Introduction
The rapid growth of biomedical research over the
past several decades has seen a concomitant expansion in the
number, complexity and diversity of experimental animals developed
as research tools (1,2) and inbred mice are among the most
widely used. The accuracy and reproducibility of scientific studies
can be greatly improved using inbred mice due to their particularly
homozygous characteristics. At least 250 inbred mouse strains are
commonly used worldwide, including C57BL/6J, C3H, C3HeB/FeJ,
BALB/c, KK, NC, CBA/J and CL/Fr mice (3–9).
Different inbred mouse strains are required for various research
types; C57BL/6J is the most widespread substrain used for studying
genetically engineered mice, C3H is a common research model for the
investigation of cancer, C3HeB/FeJ is a substrain of the C3H strain
with a high incidence of mammary tumors and BALB/c mice are widely
used for the production of monoclonal antibodies (4). To meet different research
requirements, it is important to cultivate animal models with
particular traits.
To best of our knowledge, no inbred mice have
exhibited strong radioresistance for use in radiation-damage
research at present. In the current study a variety of mice
strains, including LACA, NIH (data not shown), ICR/JCL and Kunming
(data not shown) mice, were tested in the attempt to develop a
radioresistant animal model. The desired characteristics were
challenging to achieve due to the biological characteristics of
these mice. For example, inbred mice had stable physiological and
biochemical indicators however exhibited low radioresistance; thus,
the animals did not survive exposure to the required radiation
dose. Hybrid mice exhibited high radioresistance (10), however their physiological and
biochemical indicators were unstable, resulting in poor
reproducibility. It was noted that the radioresistance of the Japan
outbreeding mouse strain ICR/JCL to ionizing radiation was higher
than that of other hybrid mice and that the Chinese inbred mouse
615 had stable hematopoietic indicators and was sensitive to
radiation. According to the laws of genetics, offspring can combine
the biological advantages of their parents. The aim of the present
study was to generate a new inbred mouse strain with high
radioresistance and stable genetic characteristics.
To develop an inbred mouse with the desired
characteristics, a female ICR/JCL mouse (white fur) was crossed
with a male 615 mouse (black fur). A new mouse strain (with
cinnamon-colored fur) was established through more than 20
continuous generations and was termed Institute of Radiation
Medicine-2 (IRM-2) mouse. The biological characteristics of the
IRM-2 mouse, including reproductive capacity, physiological and
biochemical indices and genetic characteristics, were determined.
In addition, the radiosensitivity of the IRM-2 mouse to γ-ray was
examined using a lethal dose (LD)50 test and assays of
the hematopoietic function of bone marrow.
Materials and methods
Development of IRM-2 mice
Japanese outbreeding-strain female ICR/JCL mice and
Chinese inbred-strain male 615 mice (weight: 20±2 g) were purchased
from the Academy of Military Medical Sciences (Beijing, China).
They were maintained under controlled laboratory conditions at a
temperature of 23±2°C and humidity of 55±5% with a controlled light
cycle (14 h of light and 10 h of darkness). A female ICR/JCL mouse
was crossed with a male 615 mouse to produce F1 hybrids.
Subsequently, the F1 mice were further interbred by brother-sister
mating to obtain an F2 generation. A novel mouse strain was
established through >20 continuous generations and termed the
IRM-2 mouse. The mice were bred at an animal care facility
certified by Tianjin Management Committee of Laboratory Animals in
the Institute of Radiation Medicine at Peking Union Medical College
(Beijing, China). The experimental protocol was approved by the
China Institutional Ethics Review Committee for Animal
Experimentation. IRM-2 mice, 615 mice and ICR/JCL mice used were
8–10 weeks old.
Ionizing radiation
Mice were exposed to ionizing radiation (IR) in a
Gammacell-40 137Cesium γ irradiator (Atomic Energy of Canada Inc.,
Chalk River, ON, Canada) at a rate of 0.882 Gy/min. After
irradiation, the mice were returned to the certified animal
facility.
Organ-coefficient measurement
Mice were anaesthetized by intraperitoneal
administration of 300 mg/kg chloral hydrate solution (Baomanbio,
Inc., Shanghai, China). After sacrifice by cervical dislocation,
the hearts, livers, spleens, lungs, kidneys and thymuses of the
mice were dissected out and weighed. Each organ coefficient was
calculated as organ coefficient=organ weight/body weight ×100%.
Peripheral blood cells and bone marrow
cell (BMC) counts
Whole blood was drawn from the orbital sinuses of
mice and used within 30 min of collection to perform white blood
cell (WBC), red blood cell (RBC) and platelet (PLT) counts for each
sample, using a hemocytometer (Sysmex pocH-100i; Sysmex
Corporation, Kobe, Japan). After the mice were euthanized by
cervical dislocation, BMCs were flushed from the mouse femurs as
described previously (11),
counted using the hemocytometer (Sysmex pocH-100i).
Measurement of biochemical
indices
Following a 12 h fast, the mice were euthanized by
cervical dislocation. Whole blood was sampled by eyeball
extirpation and centrifuged for 10 min at 2,500 × g at room
temperature. The concentrations of serum biochemical indices were
measured using a semi-automatic biochemical analyzer (VITALAB-II;
Vital Scientific N.V., Dieren, The Netherlands); the indices used
included blood glucose, total cholesterol, triglycerides, aspartate
aminotransferase, alanine aminotransferase, alkaline phosphatase,
total protein, albumin, blood urea nitrogen, creatinine, calcium,
phosphorus and total bilirubin.
Gene homogeneity characterization
Coat-color gene test
An IRM-2 mouse was mated with an albino BALB/c mouse
(genotype AAbbccDD) with known genes. The coat-color genes of the
IRM-2 mouse were judged based on the coat color of F1-generation
mice.
Biochemical markers test
IRM-2 mice were randomly selected from the F23 and
F38 generations of the population. Biochemical markers were
detected according to the National Standard of China
GB/T14927.1-2001 on Laboratory Animal Genetic Monitoring: Methods
for Biochemical Markers of Inbred Mice and Rats (12).
Chromosomal aberrations analysis and chromosome
G-banding karyotype
Mice were administered an intraperitoneal dose of 7
µg/g body weight colchicine (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) and euthanized after 4 h. Bone-marrow cells
were flushed from the femurs using Hank's balanced salt solution.
The cells were treated with a pre-warmed hypotonic lysis solution
(0.075 M KCl) at 37°C for 30 min. The cells were then fixed with
Carnoy's solution [3:1 (v/v) methanol/glacial acetic acid] for 20
min at room temperature. Metaphase slides were prepared by dropping
the cells onto glass slides, which were placed into an oven at 60°C
for 4 h, digested with 0.03% trypsin-EDTA solution for 20–30 sec
and then stained with Giemsa. Spontaneous chromosomal aberrations
based on 1,200 metaphases were examined under the microscope
(Eclipse 50 i, Nikon Corporation, Tokyo, Japan). Chromosomes were
identified based on unique G-banding patterns and the chromosome
karyotypes were drawn (original magnification, ×400).
Relative chromosome length
A total of five mitotic metaphases of well-dispersed
and moderate length chromosomes of mice were selected under the
microscope and were imaged. Chromosomes of five metaphases were
developed and enlarged. The length of the straighter chromosome of
each pair of homologous chromosomes was measured with a vernier
caliper and the total length of all chromosomes was calculated. The
relative chromosome length of each chromosome karyotype was
calculated as relative chromosome length=(length of each
chromosome/total length of all chromosomes)x100%. The relative
chromosome length of the five chromosome karyotype was
averaged.
Lethal dose, 50% test and dose reduction factor
analysis
Mice were exposed to a total body irradiation of
0–10 Gy (absolute lethal dose) 137Cs γ-rays. The mortality of each
mouse dose group was recorded 30 days after irradiation and the
LD50 was calculated. The radioresistant characteristics
of mice were expressed as the dose reduction factor (DRF). The DRF
was calculated as the ratio of radiation dose required to produce
the same biological effect of median lethal dose of 30 days
(LD50/30). Mice were observed for 30 days after having
been exposed to an LD50 dose of radiation to determine
LD50/30. Surviving mice were sacrificed at day 31.
Survival curve
Mice were exposed to a total body irradiation of 8
Gy 137Cs γ-rays. The number of surviving mice was recorded
continuously for 15 days after irradiation and the survival curve
of the mice was determined.
DNA content of BMCs
BMCs were flushed from mouse femurs using 0.005
mol/l CaCl2 after the mice were euthanized by cervical
dislocation. The cells were treated with a 0.2 mol/l
HClO4 solution at 90°C for 15 min and filtered. The
optical density of the BMCs was measured at 286 nm using a UV
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Colony-forming unit-spleen (CFU-S) counting
Spleens were harvested from the abdominal cavity of
mice after euthanization by cervical dislocation and stained using
picric acid. The number of CFU-S was determined by macrography
using a simple magnifier. One nodule on the spleen surface that was
apparent to the naked eye was considered as one CFU-S. One CFU-S
was treated as one CFU-S unit.
Statistical analysis
All experiments were performed at least twice. Data
are presented as mean ± standard deviation. Differences between
groups were calculated using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
The growth and development of IRM-2
mice
The IRM-2 mice were derived from crosses between
ICR/JCL and 615 parents (Fig. 1).
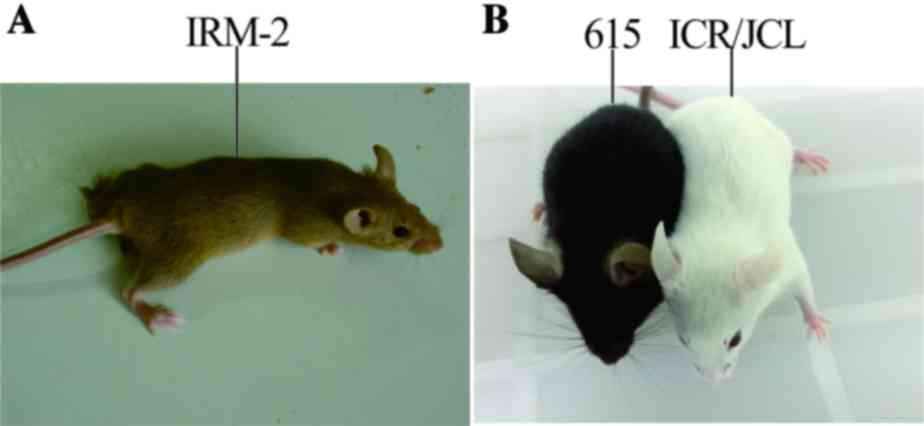
The specific characteristics of the mice included cinnamon-colored
fur, large semicircular ears, protruding eyes and black irises. The
breeding performance of IRM-2 mice was characterized as follows: i)
Number of pups per female/lifetime=45; ii) litter size=7.8; iii)
number of litters=5.8; iv) age at first productive mating=7.5
weeks; v) age at last productive mating=32.6 weeks; and vi) life
span=77.1 weeks.
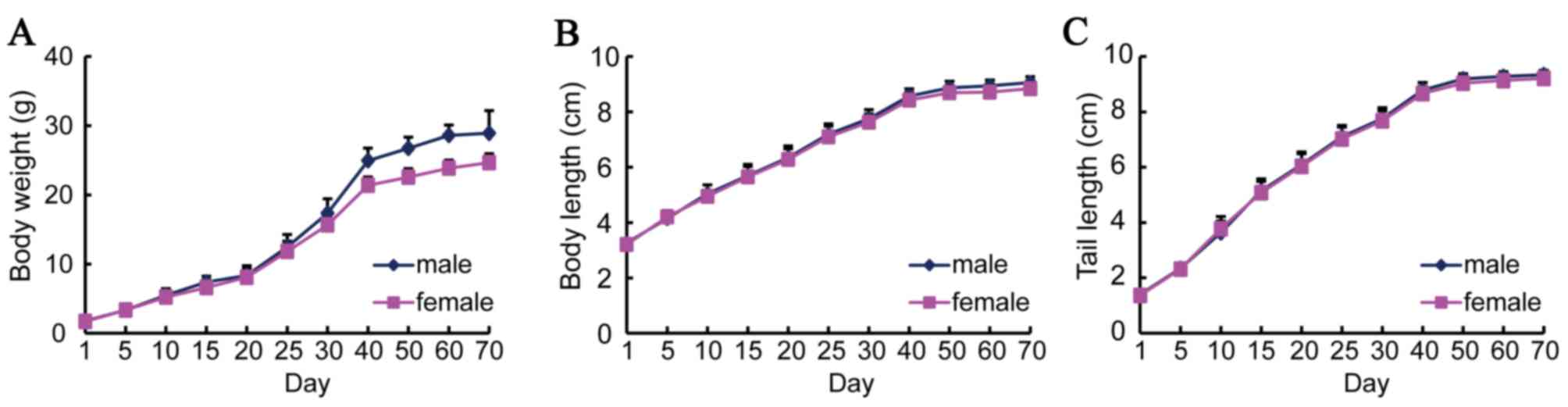
The body weight, body length and tail length of the
IRM-2 mice were measured from birth to 70 days. During the period
of rapid growth from 25 to 40 days old, the body weight of the mice
increased twice as much as that between birth and 25 days (Fig. 2A). After this period, the increase
in body weight slowed and the mice reached their maximum weight of
25 g 70 days following birth. The increases in body length and tail
length were similar to that of body weight (Fig. 2B and C). The growth curves were
plotted for male and female mice separately, however, no
significant differences were identified between the males and
females.
Organ coefficients of mice
The organ coefficients of IRM-2, 615 and ICR/JCL
mice were measured. The organ coefficients of the thymus of
same-gender IRM-2 mice was significantly smaller than that of
same-gender 615 mice and ICR/JCL mice (P<0.0001; Table I). No significant differences were
identified in the organ coefficients of other organs, with the
exception of the thymus, between the males and females of IRM-2
mice, 615 mice and ICR/JCL mice (Table
I).
 | Table I.Organ coefficients of mice (%)
presented as the mean ± standard deviation; n=40. |
Table I.
Organ coefficients of mice (%)
presented as the mean ± standard deviation; n=40.
|
| IRM-2 | 615 | ICR/JCL |
|---|
|
|
|
|
|
|---|
| Organ | Male | Female | Male | Female | Male | Female |
|---|
| Thymus | 0.13±0.03 | 0.28±0.05 |
0.21±0.02a |
0.52±0.10a | 0.25±0.1a |
0.45±0.12a |
| Spleen | 0.33±0.04 | 0.43±0.04 | 0.42±0.10 | 0.41±0.11 | 0.42±0.09 | 0.45±0.13 |
| Heart | 0.47±0.03 | 0.47±0.02 | 0.53±0.11 | 0.55±0.12 | 0.58±0.13 | 0.55±0.15 |
| Lung | 0.57±0.06 | 0.68±0.05 | 0.72±0.12 | 0.85±0.13 | 0.76±0.15 | 0.85±0.18 |
| Kidney | 1.64±0.04 | 1.23±0.05 | 1.23±0.13 | 1.03±0.12 | 1.50±0.15 | 1.28±0.12 |
| Liver | 5.53±0.35 | 4.68±0.31 | 4.22±0.50 | 4.25±0.53 | 6.01±0.58 | 5.20±0.50 |
Hematic and bone-marrow phases of
IRM-2 mice
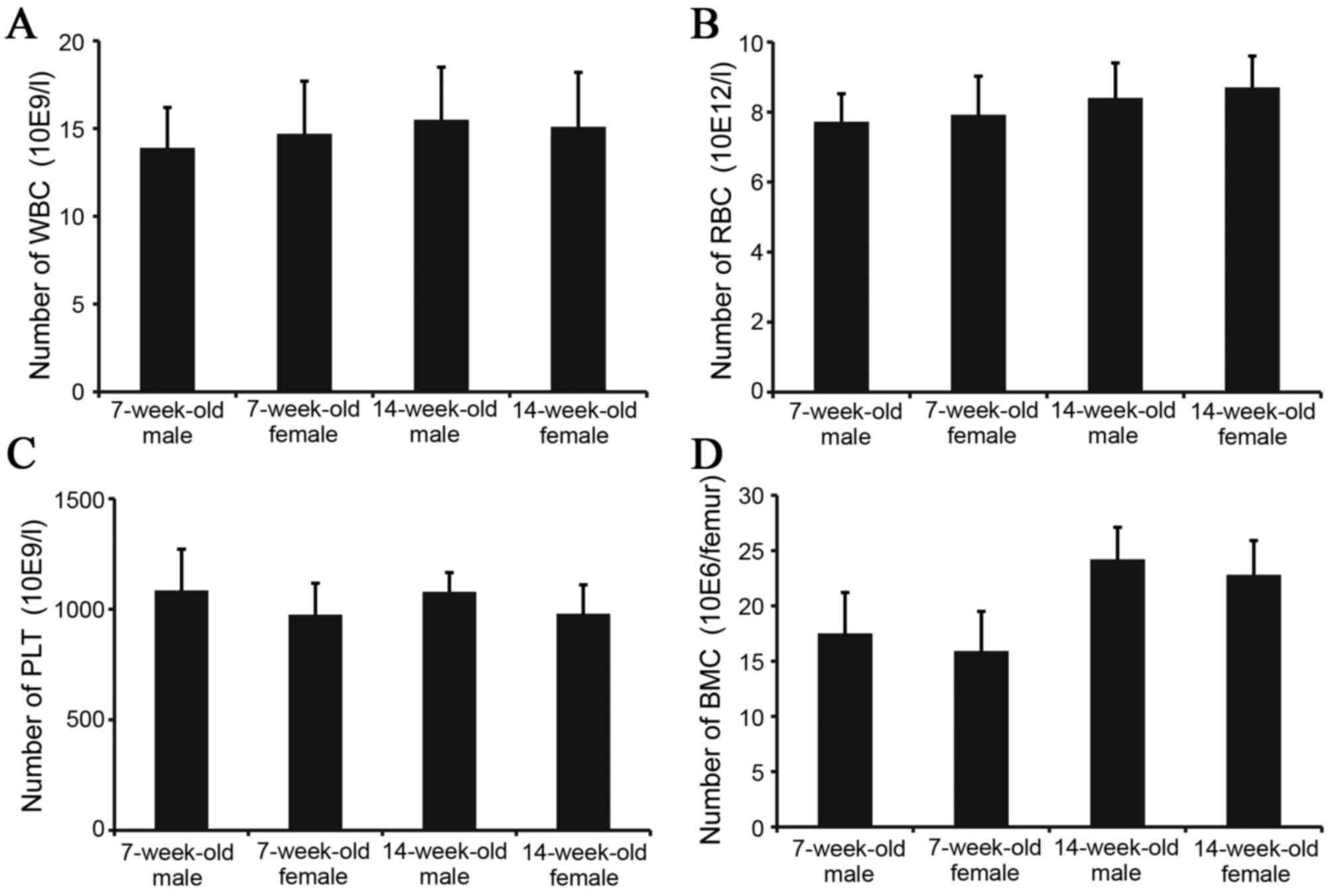
To investigate the biological characteristics of
IRM-2 mice, images of peripheral blood and bone marrow were
recorded. No significant differences were identified in peripheral
WBC, RBC and PLT or in the number of BMCs between 7 and 14-week-old
IRM-2 mice (Fig. 3).
Serum biochemical indices of mice
Biochemical indices are important biological
indicators for characterizing laboratory animals. A total of 13
serum biochemical indices of male and female IRM-2, 615 and ICR/JCL
mice were determined. All the tested indices were stable; the
individual differences of those indices were low and no differences
were identified between male and female IRM-2 mice, 615 mice or
ICR/JCL mice (Table II).
 | Table II.Biochemical indices of mice presented
as the mean ± standard deviation; n=10. |
Table II.
Biochemical indices of mice presented
as the mean ± standard deviation; n=10.
|
|
| IRM-2 | 615 | ICR/JCL |
|---|
|
|
|
|
|
|
|---|
| Index | Unit | Male | Female | Male | Female | Male | Female |
|---|
| ALB | g/l | 18.95±1.06 | 21.22±1.11 | 20.26±1.89 | 23.52±2.06 | 21.65±1.69 | 24.21±1.35 |
| ALP | U/l | 116.50±10.52 | 117.70±13.06 | 115.52±12.27 | 120.72±12.19 | 118.16±11.62 | 121.05±12.80 |
| ALT | U/l | 44.10±6.21 | 39.40±4.88 | 40.23±5.08 | 42.76±4.65 | 45.12±6.72 | 44.63±5.06 |
| AST | U/l | 113.60±11.16 | 128.20±12.38 | 116.63±12.45 | 130.23±13.78 | 118.25±12.86 | 131.50±14.03 |
| BUN | mmol/l | 5.66±0.38 | 5.28±1.19 | 6.03±0.56 | 6.12±0.95 | 5.82±0.67 | 6.05±1.15 |
| Ca | mg/dl | 7.02±0.61 | 6.80±1.03 | 6.91±0.86 | 7.26±1.06 | 7.15±0.95 | 6.96±1.12 |
| CHO | mmol/l | 3.59±0.30 | 3.94±0.25 | 3.78±0.58 | 4.02±0.65 | 3.96±0.67 | 4.10±0.87 |
| Cre | µmol/l | 51.08±6.02 | 52.19±7.26 | 52.39±8.22 | 54.38±8.26 | 52.81±7.56 | 55.63±7.95 |
| GLU | mmol/l | 4.77±0.80 | 4.19±0.81 | 4.23±1.02 | 4.65±0.95 | 5.07±0.86 | 4.89±1.23 |
| P | mg/dl | 7.77±0.87 | 7.40±0.66 | 7.50±0.93 | 8.26±1.05 | 7.56±0.86 | 7.90±0.98 |
| TBIL | µmol/l | 3.83±0.96 | 3.87±1.22 | 3.72±0.93 | 4.15±0.88 | 3.90±1.02 | 4.21±1.16 |
| TG | mmol/l | 0.78±0.13 | 0.79±0.15 | 0.85±0.23 | 0.76±0.25 | 0.87±0.32 | 0.90±0.35 |
| TP | g/l | 28.18±1.25 | 29.45±3.95 | 26.65±2.72 | 30.05±4.17 | 28.85±2.53 | 32.58±3.63 |
Coat-color gene test of IRM-2
mice
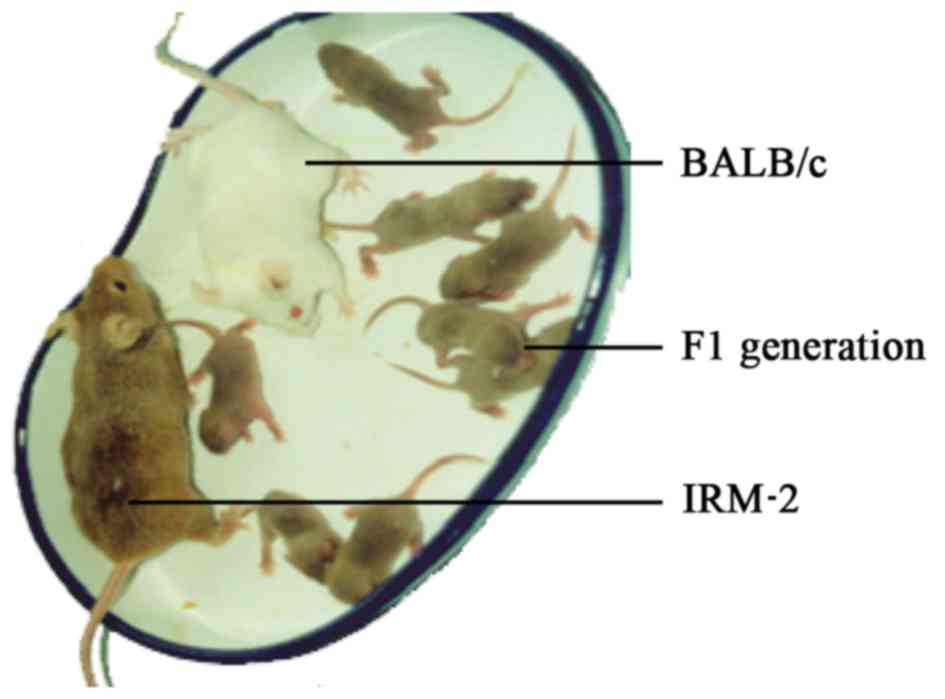
To characterize the coat genotype of IRM-2 mice, an
IRM-2 mouse was mated with an albino BALB/c mouse (genotype
AAbbccDD) with known genes. The coat-color genes of the IRM-2 mouse
were judged based on the coat color of F1-generation mice. The coat
color of all offspring (six litters) was cinnamon,
indistinguishable from that of IRM-2 mice (Fig. 4). The results demonstrated that the
coat-color gene of IRM-2 mice was homozygous and the coat genotype
was AAbbCCDD.
Biochemical markers of mice
The biochemical markers of IRM-2 mice were measured
at generations F23 and F38 to confirm the genetic quality of the
mice. No polymorphisms or mutant genes were observed in any of the
biochemical markers tested. A total of 20 markers were detected for
the IRM-2 mice at generation F23. Of the 20 markers analyzed, seven
differed between the IRM-2 mice and the 615 mice, including
alkaline phosphatase 1 (Akp1), carbonic anhydrase 2 (Car2), kidney
catalase 2 (Ce2), major urinary protein 1 (Mup1), peptidase 3
(Pep3), phosphoglucomutase 1 (Pgm1) and tosyl arginine
methylesterase 1 (Tam1) (Table
III). Of the 20 markers analyzed, 12 were measured in the IRM-2
mice at generation F38. Of these 12, four differed among the IRM-2,
615 and ICR/JCL mice, including Akp1, Car2, Ce2 and Pgm1 (these
were among the seven markers that differed in the F23 generation;
Table IV). These results indicate
that the IRM-2 mice had their own specific genotype and were
homozygous.
 | Table III.Biochemical markers of IRM-2 mice at
generation F23 (n=8). |
Table III.
Biochemical markers of IRM-2 mice at
generation F23 (n=8).
|
| Genotype |
|---|
|
|
|
|---|
| Biochemical
markers | IRM-2 | 615 | ICR/JCL |
|---|
| Akp1 | b | a | a |
| Amy1 | a | a | a |
| Car2 | b | a | b |
| Ce2 | a | b | b |
| Es1 | b | b | b |
| Es2 | b | b | b |
| Es3 | c | c | c |
| Es10 | a | a | a |
| Es11 | a | a | a |
| Gpd1 | b | b | b |
| Gpi1 | a | a | a |
| Hbb | s | s | s |
| Idh1 | a | a | a |
| Ldr1 | a | a | a |
| Mup1 | a | b | a |
| Pep3 | b | a | a |
| Pgm1 | a | b | b |
| Pgm2 | a | a | a |
| Tam1 | a | c | c |
| Trf | b | b | b |
 | Table IV.Biochemical markers of IRM-2 mice at
generation F38 (n=8). |
Table IV.
Biochemical markers of IRM-2 mice at
generation F38 (n=8).
|
| Genotype |
|---|
|
|
|
|---|
| Biochemical
markers | IRM-2 | 615 | ICR/JCL |
|---|
| Akp1 | b | a | a |
| Car2 | b | a | b |
| Ce2 | a | b | b |
| Es1 | b | b | b |
| Es3 | c | c | c |
| Es11 | a | a | a |
| Gpd1 | b | b | b |
| Gpi1 | a | a | a |
| Hbb | s | s | s |
| Idh1 | a | a | a |
| Pgm1 | a | b | b |
| Trf | b | b | b |
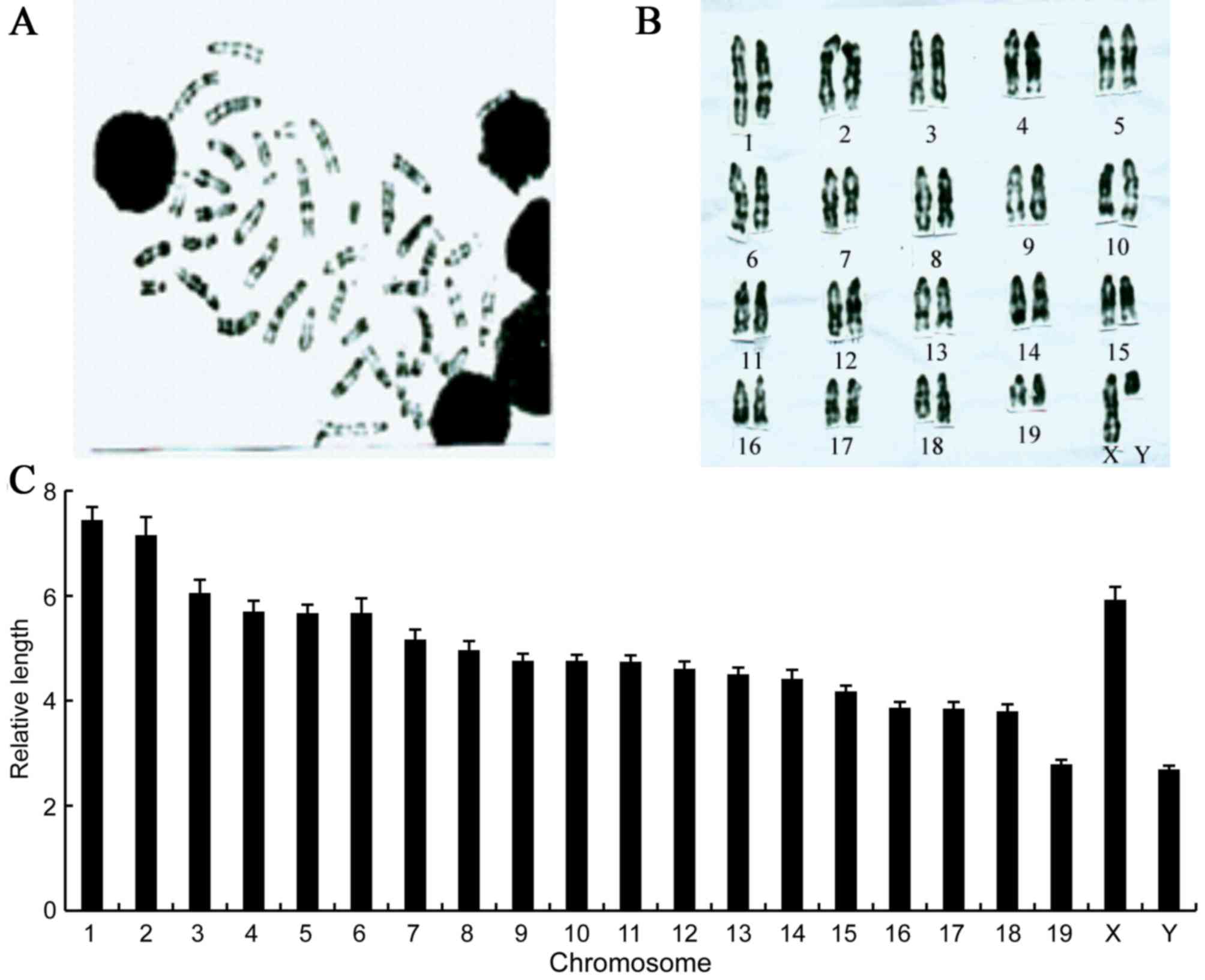
Chromosome analysis of IRM-2 mice
The cytogenetic analysis of chromosomes is an
essential part of experimental animal characterization. The
chromosomes of the IRM-2 mice were identified based on differences
in the shading of the G-bands (Fig.
5A). The IRM-2 mice had 40 chromosomes, all of which were
telocentric. The G-banding technique was applied to karyotype the
IRM-2 mice as follows: Male 40, XY (Fig. 5B); female 40, XX.
The length of homologous chromosomes in the IRM-2
mice was measured and the relative length of the chromosomes was
calculated. The relative length of all the chromosomes differed:
Chromosome 1 was the longest and chromosomes 19 and Y were the
shortest (Fig. 5C).
In addition, spontaneous chromosomal aberrations
based on 1,200 metaphases were examined. The chromosomal aberration
frequency of the IRM-2 mice was the lowest, 0.5%, compared with
that of 615 mice and ICR/JCL mice, 1.2 and 1.3% respectively
(Table V).
 | Table V.Spontaneous chromosomal aberration
frequency of mice (n=20). |
Table V.
Spontaneous chromosomal aberration
frequency of mice (n=20).
|
|
| Chromosomal
aberration |
|
|
|---|
|
|
|
|
|
|
|---|
| Mice | No. of
metaphases | Chromosomal
fracture | Acentric | Unbalanced
translocation | Total
aberration | Aberration
frequency (%) |
|---|
| IRM-2 | 1,200 | 4 | 1 | 1 | 6 | 0.5 |
| 615 | 1,200 | 10 | 2 | 2 | 14 | 1.2 |
| ICR/JCL | 1,200 | 12 | 3 | 1 | 16 | 1.3 |
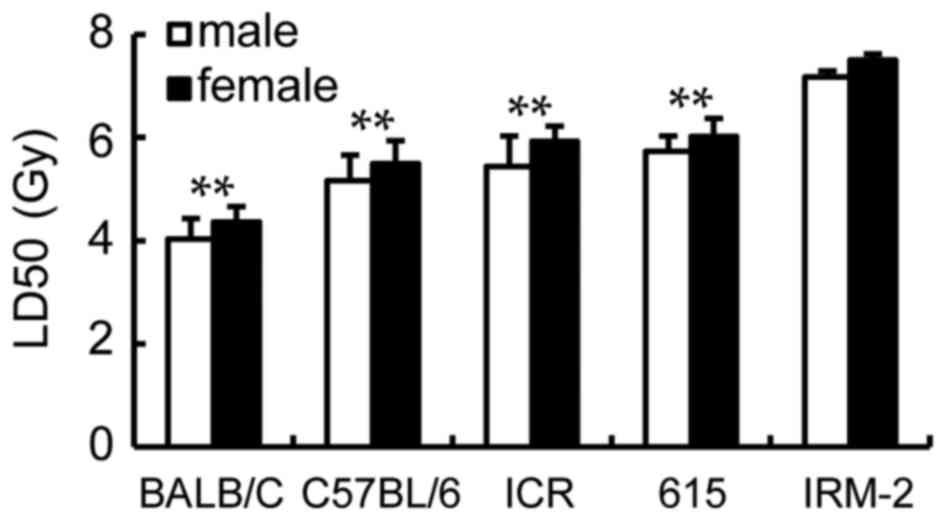
LD50 of mice strains
following exposure to γ-ray irradiation and DRF analysis
LD50 of the ICR/JCL mice and 615 mice
were calculated for 5.44 Gy (male), 5.93 Gy (female) and 5.73 Gy
(male), 6.02 Gy (female) respectively (Fig. 6). However, it was increased to 7.17
Gy (male) and 7.5 Gy (female) for the IRM-2 mice, significantly
higher than those of the parents (the 615 mice and ICR/JCL mice),
BALB/c mice or C57BL/6 mice (P<0.0001; Fig. 6).
The DRF calculated by LD50/30 for the
ICR/JCL mice and 615 mice compared with the C57BL/6 mice had a
value of 1.05 (male), 1.08 (female) and 1.11 (male), 1.10 (female)
respectively; the IRM-2 mice had the maximum DRF of 1.39 (male) and
1.37 (female).
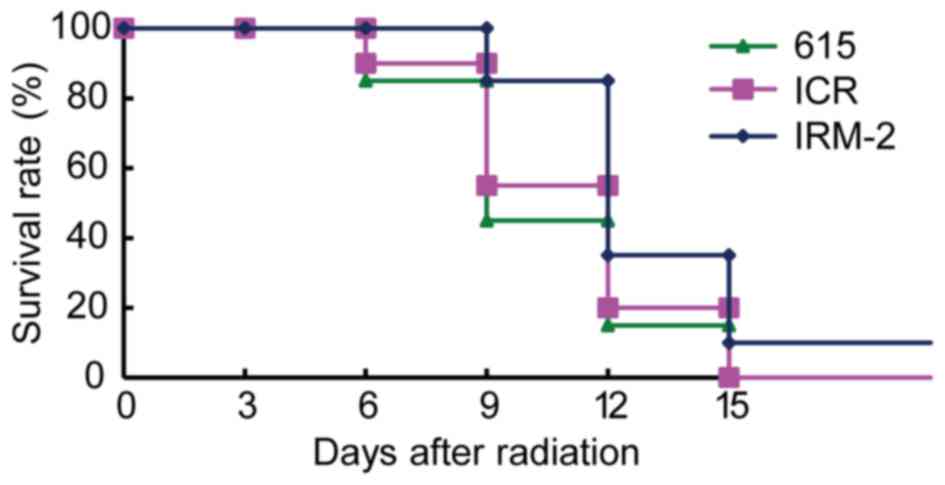
Survival curve
To test the radioresistance of the IRM-2 mice, the
survival curve of mice after total body irradiation with 8 Gy doses
of 137Cs γ-rays was plotted. As demonstrated in Fig. 7, total body radiation resulted in
55 and 45% mortality in 615 mice and ICR/JCL mice respectively by
day 9 after irradiation and the rest of the mice died at day 15
after irradiation. The mortality of IRM-2 mice was 15% at day 9 and
90% at day 15. The survival rate of IRM-2 mice was higher than that
of ICR/JCL mice and 615 mice 9 to 15 days after irradiation
(P=0.0405 vs. ICR/JCL mice and P=0.0112 vs. 615 mice; Fig. 7).
Alterations in leukocytes and the
hemopoietic system following exposure to radiation
Leukocyte counts decreased rapidly in the three mice
strains 2 days after exposure to 4 Gy radiation, falling to ~20% of
their pre-irradiation value; subsequently, the counts gradually
increased and were restored to ~45% of their pre-irradiation value
at 21 days (Fig. 8A). The number
of leukocytes in the IRM-2 mice was higher than that in the ICR/JCL
mice and 615 mice 12–21 days after the radiation exposure (P=0.0396
vs. ICR/JCL mice and P=0.0076 vs. 615 mice; Fig. 8A).
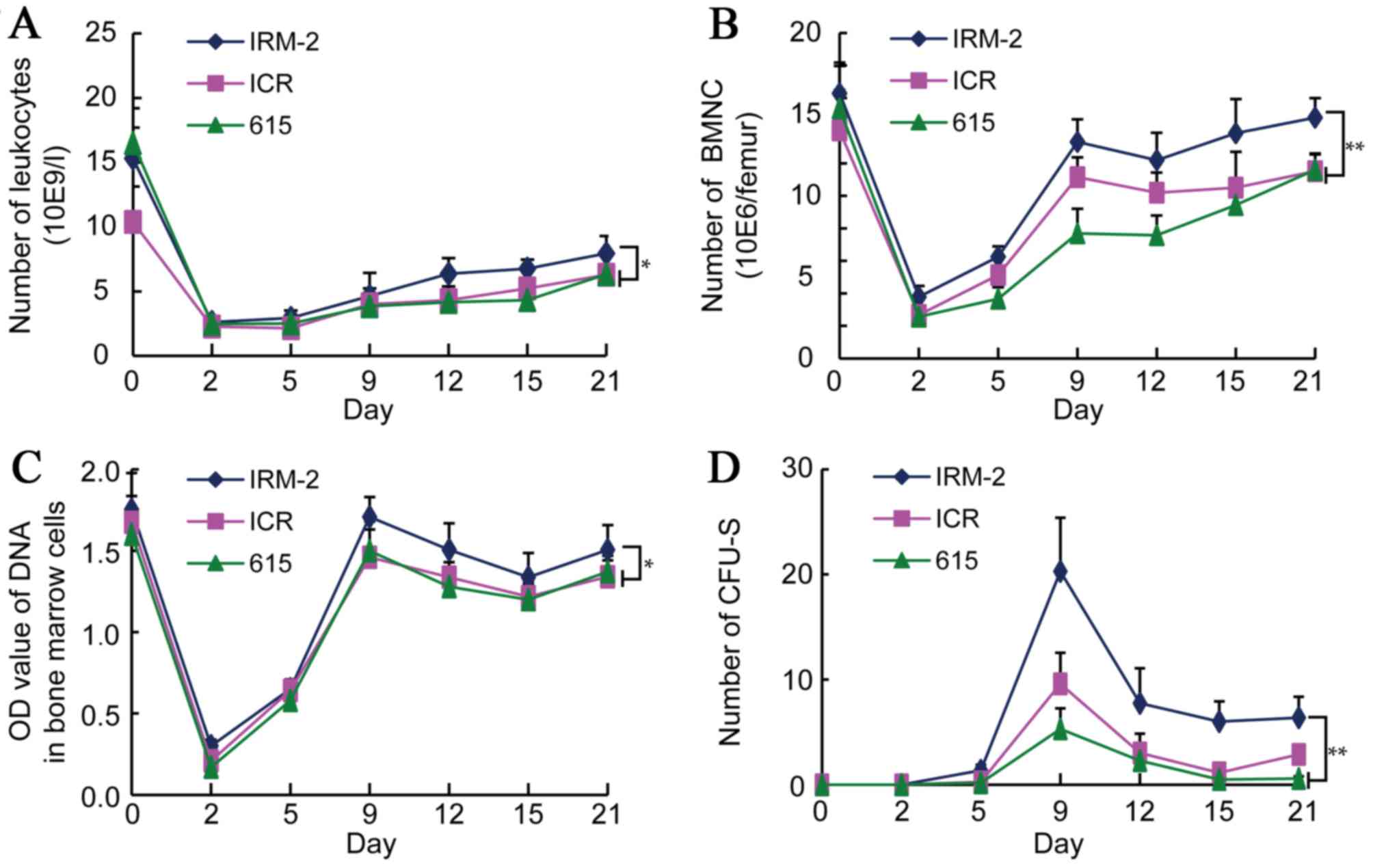 | Figure 8.Changes in the number of leukocytes
and in the hematopoietic function of mice irradiated with
γ-radiation. The function of the hematopoietic system was examined
to test the radiosensitivity of IRM-2 mice. IRM-2, ICR/JCL and 615
mice were irradiated with 4 Gy γ-radiation (n=8). (A) Whole blood
was extracted from the orbital sinuses on 2, 5, 9, 15 and 21 days
after irradiation and the leukocytes were counted. *P<0.05 vs.
IRM-2 mice 12 to 21 days after radiation. (B) Whole blood was
extracted from the orbital sinuses on 2, 5, 9, 15 and 21 days after
irradiation and the BMNCs were counted. (C) BMCs were flushed from
mouse femurs on 2, 5, 9, 15 and 21 days after irradiation and
treated with a 0.2 mol/l HClO4 solution. The DNA content
of the BMCs was measured at 286 nm. (D) Spleens were harvested from
the abdominal cavity of mice on 2, 5, 9, 15 and 21 days after
irradiation and CFU-S was counted under a microscope. *P<0.05,
**P<0.01 vs. IRM-2 mice 9 to 21 days after radiation. BMNCs,
bone marrow nucleated cells; BMCs, bone marrow cells; CFU-S,
colony-forming unit-spleen. |
To detect the hematopoietic function of mice after 4
Gy radiation, changes in the numbers of the bone marrow nucleated
cells (BMNCs), and the DNA content of BMCs and CFU-S were
determined. The number of BMNCs in the three mice strains was
decreased significantly to ~20% of the pre-irradiation value 2 days
after radiation, after which the value began to increase again in
the following days. A rapid increase in the BMNC count was
identified 2–9 days after irradiation, reaching ~80% of the
pre-irradiation value and remaining at a stable level (Fig. 8B). The number of BMNC of the IRM-2
mice was significantly higher than that of the ICR/JCL and 615 mice
9–21 days after irradiation (P<0.0001 vs. ICR/JCL and 615 mice;
Fig. 8B), indicating that the
hematopoietic function of the bone marrow of IRM-2 mice recovered
quickly.
The DNA content of the BMCs of the three mice
strains decreased significantly 2 days after 4 Gy radiation,
decreasing to ~20% of the pre-irradiation value. The DNA content
then recovered rapidly, reaching ~90% of the pre-irradiation value
on day 9 and decreasing slightly in the following days (Fig. 8C). The DNA content of the IRM-2
mice was significantly higher than that of the ICR/JCL and 615 mice
9–21 days after irradiation (P=0.0213 vs. ICR/JCL mice and P=0.0447
vs. 615 mice; Fig. 8C), similarly
suggesting that the hematopoietic function of the bone marrow of
the IRM-2 mice recovered quickly.
CFU-S were not identified in the three mice strains
prior to exposure to radiation and developed 5 days after 4 Gy
irradiation. CFU-S counts reached maximum values 9 days after
irradiation and decreased rapidly over the following days. Few
CFU-S were observed 21 days after radiation (Fig. 8D). The CFU-S counts of the IRM-2
mice were higher than those of the ICR/JCL and 615 mice 9–21 days
after irradiation (P=0.0004 vs. ICR/JCL mice and P<0.0001 vs.
615 mice; Fig. 8D), indicating
that the extramedullary hematopoietic system had a protective
effect against radiation exposure in the IRM-2 mice.
Discussion
As one of the most instructive experimental animals,
inbred mice have been used in a wide range of research fields. In
the present study, a novel inbred mouse strain, IRM-2, was
generated by crossing ICR/JCL mice (maternal strain) with 615 mice
(paternal strain). At the time of writing, the IRM-2 mouse is at
generation F88 and has therefore met the inbred-strain requirement
of having been bred for >20 generations. To determine the
quality of a new animal strain, its basic biological
characteristics have to be known. The IRM-2 inbred mice exhibited
good traits: Strong reproductive capacity, stable physiological and
biochemical indices and possessing few differences between
individuals, thereby meeting the basic requirements of experimental
animals.
Routine maintenance of experimental animals may
ensure that valuable animal resources change as little as possible
over time, thus ensuring that the biological research performed
with experimental animals is as accurate and reproducible as
possible (13). Although inbred
mice genes are genetically stable, 5% of the original
heterozygosity remains present even after 20 generations of
inbreeding (14). Spontaneous
mutations may occur during a long-term breeding process, possibly
altering the biological characteristics of inbred mice (15). Therefore, to ensure that the IRM-2
mice remain well genetically characterized, a genetic monitoring
program is necessary.
A coat-color gene test and a biochemical marker test
were used to identify the genetic homogeneity of IRM-2 mice. The
coat color of mice is genetically controlled by four locus alleles:
a, b, c and d, which are located on chromosomes 2, 4, 7 and 9
respectively. In the majority of cases, there were four coat color
phenotypes: AABBCCDD (wild-type), aabbCCDD (brown), aaBBCCDD
(black) and AAbbCCDD (cinnamon). The coat color of the IRM-2 mice
remained a constant cinnamon color during the breeding program. The
following two features of biochemical markers are characteristic of
inbred animals: i) Encoding biochemical marker genes are homozygous
without any heterozygosity and ii) marker genes are highly
consistent, with no difference between individuals. None of the
biochemical markers tested from the IRM-2 mice at generations F23
and F38 exhibited polymorphisms or mutations. Collectively, the
genetic monitoring data suggest that the IRM-2 mice were
genetically homozygous and consistent.
The purpose of the present study was to generate a
radioresistant animal model. To test the radiosensitivity of the
resulting IRM-2 mice, the function of bone marrow was examined.
Bone marrow, the hematopoietic system, is particularly sensitive to
IR (16,17). Once bone marrow is exposed to
radiation-induced injury, the body produces symptoms, including
hemorrhage, infection or anemia, that can be life-threatening. The
parameters of BMNCs and the DNA content of bone marrow may be
regarded as quantitative indices of radiation-induced injury to
bone marrow and its recovery. BMNCs include granulocytes,
megakaryocytes, lymphocytes and monocytes. The DNA content of BMCs
reflects the proliferation of BMNCs.
In the present study, the values of the number of
BMNCs and DNA content of bone marrow progressively decreased in the
early phase of radiation injury; in the recovery phase (2–21 days),
they increased to their normal leve1. The increase in the DNA
content of BMCs implied that proliferation activity of BMNC was
increased. The tolerance of cells to radiation is increased by
enhancing the ability of cells to repair DNA damage and the ability
to promote the body's hematopoietic function, which aids the
recovery and regeneration of BMNC and peripheral blood leukocytes
(18). The number of BMNCs and the
DNA content of the bone marrow from the IRM-2 mice were
significantly higher than those of the 615 and ICR/JCL mice at
different time points following irradiation, which suggested that
the repair function of the bone marrow from the IRM-2 mice treated
with radiation was robust.
When the hematopoietic microenvironment is severely
damaged following exposure to high doses of radiation, a
compensatory protective mechanism is induced; extramedullary
hematopoiesis. Residual hematopoietic stem cells can migrate into
the spleen to proliferate and spleen colonies develop in a process
termed CFU-S. Due to the fact that this process enables
self-renewal and multiple cell differentiation, CFU-S reflects the
function of hematopoietic stem cells. The results of the present
study demonstrated that the CFU-S counts of the IRM-2 mice after
exposure to 4 Gy radiation were significantly higher than those of
the parent mice. Collectively, IRM-2 mice exhibit high resistance
to radiation due to bone marrow hematopoiesis and extramedullary
hematopoiesis.
The dose of a substance that results in death in P%
of a test population is termed the LDP. In radiation
research field, the relevant LDP is the radiation dose
that is lethal to 50% of test subjects in a designated period:
Lethal dose 50 or LD50. When comparing LD50
between two test animals, the DRF is the common parameter of
interest. In the present investigation, LD50 of the
IRM-2 mice increased to 7.17 Gy (male) and 7.5 Gy (female), which
were significantly higher than those of the parents, the 615 mice
and ICR/JCL mice, giving a dose DRF value of 1.39 (male) and 1.37
(female). These data indicated that the IRM-2 mice had developed
resistance to ionizing irradiation.
In the present study, the basic biological
characteristics and the radiosensitivity of the IRM-2 mice were
determined. Further systematic and comprehensive characterizations
are required. To date, inbred IRM-2 mice have been used in studies
of the biological effects of radiation, anticancer drug screening
and nuclear medicine imaging research (19–23).
The novel inbred strain characterized in the current study
potentially constitutes a valuable mouse model for the study of
radioresistance.
Acknowledgements
The present study was supported by the Special
Foundation of the Ministry of Health (grant no. 201002009), the
National Natural Science Foundation of China (grant nos. 30870583,
31170804, 31240052, 31200634 and 31300695), the Natural Science
Foundation of Tianjin (grant nos. 09JCYBJC09300, 11ZCGYSY02400,
12JCYBJC15300, 12JCYBJC32900, 13JCYBJC23500 and 13JCQNJC11600) and
the PUMC Youth Fund and Fundamental Research Funds for the Central
Universities (grant nos. 2012G01 and 2012J05).
References
|
1
|
Steuber-Buchberger P, Wurst W and Kühn R:
Simultaneous Cre-mediated conditional knockdown of two genes in
mice. Genesis. 46:144–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu J and McMahon AP: Reproducible and
inducible knockdown of gene expression in mice. Genesis.
44:252–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berndt A, Sundberg BA, Silva KA, Kennedy
VE, Richardson MA, Li Q, Bronson RT, Uitto J and Sundberg JP:
Phenotypic characterization of the KK/HlJ inbred mouse strain. Vet
Pathol. 51:846–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Serpi R, Klein-Rodewald T, Calzada-Wack J,
Neff F, Schuster T, Gailus-Durner V, Fuchs H, Poutanen M, Hrabrè de
Angelis M and Esposito I: Inbred wild type mouse strains have
distinct spontaneous morphological phenotypes. Histol Histopathol.
28:79–88. 2013.PubMed/NCBI
|
|
5
|
Barden EK, Rellinger EA, Ortmann AJ and
Ohlemiller KK: Inheritance patterns of noise vulnerability and
‘protectability’ in (C57BL/6J × CBA/J) F1 hybrid mice. J Am Acad
Audiol. 23:332–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutsell BA and Newland MC: A quantitative
analysis of the effects of qualitatively different reinforcers on
fixed ratio responding in inbred strains of mice. Neurobio Learn
Mem. 101:85–93. 2013. View Article : Google Scholar
|
|
7
|
Kilikevicius A, Venckunas T, Zelniene R,
Carroll AM, Lionikaite S, Ratkevicius A and Lionikas A: Divergent
physiological characteristics and responses to endurance training
among inbred mouse strains. Scand J Med Sci Sports. 23:657–668.
2013.PubMed/NCBI
|
|
8
|
Chesler EJ, Plitt A, Fisher D, Hurd B,
Lederle L, Bubier JA, Kiselycznyk C and Holmes A: Quantitative
trait loci for sensitivity to ethanol intoxication in a
C57BL/6J×129S1/SvImJ inbred mouse cross. Mamm Genome. 23:305–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perez CJ, Dumas A, Vallières L, Guénet JL
and Benavides F: Several classical mouse inbred strains, including
DBA/2, NOD/Lt, FVB/N, and SJL/J, carry a putative loss-of-function
allele of Gpr84. J Hered. 104:565–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng A, Wang Y, Brown SA, Van Zant G and
Zhou D: Ionizing radiation and busulfan inhibit murine bone marrow
cell hematopoietic function via apoptosis-dependent and-independent
mechanisms. Exp Hematol. 31:1348–1356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hongying W, Yueying W, Lu L, Junling Z,
Deguan L and Aimin M: Comparison of the radiation sensitivity in
vitro of bone marrow cells from three mouse strains. Chin J Compar
Med. 19:56–58. 2009.
|
|
12
|
Junxu L, YueHua C, Wang Y, Feng X, Wang L,
Rong-Zhen S, Desen L and Fuying L: Detection of biochemical markers
in inbred MIJ and HFJ rats. Acta Lab Anim Sci Sin. 19:428–430.
2011.
|
|
13
|
Taft RA, Davisson M and Wiles MV: Know thy
mouse. Trends Genet. 22:649–653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan R, Flurkey K, Meng Q, Astle MC and
Harrison DE: Genetic regulation of life span, metabolism, and body
weight in pohn, a new wild-derived mouse strain. J Gerontol A Biol
Sci Med Sci. 68:27–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fahey JR, Katoh H, Malcolm R and Perez AV:
The case for genetic monitoring of mice and rats used in biomedical
research. Mamm Genome. 24:89–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J: Animal models for acquired bone
marrow failure syndromes. Clin Med Res. 3:102–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jagetia GC and Venkatesh P: Inhibition of
radiation-induced clastogenicity by Aegle marmelos (L.) correa in
mice bone marrow exposed to different doses of gamma-radiation. Hum
Exp Toxicol. 26:111–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuixia Z, Xuefen Y, Li S, Xiaodan W and
Tao X: Protective Effects of radaway radioprotection capsule on
radiation injured mice. Chin J Radiol Health. 21:259–260. 2012.
|
|
19
|
Wang YY, Zhou ZW, Shen X, et al:
Radiation-protective effect of E838 on IRM-2 and ICR/JCL mice. Chin
J Compar Med. 14:332–335. 2004.
|
|
20
|
Yueying W, Ruqin W and Zhong-Ping Z:
Radiation protective effects of four kind of estrogens on mice.
Chin J Radiol Med Prot. 25:39–40. 2005.
|
|
21
|
Li L, Peizhen H, Yujun Y, Yueying W, Junqi
W and Meijia L: Application of 99Tcm-HL91 in the hypoxia study of
malignant lymphoma. Chin J Nucl Med. 22:p118–119. 2002.
|
|
22
|
Zhou ZW, Liu PX, Li SN, et al: Inhibiting
of 9002 on the growth of xenografted tumor of IRM-2 mice. Chin J
Lab Anim Sci. 12:1732002.
|
|
23
|
Jingying Y, Qing W, Guofan L, Chuanjie M,
Peizhen H and Xuemin F: An experimental study of inflammation
imaging with ‘99Tcm-HYNIC-fMLFK’ in IRM-2 inbred mice. Chin J Nucl
Med. 23:p179–181. 2003.
|