Introduction
Pulmonary arterial hypertension (PAH) is a chronic
disorder of the small pulmonary arteries and vasculature,
characterized by increasing vascular narrowing, leading to
progressive pulmonary vascular resistance, with potentially fatal
consequences, such as right heart failure (1,2). As
a multifactorial disorder, PAH can be idiopathic or associated with
underlying conditions, including scleroderma, rheumatoid arthritis
and infection (3). The
pathobiology of PAH is complex, and involves diverse pathological
events, including vasoconstriction, thrombosis, inflammation,
structural remodeling of pulmonary vessels, in addition to
disordered production of vasoactive mediators (1,2,4,5).
Despite an improved understanding of the therapeutic development of
PAH, the efficacy of the therapies and prognosis remain
unsatisfactory. Therefore, it is essential to screen sensitive and
specific therapeutic targets and biomarkers to provide improved
clinical tools for early diagnosis and therapeutic evaluation of
PAH.
Owning to its analytical abilities and efficient and
accurate simultaneous expression of a large volume of genes,
microarray technology has been widely implemented in the
investigation of complicated disorders, including cancer,
cardiovascular diseases and diabetes (6,7). As
the pathogenesis of PAH is complex, it is suggested that various
genes and environmental factors are involved. The expression of key
genes and regulatory networks challenge the traditional methods,
while microarray technology provides a potential tool to further
explore the pathogenesis of PAH (8,9).
Additionally, high-throughput screening aids in the identification
of potential biomarkers for diagnosis, prognosis and as therapeutic
targets. Several studies have taken advantage of gene microarray to
analyze the peripheral blood cells and smooth muscle cells from PAH
patients (9,10), and identified multiple key genes
involved in PAH, such as anti-bradykinin B2 receptor, demonstrating
the powerful potential of high-throughput technology to aid in the
understanding of the pathophysiology of PAH.
miRNAs are small non-coding RNAs that have emerged
as key post-transcriptional modulators of gene expression, acting
by targeting mRNAs for translational repression or destabilization
(11). Hundreds of mammalian
miRNAs have been identified, and they are important in various
biological events and pathological processes (12,13).
For PAH, reduced plasma miR-150 has been demonstrated to be
associated with poor survival (14). Although the source and working
mechanism of miR-150 remain to be fully elucidated, it appears that
it has a pivotal role in the pathogenesis of PAH. An additional
study has reported the emerging role of miRNAs during this process;
however, miRNAs in PAH remain to be fully investigated (15).
The present study intended to comprehensively
analyze the miRNA profile of lung tissues in monocrotaline-induced
PAH rat models. The present study identified 16 downregulated
miRNAs in smooth muscle cells from PAH rats. Targeted gene
prediction and gene ontology (GO) analysis identified certain
unexpected signaling pathways, which potentially serve as
therapeutic targets for PAH.
Materials and methods
Monocrotaline-induced PAH animal
model
A total of 35 adult male Wistar rats (7 rats as
controls, 28 rats as PAH models) were purchased from the Shanghai
Laboratory Animal Center (Chinese Academy of Sciences, Shanghai,
China), weighing 350–400 g. Animals were fed standard chow and
administered a subcutaneous injection of monocrotaline (MCT) 60
mg/kg (MCT group; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) or 0.9% saline as the control group (sham; n=7). The
weight and health status were monitored regularly, and the rats
were euthanized using 3% chloral hydrate (47335-U; Sigma-Aldrich;
Merck Millipore) to induce anesthesia at days 7, 14, 21 and 28
following MCT administration. The lungs were removed and lavaged
before further treatment. All animal experiments were approved by
The Institutional Animal Care and Use Committee of Shanghai
Jiaotong University (Shanghai, China) and were in accordance with
the Guide for the Care and Use of Laboratory Animals (16).
RNA preparation and quality
control
Rat lungs were homogenized and total RNA (enriched
for small RNA) was extracted according to manufacturer's
instructions using the mirVana miRNA isolation kit (Ambion; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and RNA concentration
was quantified using NanoDrop ND-2100 (Thermo Fisher Scientific,
Inc.). The RNA integrity was assessed using Agilent 2100 (Agilent
Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's instructions. To ensure a robust analysis, total RNA
was assessed by electrophoresis and the RNA integrity number (RIN).
Only samples with acceptable XVIIIS and 28S peaks and RIN values
greater than eight were included for miRNA profile analysis.
miRNA microarray expression
profile
Total RNAs from PAH rats and controls were used for
miRNA microarray profiling. miRNA expression was determined by
Affymetrix miRNA 3.0 array (Affymetrix, Inc., Santa Clara, CA, USA)
containing probes for a total of 1,242 rat miRNAs. The sample
labeling, microarray hybridization and washing were performed
according to the manufacturer's instructions. Briefly, total RNA
were tailed with Poly A and then labeled with Biotin, followed by
hybridization onto the microarray. After washing and staining
(GeneChip Fluidics Station 450; Affymetrix, Inc.), the arrays were
processed using the Affymetrix Scanner 3000 (Affymetrix, Inc.). The
scanned images were analyzed using Expression Console software
(version, 3.1; Affymetrix, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Based on the microarray results, candidate miRNAs
were selected for further validation with RT-qPCR. TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to prepare
the total RNAs from lung tissue for miRNA quantitation, and the RNA
was reverse transcribed using the TaqMan miRNA reverse
transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and random hexamer primer. The 10 ng cDNA was used as
template for miRNA-specific stemloop primers (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. miRNA expression assays were conducted using TaqMan
primers and probes (Applied Biosystems; Thermo Fisher Scientific,
Inc.) for endogenous control small RNAs RNU48 (ID 001006) and
target miRNAs. Each sample was run in triplicate for analysis. The
primers (miRNA-specific stem-loop primers) and miRNA-specific PCR
primer/probe mix were based on the miRNA sequences obtained from
the miRBase database. Relative quantifications were performed using
the Applied Biosystems 7900HT Fast Real-time PCR system. Data were
collected using SDS software (version 2.3; Applied Biosystems;
Thermo Fisher Scientific, Inc.). Subsequent to being normalized to
RNU48, the expression levels of miRNAs were calculated using the
2-ΔΔCq method (17).
One-way analysis of variance tests were performed for global
comparisons and Tukey's post hoc tests for pair-wise comparisons
using SPSS software (version 19.0; IBM SPSS, Armonk, NY, USA).
GO analysis
GO analysis was used to perform enrichment analysis
of gene sets based on the GO classification database. For this
analysis, Fisher's exact test and the χ2 test were used to classify
the GO category, and the false discovery rate (FDR) was calculated
to correct the P-value, as smaller FDR represents smaller errors in
the P-values. P-values for the GOs of all the differentially
expressed genes were computed, and the significance of the function
was measured based on enrichment analysis.
miRNA target prediction and pathway
analysis
The target genes of the differentially expressed
miRNAs were predicted with TargetScan software, (version 6.2;
http://www.targetscan.org/vert_40/)
and miRanda (August 2010; (http://www.microrna.org/microrna/home.do). The search
was based on the 3′-UTR regions of target mRNAs with a minimum
miRNA seed length of seven. Only the genes identified
simultaneously by the two programs were regarded as potential
target genes.
To identify the significant pathway of the
differentially expressed genes, Kyoto Encyclopedia of Genes and
Genomes (KEGG) was used for the pathway enrichment analysis.
Similarly, the Fisher's exact test and χ2 test were used
to determine the P-values and FDR. Based on differential expression
values, the association between the miRNAs and genes was computed,
and the miRNA gene network was constructed according to the
interactions of miRNA and genes in the Sanger miRNA database.
miRNA-GO-network
The miRNA-GO-network is built according to the
association between significant GOs and genes and the associations
among miRNAs and genes. In the gene network, circles represent
genes and squares represent miRNAs, and their associations are
represented by edges. The center of the network is represented by
degree, which means the contribution made by one miRNA to the
surrounding genes, or the contribution made by one gene to the
surrounding miRNAs. The key miRNAs and genes in the network always
have the largest degrees.
Statistical analysis
Student's t-test or one way analysis of variance
tests were used, unless otherwise specified, to determine the
significance between PAH and healthy controls using SPSS software
(version 19.0; IBM SPSS). P<0.05 was considered to indicate a
statistically significant difference.
Results
miRNA expression profile in lung of
PAH rats
Using an Affymetrix miRNA 3.0 array, the study
investigated the global expression profile of mature miRNA in lungs
from PAH rats. Total RNAs from PAH rats and controls were used for
miRNA microarray profiling. The Affymetrix miRNA 3.0 array contains
probes for a total of 1,242 rat miRNAs, and was used to capture the
expression signaling of 440 miRNAs. Compared with the control
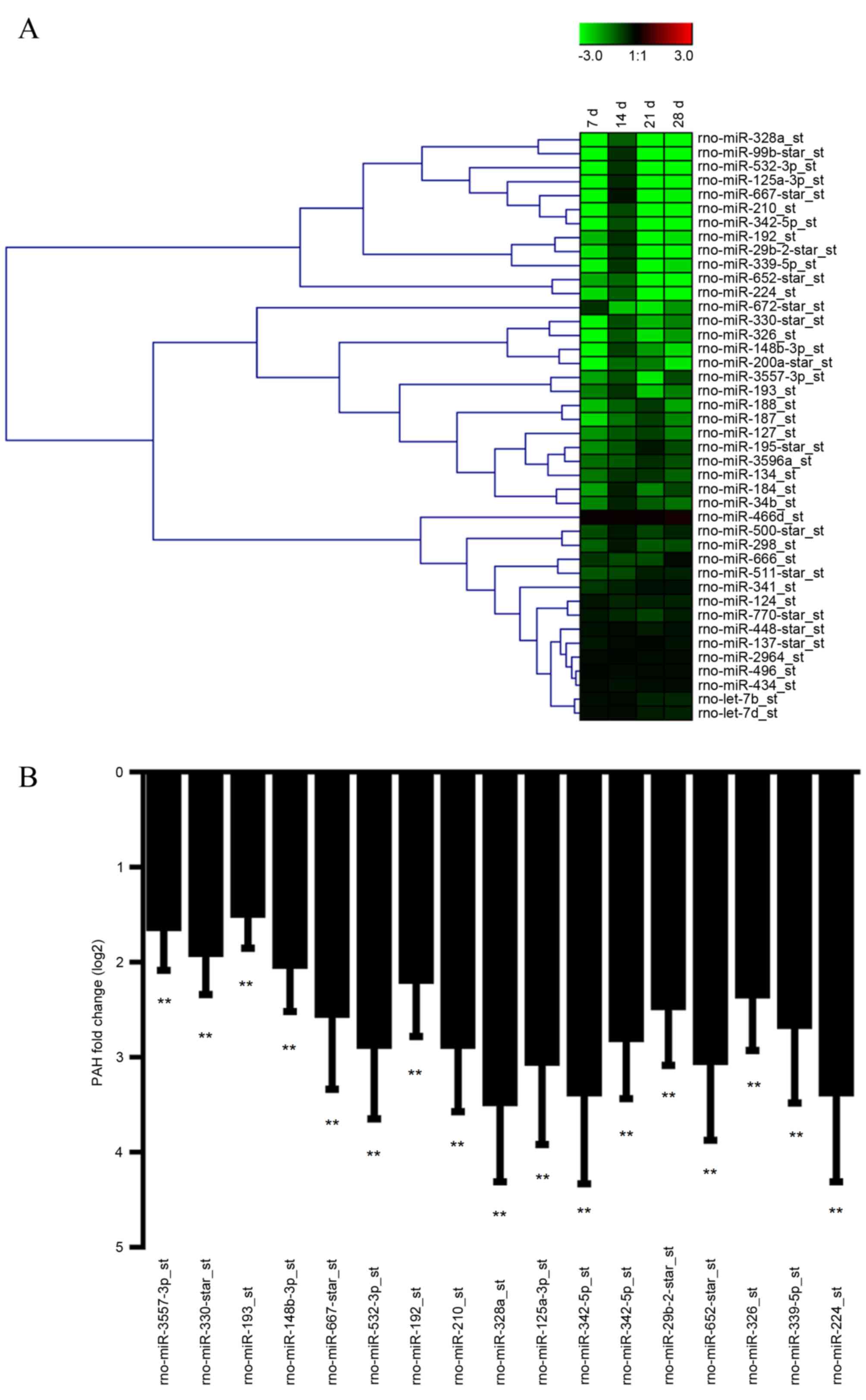
group, 42 miRNA displayed markedly reduced expression (Fig. 1A) in lungs from PAH rats. After
normalization, a total of 17 miRNAs exhibited significant
expression changes in lungs from PAH rats (P<0.01; Fig. 1B). Among them, miR-3557-3p is
constantly expressed in control animals, while no signaling was
detected in PAH rats. In addition RT-qPCR was performed to validate
the array results for all 17 miRNAs, and the data showed similar
results, confirming the expression change of the miRNAs.
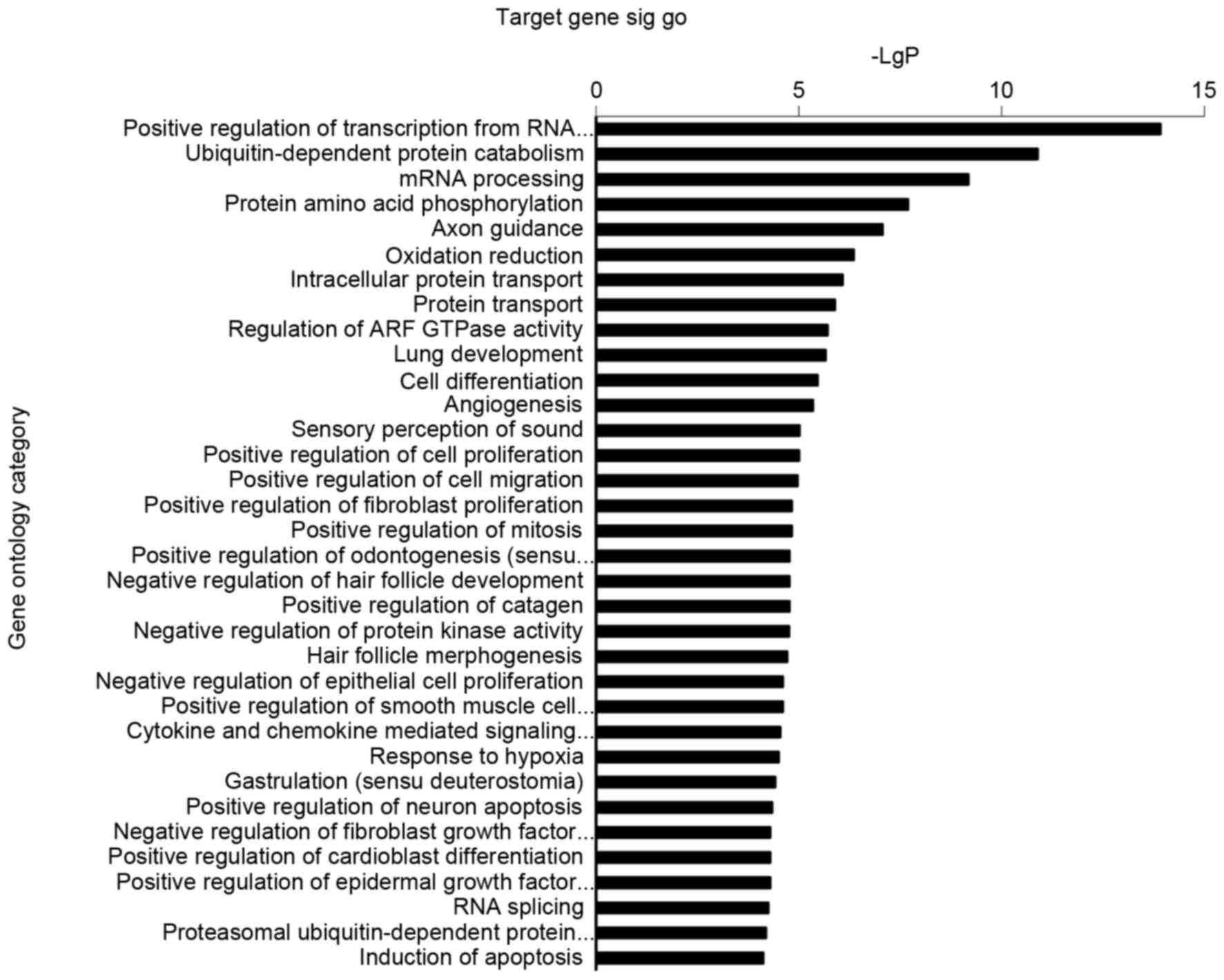
Microarray-based GO analysis
P<0.05 was considered the threshold of
significance for GOs regulated by miRNAs. In the present study, the
highly enriched GOs corresponding to downregulated miRNAs included
positive regulation of transcription from RNA polymerase I
promoter, ubiquitin-dependent protein catabolism, mRNA processing,
protein amino acid phosphorylation, oxidation reduction and
intracellular protein transport (Fig.
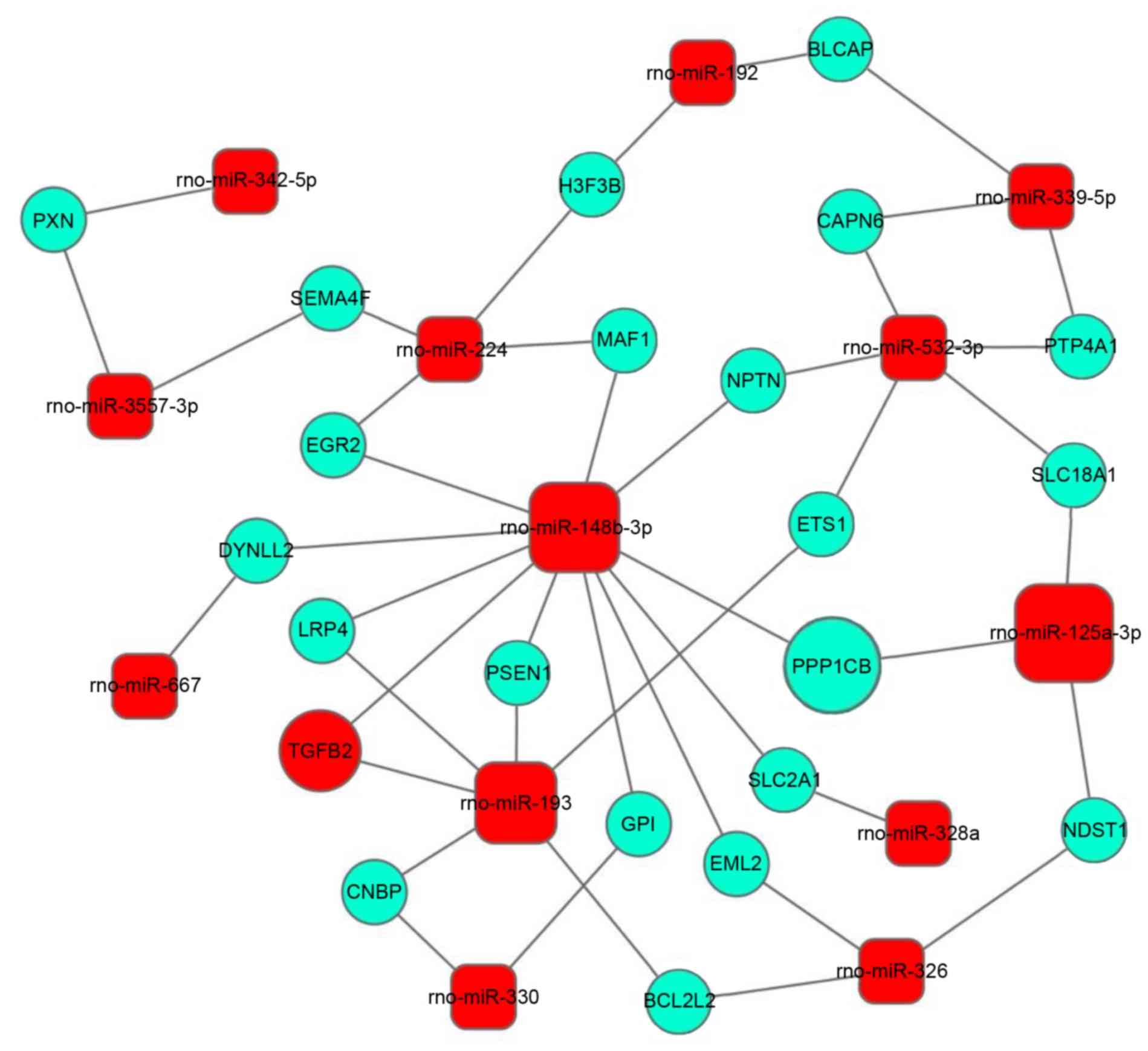
2). Subsequently, miRNA-mRNA network analysis was performed
integrating these miRNAs and GOs by outlining the interactions of
miRNA and GO-associated genes (Fig.
3). Three downregulated miRNAs (miR-125-3p, miR-148-3p and
miR-193) displayed the most marked regulatory function, and
miR-148-3p and miR-193 were demonstrated to target the highest
number of mRNAs. Among which, miR-125-3p regulates protein
phosphatase 1 catalytic subunit b (PPP1CB), which may bind tumor
protein p53 binding protein 2 (TP53BP2) to form a complex to
regulate cellular apoptosis (18).
miR-148-3p is predicted to regulate transforming growth factor β2
(TGF-β2), which serves multiple functions in diverse biological
events, therefore, may be of importance in PAH.
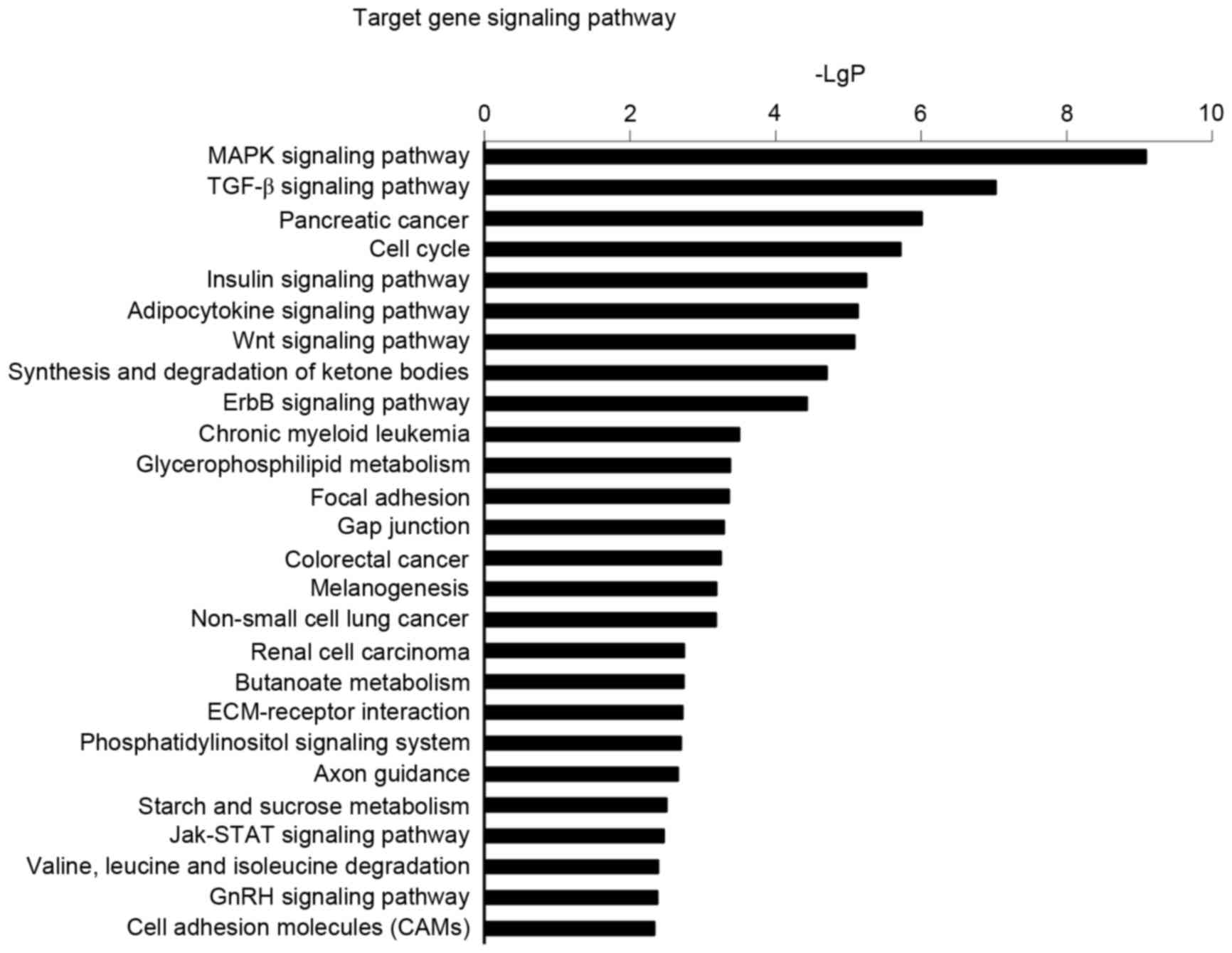
Signaling pathways regulated by
differentially expressed miRNA
In order to further elucidate the regulatory
function of miRNAs in signal transduction, functional analysis of
miRNAs was implemented by KEGG and the results demonstrated that 26
signal transduction pathways were upregulated in PAH (Fig. 4). The prominent upregulated
signaling pathways including mitogen-activated protein kinase
(MAPK), TGF-β and the cell cycle have been demonstrated to
participate in the pathogenesis of pulmonary arterial hypertension,
suggesting essential roles for these pathways in PAH.
miRNA target analysis
In total, 342 genes were identified with TargetScan
and miRanda to be the potential targets of these 15 miRNAs.
According to GO analysis and signaling pathway analysis, these
genes potentially participate in numerous biological effects,
including cell cycle, cell proliferation, differentiation and
apoptosis. Of note, axon guidance potentially served an unexpected
role in PAH pathogenesis (Fig. 2).
Similarly, the pathway analysis also indicated a significant
enrichment in axon guidance (Fig.
4), which may possibly lead to the elucidation of a novel
mechanism and therapeutic approaches.
Discussion
Multiple factors, genetic and environmental,
participate in the pathogenesis of PAH, which involves a multitude
of cellular and molecular elements (19). Proliferation of smooth muscle cells
in the small pulmonary arteries is commonly observed in all forms
of PAH. Other events, including abnormal proliferation of
endothelial cells, inflammation, thrombosis and platelet
dysfunction are also common (1,4,20).
Given the universal role of miRNA in biological regulation, it is
expected that certain miRNAs, including miR-32, 20, 245 and 122
have been reported to be involved in PAH (21). The present study identified certain
novel miRNAs by employing a comprehensive miRNA array screening,
including miR-125-3p, miR-148-3p and miR-193.
miRNAs exert their functions by regulating target
proteins. For example, in PAH the miR-210 expression is decreased,
and the downregulation of miR-204 increases expression of SHP2,
initiating a signaling cascade involving Src kinase and signal
transducer and activator of transcription 3 activation, thus
promoting pulmonary vessel wall thickening and smooth muscle
proliferation (22). As for the
newly discovered genes, although no studies have reported an
association with PAH, the target prediction provides insight into
how these miRNAs are involved in the condition. miR-125-3p is
expressed in normal lung tissue, and involved in cellular apoptosis
in lung cancer cells. One of the predicted targets of miR-125-3p is
PPP1CB, which may bind TP53BP2 to form a complex to regulate
cellular apoptosis (18), and is
also involved in pulmonary arterial hypertension (9). However, the notable association
between miR-125-3p and PAH pathogenesis requires further
research.
TGF-β has been reported to contribute to PAH
development, as endothelial TGF-β/activin receptor-like kinase
1/endoglin signaling leads to pulmonary blood vessel angiogenesis,
macrophage infiltration, and cytokine expression in the lungs
(23,24). The present study identified
miR-148-3p in PAH, and identified TGF-β as a potential target of
miR-148-3p. The association between TGF-β and miR-148-3p
necessitates further investigation, as a positive answer to this
may potentially lead to the development of novel therapeutic
approaches. Another promising topic is whether axon guidance is
involved in PAH pathogenesis, an area which remains to be
investigated fully. In addition, it is suggested that the
underlying mechanism may be an association between neuroscience and
PAH (3).
The present study identified a set of miRNAs in the
lungs from PAH animals and identified unexpected associations
between biological events and PAH pathogenesis, which may aid in
the development of provide strategic therapeutic approaches.
However, limitations were present in the study; for example, no
cell-specific identification of these miRNAs was conducted, and how
these miRNA function in PAH remains unclear. This should be the
subject of further investigations.
Acknowledgements
The present study was supported by the Shanghai
Committee of Science and Technology, China (grant no.
14ZR1434300).
References
|
1
|
Rabinovitch M, Guignabert C, Humbert M and
Nicolls MR: Inflammation and immunity in the pathogenesis of
pulmonary arterial hypertension. Circ Res. 115:165–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuder RM, Stacher E, Robinson J, Kumar R
and Graham BB: Pathology of pulmonary hypertension. Clin Chest Med.
34:639–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zamanian RT, Kudelko KT, Sung YK, de Jesus
Perez V, Liu J and Spiekerkoetter E: Current clinical management of
pulmonary arterial hypertension. Circ Res. 115:131–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nogueira-Ferreira R, Ferreira R and
Henriques-Coelho T: Cellular interplay in pulmonary arterial
hypertension: Implications for new therapies. Biochim Biophys Acta.
1843:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perros F, Humbert M and Cohen-Kaminsky S:
Pulmonary arterial hypertension: A flavor of autoimmunity. Med Sci
(Paris). 29:607–616. 2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saei AA and Omidi Y: A glance at DNA
microarray technology and applications. Bioimpacts. 1:75–86.
2011.PubMed/NCBI
|
|
7
|
Szelinger S, Pearson JV and Craig DW:
Microarray-based genome-wide association studies using pooled DNA.
Methods Mol Biol. 700:49–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menon S, Fessel J and West J: Microarray
studies in pulmonary arterial hypertension. Int J Clin Pract Suppl.
19–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu J, Wilson J, Taylor L and Polgar P: DNA
microarray and signal transduction analysis in pulmonary artery
smooth muscle cells from heritable and idiopathic pulmonary
arterial hypertension subjects. J Cell Biochem. 116:386–397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bull TM, Coldren CD, Moore M,
Sotto-Santiago SM, Pham DV, Nana-Sinkam SP, Voelkel NF and Geraci
MW: Gene microarray analysis of peripheral blood cells in pulmonary
arterial hypertension. Am J Respir Crit Care Med. 170:911–919.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hata A: Functions of MicroRNAs in
cardiovascular biology and disease. Annu Rev Physiol. 75:69–93.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rhodes CJ, Wharton J, Boon RA, Roexe T,
Tsang H, Wojciak-Stothard B, Chakrabarti A, Howard LS, Gibbs JS,
Lawrie A, et al: Reduced microRNA-150 is associated with poor
survival in pulmonary arterial hypertension. Am J Respir Crit Care
Med. 187:294–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu D, Talbot CC Jr, Liu Q, Jing ZC, Damico
RL, Tuder R, Barnes KC, Hassoun PM and Gao L: Identifying microRNAs
targeting Wnt/β-catenin pathway in end-stage idiopathic pulmonary
arterial hypertension. J Mol Med (Berl). 94:875–885. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Committee
on Educational Programs in Laboratory Animal Science, . Education
and Training in the Care and Use of Laboratory Animals: A Guide for
Developing Institutional Programs. National Academies Press;
Washington, DC: 1991
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leotta M, Biamonte L, Raimondi L,
Ronchetti D, Di Martino MT, Botta C, Leone E, Pitari MR, Neri A,
Giordano A, et al: A p53-dependent tumor suppressor network is
induced by selective miR-125a-5p inhibition in multiple myeloma
cells. J Cell Physiol. 229:2106–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Montani D, Günther S, Dorfmüller P, Perros
F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC,
et al: Pulmonary arterial hypertension. Orphanet J Rare Dis.
8:972013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huertas A, Perros F, Tu L, Cohen-Kaminsky
S, Montani D, Dorfmüller P, Guignabert C and Humbert M: Immune
dysregulation and endothelial dysfunction in pulmonary arterial
hypertension: A complex interplay. Circulation. 129:1332–1340.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Xue XY, Liu YX, Wang KF, Zang XF,
Wang J, Wang PL, Zhang J, Pan L, Zhang SY and Wang JX: Pulmonary
arterial hypertension and microRNAs-an ever-growing partnership.
Arch Med Res. 44:483–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Courboulin A, Tremblay VL, Barrier M,
Meloche J, Jacob MH, Chapolard M, Bisserier M, Paulin R, Lambert C,
Provencher S and Bonnet S: Kruppel-like factor 5 contributes to
pulmonary artery smooth muscle proliferation and resistance to
apoptosis in human pulmonary arterial hypertension. Respir Res.
12:1282011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rde C Ferreira, Montenegro SM, Domingues
AL, Bandeira AP, Silveira CA, Leite LA, Cde A Pereira, Fernandes
IM, Mertens AB and Almeida MO: TGF beta and IL13 in Schistosomiasis
mansoni associated pulmonary arterial hypertension; a descriptive
study with comparative groups. BMC Infect Dis. 14:2822014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gore B, Izikki M, Mercier O, Dewachter L,
Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Lebrin F
and Eddahibi S: Key role of the endothelial TGF-b/ALK1/endoglin
signaling pathway in humans and rodents pulmonary hypertension.
PLoS One. 9:e1003102014. View Article : Google Scholar : PubMed/NCBI
|