Introduction
Zing finger protein 580 (ZNF580) is a novel gene
that is associated with low-density lipoprotein stimulation in
vascular endothelial cells. ZNF580 gene cloning (1) and subsequent bioinformatics analysis
revealed that ZNF580 is a Cys2-His2 (C2H2)-type transcription
factor (2). C2H2-zinc finger genes
constitute the largest class of transcription factors within the
human genome; they are typically involved in critical cell
functions, such as survival and growth (3). Our previous study indicated that
ZNF580 is ubiquitously expressed in human tissues, and serves
important roles in maintaining normal cell functions, including
migration and proliferation (4,5).
ZFP580 is the murine homologue of ZNF580, research regarding cloned
ZFP580 (6) revealed that
intermittent hypoxia could induce expression, which displayed an
anti-apoptotic role during early phase ischemia-reperfusion injury
(7). However, the mechanism and
signaling pathways underlying the anti-apoptotic effects of ZFP580
have not yet been fully elucidated.
Members of the transforming growth factor-β (TGF-β)
family regulate numerous cellular functions, including cell growth,
differentiation, adhesion, migration and apoptosis. TGF-β1 is a
multifunctional cytokine that regulates apoptosis in a cell
type-specific and context-dependent manner, with proapoptotic or
anti-apoptotic actions depending on the target cell type and the
pathophysiologic milieu (8–10).
Despite its proapoptotic role in several cell types, TGF-β1
demonstrated cardioprotective effects during reperfusion injury or
cardiac inflammatory disease in myocytes (11–13),
and exerted an anti-apoptotic role in hypoxia-reoxygenation-induced
myocardial cell injury (12,14,15).
Mothers against decapentaplegic homolog (Smad)
proteins are the primary intracellular mediators of the TGF-β1
signaling pathway. Smad2 and Smad3 are particularly important in
the transcriptional response to TGF-β1, in various physiological
scenarios. Our previous study determined that ZNF580 is involved in
the TGF-β1 signaling pathway as a binding partner of Smad2
(16,17). However, the actual relationship
between ZFP580 and Smad proteins in cobalt chloride
(CoCl2)-induced apoptosis has not been fully elucidated.
Therefore, the present study aimed to investigate the involvement
of ZFP580 in TGF-β1-mediated cytoprotection during
CoCl2-induced apoptosis, and its association with Smad2
in embryonic rat heart H9c2 cells.
Materials and methods
Materials
SB431542, CoCl2,
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
and 2,7-dichlorofluorescin diacetate (DCFH-DA) were provided by
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Recombinant
human TGF-β1 was purchased from PeproTech, Inc. (Rocky Hill, NJ,
USA). Anti-ZNF580 (catalog no. ab59015), anti-hypoxia inducible
factor (HIF)-1α (ab463), anti-phosphorylated Smad (p-Smad)-2
(phospho S467; ab53100), anti-p-Smad3 (phospho S425; ab51177), and
anti-active caspase-3 (ab2302) primary antibodies were purchased
from Abcam (Cambridge, UK). Anti-Smad2 (sc-393312), anti-Smad3
(sc-101154), anti-B-cell lymphoma 2 (Bcl-2; sc-783),
anti-Bcl-2-associated X protein (Bax; sc-526), and anti-β-actin
(sc-47778) antibodies were purchased from Santa Cruz Biotechnology
Inc. (Dallas, TX, USA). The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis Detection kit was supplied
by Promega Corp. (Madison, WI, USA), and the high-glucose
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). The carbon dioxide independent DMEM was
purchased from the Medical Gas Company (Tianjin, China).
Cell culture and hypoxia
induction
The embryonic rat heart-derived cell line (H9c2) was
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). H9c2 cells were cultured in
DMEM supplemented with 10% FBS and 1% penicillin-streptomycin in a
humidified atmosphere containing 5% CO2 at 37°C. The
hypoxia model was established using the hypoxia-inducing agent
CoCl2, according to a previous study (18).
Cell viability assessment under
various hypoxic conditions
Cells at a density of 1×104/200 µl were plated in
DMEM supplemented with 10% FBS, in 96-well microtiter plates
(Corning Life Sciences, USA) and incubated for 24 h at 37°C. Cells
were subsequently incubated in serum-free
CO2-independent DMEM supplemented with various
concentrations of CoCl2 (200, 400, 600, 800 and 1,000
µM) for 24 h, or with 600 µM CoCl2 for various time
intervals (0, 4, 8, 12, 16, 20 and 24 h). Normoxic control cells
were incubated under the same conditions but in a normal atmosphere
Normoxic cells received normal serum and no CoCl2
treatment, and the hypoxic cells were then incubated in a hypoxic
chamber. MTT was added at a final concentration of 0.5 mg/ml. After
a 4-h incubation at 37°C, the reaction was halted by adding 200 µl
dimethyl sulfoxide, and the relative optical density was measured
at 490 nm by a microplate spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA). Cell viability was calculated according
to the following equation: Cell viability (%) =
(ODtreatment-ODblank)/(ODcontrol-ODblank)
× 100. Experiments were performed in triplicate. To analyze TGF-β1
function under hypoxic conditions, cell viability was measured
following pretreatment with 2 ng/ml TGF-β1 for 30 min, prior to
exposure to 600 µM CoCl2 for 8, 16 or 24 h. To analyze
the role of Smad2/3 in the TGF-β1-mediated cytoprotection against
CoCl2-induced hypoxia, H9c2 cells were pretreated with
SB431542 (20 µM, diluent with DMSO, at 37°C), a selective inhibitor
of TβR1-Smad2/3, prior to stimulation with TGF-β1, and were
subsequently exposed to 600 µM CoCl2 for 24 h.
RNA extraction, cDNA synthesis, and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA from H9c2 cells treated with 600 µM
CoCl2 at various time intervals (0, 4, 8, 12, 16, 20 or
24 h) was isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
First-strand cDNA samples were synthesized using a TransScript
First-Strand cDNA Synthesis Supermix kit according to the
manufacturer's protocol (Beijing Transgen Biotech, Co., Ltd.,
Beijing, China). GAPDH RNA levels were quantified in all of the
samples as an internal control, and mRNA levels were calculated
relative to GAPDH mRNA. qPCR was performed in a 25 µl volume with
SYBR® Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Each gene analysis was repeated at least
3 times, and all RT-qPCR experiments were performed in triplicate
using the ABI 7500 Real-Time PCR platform (Applied Biosystems;
Thermo Fisher Scientific, Inc.) The specific primers used were as
follows: Forward, 5′-ACATCATTTCGTCTTTTCTTCTG-3′ and reverse,
5′-GGTGCTTTTGTCATTTCTTCCAC-3′ for ZFP580. The PCR conditions for
ZFP580 were 15 sec at 95°C, 34 sec at 63°C and 45 sec at 72°C for
40 cycles. Expression of glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), a housekeeping gene, served as an internal control. The
fold-change in expression of the gene of interest between the two
samples was calculated using the ΔΔCq method (19).
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% NP-40,
and protease inhibitor cocktail] for 30 min and centrifuged at
12,000 × g for 10 min prior to supernatant collection. The protein
concentration was quantified using the bicinchoninic acid assay.
Equal amounts (60 µg) of protein were loaded into each lane and
separated by 10% SDS-PAGE, then transferred to polyvinylidene
fluoride (PVDF) membranes (Roche Diagnostics GmbH, Mannheim,
Germany). After blocking in 5% fat-free milk in Tris-buffered
saline-Tween-20 (TBST) for 1 h at room temperature, the PVDF
membranes were incubated with primary antibodies (1:2,000)
overnight at 4°C. The membranes were washed with TBST and then
incubated with horseradish peroxidase-conjugated anti-rabbit
(L3012, Signalway Antibody, Nanjing, China) or anti-mouse (L3032,
Signalway Antibody) secondary antibodies in TBST (1:5,000) for 1 h
at room temperature, and then visualized by a super enhanced
chemiluminescence detection reagent (Beyotime Institute of
Biotechnology, Haimen, China). The signals were detected using
Image Station 4000R (Kodak, Rochester, NY, USA). Quantification of
results was performed using ImageJ version 1.44 (National
Institutes of Health, Bethesda, MD, USA). Each experiment was
repeated at least three times.
Flow cytometric analysis
The aforementioned treated H9c2 cells were collected
as previously described (20).
Briefly, cell supernatants were incubated with 5 µl Annexin V-FITC
and 10 µl propidium iodide (PI) for 15 min in the dark. Following
incubation, 400 µl binding buffer was added to each sample and the
samples were then filtered through a 50 µm nylon mesh. Fluorescence
was analyzed by flow cytometry Epics Elite flow cytometer (BD
Immunocytometry Systems, San Jose, CA, USA). Cells display
phosphatidylserine on their outer cell membranes, which is readily
detected by Annexin V during the early stages of apoptosis. The
plasma membrane becomes increasingly permeable during the later
stages of apoptosis, and PI may move across the cell membrane to
bind to cellular DNA. The data were analyzed using FlowJo 7.6
software (FlowJo, LLC, Ashland, OR, USA). Cells in the fourth
quadrant indicated the presence of apoptotic cells.
Lentiviral infection
Lentiviral vectors expressing small interfering RNAs
(siRNAs) directed against ZFP580 (Lenti-RNAi) or a negative control
(Lenti-NC) were constructed by Shanghai GenePharma Co., Ltd.
(Shanghai, China). H9c2 cells were cultured to 30–40% confluence
and were then infected with either the Lenti-RNAi or Lenti-NC for
72 h at 37°C [multiple of infection (MOI)=50]. The infected cells
were treated with or without 2 ng/ml TGF-β1 for 30 min prior to
exposure to 600 µM CoCl2 for 24 h.
Fluorescence assay of intracellular
reactive oxygen species (ROS)
Intracellular ROS generation was determined using
the ROS-dependent oxidative conversion of cell-permeable DCFH-DA to
the fluorescent dichlorofluorescein. H9c2 cells were treated as
aforementioned, washed 3 times with PBS, and incubated with a 10 µM
DCFH-DA solution in serum-free medium at 37°C, for 30 min, in the
dark. Cells were washed 3 times with PBS, and ROS concentrations
were quantified using a Leica TSP SP8 confocal microscope at a
wavelength of 485 nm (Leica Microsystems GmbH, Wetzlar, Germany)
and a SpectraMax M2 microplate reader at a wavelength of 530 nm
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
All data are presented as the mean ± standard error.
Data were analyzed using SPSS software version 18.0 (SPSS, Inc.,
Chicago, IL, USA). Statistically significant differences were
determined using one-way analysis of variance, followed by the
least significant difference test. P<0.05 was considered to
indicate a statistically significant difference.
Results
CoCl2 reduces cell
viability and increases expression of ZFP580 and HIF-1α in H9c2
myocardial cells
MTT assay revealed that CoCl2 treatment
decreased cell viability in a concentration- and time- dependent
manner (Fig. 1A and B). Cell
viability decreased following a 24 h incubation with increasing
concentrations of CoCl2 (400, 600, 800 or 1,000 µM); 600
µM CoCl2 was sufficient to decrease viability by ~50%.
Based on these results a concentration of 600 µM CoCl2
was selected for a time-course experiment investigating the effects
of CoCl2 on cell viability at different time intervals
(0, 4, 8, 12, 16, 20 or 24 h). The results indicated that cell
viability was significantly decreased at 8, 12, 16, 20 and 24 h
after CoCl2 treatment compared with the control group
(P<0.05; Fig. 1B). Western blot
analysis indicated that the protein expression levels of HIF-1α, a
marker of hypoxia, rose during hypoxia and peaked at 12 h; however,
HIF-1α mRNA levels, as determined by RT-qPCR, did not significantly
increase during the same time period (Fig. 1C and D); this result is consistent
with previously reported research (21,22).
CoCl2 treatment increased ZFP580 expression at mRNA and
protein levels; ZFP580 mRNA expression peaked at 12 h, and protein
expression peaked at 16 h (Fig. 1C and
E). Notably, cell viability steadily decreased in
hypoxia-induced cells, whereas the expression of ZFP580 increased
and then decreased after 16 h. This may be due to the role of
ZFP580 as an anti-injury marker (7), where it is first activated then
degraded with increasing hypoxia. These findings indicated that a
CoCl2-induced hypoxia model of H9c2 cells had been
successfully established; in addition, it was observed that
CoCl2-induced hypoxia increases ZFP580 expression.
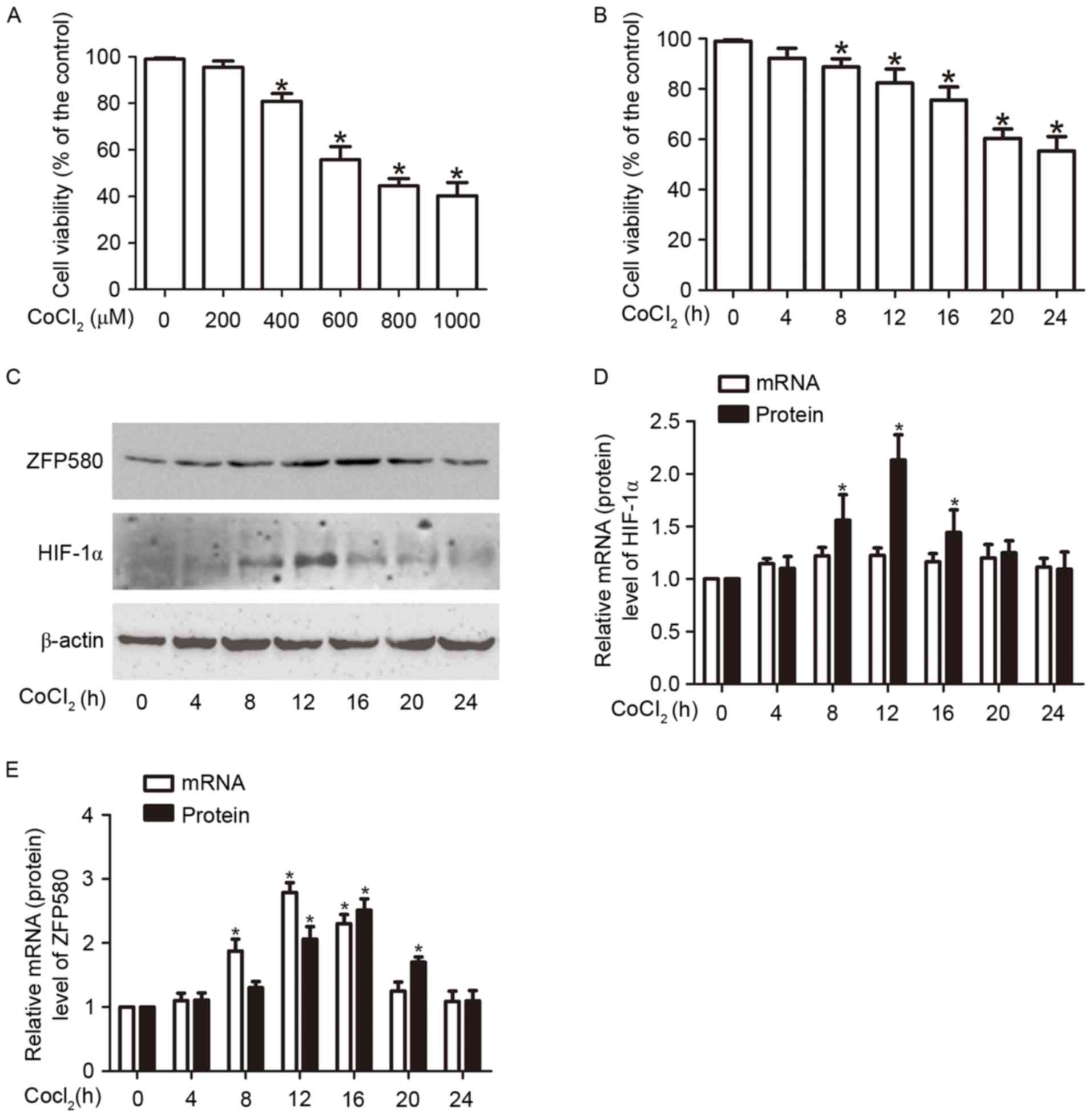 | Figure 1.Effects of CoCl2 on cell
viability and the expression of ZFP580 or HIF-1α n H9c2 myocardial
cells. (A) H9c2 cells were treated with 0, 200, 400, 600, 800 or
1,000 µM CoCl2 for 24 h. The concentration-dependent
effects of CoCl2 on cell survival were assessed by MTT
assay. (B) H9c2 cells were treated with 600 µM CoCl2 for
0, 4, 8, 12, 16, 20 or 24 h. The time-dependent effects of
CoCl2 on cell survival were assessed by MTT assay. H9c2
cells were treated with 600 µM CoCl2 for 0 to 24 h, and
expression of (C) HIF-1α and ZFP580 protein, or (D) HIF-1α and (E)
ZFP580 mRNA were determined by western blot analysis or reverse
transcription-quantitative polymerase chain reaction, respectively.
Three experiments were performed for each group, and each
experiment was replicated twice. The results are presented as the
mean ± standard error. *P<0.05 vs. control. CoCl2,
cobalt chloride; HIF-1α, hypoxia-inducible factor-1α; ZFP580, zinc
finger protein 580; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide. |
TGF-β1 attenuates
CoCl2-induced cytotoxicity and upregulates ZFP580
protein expression
To analyze TGF-β1 function under hypoxic conditions,
H9c2 cells were pretreated with 2 ng/ml TGF-β for 30 min prior to
exposure to 600 µM CoCl2 for 8, 16 or 24 h. MTT assay
revealed that pretreatment with TGF-β1 reduced
CoCl2-induced cytotoxicity and increased cell viability
by ~10% at 16 and 24 h, compared with that of the untreated hypoxic
group (Fig. 2A; P<0.05).
Western blot analysis indicated that ZFP580 protein expression was
upregulated by pretreatment with TGF-β1 and subsequent exposure to
CoCl2 (Fig. 2B and C).
ZFP580 expression was upregulated at 8 and 24 h following TGF-β1
and CoCl2 treatment (P<0.05); however, there was no
difference in ZFP580 expression at the TGF-β1-treated 16 h
time-point, regardless of CoCl2 treatment. It is
possible that ZFP580 protein levels had plateaued at this time
point. At 24 h there were clear differences in both cell viability
and ZFP580 expression in the TGF-β1 pretreatment group, with or
without CoCl2 treatment; therefore, at 24 h, 600 µM
CoCl2 treatment was selected for subsequent experimental
conditions. From these findings it was hypothesized that ZFP580 is
involved in the TGF-β1-mediated defensive mechanisms against
CoCl2-induced injury.
 | Figure 2.TGF-β1 attenuated
CoCl2-induced cytotoxicity and upregulated ZFP580
protein expression. (A) MTT results indicated that pretreatment
with TGF-β1 increased cell viability after CoCl2
treatment. (B and C) H9c2 cells were pretreated with TGF-β1 and
then exposed to 600 µM CoCl2 for 8, 16 or 24 h, the
ZFP580 protein expression was determined by western blot analysis.
Three experiments were performed for each group, and each
experiment was repeated twice. The results are presented as the
mean ± standard error. *P<0.05 vs. control,
#P<0.05 vs. the CoCl2 treatment control
group. TGF-β1, transforming growth factor-β1; CoCl2,
cobalt chloride; ZFP580, zinc finger protein 580; MTT,
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide. |
Inhibition of Smad2/3 activation
attenuates ZFP580 protein expression and TGF-β1-mediated
cytoprotection against CoCl2-induced apoptosis
Smad2 and Smad3 are important cofactors in TGF-β1
signal transduction. To analyze the role of Smad2/3 in the
TGF-β1-mediated cytoprotection against CoCl2-induced
hypoxia, H9c2 cells were first pretreated with SB431542, a
well-known selective inhibitor of TGF-β type I receptor (TβR-I) and
Smad2/3 activation. Following SB431542 treatment, cells were
stimulated with TGF-β1, and subsequently exposed to
CoCl2. Western blot analysis indicated that SB431542
partially blocked the TGF-β1-induced upregulation of ZFP580
expression (Fig. 3A and B). Flow
cytometric analysis demonstrated that pretreatment with TGF-β1
significantly decreased the proportion of apoptotic cells after
CoCl2 treatment (20.64±2.14 vs. 35.68±1.30%; P<0.05);
whereas inhibition of Smad2/3 activation appeared to reduce these
protective effects by increasing the amount of apoptosis by ~8%
(from 23.46±3.33 to 31.97±2.00%; P<0.05; Fig. 3C and D). These findings
demonstrated that ZFP580, as a downstream target of the
TGF-β1/Smad2/3 signaling pathway, may be associated with the
protective role mediated by TGF-β1 against CoCl2-induced
cell apoptosis.
 | Figure 3.Inhibition of TβR1-Smad2/3 activation
attenuated ZFP580 protein expression and the protective effects of
TGF-β1 against CoCl2-induced apoptosis. H9c2 cells were
pretreated with SB431542, a selective inhibitor of TβR1-Smad2/3,
prior to stimulation with TGF-β1, and were subsequently exposed to
600 µM CoCl2 for 24 h. (A) Protein expression and (B)
histogram analysis detected Smad2/3 activation and ZFP580
expression in H9c2 cells with or without SB431542 pretreatment. (C)
Cells (1×104) were subjected to AnnexinV-FITC/PI
staining and analyzed by flow cytometry. C1, Control group; C2,
TGF-β1 induction group; C3, SB431542 pretreatment and TGF-β1
induction group; C4, DMSO pretreatment and TGF-β1 induction group,
all exposed to CoCl2. (D) Representative histograms for
the cell apoptosis rates in H9c2 cells. Three experiments were
performed for each group, and each experiment was repeated twice.
The results are presented as the mean ± standard error. *P<0.05.
TGF-β1, transforming growth factor-β1; TβR1, TGF-β type I receptor;
Smad, mothers against decapentaplegic homolog; ZFP580, zinc finger
protein 580; CoCl2, cobalt chloride; DMSO, dimethyl
sulfoxide; FITC, fluorescein isothiocyanate; PI, propidium
iodide. |
ZFP580 serves an important role in the
protective effects of TGF-β1 against CoCl2-induced
apoptosis and ROS generation
To evaluate the potential role of ZFP580 in the
TGF-β1/Smad2/3-mediated protection against CoCl2-induced
apoptosis, it was hypothesized that TGF-β1 pretreatment of
CoCl2-induced hypoxic cells may stimulate the expression
of ZFP580. H9c2 cells were transfected with lentiviral vectors
expressing siRNAs directed against ZFP580 (Lenti-RNAi) or a
negative control (Lenti-NC) and were subsequently pretreated with
or without TGF-β1, prior to exposure to CoCl2. Flow
cytometric analysis indicated that suppression of ZFP580
significantly increased the proportion of apoptotic cells compared
with the number of apoptotic cells in the Lenti-NC group
(35.90±2.92 vs. 46.13±1.68%; P<0.05). Furthermore, the
anti-apoptotic role of TGF-β1 was reduced by Lenti-RNAi
transfection (Fig. 4A and B).
Analysis of ROS generation revealed that CoCl2
significantly induced ROS production in Lenti-RNAi cells compared
with in Lenti-NC cells (P<0.05; Fig. 4C-1, C-2, and D). TGF-β1
significantly decreased CoCl2-induced ROS generation, an
effect that was reduced by Lenti-RNAi transfection (Fig. 4C-3, C-4, and D). These results
indicated that ZFP580 serves an important role in the
TGF-β1-mediated cytoprotective effects against
CoCl2-induced apoptosis and ROS generation.
 | Figure 4.ZFP580 serves an important role in
the protective effects of TGF-β1 against CoCl2-induced
apoptosis and ROS generation. H9c2 cells were transfected with
Lenti-RNAi or Lenti-NC lentiviral vectors for 72 h, followed by
treatment with or without TGF-β1 and subsequent exposure to 600 µM
CoCl2 for 24 h. (A and B) Cells (1×104) were
subjected to AnnexinV-FITC/PI staining and analyzed by flow
cytometry. (C) DCFH-DA (10 mM) fluorescence probe images were
captured using a confocal microscope (Magnification, ×200; bars, 25
µm). (D) Intracellular ROS generation was measured by a microplate
reader in each of the indicated treatment groups. A1 and C1,
Lenti-RNAi with CoCl2 exposure; A2 and C2, Lenti-NC with
CoCl2 exposure; A3 and C3, Lenti-RNAi with TGF-β1
pretreatment followed by CoCl2 exposure; A4 and C4,
Lenti-NC with TGF-β1 pretreatment followed by CoCl2
exposure. Three experiments were performed for each group, and each
experiment was repeated twice. The results are presented as the
mean ± standard error. *P<0.05. ZFP580, zinc finger protein 580;
TGF-β1, transforming growth factor-β1; CoCl2, cobalt
chloride; ROS, reactive oxygen species; Lenti-RNAi, small
interfering RNA lentiviral vectors directed against ZFP580;
Lenti-NC, negative control lentiviral vectors; FITC, fluorescein
isothiocyanate; PI, propidium iodide; DCFH-DA,
2,7-dichlorofluorescin diacetate. |
ZFP580 is involved in the
anti-apoptotic effects of TGF-β1 through inhibition of the
mitochondrial apoptotic pathway
The role of ZFP580 in the TGF-β1/Smad2/3 signaling
pathway during CoCl2-induced apoptosis was investigated.
Western blotting indicated that TGF-β1 significantly increased the
expression of the anti-apoptotic protein Bcl-2, and decreased the
expression of the proapoptotic protein Bax, in Lenti-NC groups
compared with Lenti-RNAi groups (Fig.
5). Furthermore, the Bax/Bcl-2 ratio and levels of active
caspase-3 were decreased (P<0.05). However, suppression of
ZFP580 reduced the anti-apoptotic effects of TGF-β1, increased the
Bax/Bcl-2 ratio, and promoted caspase-3 activation (P<0.05).
These results demonstrated that ZFP580 is involved in the
protective effects of TGF-β1 against CoCl2-induced H9c2
cell apoptosis, and may serve an important anti-apoptotic role
through inhibition of the mitochondrial apoptotic pathways.
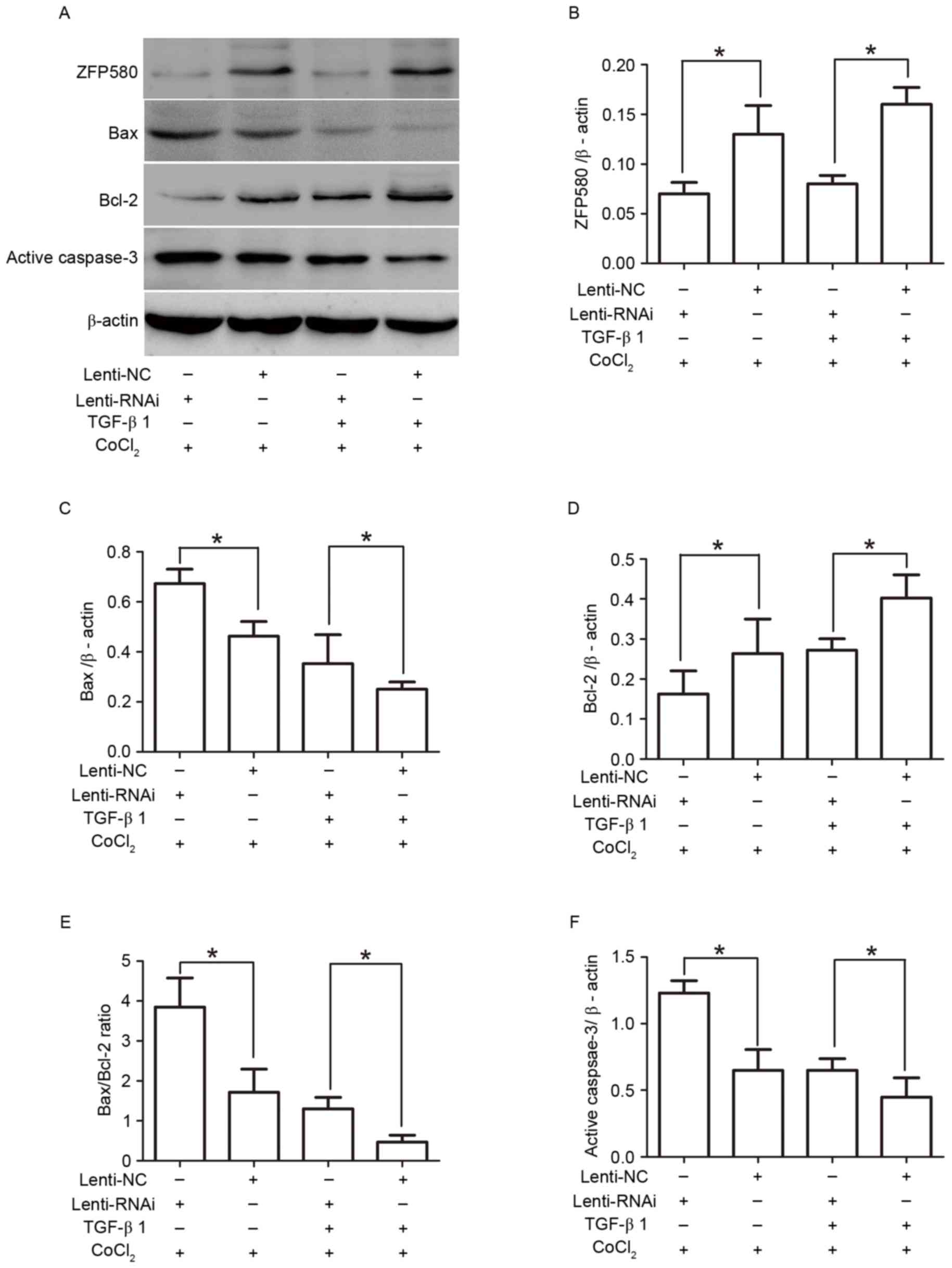 | Figure 5.ZFP580 is involved in the
anti-apoptotic effects of TGF-β1 through inhibition of the
mitochondrial apoptosis pathway. H9c2 cells were transfected with
Lenti-RNAi or Lenti-NC lentiviral vectors for 72 h, followed by
treatment with or without TGF-β1 and subsequent exposure to 600 µM
CoCl2 for 24 h. (A) Western blot analysis, and histogram
analysis of (B) ZFP580, (C) Bax, (D) Bcl-2, (E) Bax/Bcl-2 ratio,
and (F) active caspase-3 in each of the indicated treatment groups.
Three experiments were performed for each group, and each
experiment was replicated twice. The results are presented as the
mean ± standard error. *P<0.05. ZFP580, zinc finger protein 580;
TGF-β1, transforming growth factor-β1; Lenti-RNAi, small
interfering RNA lentiviral vectors directed against ZFP580;
Lenti-NC, negative control lentiviral vectors; CoCl2,
cobalt chloride; ROS, reactive oxygen species; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein. |
Discussion
ZFP580 is a novel zinc finger protein with three
C-terminal C2H2 zinc finger domains and an N-terminal proline-rich
domain. The zinc finger is the most abundant protein motif in
mammalian cells, and it is involved in the expression and
regulation of numerous eukaryotic genes. C2H2 zinc finger proteins
in particular often have a crucial role in physiological cellular
functions, such as growth and differentiation (3). Previous research has indicated that
ZFP580 may be upregulated by hypoxic preconditioning, and may serve
a critical anti-apoptotic role in myocardial ischemia and
reperfusion injury (7). However,
little is known regarding the regulation of ZFP580 expression and
function under hypoxic conditions. In the present study, a
CoCl2 -induced hypoxia model was established in H9c2
myocardial cells. The results demonstrated that CoCl2
treatment decreased cell viability and increased the expression of
HIF-1α protein, but not HIF-1α mRNA. These findings are consistent
with those of previous studies (21,22).
Conversely, ZFP580 mRNA and protein expression were upregulated by
CoCl2 treatment. The observed ZFP580 mRNA expression
trend was similar to that of the HIF-1α protein, which is a protein
marker of hypoxia. Therefore, it was hypothesized that ZFP580 may
serve a role in hypoxia, and its expression may be regulated under
hypoxic conditions.
Hypoxia regulates the expression of numerous genes
and the secretion of multiple cytokines. Various growth factors,
including hepatocyte growth factor, basic fibroblast growth factor
and TGF-β1, have important roles in tissues exposed to hypoxia or
ischemia (9). The present study
focused on TGF-β1 due to previous research indicating that this
growth factor exerts cardioprotective effects and mediates
important anti-apoptotic effects in vitro and in vivo
(9,10,23).
Our previous study demonstrated that the human gene ZNF580 was
involved in the TGF-β1 signaling pathway and interacted with the
TGF-β signal molecule Smad2 (16,17).
Therefore, it was hypothesized that ZFP580 may be involved in the
cardioprotective effects of TGF-β1. In the present study, MTT assay
revealed that TGF-β1 reduced chemical hypoxia-induced cytotoxicity
and increased cell viability. Furthermore, it was discovered that
TGF-β1 upregulates ZFP580 expression in H9c2 cells with or without
chemical hypoxia, however expression was highest under hypoxic
conditions. These findings support the hypothesis that ZFP580 may
be a downstream target of the TGF-β1 signaling pathway and may
mediate the protective effects of TGF-β1.
Following TGF-β1 binding to its cognate receptors,
downstream intracellular signaling involves activation of the
canonical pathway involving Smad 2/3, or the non-canonical
signaling pathways, including extracellular signal-related kinase
(ERK)1/2 and phosphoinositide-3-kinase-Akt (11,24).
Previous research has reported that activation of the
TGF-β1-dependent Smad2/3 pathway is correlated with the
cardioprotective effects of TGF-β1 against
ischemia/reperfusion-induced apoptosis (25). The present study indicated that
TGF-β1 could significantly decrease CoCl2-induced
apoptosis, however these effects were reduced following
pretreatment with SB431542, an inhibitor of TβR-I and Smad2/3
phosphorylation. These findings indicated that TGF-β1 was able to
limit chemical hypoxia-induced H9c2 cell injury via activation of
Smad2/3. Furthermore, it was demonstrated that inhibition of
TGFβR-I-Smad2/3 activation partially blocked the TGF-β1-induced
upregulation of ZFP580 expression. These results supported the
hypothesis that ZFP580 is involved in the protective effect of
TGF-β1 against CoCl2-induced apoptosis, as a downstream
target of the TGF-β1/Smad2/3 signaling pathway. ZFP580 has a
similar structure to that of the Sp1-like/krüppel-like factor (KLF)
transcription factor superfamily. Li et al reported that
TGF-β1 could increase the phosphorylation of KLF4 via Smad
signaling pathways in vascular smooth muscle cells (26). Hence, it was further hypothesized
that ZFP580 may be regulated by TGF-β1 through canonical Smad
signaling pathways. Our previous study demonstrated that ZFP580 has
an anti-apoptotic function as a downstream target of the ERK
pathway in myocardial ischemia and reperfusion injury (7). However, whether other non-canonical
signaling pathways have critical roles in the regulation of ZFP580
by TGF-β1 is worth exploring further.
Hypoxia/ischemia may induce a series of pathological
changes, including apoptosis and necrosis. Three major pathways can
regulate apoptosis: Extracellular Fas protein; mitochondria; and
endoplasmic reticulum. CoCl2 was previously reported to
promote hypoxic/ischemic responses by increasing ROS generation,
dissipating the mitochondrial membrane potential, activating
caspase-3, decreasing cell viability and inducing apoptosis
(20,27,28).
A study in rat pheochromocytoma PC12 cells indicated that
CoCl2-induced apoptosis may be associated with the
mitochondrial-mediated apoptosis pathway (29). This mitochondrial pathway is
predominantly mediated by members of the Bcl-2 family, and is
triggered as a result of cell injury induced by DNA damage or cell
distress. This leads to mitochondrial disruption and the release of
apoptotic mediators, such as apoptosis inducing factor and
cytochrome c into the cytoplasm, thereby inducing activation
of the caspase cascade (30).
Furthermore, numerous reports have demonstrated that Bax, Bcl-2 and
caspase-3 act as downstream molecules of TGF-β1 signaling in some
cases of apoptosis (8,31,32).
Therefore, the present study investigated the protective mechanisms
mediated by ZFP580 and TGF-β1 against CoCl2-induced
apoptosis in the mitochondrial-mediated apoptotic pathway. The
results demonstrated that TGF-β1 upregulated the anti-apoptotic
protein Bcl-2, downregulated the proapoptotic protein Bax,
suppressed ROS generation and reduced the activation of caspase-3,
which is a final executioner protein in the apoptotic cascade.
Conversely, suppression of ZFP580 expression by RNA interference
enhanced CoCl2-induced cell apoptosis and reduced the
anti-apoptotic role of TGF-β1. These findings suggested that ZFP580
may be a component of the anti-apoptotic process mediated by the
TGF-β1/Smad signaling pathway.
In conclusion, the present study provided
experimental evidence that ZFP580 may function as a novel
cytoprotective regulator under hypoxic conditions. It was
demonstrated that ZFP580 serves an essential role in mediating the
cardioprotective effect of TGF-β1 against chemical hypoxia-induced
cell apoptosis by inhibiting the mitochondrial apoptotic
pathway.
Acknowledgements
The authors would like to thank colleagues in the
Department of Physiology and Pathophysiology, Logistics University
of Chinese People's Armed Police Force for their excellent
technical assistance and encouragement. They would also like to
thank Dr Xin Zhou and Dr WenJie Ji (Tianjin Key Laboratory of
Cardiovascular Remodeling and Target Organ Injury, Pingjin Hospital
Heart Center, Tianjin, China) for their help in the flow cytometry
study. This study was supported by funds from the National Natural
Science Foundation of China (grant no. 81173588); the Tianjin
Natural Science Foundation (grant no. 12JCYBJC15900); and the
Tianjin Key Laboratory for the Prevention and Control of
Occupational and Environmental Hazard (grant no. TJC1409).
Glossary
Abbreviations
Abbreviations:
|
TGF-β1
|
transforming growth factor-β1
|
|
CoCl2
|
cobalt chloride
|
|
TβR-I
|
TGF-β type I receptor
|
References
|
1
|
Zhang WC, Chen BS, Zeng WW and Wu G:
Cloning and tissue expression of a novel gene down-regulated by low
density lipoprotein. Basic Med Sci Clin. 23:279–282. 2003.
|
|
2
|
Zhang WC, Sun HY and Luo YY: Construction
of eukaryotic expression vector for ZNF580 and EGFP fusion protein
and its expression and localization in HEK293 cells. Acta Acad Med
CPAF. 17:161–165. 2008.
|
|
3
|
Thomas JH and Emerson RO: Evolution of
C2H2-zinc finger genes revisited. BMC Evol Biol. 9:512009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DangLi R, HeKong W, JiQin L, MingHua Z and
WenCheng Z: ROS-induced ZNF580 expression: A key role for
H2O2/NF-κB signaling pathway in vascular endothelial inflammation.
Mol Cell Biochem. 359:183–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun HY, Wei SP, Xu RC, Xu PX and Zhang WC:
Sphingosine-1-phosphate induces human endothelial VEGF and MMP-2
production via transcription factor ZNF580: Novel insights into
angiogenesis. Biochem Biophys Res Commun. 395:361–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y and Zhang W: Cloning and analyzing
of the cDNA sequence of N-terminal region and C-terminal region of
zinc finger protein (ZFP580) gene. Life Science Journal. 5:68–73.
2008.
|
|
7
|
Meng XY, Yu HL, Zhang WC, Wang TH, Mai X,
Liu HT and Xu RC: ZFP580, a novel zinc-finger transcription factor,
is involved in cardioprotection of intermittent high-altitude
hypoxia against myocardial ischemia-reperfusion injury. PLoS One.
9:e946352014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Li D, Saldeen T and Mehta JL:
TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via
inhibition of upregulation of MMP-1. Am J Physiol Heart Circ
Physiol. 284:H1612–H1617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dandapat A, Hu CP, Li D, Liu Y, Chen H,
Hermonat PL and Mehta JL: Overexpression of TGFbeta1 by
adeno-associated virus type-2 vector protects myocardium from
ischemia-reperfusion injury. Gene Ther. 15:415–423. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frantz S, Hu K, Adamek A, Wolf J, Sallam
A, Maier SK, Lonning S, Ling H, Ertl G and Bauersachs J:
Transforming growth factor beta inhibition increases mortality and
left ventricular dilatation after myocardial infarction. Basic Res
Cardiol. 103:485–492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Azayzih A, Gao F, Goc A and Somanath
PR: TGFβ1 induces apoptosis in invasive prostate cancer and bladder
cancer cells via Akt-independent, p38 MAPK and JNK/SAPK-mediated
activation of caspases. Biochem Biophys Res Commun. 427:165–170.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu MY, He YJ, Lv X, Liu ZH, Shen Y, Ye GR,
Deng YM and Shu JC: Transforming growth factor-β1 reduces apoptosis
via autophagy activation in hepatic stellate cells. Mol Med Rep.
10:1282–1288. 2014.PubMed/NCBI
|
|
13
|
Sánchez-Capelo A: Dual role for TGF-beta1
in apoptosis. Cytokine Growth Factor Rev. 16:15–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baxter GF, Mocanu MM, Brar BK, Latchman DS
and Yellon DM: Cardioprotective effects of transforming growth
factor-beta1 during early reoxygenation or reperfusion are mediated
by p42/p44 MAPK. J Cardiovasc Pharmacol. 38:930–939. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang BC, Zander DS and Mehta JL:
Hypoxia-reoxygenation- induced apoptosis in cultured adult rat
myocytes and the protective effect of platelets and transforming
growth factor-beta(1). J Pharmacol Exp Ther. 291:733–738.
1999.PubMed/NCBI
|
|
16
|
Luo Y, Zhao Y, Li X, Zhao J and Zhang W:
ZNF580 mediates eNOS expression and endothelial cell
migration/proliferation via the TGF-β1/ALK5/Smad2 pathway. Mol Cell
Biochem. 393:199–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo Y, Hu W, Xu R, Hou B, Zhang L and
Zhang W: ZNF580, a novel C2H2 zinc-finger transcription factor,
interacts with the TGF-β signal molecule Smad2. Cell Biol Int.
35:1153–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong XX, Wu D, Wang X, Chen HL, Chen JX,
Wang XX, Wang XL, Gan L, Guo ZY, Shi GX, et al: Ghrelin protects
against cobalt chloride-induced hypoxic injury in cardiac H9c2
cells by inhibiting oxidative stress and inducing autophagy.
Peptides. 38:217–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantification PCR
and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen SL, Yang CT, Yang ZL, Guo RX, Meng
JL, Cui Y, Lan AP, Chen PX and Feng JQ: Hydrogen sulphide protects
H9c2 cells against chemical hypoxia-induced injury. Clin Exp
Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Lei J, Zou J, Xiao H, Chen A, Liu
X, Liu Y, Jiang L, Xiao Z and Xiao X: Mipu1, a novel direct target
gene, is involved in hypoxia inducible factor 1-mediated
cytoprotection. PLoS One. 8:e828272013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mehta JL, Chen HJ and Li DY: Protection of
myocytes from hypoxia-reoxygenation injury by nitric oxide is
mediated by modulation of transforming growth factor-beta1.
Circulation. 105:2206–2211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Chen L, Ni H, Wang G, Ding W, Cong
H, Ju S, Yang S and Wang H: APRIL depletion induces cell cycle
arrest and apoptosis through blocking TGF-β1/ERK signaling pathway
in human colorectal cancer cells. Mol Cell Biochem. 383:179–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vivar R, Humeres C, Ayala P, Olmedo I,
Catalán M, García L, Lavandero S and Díaz-Araya G: TGF-β1 prevents
simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis
by activation of both canonical and non-canonical signaling
pathways. Biochim Biophys Acta. 1832:754–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HX, Han M, Bernier M, Zheng B, Sun SG,
Su M, Zhang R, Fu JR and Wen JK: Kruppel-like factor 4 promotes
differentiation by transforming growth factor-beta
receptor-mediated Smad and p38 MAPK signaling in vascular smooth
muscle cells. J Biol Chem. 285:17846–17856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan A, Liao X, Mo L, Yang C, Yang Z, Wang
X, Hu F, Chen P, Feng J, Zheng D and Xiao L: Hydrogen sulfide
protects against chemical hypoxia-induced injury by inhibiting
ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells.
PLoS One. 6:e259212011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Liao SG, He Y, Li J, Zhong RF, He
X, Liu Y, Xiao TT, Lan YY, Long QD and Wang YL: Protective effects
of fractions from Pseudostellaria heterophylla against cobalt
chloride-induced hypoxic injury in H9c2 cell. J Ethnopharmacol.
147:540–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG,
Choi NK, Oh WM, Oh HK, Kim SH, Lee JH, et al: Inhibition by
epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12
cells. Life Sci. 80:1355–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan CY, Ban H, Kim YH and Lee SK: The heat
shock protein 27 (Hsp27) operates predominantly by blocking the
mitochondrial-independent/extrinsic pathway of cellular apoptosis.
Mol Cells. 27:533–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bakhshayesh M, Zaker F, Hashemi M, Katebi
M and Solaimani M: TGF-β1-mediated apoptosis associated with
SMAD-dependent mitochondrial Bcl-2 expression. Clin Lymphoma
Myeloma Leuk. 12:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Surachetpong S, Jiranantasak T,
Rungsipipat A and Orton EC: Apoptosis and abundance of Bcl-2 family
and transforming growth factor β1 signaling proteins in canine
myxomatous mitral valves. J Vet Cardiol. 15:171–180. 2013.
View Article : Google Scholar : PubMed/NCBI
|



















