Bone regeneration has been extensively investigated
during the past several decades, resulting in therapeutic
progression in this field. However, critical bone defects,
particularly in patients with an unfavorable healing
microenvironment, remain a primary concern for surgeons (1–3).
Various mouse models have been developed for the investigation of
various injuries and pathological processes associated with bone
regeneration, and numerous important molecular signaling pathways
have been elucidated and therapies developed (1,4–6).
Among all the different mouse models, surgically-induced models are
prevalent in bone regeneration research (7). Regenerative medical therapies
associated with bone healing employ an extensive range of various
strategies that aim to repair, augment, substitute or regenerate
lost tissue (4). To determine the
effect of these various treatment therapies, mouse models that use
surgical induction of a particular condition are frequently
performed, due to their similarity to the trauma and the patient
recovery process (8–10). These models are well established in
combination with tissue engineering strategies, for analysis of the
function of growth factors, scaffolds and stem cells (11,12).
Furthermore, these mouse models may be performed in genetically
modified mice, which is an important method using gene-targeting to
investigate the genes involved in bone regeneration (13,14).
This review briefly evaluates surgically-induced mouse models, with
focus on the most important models currently used and the potential
development of novel models in the future.
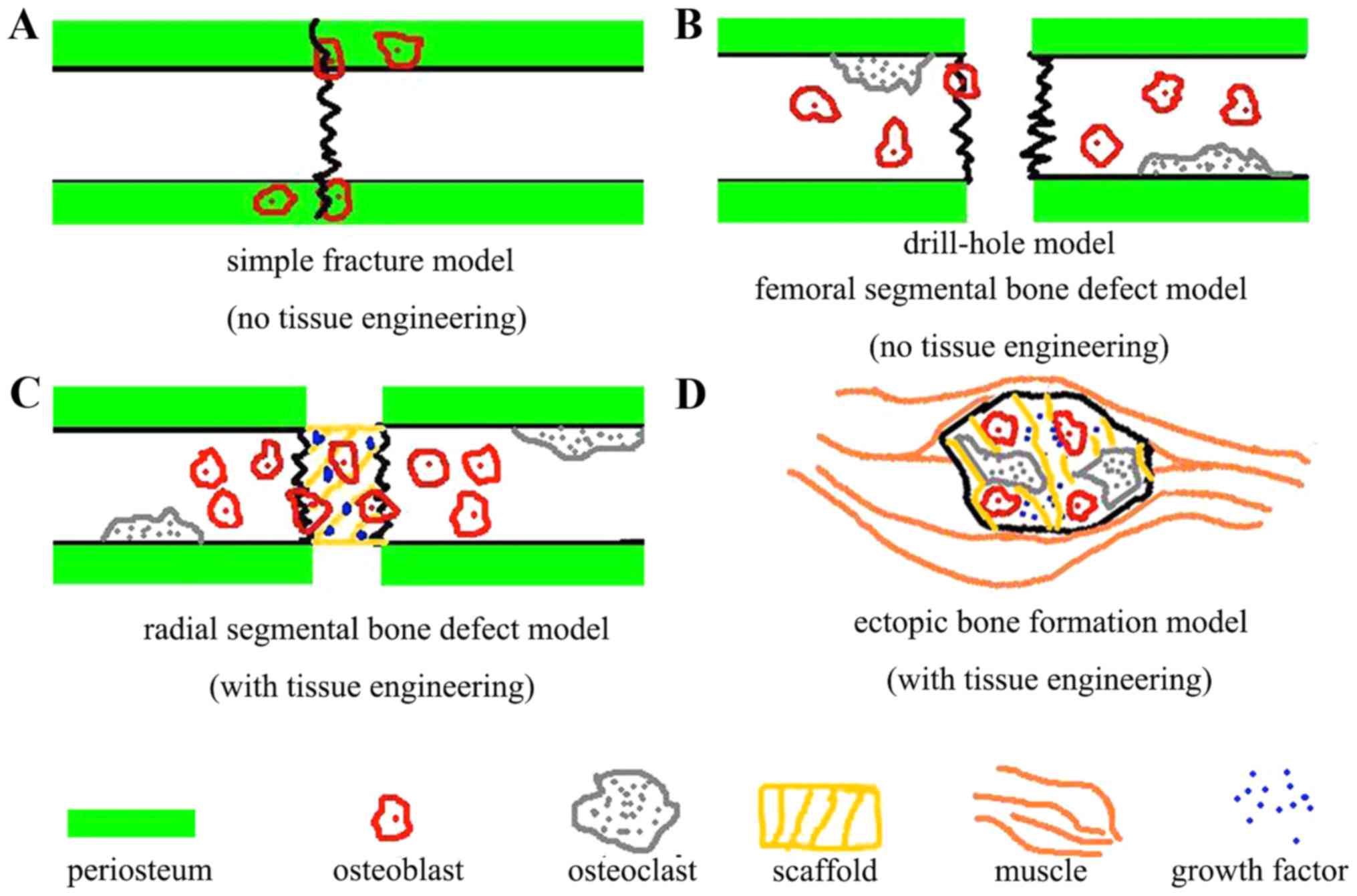
The surgically induced mouse models were divided
into three different groups based on the severity of trauma and the
mouse phenotypes: Simple fracture models, bone defect models and
ectopic bone formation models.
Simple fracture models are used to determine the
effect of various drugs and gene modifications in fracture healing.
The fracture model may be further classified by anatomic location,
with the fibula (15,16) and femur (17,18)
among the most common sites. The fracture may be created by blunt
trauma or using ophthalmic forceps (15,16).
For the simple blunt fracture model, three-point bending equipment
is used to create a fracture. The simple fracture model in the
femur is more complex, as it requires a needle to be implanted into
the intramedullary cavity via the intercondylar notch to ‘fix’ the
fracture prior to creation. This is not required in the fibular
fracture model (19–21). These models are technically simple
compared with other models and are frequently used for
identification of bone regeneration associated factors (22,23).
Critical sized bone defects are a challenging
clinical scenario for surgeons and frequently result in a delayed
bone union or a nonunion in numerous cases (24). Therefore, surgically-induced bone
defect mouse models have been extensively used for analysis of
growth factors (25,26). It has previously been reported that
deficiency of progranulin (PGRN), which is a downstream mediator of
bone morphogenetic protein-2 (BMP-2) involved in bone healing,
delayed bone healing, whereas recombinant PGRN enhanced bone
regeneration. Furthermore, PGRN was required for BMP-2 induction of
osteoblastogenesis and ectopic bone formation (25). When the bone defect models have
been used in biomaterial research (27,28),
the results indicated that osteoinduction and appropriate
degradation were important in accelerating and promoting bone
augmentation. This strategy appears promising as 3D temporal
scaffolds for potential orthopedic applications (28). In addition, this type of model may
be used in stem cell research (29–31).
The findings of the experiment indicated that human muscle-derived
stem cells (hMDSCs; Stem Cell Research Center, University of
Pittsburgh, Pittsburgh, PA, USA) are mesenchymal stem cells of
muscle origin and that BMP2 is more efficient than BMP4 in
promoting the bone regenerative capacity of the hMDSCs in
vivo (31). Local or systemic
delivery of drugs may be tested using these models. Altering the
genotype of the mouse involved with these models may also enable
researchers to understand the molecular signaling pathways involved
in fracture healing and bone regeneration. According to the size
and pattern of the bone defect, these models are further divided
into drill-hole or critical-size bone defect models.
The critical bone defect model is used to simulate a
greater degree of bone loss than the drill-hole model and is
frequently used to study non-unions. A review of the literature
revealed that two of the most frequently used methods to establish
a critical bone defect include the use of either the cranial bone
of the skull (38,39) or long bones of the extremities,
including the femur (25,40,41)
and radius. There are various differences in the methods used to
establish these models. To create the cranial defect, the
pericranium is removed and a trephine is used to create a circular
bone defect in the skull, with meticulous care taken to avoid
damaging the underlying dura mater (38,39).
A drill bit is used to create the defect in the long bone defect
models (25,41); however, a drill bit cannot be used
to create cranial defects as the dura mater is in close proximity
to the inferior aspect of the skull. In the mouse, a critical-size
cranial defect is defined as a bone deficit ≥5 mm (42,43).
This model has been used for the investigation of molecular
signaling pathways associated with bone healing, by using knockout
and overexpressing mice, and determining the effects of treatments
aimed at the promotion of bone regeneration (44–46).
For instance, critical-size bone defect models reveal accelerated
bone formation and bone remodeling in the absence of the Toll-like
receptor 4 signaling pathway. This phenotype is associated with
alterations of local inflammatory cytokines and expression of
osteoclastogenic factors (44).
The femoral bone defect model was originally established to
investigate the pathways involved in non-unions (40,47,48),
and has since been used to study various treatments to promote bone
healing (49). In our previous
study, a 0.5 mm femoral bone defect was used to investigate bone
healing. It was demonstrated that wild-type mice of the control
group were able to fully heal the 0.5 mm bone defect, however PGRN
knockout mice exhibited impaired bone healing (25). The mouse model was relatively
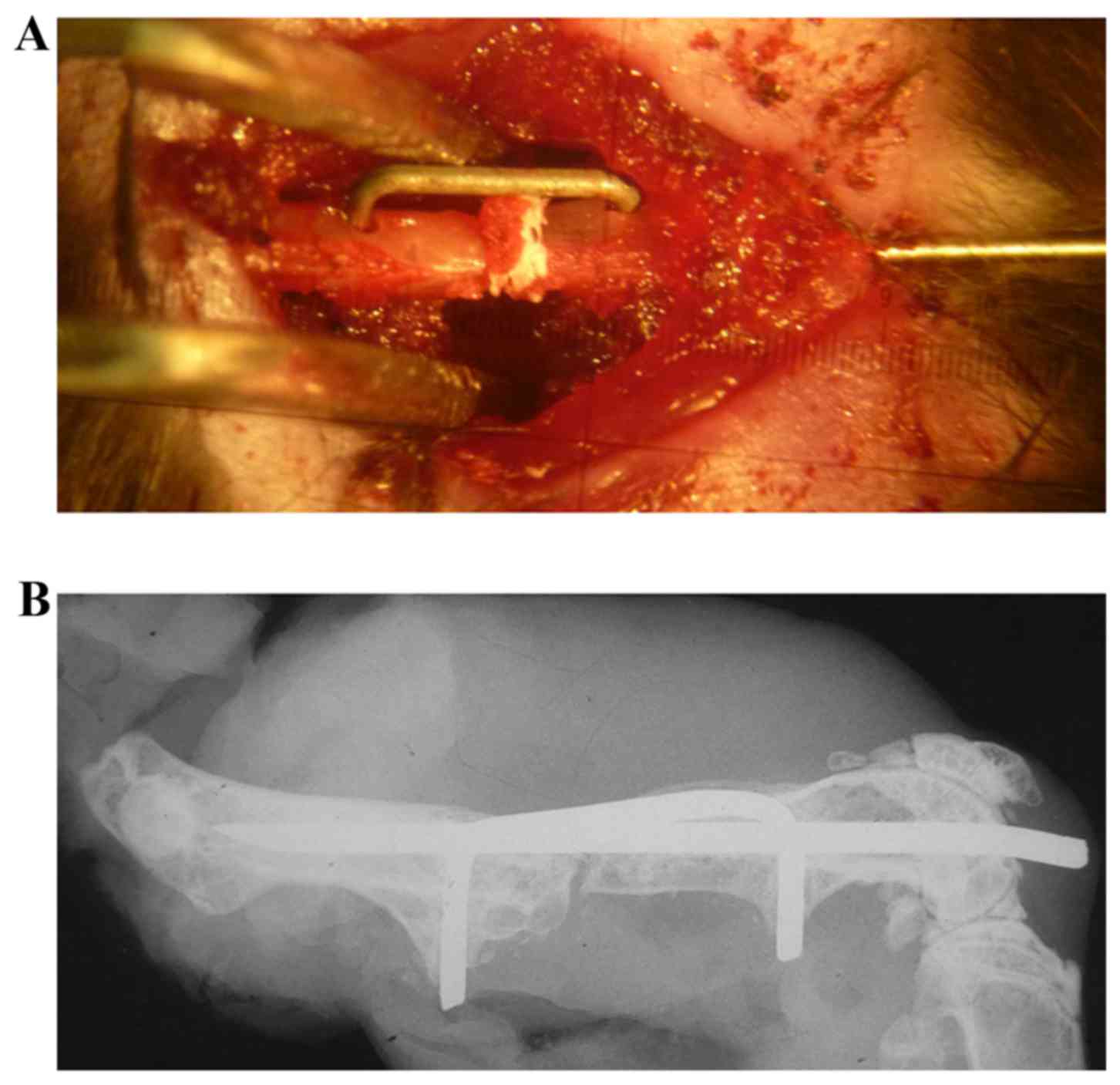
complicated to create, as an intramedullary needle and a
custom-made clip were implanted into the femur to fix the bone
defect (Fig. 1). The use of metal
devices may interfere with the bone signal when using micro
computed tomography (CT; data not shown), and should be removed to
minimize any of these artifacts (50). However, the removal process may
result in damage to the original structure of the bone defect
position.
The radial bone defect model has been extensively
used for determining the effects of tissue engineering in bone
repair (51–53). This is a non-union model and the
bone defect will not recover spontaneously without additional
treatment, which enables the use of gain-of-function studies
(54). The bone defect of the
radius is stable, supported by an intact ulna and scaffold carrying
growth factors, to aid the implantation of cells. Furthermore, this
model has previously been established in genetically modified mice
to study molecular signaling pathways of fracture healing. The
present study established this model in tumor necrosis factor-α
receptor (TNFR)-deficient mice (Jackson Laboratory, Bar Harbor, ME,
USA) to investigate the role of TNFR in the effect of recombinant
PGRN protein in the promotion of bone repair (25).
Ectopic bone is bone that forms in locations where
bone formation does not typically occur. Several molecules have
been identified to be involved in the process of ectopic bone
formation. It has previously been demonstrated that ectopic bone
formation may occur in PGRN knockout mice (New York University
Medical Center, New York, NY, USA) (78). BMPs are extensively used to induce
ectopic bone formation (55,56).
Molecules and signaling pathways associated with these growth
factors are investigated using models of ectopic bone formation
(35,57). These models are typically either
subcutaneous or intramuscular in location (25,56,58).
For subcutaneous ectopic bone formation models, implants carrying
genetically modified stem cells and/or growth factors are
surgically implanted into a pocket beneath the skin, and bone
formation is detected at indicated time points (59). Intramuscular ectopic bone formation
can be established in paravertebral (51,60,61),
thigh (62) or calf muscles
(63). These models may be used to
determine the effect of various therapies on BMP-induced bone
formation, and may aid the identification of novel therapeutic
strategies (25,59,64).
The data from this type of model demonstrates that a focused
approach to develop targeted differentiation protocols in adult
progenitor cells may be achieved via the identification and
subsequent stimulation of genes, proteins and signaling pathways
associated with calcium phosphate mediated osteoinduction (64).
Mouse models have numerous advantages compared with
larger animal models, and are used for a broad range of
applications (Table I) (65). Mice are docile, tolerate the
surgical procedures and are able to ambulate with the implanted
limb within a short time following surgery (66). Additionally, genetic alterations
are easily created in mice and therefore certain genes can be
targeted for knockout or overexpression. This allows the
investigation of the effect of drug therapies on bone regeneration
and the identification of the underlying molecular mechanisms
involved. Furthermore, various mouse models have been well
established in the literature, and researchers may select an
appropriate model based on the aim of the experiment.
However, surgically-induced mouse models have
limitations. In numerous cases, genetic modification results in a
defect during development, which may involve bone growth (67,68).
This may subsequently interfere with bone healing, and therefore
artificially alter the results of the experiment. In these cases,
inducible genetically modified mice may be used to eliminate any
effect on bone development (69).
The use of surgically-induced mouse models of bone
regeneration have the potential to be improved. Firstly, more
efficient devices may be developed for fixation of these models.
Fixation devices that are used near the surgical site should be
free of degrading particles to result in a more purified
microenvironment for bone regeneration. Novel devices are required
for more convenient fixation and less damage to the surrounding
soft tissue, so that the blood supply to the area of healing is
protected. Imaging modalities used for these small areas of bone
regeneration also require improvement, including micro CT and
magnetic resonance imaging. Finally, inducible transgenic mice
should be used more frequently in the establishment of these
models, as this would eliminate any alterations in bone formation
that occur during development.
This study was partially supported by the National
Natural Science Foundation of China (grant no. 81401014), the Star
of Jinan Youth Science and Technology Project (grant no. 20100114),
the Young Scientists Awards Foundation of Shandong Province (grant
no. BS2013YY049) and the International Cooperation Projects of
Jinan City (grant no. 201305053).
|
1
|
Gomes PS and Fernandes MH: Rodent models
in bone-related research: The relevance of calvarial defects in the
assessment of bone regeneration strategies. Lab Anim. 45:14–24.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hobby B and Lee MA: Managing atrophic
nonunion in the geriatric population: Incidence, distribution and
causes. Orthop Clin North Am. 44:251–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edwards BJ, Bunta AD, Lane J, Odvina C,
Rao DS, Raisch DW, McKoy JM, Omar I, Belknap SM, Garg V, et al:
Bisphosphonates and nonhealing femoral fractures: Analysis of the
FDA adverse event reporting system (FAERS) and international safety
efforts: A systematic review from the research on adverse drug
events and reports (RADAR) project. J Bone Joint Surg Am.
95:297–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleinschmidt K, Ploeger F, Nickel J,
Glockenmeier J, Kunz P and Richter W: Enhanced reconstruction of
long bone architecture by a growth factor mutant combining positive
features of GDF-5 and BMP-2. Biomaterials. 34:5926–5936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Zara J, Siu RK, Ting K and Soo C:
The role of NELL-1, a growth factor associated with
craniosynostosis, in promoting bone regeneration. J Dent Res.
89:865–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao YP, Tian QY and Liu CJ: Progranulin
deficiency exaggerates, whereas progranulin-derived Atsttrin
attenuates, severity of dermatitis in mice. FEBS Lett.
587:1805–1810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szpalski C, Barr J, Wetterau M, Saadeh PB
and Warren SM: Cranial bone defects: Current and future strategies.
Neurosurgical Focus. 29:E82010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wahl EC, Aronson J, Liu L, Skinner RA,
Ronis MJ and Lumpkin CK Jr: Distraction osteogenesis in TNF
receptor 1 deficient mice is protected from chronic ethanol
exposure. Alcohol. 46:133–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Péault B, Chen W, Li W, Corselli
M, James AW, Lee M, Siu RK, Shen P, Zheng Z, et al: The Nell-1
growth factor stimulates bone formation by purified human
perivascular cells. Tissue Eng Part A. 17:2497–2509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mashiba T, Iwata K, Komatsubara S and
Manabe T: Animal models for bone and joint disease. Animal fracture
model and fracture healing process. Clin calcium. 21:235–241.
2011.PubMed/NCBI
|
|
11
|
Burg KJ, Porter S and Kellam JF:
Biomaterial developments for bone tissue engineering. Biomaterials.
21:2347–2359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giannoudis PV and Pountos I: Tissue
regeneration. The past, the present and the future. Injury.
36:(Suppl 4). S2–S5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maes C, Carmeliet G and Schipani E:
Hypoxia-driven pathways in bone development, regeneration and
disease. Nat Rev Rheumatol. 8:358–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosen V: BMP2 signaling in bone
development and repair. Cytokine Growth Factor Rev. 20:475–480.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng L, Ye F, Yang R, Lu X, Shi Y, Li L,
Fan H and Bu H: Osteoinduction of hydroxyapatite/beta-tricalcium
phosphate bioceramics in mice with a fractured fibula. Acta
Biomater. 6:1569–1574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kayal RA, Siqueira M, Alblowi J, McLean J,
Krothapalli N, Faibish D, Einhorn TA, Gerstenfeld LC and Graves DT:
TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during
fracture healing and stimulates chondrocyte apoptosis through
FOXO1. J Bone Miner Res. 25:1604–1615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holstein JH, Karabin-Kehl B, Scheuer C,
Garcia P, Histing T, Meier C, Benninger E, Menger MD and Pohlemann
T: Endostatin inhibits Callus remodeling during fracture healing in
mice. J Orthop Res. 31:1579–1584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holstein JH, Matthys R, Histing T, Becker
SC, Fiedler M, Garcia P, Meier C, Pohlemann T and Menger MD:
Development of a stable closed femoral fracture model in mice. J
Surg Res. 153:71–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Neill KR, Stutz CM, Mignemi NA, Burns
MC, Murry MR, Nyman JS and Schoenecker JG: Micro-computed
tomography assessment of the progression of fracture healing in
mice. Bone. 50:1357–1367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Einhorn TA: Enhancement of
fracture-healing. J Bone Joint Surg Am. 77:940–956. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kellum E, Starr H, Arounleut P, Immel D,
Fulzele S, Wenger K and Hamrick MW: Myostatin (GDF-8) deficiency
increases fracture callus size, Sox-5 expression, and callus bone
volume. Bone. 44:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wigner NA, Kulkarni N, Yakavonis M, Young
M, Tinsley B, Meeks B, Einhorn TA and Gerstenfeld LC: Urine matrix
metalloproteinases (MMPs) as biomarkers for the progression of
fracture healing. Injury. 43:274–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerstenfeld LC, Cho TJ, Kon T, Aizawa T,
Tsay A, Fitch J, Barnes GL, Graves DT and Einhorn TA: Impaired
fracture healing in the absence of TNF-alpha signaling: The role of
TNF-alpha in endochondral cartilage resorption. J Bone Miner Res.
18:1584–1592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haddock NT, Wapner K and Levin LS:
Vascular bone transfer options in the foot and ankle: A
retrospective review and update on strategies. Plast Reconstr Surg.
132:685–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao YP, Tian QY, Frenkel S and Liu CJ:
The promotion of bone healing by progranulin, a downstream molecule
of BMP-2, through interacting with TNF/TNFR signaling.
Biomaterials. 34:6412–6421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ben-David D, Srouji S, Shapira-Schweitzer
K, Kossover O, Ivanir E, Kuhn G, Müller R, Seliktar D and Livne E:
Low dose BMP-2 treatment for bone repair using a PEGylated
fibrinogen hydrogel matrix. Biomaterials. 34:2902–2910. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Annibali S, Cicconetti A, Cristalli MP,
Giordano G, Trisi P, Pilloni A and Ottolenghi L: A comparative
morphometric analysis of biodegradable scaffolds as carriers for
dental pulp and periosteal stem cells in a model of bone
regeneration. J Craniofac Surg. 24:866–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang F, Wang J, Hou J, Guo H and Liu C:
Bone regeneration using cell-mediated responsive degradable
PEG-based scaffolds incorporating with rhBMP-2. Biomaterials.
34:1514–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H and Xing L: Ubiquitin e3 ligase
itch negatively regulates osteoblast differentiation from
mesenchymal progenitor cells. Stem cells. 31:1574–1583. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fricain JC, Schlaubitz S, Le Visage C,
Arnault I, Derkaoui SM, Siadous R, Catros S, Lalande C, Bareille R,
Renard M, et al: A nano-hydroxyapatite-pullulan/dextran
polysaccharide composite macroporous material for bone tissue
engineering. Biomaterials. 34:2947–2959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao X, Usas A, Lu A, Tang Y, Wang B, Chen
CW, Li H, Tebbets JC, Cummins JH and Huard J: BMP2 is superior to
BMP4 for promoting human muscle-derived stem cell-mediated bone
regeneration in a critical-sized calvarial defect model. Cell
transplantat. 22:2393–2408. 2013. View Article : Google Scholar
|
|
32
|
Tanaka K, Tanaka S, Sakai A, Ninomiya T,
Arai Y and Nakamura T: Deficiency of vitamin A delays bone healing
process in association with reduced BMP2 expression after
drill-hole injury in mice. Bone. 47:1006–1012. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katae Y, Tanaka S, Sakai A, Nagashima M,
Hirasawa H and Nakamura T: Elcatonin injections suppress systemic
bone resorption without affecting cortical bone regeneration after
drill-hole injuries in mice. J Orthop Res. 27:1652–1658. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Behr B, Leucht P, Longaker MT and Quarto
N: Fgf-9 is required for angiogenesis and osteogenesis in long bone
repair. Proc Natl Acad Sci USA. 107:11853–11858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang N, Song WX, Luo J, Luo X, Chen J,
Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al: BMP-9-induced
osteogenic differentiation of mesenchymal progenitors requires
functional canonical Wnt/beta-catenin signalling. J Cell Mol Med.
13:2448–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He YX, Zhang G, Pan XH, Liu Z, Zheng LZ,
Chan CW, Lee KM, Cao YP, Li G, Wei L, et al: Impaired bone healing
pattern in mice with ovariectomy-induced osteoporosis: A drill-hole
defect model. Bone. 48:1388–1400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jawad MU, Fritton KE, Ma T, Ren PG,
Goodman SB, Ke HZ, Babij P and Genovese MC: Effects of sclerostin
antibody on healing of a non-critical size femoral bone defect. J
Orthop Res. 31:155–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meszaros LB, Usas A, Cooper GM and Huard
J: Effect of host sex and sex hormones on muscle-derived stem
cell-mediated bone formation and defect healing. Tissue Eng Part A.
18:1751–1759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Behr B, Sorkin M, Lehnhardt M, Renda A,
Longaker MT and Quarto N: A comparative analysis of the osteogenic
effects of BMP-2, FGF-2, and VEGFA in a calvarial defect model.
Tissue Eng Part A. 18:1079–1086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu K, Li D, Huang X, Lv K, Ongodia D, Zhu
L, Zhou L and Li Z: A murine femoral segmental defect model for
bone tissue engineering using a novel rigid internal fixation
system. J Surg Res. 183:493–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Manassero M, Viateau V, Matthys R,
Deschepper M, Vallefuoco R, Bensidhoum M and Petite H: A novel
murine femoral segmental critical-sized defect model stabilized by
plate osteosynthesis for bone tissue engineering purposes. Tissue
Eng Part C Methods. 19:271–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Krebsbach PH, Mankani MH, Satomura K,
Kuznetsov SA and Robey PG: Repair of craniotomy defects using bone
marrow stromal cells. Transplantation. 66:1272–1278. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee JY, Musgrave D, Pelinkovic D,
Fukushima K, Cummins J, Usas A, Robbins P, Fu FH and Huard J:
Effect of bone morphogenetic protein-2-expressing muscle-derived
cells on healing of critical-sized bone defects in mice. J Bone
Joint Surg Am. 83-A:1032–1039. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang D, Gilbert JR, Cray JJ Jr, Kubala AA,
Shaw MA, Billiar TR and Cooper GM: Accelerated calvarial healing in
mice lacking Toll-like receptor 4. PLoS One. 7:e469452012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Levi B, Hyun JS, Montoro DT, Lo DD, Chan
CK, Hu S, Sun N, Lee M, Grova M, Connolly AJ, et al: In vivo
directed differentiation of pluripotent stem cells for skeletal
regeneration. Proc Natl Acad Sci USA. 109:20379–20384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lo DD, Mackanos MA, Chung MT, Hyun JS,
Montoro DT, Grova M, Liu C, Wang J, Palanker D, Connolly AJ, et al:
Femtosecond plasma mediated laser ablation has advantages over
mechanical osteotomy of cranial bone. Lasers Surg Med. 44:805–814.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garcia P, Holstein JH, Maier S,
Schaumlöffel H, Al-Marrawi F, Hannig M, Pohlemann T and Menger MD:
Development of a reliable non-union model in mice. J Surg Res.
147:84–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zwingenberger S, Niederlohmann E, Vater C,
Rammelt S, Matthys R, Bernhardt R, Valladares RD, Goodman SB and
Stiehler M: Establishment of a femoral critical-size bone defect
model in immunodeficient mice. J Surg Res. 181:e7–e14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin EA, Liu CJ, Monroy A, Khurana S and
Egol KA: Prevention of atrophic nonunion by the systemic
administration of parathyroid hormone (PTH 1–34) in an experimental
animal model. J Orthop Trauma. 26:719–723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Holstein JH, Orth M, Scheuer C, Tami A,
Becker SC, Garcia P, Histing T, Mörsdorf P, Klein M, Pohlemann T
and Menger MD: Erythropoietin stimulates bone formation, cell
proliferation, and angiogenesis in a femoral segmental defect model
in mice. Bone. 49:1037–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kimelman-Bleich N, Pelled G, Sheyn D,
Kallai I, Zilberman Y, Mizrahi O, Tal Y, Tawackoli W, Gazit Z and
Gazit D: The use of a synthetic oxygen carrier-enriched hydrogel to
enhance mesenchymal stem cell-based bone formation in vivo.
Biomaterials. 30:4639–4648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moutsatsos IK, Turgeman G, Zhou S,
Kurkalli BG, Pelled G, Tzur L, Kelley P, Stumm N, Mi S, Müller R,
et al: Exogenously regulated stem cell-mediated gene therapy for
bone regeneration. Mol Ther. 3:449–461. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kimelman-Bleich N, Pelled G, Zilberman Y,
Kallai I, Mizrahi O, Tawackoli W, Gazit Z and Gazit D: Targeted
gene-and-host progenitor cell therapy for nonunion bone fracture
repair. Mol Ther. 19:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tai K, Pelled G, Sheyn D, Bershteyn A, Han
L, Kallai I, Zilberman Y, Ortiz C and Gazit D: Nanobiomechanics of
repair bone regenerated by genetically modified mesenchymal stem
cells. Tissue Eng Part A. 14:1709–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bergeron E, Leblanc E, Drevelle O, Giguère
R, Beauvais S, Grenier G and Faucheux N: The evaluation of ectopic
bone formation induced by delivery systems for bone morphogenetic
protein-9 or its derived peptide. Tissue Eng Part A. 18:342–352.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kamiya N: The role of BMPs in bone
anabolism and their potential targets SOST and DKK1. Curr Mol
Pharmacol. 5:153–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen L, Jiang W, Huang J, He BC, Zuo GW,
Zhang W, Luo Q, Shi Q, Zhang BQ and Wagner ER: Insulin-like growth
factor 2 (IGF-2) potentiates BMP-9-induced osteogenic
differentiation and bone formation. J Bone Miner Res. 25:2447–2459.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wagner-Ecker M, Voltz P, Egermann M and
Richter W: The collagen component of biological bone graft
substitutes promotes ectopic bone formation by human mesenchymal
stem cells. Acta Biomater. 9:7298–7307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Frescaline G, Bouderlique T, Mansoor L,
Carpentier G, Baroukh B, Sineriz F, Trouillas M, Saffar JL, Courty
J, Lataillade JJ, et al: Glycosaminoglycan mimetic associated to
human mesenchymal stem cell-based scaffolds inhibit ectopic bone
formation, but induce angiogenesis in vivo. Tissue Eng Part A.
19:1641–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hasharoni A, Zilberman Y, Turgeman G, Helm
GA, Liebergall M and Gazit D: Murine spinal fusion induced by
engineered mesenchymal stem cells that conditionally express bone
morphogenetic protein-2. J Neurosurg Spine. 3:47–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sheyn D, Pelled G, Zilberman Y, Talasazan
F, Frank JM, Gazit D and Gazit Z: Nonvirally engineered porcine
adipose tissue-derived stem cells: Use in posterior spinal fusion.
Stem cells. 26:1056–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Medici D, Shore EM, Lounev VY, Kaplan FS,
Kalluri R and Olsen BR: Conversion of vascular endothelial cells
into multipotent stem-like cells. Nat Med. 16:1400–1406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shimono K, Tung WE, Macolino C, Chi AH,
Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto
M, Pacifici M and Iwamoto M: Potent inhibition of heterotopic
ossification by nuclear retinoic acid receptor-γ agonists. Nat Med.
17:454–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Eyckmans J, Roberts SJ, Bolander J,
Schrooten J, Chen CS and Luyten FP: Mapping calcium phosphate
activated gene networks as a strategy for targeted osteoinduction
of human progenitors. Biomaterials. 34:4612–4621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Aalami OO, Nacamuli RP, Lenton KA, Cowan
CM, Fang TD, Fong KD, Shi YY, Song HM, Sahar DE and Longaker MT:
Applications of a mouse model of calvarial healing: Differences in
regenerative abilities of juveniles and adults. Plast Reconstr
Surg. 114:713–720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang T, Yu H, Gong W, Zhang L, Jia T,
Wooley PH and Yang SY: The effect of osteoprotegerin gene
modification on wear debris-induced osteolysis in a murine model of
knee prosthesis failure. Biomaterials. 30:6102–6108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Seto J, Busse B, Gupta HS, Schäfer C,
Krauss S, Dunlop JW, Masic A, Kerschnitzki M, Zaslansky P, Boesecke
P, et al: Accelerated growth plate mineralization and foreshortened
proximal limb bones in fetuin-A knockout mice. PLoS One.
7:e473382012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xie C, Xue M, Wang Q, Schwarz EM, O'Keefe
RJ and Zhang X: Tamoxifen-inducible CreER-mediated gene targeting
in periosteum via bone-graft transplantation. J Bone Joint Surg Am.
90:(Suppl 1). S9–S13. 2008. View Article : Google Scholar
|
|
70
|
Bockamp E, Maringer M, Spangenberg C, Fees
S, Fraser S, Eshkind L, Oesch F and Zabel B: Of mice and models:
Improved animal models for biomedical research. Physiol Genomics.
11:115–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matsushita Y, Sakamoto K, Tamamura Y,
Shibata Y, Minamizato T, Kihara T, Ito M, Katsube K, Hiraoka S,
Koseki H, et al: CCN3 protein participates in bone regeneration as
an inhibitory factor. J Biol Chem. 288:19973–19985. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gualeni B, de Vernejoul MC, Marty-Morieux
C, De Leonardis F, Franchi M, Monti L, Forlino A, Houillier P,
Rossi A and Geoffroy V: Alteration of proteoglycan sulfation
affects bone growth and remodeling. Bone. 54:83–91. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
do Soung Y, Gentile MA, le Duong T and
Drissi H: Effects of pharmacological inhibition of cathepsin K on
fracture repair in mice. Bone. 55:248–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Colnot C, Zhang X and Tate Knothe ML:
Current insights on the regenerative potential of the periosteum:
Molecular, cellular, and endogenous engineering approaches. J
Orthop Res. 30:1869–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu YY, Bahney C, Hu D, Marcucio RS and
Miclau T III: Creating rigidly stabilized fractures for assessing
intramembranous ossification, distraction osteogenesis, or healing
of critical sized defects. J Vis Exp pii. 35522012.
|
|
76
|
Bose S, Roy M and Bandyopadhyay A: Recent
advances in bone tissue engineering scaffolds. Trends Biotechnol.
30:546–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yun YR, Jang JH, Jeon E, Kang W, Lee S,
Won JE, Kim HW and Wall I: Administration of growth factors for
bone regeneration. Regen Med. 7:369–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhao YP, Tian QY, Liu B, Cuellar J,
Richbourgh B, Jia TH and Liu CJ: Progranulin knockout accelerates
intervertebral disc degeneration in aging mice. Sci Rep.
5:91022015. View Article : Google Scholar : PubMed/NCBI
|