Introduction
Colorectal carcinoma (CRC) is one of the most
frequent malignancies affecting men and women worldwide. According
to a previous statistic, a total of 102,480 new cases of CRC were
estimated within the United States in 2013. In addition, 50,830
cases of CRC-associated mortality were estimated, making it the
third leading cause of cancer-associated mortality worldwide
(1). Although a level of
progression has been achieved in treating CRC in past decades, the
overall survival rate of patients suffering from CRC has remained
poor (2,3). Therefore, the identification of novel
strategies for the treatment and prevention of CRC is urgently
required.
Global evidence has established that inflammation is
a well-recognized risk factor for cancer development (4–6).
Anti-inflammatory agents have been shown to be associated with
reduced risks of developing CRC and improved survival rates in
patients with CRC (7,8). Inflammation also has effects on tumor
biology. For example, the local intratumoral inflammatory response,
as evidenced by a high density of tumor-infiltrating lymphocytes,
is considered to be a prognostic indicator for several types of
malignancy, including CRC (9,10).
By contrast, systemic inflammation has always been associated with
poor prognosis in CRC (11,12).
Therefore, the development and progression of CRC may be closely
associated with immune regulatory processes.
T cell immunoglobulin mucin-3 (Tim-3) belongs to the
Tim family, the members of which are cell surface receptors
differentially expressed on mature T lymphocytes and macrophages.
Specifically, Tim-3 is expressed in the Th1 subset, however, it is
not expressed on Th2 cells (13,14).
The expression of Tim-3 is also present on macrophages, dendritic
cells and mast cells (15,16). The mechanisms underlying the immune
regulatory reaction of Tim-3 are associated with controlling the
functionality of T cell subsets, which occurs by inducing
activating or apoptotic signals following interaction with its
ligand, galectin-9 (17). Of note,
with the exception of the immune response, increasing evidence has
suggested that Tim-3 has functional roles in tumor biology. The
expression of Tim-3 in peripheral blood monocytes and in tumor
tissues has been suggested to be prognostic in prostate cancer
(18). Tim-3 may affect
development and progression, and be a therapeutic target in
prostate cancer (19). The role of
Tim-3 in human tumorigenesis is not limited to prostate cancer. Its
tumor involvement in humans has been widely reported in various
types of cancer, including clear cell renal cell carcinoma,
hepatocellular carcinoma and melanoma (20–23).
Targeting Tim-3 pathways has been suggested to reverse T cell
exhaustion and restore antitumor immunity (24), therefore, Tim-3-targeted antitumor
immunotherapy has been suggested as a prospective therapeutic
strategy (21).
Despite the emerging evidence that Tim-3 may be
critical in tumorigenesis, the role of Tim-3 in CRC remains to be
fully elucidated. Considering CRC is closely associated with
regulatory processes in inflammation, the present study
hypothesized that Tim-3 may be a critical molecular involved in the
development and progression of CRC. Therefore, the present study
aimed to investigate whether Tim-3 is aberrantly expressed in
clinical CRC samples and to assess the biological activities of
Tim-3 in CRC cell lines.
Materials and methods
Human samples
Tissue samples from 30 cases of CRC and their
adjacent non-cancerous tissues were collected from patients who
underwent surgical tumor resection at Tianjin Medical University
General Hospital (Tianjin, China) between January 1, 2014 and
January 1, 2015. Slides from 112 paraffin-embedded CRC cases were
also obtained from The Department of pathology, Tianjin Medical
University General Hospital. All patients confirmed involvement in
the present study, and written consent was obtained. This research
was approved by the ethics committee of Tianjin Medical University
General Hospital.
Histological and immunohistochemical
(IHC) analysis
Following dissection from the patients, the tumor
tissues were fixed in formaldehyde solution and embedded in
paraffin to produce 4 µm slices. Following antigen retrieval in 0.1
M citric acid buffer (pH 6.0) in a microwave, the slices were
incubated with primary antibody against Tim-3 (cat. no. ab185703;
Abcam, Cambridge, UK) at 4°C overnight. On the subsequent day, the
slices were washed with Tris-buffered saline three times and
incubated with secondary antibody (1:1,000; cat. no. sc-3836; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at 37°C for 1 h,
following which the slices were developed with 0.05%
diaminobenzidine supplemented with 0.01%
H2O2. As a negative control, normal goat
serum (Beyotime Institute of Biotechnology, Haimen, China) was used
in place of the specific primary antibody. Images were captured
with a Nikon light microscope (Nikon, Tokyo, Japan; magnification,
×400).
Cell culture and antibodies
The human colorectal adenocarcinoma cell lines, COLO
205 and HT-29, the CRC cell line, HCT116 and the human embryonic
kidney cell line, 293T, were purchased from America Type Culture
Collection (Manassas, VA, USA). All cells were cultured in the
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplied with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a humidified
incubator with 5% CO2 at 37°C. For the transfection
assays, the cells were grown until 60% confluent and transfected
with small interfering (si)RNAs using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. GAPDH was included as an internal control,
and its primary antibody and secondary antibodies were purchased
from Santa Cruz Biotechnology, Inc.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the human tissues and cultured cells
were extracted using TRIzol solution (Takara Biotechnology Co.,
Ltd., Dalian, China) and quantified using a Nanodrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). A 1 µg sample
of mRNA was reverse transcribed using PrimeScript RT Master Mix
(Perfect Real Time) kit (Takara Biotechnology Co., Ltd.) and qPCR
was performed in an ABI PRISM 7900 Real Time system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the SYBR Premix
Ex Taq kit (Takara Biotechnology Co., Ltd.). The primers used were
as follows: Tim-3, forward 5′-GCTACTACTTACAAGGTCCTCAG-3′ and
reverse 5′-ATTCACATCCCTTTCATCAGTC-3′; GAPDH, forward
5′-GTGGACATCCGCAAAGAC-3′ and reverse 5′-AAAGGGTGTAACGCAACTA-3′. U
Initial denaturation was performed at 95°C for 30 sec, and PCR by
40 cycles of 95°C for 5 sec and 60°C for 35 sec. All experiments
were performed in triplicate at least three times. Values were
calculated used the 2−ΔΔCq method (25)
Western blot analysis
The cells were lysed with lysis buffer supplemented
with protease inhibitors. The proteins obtained from the CRC cell
lines were quantified using a Bicinchoninic Acid kit (Thermo Fisher
Scientific, Inc.). Subsequently, a total of 50 µg protein was
loaded onto a 10% SDS-PAGE gel for separation, and then transferred
onto a nitrocellulose membrane. Following blocking in 5% milk for 1
h at room temperature, the membrane was incubated with primary
antibodies against Tim-3 (1:1,000; cat. no. ab185703; Abcam) and
GAPDH (1:1,000; cat. no. sc-365062; Santa Cruz Biotechnology, Inc.)
at 4°C overnight. The following day, the membrane was washed with
TBS with Tween 20 three times and incubated with secondary
antibodies (1:1,000; cat. no. sc-3836; Santa Cruz Biotechnology,
Inc.) for another 1 h at 37°C. Protein expression was quantified
using enhanced chemiluminescence (Thermo Fisher Scientific, Inc.)
and images were captured using the LAS3000 imaging system (Fujifilm
Corporation, Tokyo, Japan).
Cell viability assay
The HCT116 and HT-29 cells were seeded in 96-well
plates (5×103 cells/well) and allowed to grow overnight
at 37°C. The cells were then transfected with either control siRNA
or specific siRNA against Tim-3 (synthesized by GenePharma Co.,
Ltd., Shanghai, China) and grown for another 72 h. Subsequently,
cell viability assays were performed consecutively for 5 days using
MTT solution. Following the addition of 2 mg/ml MTT for 4 h at
37°C, the medium was discarded and 200 µl of DMSO was added to each
well. The solution was added into each well on each of the
monitored days. The cells were incubated for an additional 5 min on
a shaker and the optical density was determined at 570 nm.
Cell migration and invasion
assays
The HCT116 and HT-29 cells were cultured in 24-well
plates and transfected with the specific Tim-3 siRNA or control
siRNA. At 48 h post-transfection, the cells were harvested in
serum-free medium as a single cell suspension, and 150 µl volume of
cell suspension (3×104 cells) was seeded into the upper
Transwell chamber (Corning Incorporated, Corning, NY, USA). A 600
µl volume of medium supplemented with 10% FBS was added to the
lower chamber. For the invasion assay, the chamber was coated with
Matrigel and incubated for 6 h at 37°C to solidify prior to seeding
the cells into the chamber. Subsequently, the cells were incubated
for 24 h, then, fixed with ice-cold methanol for 20 min and then
stained with 0.1% crystal violet for another 5 min. Images were
captured under a Nikon light microscope (magnification, ×200). The
number of cells from each well were counted in 10 randomly selected
fields.
Wound healing assay
The HCT116 and HT-29 CRC cells (1×106)
were seeded into 6-well plates and transfected with specific siRNA
against Tim-3 (0, 0.3, 0.6, 0.9, 1.2 and 1.5 µm). At 48 h
post-transfection, the cells were washed twice with PBS and a 10 µl
sterile pipette tip was then used to scratch a cross in the center
of each well. Following scratching, the cells were rinsed with PBS
again, and immediately placed in serum-free medium. The cells were
then allowed to migrate for another 24 h, following which the
scratches in each group were observed and images were captured.
Each assay was repeated in triplicate at least three times.
Statistical analysis
SPSS software (Chicago, IL, USA) was used for
statistical analysis. Student's t-test was used for simple
comparisons between different groups. Regression analysis was used
to evaluate dose-response associations. Values are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Tim-3 is overexpressed in CRC and
associated with tumor progression
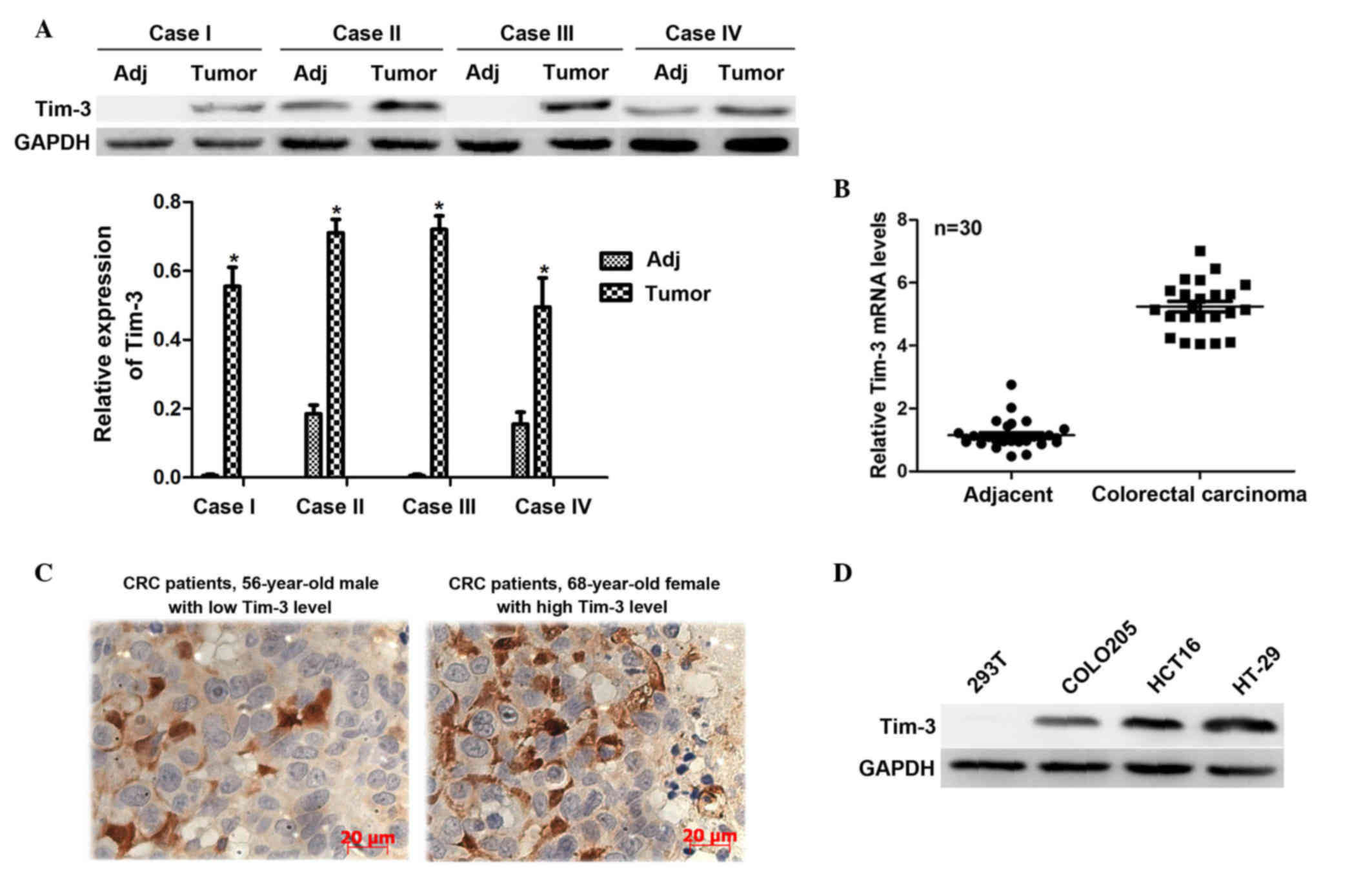
The present study first investigated the expression
levels of Tim-3 in 30 clinical CRC tissues and their adjacent
non-cancerous tissues. The protein levels were analyzed using
western blot analysis. It was shown that the protein levels of
Tim-3 in the cancerous samples were significantly higher, compared
with those in the paired non-cancerous samples in the
representative four cases (Fig.
1A). The total RNA extracted from the CRC tissues and their
adjacent non-cancerous tissues were subjected to RT-qPCR analysis.
It was observed that the mRNA levels of Tim-3 in the CRC tissues
were >5-fold higher than those in the adjacent tissues (Fig. 1B). These results suggested that
Tim-3 was expressed at a high level in the clinical CRC tissues.
Furthermore, to assess the association between the clinical
expression of Tim-3 and clinicopathological parameters, IHC
staining was performed to detect the Tim-3 antigen in the slides
from the 112 CRC cases. Based on the IHC results, the staining
intensity of Tim-3 was classified as low expression or high
expression for each case (Fig.
1C). Statistical analysis revealed that the expression levels
of Tim-3 were significantly positively correlated with tumor size
(P=0.007; R2=0.258), TNM staging (P<0.001;
R2=0.367) and distant metastasis (P<0.001;
R2=0.339), however, expression was not correlated with
demographic data, including age and gender (Table I). This data suggested that the
expression of Tim-3 is associated with tumor progression in CRC. In
addition, COLO 205 and HT-29 are two representative human CRC cell
lines, and HCT116 is a typical CRC cell line. Proteins from these
cell lines were extracted to examine the expression levels of Tim-3
in vitro. Compared with the 293T control cells, Tim-3 was
overexpressed in the CRC cell lines, particularly in the HCT116 and
HT-29 cells (Fig. 1D). Therefore,
these two cell lines were selected for subsequent analyses. These
data suggested the close association between the expression of
Tim-3 and oncogenic activity in CRC.
 | Table I.Correlation of the expression of Tim-3
with clinicopathological parameters in 112 cases of colorectal
carcinoma. |
Table I.
Correlation of the expression of Tim-3
with clinicopathological parameters in 112 cases of colorectal
carcinoma.
|
|
| Expression of
Tim-3 |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameter | No. | Low (n=65) | High(n=47) | P-value | Correlation
coefficient |
|---|
| Age (years) |
|
|
| 0.220 |
|
<50 | 29 | 14 | 15 |
| −0.117 |
| ≥50 | 83 | 51 | 32 |
|
| Gender |
|
|
| 0.775 |
| Male | 68 | 43 | 25 |
| −0.027 |
|
Female | 44 | 29 | 15 |
|
| Tumor size (cm) |
|
|
| 0.007 |
| ≤2 | 71 | 54 | 27 |
| 0.258 |
| 2–5 | 29 | 11 | 18 |
|
|
>5 | 2 | 0 | 2 |
|
| TNM stage |
|
|
| <0.001 |
| I and
II | 85 | 58 | 27 |
| 0.367 |
| III and
IV | 27 | 7 | 20 |
|
| Distant
metastasis |
|
|
| <0.001 |
| No | 93 | 61 | 32 |
| 0.339 |
|
Yes | 19 | 4 | 15 |
|
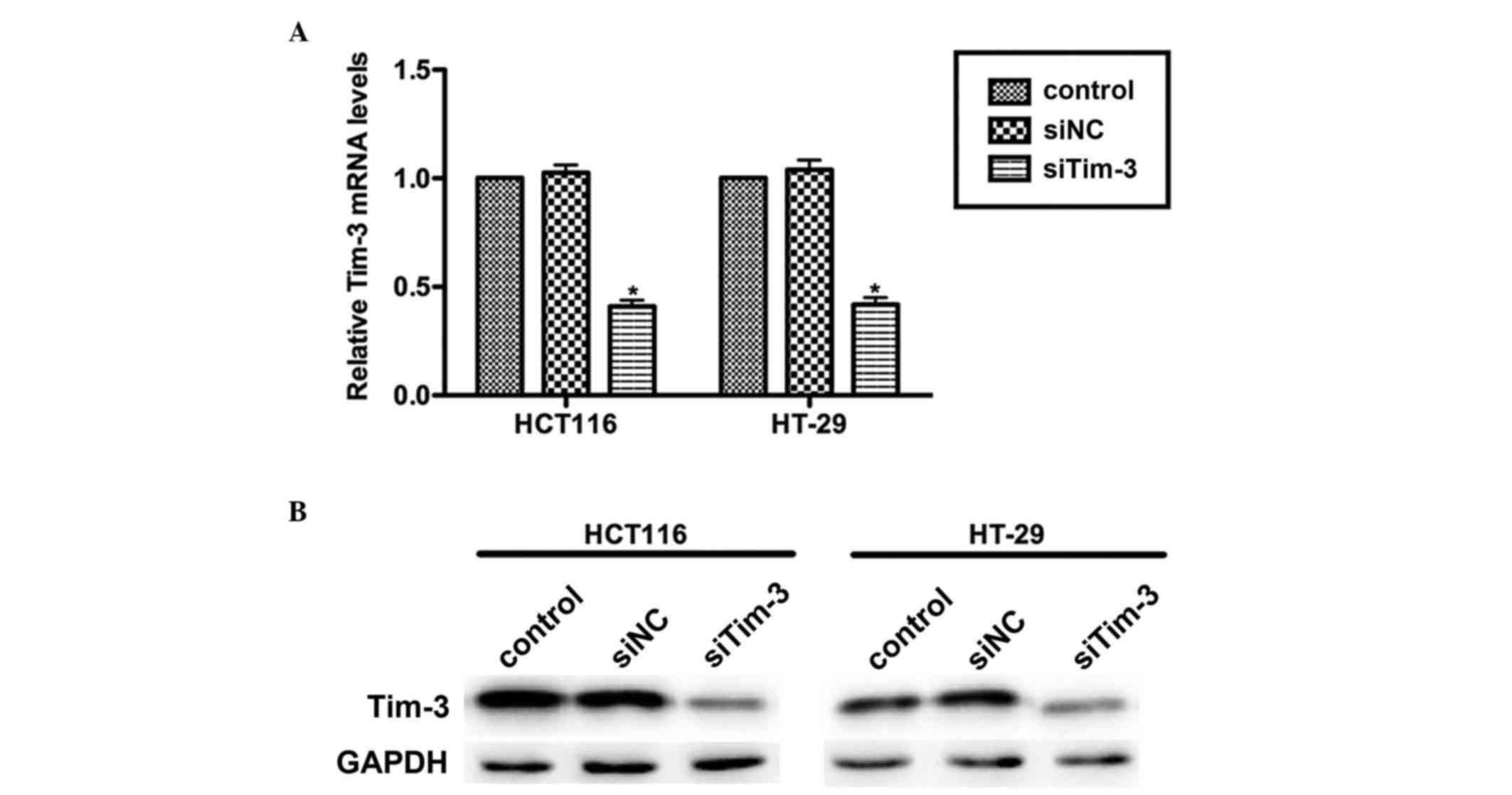
Knockdown of Tim-3 with specific siRNA
in cultured CRC cells
CRC is a common malignancy worldwide and has high
rates of metastasis. The present study aimed to assess whether
Tim-3 is involved in the progressive activities of CRC. Specific
siRNA against Tim-3 (siTim-3) was designed to knock down the
expression of Tim-3 in cultured CRC cell lines. At 72 h
post-transfection, the RNAs and proteins were extracted and
subjected to RT-qPCR and western blot analyses, respectively. As
shown in Fig. 2A, the mRNA levels
of Tim-3 in the HCT116 and HT-29 cells were significantly decreased
by siTim-3 by up to ~50%, compared with the control groups. The
protein levels of Tim-3 were also markedly decreased when the two
cell lines were transfected with siTim-3 (Fig. 2B). These results revealed the high
specificity of siRNA and the efficiency of transfection.
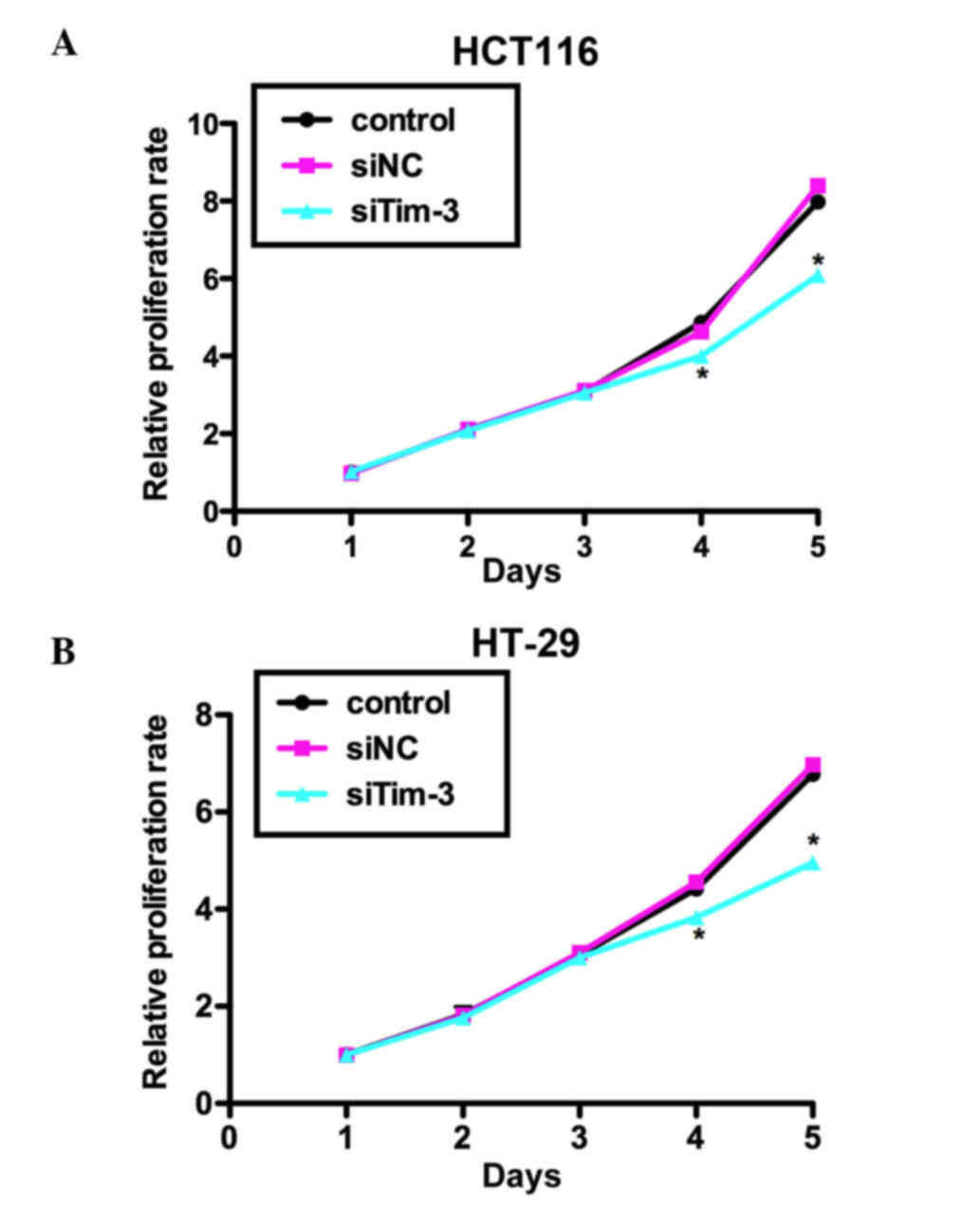
Knockdown of Tim-3 suppresses cell
proliferation in CRC cells
The present study performed an MTT assay to examine
the role of Tim-3 on cell proliferation in the HCT116 and HT-29 CRC
cell lines. As shown in Fig. 3A,
no significant differences were observed between the HCT116 cells
of the three groups in the first 3 days. However, on day 4, the
proliferation rate was decreased by 18% in the siTim-3-treated
group, whereas the control siRNA-transfected group remained stable.
The suppression was more marked on day 5 by up to ~25%. Similar
results were observed in the HT-29 cells, in which cell
proliferation rate was decreased by 19% on day 4 and 31.25% on day
5 by siTim-3 (Fig. 3B). These data
suggested that the knockdown of Tim-3 inhibited the proliferative
activity of the HCT116 and HT-29 CRC cell lines.
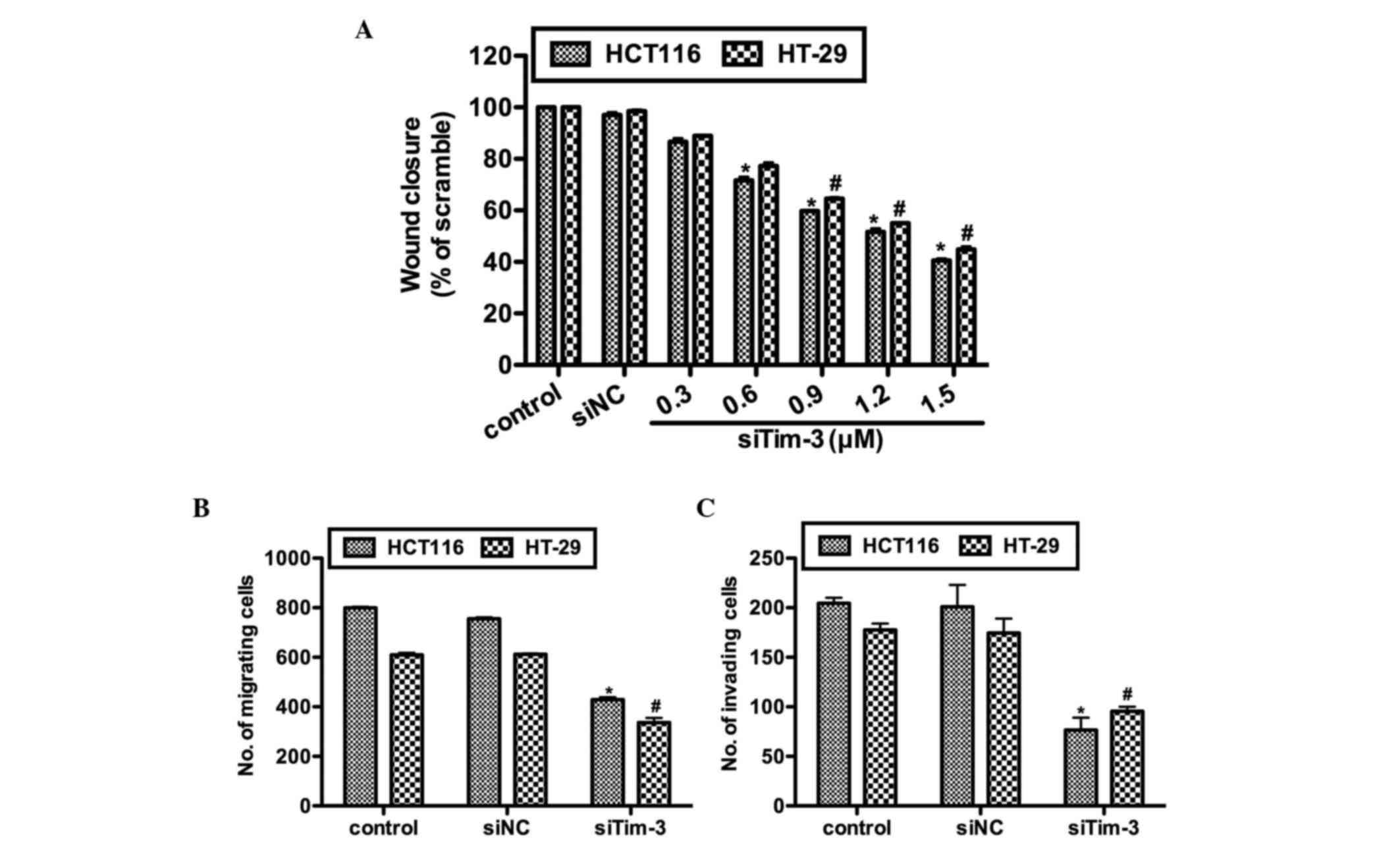
Knockdown of Tim-3 inhibits cell
migration and invasion in CRC cells
The present study further investigated the effects
of Tim-3 knockdown on cell migration and invasion in vitro.
Prior to the wound healing assay and Transwell assays, the HCT116
and HT-29 cells were transfected with either control siRNA or
various concentrations of siTim-3. As shown in Fig. 4A, no notable differences were
observed between the control groups. However, in the siTim-3
groups, the rates of wound closure were significantly decreased in
the two cell lines and these inhibitory effects were
dose-dependent. The results of the Transwell assay confirmed the
above observations. In the siTim-3-transfected HCT116 cells, the
percentage of cells found to migrate to the inferior surface of the
membrane were only 55 and 45% of the control groups in the
migration and invasion assays, respectively. The HT-29 cells also
exhibited lower migration and invasion rates when transfected with
siTim-3, compared with the control groups (Fig. 4B and C). These results revealed
that the knockdown of Tim-3 decreased the migration and invasion
abilities of the cells in vitro.
Discussion
CRC is the third leading cause of cancer-associated
mortality each year. Surgery and 5-fluorouracil-based adjuvant
chemotherapy are recommended for patients with high-risk stage II
and stage III CRC (26). However,
the prognosis of CRC has remained poor over past decades. Previous
ivestigations have predominantly focused on the immunotherapy of
cancer, particularly those closely associated with inflammation
(21). However, despite the wide
recognition of CRC as an inflammation-associated malignancy,
progression in immunotherapy has not been substantial in treating
CRC until now.
The present study is the first, to the best of our
knowledge, to report that Tim-3 is critical in CRC cell
proliferation, migration and invasion. Tim-3 is an immune
regulatory molecule, which triggers downstream cascade events upon
stimulation by its ligand, galectin-9. Emerging evidence has
demonstrated the importance of Tim-3 in human tumorigenesis.
However, no studies have been performed to examine the role of
Tim-3 in CRC. A previous study reported the expression profile of
Tim-3 in pediatric Crohn's disease, in which Tim-3 was expressed at
high levels in tissue samples of Crohn's disease and its expression
was correlated with the pediatric Crohn's disease activity index
(27). This report is important as
Crohn's disease is widely-recognized to trigger an immune response
and progress to CRC if not controlled. However, no further
investigations have been performed with respect to the role of
Tim-3 in CRC.
Tim-3 has previously been reported to be expressed
at high levels in prostate cancer, hepatocellular carcinoma, renal
cell carcinoma and melanoma (18–20,22,23).
In line with these reports, the present study found that the
expression of Tim-3 was significantly higher in CRC cancerous
samples, compared with adjacent non-cancerous samples. Of note,
following the knockdown of Tim-3 with specific siRNA, it was found
that cell proliferation was significantly inhibited whereas the
proliferation rates of the control cells were unaffected. Wound
healing abilities, which reflect cell migration potential, were
also inhibited in two CRC cell lines, in a dose-dependent manner,
and their migration and invasion abilities were also inhibited, as
determined using Transwell assays. These in vitro results
confirmed the results in vivo that the expression of Tim-3
was statistically associated with tumor TNM staging, distant
metastasis and tumor size (Table
I). However, these observations also suggested that Tim-3
promoted CRC cell oncogenic activities, including proliferation,
migration and invasion, and confirmed the that Tim-3-targeted
therapy may be anti-neoplastic (21).
The mechanism underlying Tim-3-mediated tumor
progression remains to be fully elucidated. According to previous
data, the interleukin-6 (IL-6)-signal transducer and activator of
transcription (STAT)3 pathway is critical in tumor growth and
metastasis in human hepatocellular carcinoma (28). DNA damage induces the IL-STAT3
pathway which has growth-promoting effects in human tumors
(29). Inhibiting IL-6 reverses
the Tim-3-mediated effects on HCC cell growth in vitro
(23). Therefore, the IL-STAT3
pathway may be critical in the biological activities of Tim-3.
However, another previous report demonstrated that the mechanisms
involving the biological activities of Tim-3 may be distinct in
different scenarios. It was observed that the suppression of
downstream GATA binding protein 3 (GATA3) was an important
mechanism by which Tim-3 triggered metastasis in renal cell
carcinoma. However, distinct from inhibiting Tim-3, the same report
documented that GATA3 was activated by Tim-3 in facilitating
systemic lupus erythematosus. Therefore, Tim-3 may exert
disease-promoting effects through inconsistent pathways. The
mechanisms underlying the Tim-3-mediated progression of CRC may
also be unique and require further investigation in the future.
In conclusion, the present study was the first to
investigate the expression and involvement of Tim-3 in the
progression of human CRC. Tim-3 was expressed at high levels in CRC
tissues. The knockdown of Tim-3 significantly reduced cell
proliferation, migration and invasion, and these results were
consistent with those from the clinical tissues. Therefore,
interference of Tim-3 may offer potential in CRC therapy. However,
further investigations are required to reveal the detailed
mechanisms.
Acknowledgements
The authors would like to thank Dr Peng Zhang for
his professional technical support during the IHC assay.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen C, Wang L, Liao Q, Huang Y, Ye H,
Chen F, Xu L, Ye M and Duan S: Hypermethylation of EDNRB promoter
contributes to the risk of colorectal cancer. Diagn Pathol.
8:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qu YL, Wang HF, Sun ZQ, Tang Y, Han XN, Yu
XB and Liu K: Up-regulated miR-155-5p promotes cell proliferation,
invasion and metastasis in colorectal carcinoma. Int J Clin Exp
Pathol. 8:6988–6994. 2015.PubMed/NCBI
|
|
4
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooper K, Squires H, Carroll C,
Papaioannou D, Booth A, Logan RF, Maguire C, Hind D and Tappenden
P: Chemoprevention of colorectal cancer: Systematic review and
economic evaluation. Health Technol Assess. 14:1–206. 2010.
View Article : Google Scholar
|
|
8
|
Goh CH, Leong WQ, Chew MH, Pan YS, Tony
LK, Chew L, Tan IB, Toh HC, Tang CL, Fu WP and Chia WK:
Post-operative aspirin use and colorectal cancer-specific survival
in patients with stage I–III colorectal cancer. Anticancer Res.
34:7407–7414. 2014.PubMed/NCBI
|
|
9
|
Pagès F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canna K, McMillan DC, McKee RF, McNicol
AM, Horgan PG and McArdle CS: Evaluation of a cumulative prognostic
score based on the systemic inflammatory response in patients
undergoing potentially curative surgery for colorectal cancer. Br J
Cancer. 90:1707–1709. 2004.PubMed/NCBI
|
|
12
|
Guthrie GJ, Roxburgh CS, Farhan-Alanie OM,
Horgan PG and McMillan DC: Comparison of the prognostic value of
longitudinal measurements of systemic inflammation in patients
undergoing curative resection of colorectal cancer. Br J Cancer.
109:24–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th1-specific cell surface protein Tim-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horlad H, Ohnishi K, Ma C, Fujiwara Y,
Niino D, Ohshima K, Jinushi M, Matsuoka M, Takeya M and Komohara Y:
TIM-3 expression in lymphoma cells predicts chemoresistance in
patients with adult T-cell leukemia/lymphoma. Oncol Lett.
12:1519–1524. 2016.PubMed/NCBI
|
|
16
|
Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X,
Deng L, Wang Y, Zhang X, Jiang T and Lu X: Targeting PD-1 and Tim-3
pathways to reverse CD8 T-cell exhaustion and enhance ex vivo
T-cell responses to autologous dendritic/tumor vaccines. J
Immunother. 39:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: Emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piao YR, Piao LZ, Zhu LH, Jin ZH and Dong
XZ: Prognostic value of T cell immunoglobulin mucin-3 in prostate
cancer. Asian Pac J Cancer Prev. 14:3897–3901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piao YR, Jin ZH, Yuan KC and Jin XS:
Analysis of Tim-3 as a therapeutic target in prostate cancer.
Tumour Biol. 35:11409–11414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng H, Guo X, Tian Q, Li H and Zhu Y:
Distinct role of Tim-3 in systemic lupus erythematosus and clear
cell renal cell carcinoma. Int J Clin Exp Med. 8:7029–7038.
2015.PubMed/NCBI
|
|
21
|
Ngiow SF, Teng MW and Smyth MJ: Prospects
for TIM3-targeted antitumor immunotherapy. Cancer Res.
71:6567–6571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wiener Z, Kohalmi B, Pocza P, Jeager J,
Tolgyesi G, Toth S, Gorbe E, Papp Z and Falus A: TIM-3 is expressed
in melanoma cells and is upregulated in TGF-beta stimulated mast
cells. J Invest Dermatol. 127:906–914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan W, Liu X, Ma H, Zhang H, Song X, Gao
L, Liang X and Ma C: Tim-3 fosters HCC development by enhancing
TGF-β-mediated alternative activation of macrophages. Gut.
64:1593–1604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chono H, Saito N, Tsuda H, Shibata H,
Ageyama N, Terao K, Yasutomi Y, Mineno J and Kato I: In vivo safety
and persistence of endoribonuclease gene-transduced CD4+ T cells in
cynomolgus macaques for HIV-1 gene therapy model. PLoS One.
6:e235852011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smolle MA, Pichler M, Haybaeck J and
Gerger A: Genetic markers of recurrence in colorectal cancer.
Pharmacogenomics. 16:1315–1328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MJ, Lee WY and Choe YH: Expression of
TIM-3, human β-defensin-2, and FOXP3 and correlation with disease
activity in pediatric crohn's disease with infliximab therapy. Gut
Liver. 9:370–380. 2015.PubMed/NCBI
|
|
28
|
Yang X, Liang L, Zhang XF, Jia HL, Qin Y,
Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, et al: MicroRNA-26a
suppresses tumor growth and metastasis of human hepatocellular
carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology.
58:158–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yun UJ, Park SE, Jo YS, Kim J and Shin DY:
DNA damage induces the IL-6/STAT3 signaling pathway, which has
anti-senescence and growth-promoting functions in human tumors.
Cancer Lett. 323:155–160. 2012. View Article : Google Scholar : PubMed/NCBI
|