation of JNK and p38 MAPK. As expected, NAC and apocynin
effectively suppressed AOPPs-induced upregulation of sclerostin and
RANKL (Fig. 5).
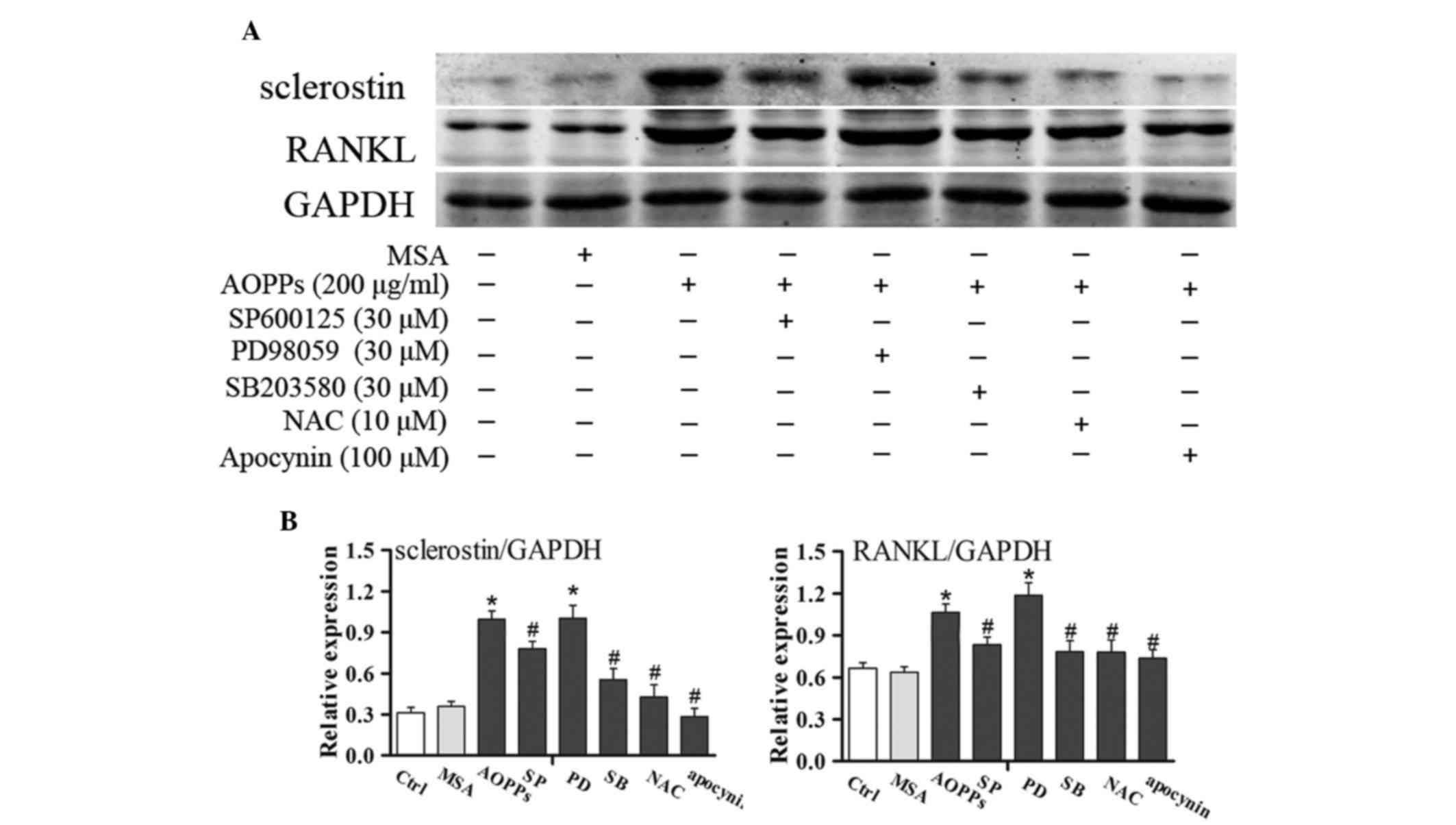 | Figure 5.Effects of mitogen-activated protein
kinases (MAPKs) signaling on advanced oxidation protein products
(AOPPs)-induced upregulation of sclerostin and receptor activator
of nuclear factor kappa-B ligand (RANKL) in MLO-Y4 cells. (A)
Overnight serum-deprived MLO-Y4 cells were pretreated with
SP600125, PD98059, SB203580, N-acetylcysteine (NAC) or apocynin for
30 min, and were then treated with 200 µg/ml AOPPs for 24 h.
Sclerostin and RANKL expression were determined by western
blotting. (B) Blots were statistically analyzed. Data are presented
as the mean ± standard deviation of three independent experiments.
Analysis of variance; *P<0.05 vs. control; #P<0.05
vs. AOPPs-treated group. Ctrl, untreated cells; MSA, mouse serum
albumin; AOPPs, AOPPs-MSA; SP, SP600125; PD, PD98059; SB, SB203580;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
AOPPs are known to induce apoptosis in several cell
types (19–21); however, their effects on osteocytes
remain unclear. The present study demonstrated that AOPPs induced
sustained activation of the JNK/p38 signaling pathways in a
ROS-dependent manner, and consequently induced apoptosis of
osteocytic MLO-Y4 cells. In addition, the results provided evidence
to suggest that treatment of MLO-Y4 cells with AOPPs increased
sclerostin and RANKL expression, and this upregulation was also
associated with the JNK/p38 signaling pathways.
Osteocytes, which are the most abundant cell type in
bones, are located deep within the mineralized matrix and
communicate with each other and with other cells on the bone
surface via cellular processes that are projected along the
canaliculi (22,23). It has previously been reported that
dysregulation of the osteocyte network is likely to increase bone
fragility and account for the higher incidence of fractures in
glucocorticoid-consuming patients (24). In the present study, treatment of
MLO-Y4 cells with AOPPs induced significant apoptosis in a dose-
and time-dependent manner. AOPPs were able to activate JNK and p38,
but not ERK MAPK in MLO-Y4 cells. Proteins in the MAPK family are
involved in various cellular responses, in particular, JNK and p38
are important mediators of apoptosis induced by stressful stimuli
(25,26). Following pretreatment with the JNK
and p38 specific inhibitors, SP600125 and SB203580, the present
study successfully demonstrated that AOPPs-induced apoptosis of
MLO-Y4 cells was markedly ameliorated. These results indicated that
JNK and p38 MAPK signaling are upstream pathways of AOPPs-induced
apoptosis of MLO-Y4 cells. Notably, the present study also
determined the effects of the NADPH oxidase inhibitor apocynin on
AOPPs-induced apoptosis in MLO-Y4 cells. As expected, apocynin
exerted an inhibitory effect on AOPPs-induced apoptosis in MLO-Y4
cells; however, the detailed signaling cascade involved was not
under investigation in the present study.
ROS are known to serve critical roles in apoptosis
(26–28), and in the present study, AOPPs were
able to induce generation of a detectable level of intracellular
ROS, as determined using the Cellular Reactive Species Detection
Assay kit (Deep Red Fluorescence). Furthermore, it has previously
been indicated that accumulated ROS induces sustained JNK and p38
MAPK activation, consequently leading to cell death (26). The results of the present study
indicated that sustained phosphorylation of JNK and p38 MAPK was
induced by ROS generation following AOPPs treatment. Notably,
inhibition of ROS by NAC, a ROS scavenger, markedly abolished the
effect of AOPPs on JNK and p38 MAPK activation, thus suggesting
that AOPPs activate JNK and p38 MAPK signaling in a ROS-dependent
manner.
Sclerostin and RANKL are two important proteins
involved in bone remodeling. Encoded by the SOST gene, sclerostin
is almost exclusively produced by osteocytes and binds to
lipoprotein-receptor related protein (LRP-5) or −6 (LRP-6) domains.
Sclerostin antagonizes LRP5/6-mediated canonical Wnt signaling
within the osteoblast, thus inhibiting osteoblast activity and
promoting their apoptosis (29).
Furthermore, sclerostin promotes osteoclast formation and activity
via upregulation of RANKL production by osteocytes (30). In a previous study, overexpression
of RANKL in transgenic mice led to severe cortical bone porosity,
whereas an anti-RANKL antibody significantly improved cortical
porosity in ovariectomized cynomolgus monkeys (31). The present study demonstrated that
significant upregulation of sclerostin and RANKL expression was
observed in AOPPs-challenged osteocytic MLO-Y4 cells. Furthermore,
as determined following treatment with MAPK inhibitors, the
AOPPs-induced upregulation of sclerostin and RANKL expression in
MLO-Y4 cells was dependent on activation of the JNK/p38 MAPK
signaling pathways.
There are some limitations to the present study.
Firstly, the characteristics of the cultured osteocytic MLO-Y4
cells may not be identical to original osteocytes in vivo;
therefore, further in vivo experiments are required to
verify the present findings. In addition, it was not determined as
to whether the AOPPs-induced production of sclerostin and RANKL was
from the apoptotic MLO-Y4 cells or from the live cells.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that AOPPs induced
apoptosis of osteocytic MLO-Y4 cells via ROS-dependent JNK/p38 MAPK
signaling. Furthermore, AOPPs stimulated the upregulation of
sclerostin and RANKL in MLO-Y4 cells, which was also associated
with activation of the JNK/p38 MAPK signaling pathways. These
findings raise the possibility that AOPPs contribute to the
development of osteoporosis under certain medical conditions with
excessive oxidative stress. Therefore, modulation of apoptotic
pathways via the MAPK signaling cascade may be considered a
therapeutic strategy for the prevention and treatment of secondary
osteoporosis.
References
|
1
|
Witko-Sarsat V, Friedlander M,
Capeillère-Blandin C, Nguyen-Khoa T, Zingraff J, Jungers P and
Descamps-Latscha B: Advanced oxidation protein products as a novel
marker of oxidative stress in uremia. Kidney Int. 49:1304–1313.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Witko-Sarsat V, Friedlander M, Khoa T
Nguyen, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM,
Jungers P, Drüeke T and Descamps-Latscha B: Advanced oxidation
protein products as novel mediators of inflammation and monocyte
activation in chronic renal failure1, 2. J Immunol. 161:2524–2532.
1998.PubMed/NCBI
|
|
3
|
Tucker PS, Dalbo VJ, Han T and Kingsley
MI: Clinical and research markers of oxidative stress in chronic
kidney disease. Biomarkers. 18:103–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krzystek-Korpacka M, Neubauer K, Berdowska
I, Boehm D, Zielinski B, Petryszyn P, Terlecki G, Paradowski L and
Gamian A: Enhanced formation of advanced oxidation protein products
in IBD. Inflamm Bowel Dis. 14:794–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baskol G, Demir H, Baskol M, Kilic E, Ates
F, Karakukcu C and Ustdal M: Investigation of protein oxidation and
lipid peroxidation in patients with rheumatoid arthritis. Cell
Biochem Funct. 24:307–311. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piwowar A, Knapik-Kordecka M and Warwas M:
AOPP and its relations with selected markers of
oxidative/antioxidative system in type 2 diabetes mellitus.
Diabetes Res Clin Pract. 77:188–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Painter SE, Kleerekoper M and Camacho PM:
Secondary osteoporosis: A review of the recent evidence. Endocr
Pract. 12:436–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong ZM, Bai L and Chen JT: Advanced
oxidation protein products inhibit proliferation and
differentiation of rat osteoblast-like cells via NF-kappaB pathway.
Cell Physol Biochem. 24:105–114. 2009. View Article : Google Scholar
|
|
9
|
Sun N, Yang L, Li Y, Zhang H, Chen H, Liu
D, Li Q and Cai D: Effect of advanced oxidation protein products on
the proliferation and osteogenic differentiation of rat mesenchymal
stem cells. Int J Mol Med. 32:485–491. 2013.PubMed/NCBI
|
|
10
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakashima T, Hayashi M, Fukunaga T, Kurata
K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et
al: Evidence for osteocyte regulation of bone homeostasis through
RANKL expression. Nat Med. 17:1231–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenov M, Tamai K and Xi H: SOST is a
ligand for LRP5/LRP6 and a wnt signaling inhibitor. J Biol Chem.
280:26770–26775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krause C, Korchynskyi O, de Rooij K,
Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides
AN, Mueller TD, Löwik CW and ten Dijke P: Distinct modes of
inhibition by sclerostin on bone morphogenetic protein and Wnt
signaling pathways. J Biol Chem. 285:41614–41626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
15
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thouverey C and Caverzasio J: Focus on the
p38 MAPK signaling pathway in bone development and maintenance.
Bonekey Rep. 4:7112015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SS, Huang QT, Zhong M and Yin Q:
AOPPs (advanced oxidation protein products) promote apoptosis in
trophoblastic cells through interference with NADPH oxidase
signaling: Implications for preeclampsia. J Matern Fetal Neonatal
Med. 28:1747–1755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou LL, Cao W, Xie C, Tian J, Zhou Z,
Zhou Q, Zhu P, Li A, Liu Y, Miyata T, et al: The receptor of
advanced glycation end products plays a central role in advanced
oxidation protein products-induced podocyte apoptosis. Kidney Int.
82:759–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie F, Sun S, Xu A, Zheng S, Xue M, Wu P,
Zeng JH and Bai L: Advanced oxidation protein products induce
intestine epithelial cell death through a redox-dependent, c-jun
N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated
pathway. Cell Death Dis. 5:e10062014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonewald LF: The amazing osteocyte. J Bone
Res. 26:229–238. 2011. View
Article : Google Scholar
|
|
23
|
Compton JT and Lee FY: A review of
osteocyte function and the emerging importance of sclerostin. J
Bone Joint Surg Am. 96:1659–1668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seibel MJ, Cooper MS and Zhou H:
Glucocorticoid-induced osteoporosis: Mechanisms, management and
future perspectives. Lancet Diabetes Endocrinol. 1:59–70. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Osone S, Hosoi H, Kuwahara Y, Matsumoto Y,
Iehara T and Sugimoto T: Fenretinide induces sustained-activation
of JNK/p38 MAPK and apoptosis in a reactive oxygen
species-dependent manner in neuroblastoma cells. Int J Cancer.
112:219–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park GB, Choi Y, Kim YS, Lee HK, Kim D and
Hur DY: ROS-mediated JNK/p38-MAPK activation regulates bax
translocation in sorafenib-induced apoptosis of EBV-transformed B
cells. Int J Oncol. 44:977–985. 2014.PubMed/NCBI
|
|
27
|
Lee SY, Kang SU, Kim KI, Kang S, Shin YS,
Chang JW, Yang SS, Lee K, Lee JS, Moon E and Kim CH: Nonthermal
plasma induces apoptosis in ATC cells: Involvement of JNK and p38
MAPK-dependent ROS. Yonsei Med J. 55:1640–1647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palit S, Kar S, Sharma G and Das PK:
Hesperetin induces apoptosis in breast carcinoma by triggering
accumulation of Ros and activation of ASK1/JNK Pathway. J Cell
Physiol. 230:1729–1739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Winkler DG, Sutherland MK, Geoghegan JC,
Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR,
Staehling-Hampton K, et al: Osteocyte control of bone formation via
sclerostin, a novel BMP antagonist. EMBO J. 22:6267–6276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wijenayaka AR, Kogawa M, Lim HP, Bonewald
LF, Findlay DM and Atkins GJ: Sclerostin stimulates osteocyte
support of osteoclast activity by a RANKL-dependent pathway. PloS
one. 6:e259002011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kostenuik PJ, Smith SY, Jolette J,
Schroeder J, Pyrah I and Ominsky MS: Decreased bone remodeling and
porosity are associated with improved bone strength in
ovariectomized cynomolgus monkeys treated with denosumab, a fully
human RANKL antibody. Bone. 49:151–161. 2011. View Article : Google Scholar : PubMed/NCBI
|