Introduction
Chronic kidney disease (CKD) carries a high risk of
mortality, the immediate cause of which is usually cardiovascular
complications (1,2). The main complications observed in
patients with CKD include impaired angiogenesis, atherosclerosis,
arterial stiffness, vascular calcifications and neointimal
hyperplasia (3,4). Endothelial progenitor cell (EPC)
dysfunction is a key contributor to this pathogenesis, as the
potential of EPCs to promote vascular repair by differentiating
into endothelial cells is impaired (5).
Among the factors potentially influencing EPC
function in patients with CKD, the constituents of the uremic
milieu, particularly protein-bound uremic toxins, are likely to be
important, since they cannot be removed by dialysis therapy
(6–8). Para-cresol (p-cresol)
is a protein-bound uremic toxin that originates from bacterial
amino acid L-tyrosine fermentation in the large intestine mucosa
(9). P-cresol has been
demonstrated to inhibit proliferation of various cell types in
vitro, including EPCs (10–12),
indicating that p-cresol may be among the factors mediating
the high incidence of cardiovascular complications in patients with
CKD. However, the mechanism remains unclear.
Reactive oxygen species (ROS) such as superoxide
anion and hydrogen peroxide have been demonstrated to function as
signaling molecules that regulate EPC proliferation (13). P-cresol has antioxidant
activity, and a previous study has demonstrated that its
antiproliferative effect in platelets was related to inhibition of
ROS (14). Therefore, the
hypothesis that p-cresol may inhibit EPC proliferation via
its antioxidant activity was tested. The present study aimed to
further elucidate the mechanism of p-cresol and its toxicity
to the cardiovascular system.
Materials and methods
Culture and determination of human
late EPCs
Culture and determination of human late EPCs were
performed as reported previously (10). Briefly, 20 ml human peripheral
blood was diluted 1:1 with phosphate-buffered saline (PBS) and
suspended in an equal volume of Ficoll density-gradient media
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Cells were
centrifuged for 30 min at room temperature at a speed of 740 × g.
Recovered cells were then washed twice with PBS and resuspended in
Gibco Medium 199 (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 20% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) and endothelial cell growth medium (EGM)-2
SingleQuot (Lonza, Basel, Switzerland). Late EPC colonies appeared
following ~2–4 weeks of culture in a 5% CO2 incubator at
37°C. These cells were then harvested and cultured for later
experiments. These cells were confirmed to be EPCs as cell
determination demonstrated that these cells could incorporate
acetylated low density lipoprotein and exhibited Ulex europaeus
agglutinin I binding affinity. The cells were also negative for
prominin 1 (CD133) expression, but expressed CD34 and vascular
endothelial factor receptor 2.
Measurement of ROS production in
EPCs
Intracellular oxidant formation was assessed in EPCs
using 5-(and-6)-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate
(carboxy-H2DCFDA; Invitrogen; Thermo Fisher Scientific, Inc.), a
non-fluorescent and cell-permeable analog of fluorescein that is
converted into carboxy-2′,7′-dichlorodihydrofluorescein following
intracellular deacetylation and is oxidized to highly fluorescent
carboxy-dichlorofluorescein (carboxy-DCF). Following incubation
with 10 µM carboxy-H2DCFDA in warm Hank's Buffered Salt Solution
(HBSS, Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min,
cells were washed with PBS for 30 sec and immediately viewed by
fluorescence microscopy or analyzed by fluorescence-activated cell
sorting (FACS) analysis.
Cell proliferation assay
Effects of ROS inhibitors on growth factor-dependent
EPC proliferation were determined using the water-soluble
tetrazolium-1 (WST-1) assay. EPCs at a concentration of 10,000
cells per well were seeded on 96-well culture plates in Medium 199
(Thermo Fisher Scientific, Inc.) supplemented with 20% FBS (Thermo
Fisher Scientific, Inc.) and EGM-2 SingleQuot (Lonza) in a 5%
CO2 incubator at 37°C and cultured for 24 h. Cells were
then washed once with warm HBSS (Invitrogen; Thermo Fisher
Scientific, Inc.) and the media was subsequently replaced with
Medium 199 (Thermo Fisher Scientific, Inc.) containing 0.5% FBS
(Thermo Fisher Scientific, Inc.). Following culture for 6 h, EPCs
were treated with 20 µM N-acetylcysteine (NAC) or 20 µM
dibenziodolium (DPI) for 1 h, then stimulated for 24 h with
different concentrations of growth factors [0–10 ng/ml, including
epidermal growth factor (EGF), vascular endothelial growth factor
(VEGF), fibroblast growth factor (FGF), and insulin-like growth
factor (IGF); Lonza]. Cell proliferation assay reagent WST-1 (Roche
Applied Science, Penzberg, Germany) was then added to each well (10
µl) and incubated for 4 h. Absorbance at 450 nm was measured using
an enzyme-linked immunosorbent assay reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using TRIzol
reagent (Thermo Fisher Scientific, Inc.). RNA was then reverse
transcribed to cDNA in a total volume of 20 µl using a
Moloney-Murine Leukemia Virus Reverse Transcriptase kit (Promega
Corporation, Madison, WI, USA). The mRNA levels of antioxidant
proteins, including superoxide dismutase 1 (SOD-1), superoxide
dismutase 2 (SOD-2), glutathione peroxidase 1 (Gpx-1), glutathione
peroxidase 4 (Gpx-4), and catalase (CAT), were analyzed by RT-qPCR
using specific primers, according to a previous report (15). The qPCR reaction was carried out
using a commercial SYBR-Green reaction mix (Takara Bio, Inc., Otsu,
Japan). Thermal cycling was performed in an ABI 5700 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Reaction conditions were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 31 sec. mRNA
expression levels were quantified using the 2∆∆Cq method as
described previously (16).
β-actin was used as internal control for each sample.
Preparation of cell lysates and
western blotting
Following 72 h exposure to p-cresol in complete M199
medium, EPCs were rinsed twice with ice-cold PBS and proteins were
extracted using the ProteoJET Mammalian Cell Lysis Reagent
(Fermentas; Thermo Fisher Scientific, Inc.). The lysate solution
was then centrifuged at 14,000 × g for 15 min at 4°C, and the
supernatant (soluble fraction) was transferred into new tubes and
stored at −80°C until needed. Equal amounts of protein sample (80
µg) were separated by 12 or 15% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. The membranes were incubated at
room temperature for 1 h in TBS/0.1% Tween-20 (TBST) containing 5%
milk to block nonspecific binding and subsequently incubated
overnight at 4°C with the following primary antibodies:
Anti-cytochrome b-245 α chain (CYBA; catalog no. sc-130550; 1:500),
anti-cytochrome b-245 β chain (CYBB; catalog no. sc-130543; 1:500)
and anti-nicotinamide adenine dinucleotide phosphate (NAPDH)
oxidase 4 (NOX4; catalog no. sc-30141; 1:500), all purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), in TBST
containing 5% bovine serum albumin (Thermo Fisher Scientific,
Inc.). The membranes were then washed three times with TBST and
incubated for 1 h at room temperature in horseradish
peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary
antibodies (1:5,000 dilution in TBST containing 5% non-fat milk).
The immunoreactive proteins were detected using an enhanced
chemiluminescence western blotting detection system (Merck
Millipore, Darmstadt, Germany). β-actin served as the loading
control. Each experiment was repeated three times, with a
representative blot shown for each experiment.
Statistical analysis
Results are expressed as the mean ± standard
deviation from at least three independent experiments.
Statistically significant differences among different treatment
groups at a single point in time were determined by one-way
analysis of variance, followed by two-tailed Student's t-tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of ROS on growth
factor-induced EPC proliferation
To assess the role of ROS in growth factor-induced
EPC proliferation, ROS levels were measured in EPCs treated with
different concentrations of EGM-2 SingleQuot. The results
demonstrated that ROS levels in EPCs were visibly increased
following 6 h growth factor treatment, as measured by the
carboxy-H2DCFDA fluorescence assay and FACS analysis (Fig. 1A). Decomposition of cellular ROS by
treatment of EPCs with the antioxidants NAC or DPI significantly
reduced cell viability compared with EPCs cultured without
antioxidant in the same concentration of growth factors (P<0.05,
Fig. 1B and C, respectively).
These observations underscore the critical role of ROS in driving
the proliferative properties of EPCs.
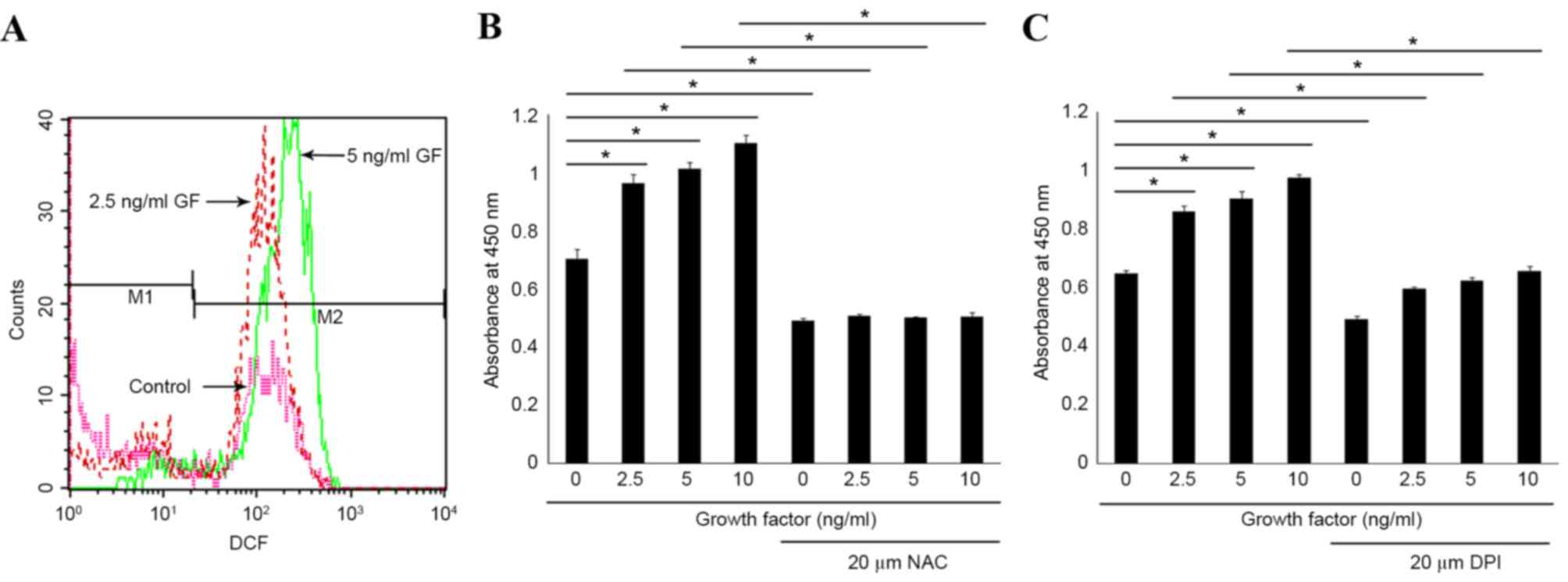 | Figure 1.Analysis of ROS production and its
effect on EPC cell proliferation. (A) DCF fluorescence in EPCs
treated with different concentrations of growth factors for 6 h was
measured by fluorescence-activated cell sorting analysis. A
representative histogram of fluorescence intensity is presented
here for 0 (control, magenta line), 2.5 (red line) and 5 (green
line) ng/ml growth factors treatments. EPCs were pretreated with
(B) NAC or (C) DPI for 1 h and then stimulated with different
concentrations of growth factors for 24 h. Cell proliferation was
then determined using a WST-1 assay. Data were expressed as mean ±
standard deviation of three independent experiments (n=8 samples
per group). *P<0.05, with comparisons indicated by lines. ROS,
reactive oxygen species; EPC, endothelial progenitor cells; DCF,
dichlorofluorescein; M1, unstressed cells; M2, cells with increased
ROS production; GF, growth factors; NAC, N-acetylcysteine; DPI,
dibenziodolium; WST-1, water-soluble tetrazolium-1. |
Effects of p-cresol on ROS expression
in human EPCs
Since growth factor stimulation induced ROS
production in EPCs within 6 h of exposure, the effect of p-cresol
on ROS production in the same timeframe was analyzed. Treatment of
EPCs with p-cresol resulted in a dose-dependent decrease in ROS
levels in EPCs, as demonstrated by a significant reduction in
DCF-positive cells in the p-cresol-treated cells compared with the
control cells (P<0.05, Fig.
2).
Effects of p-cresol on antioxidant
gene expression
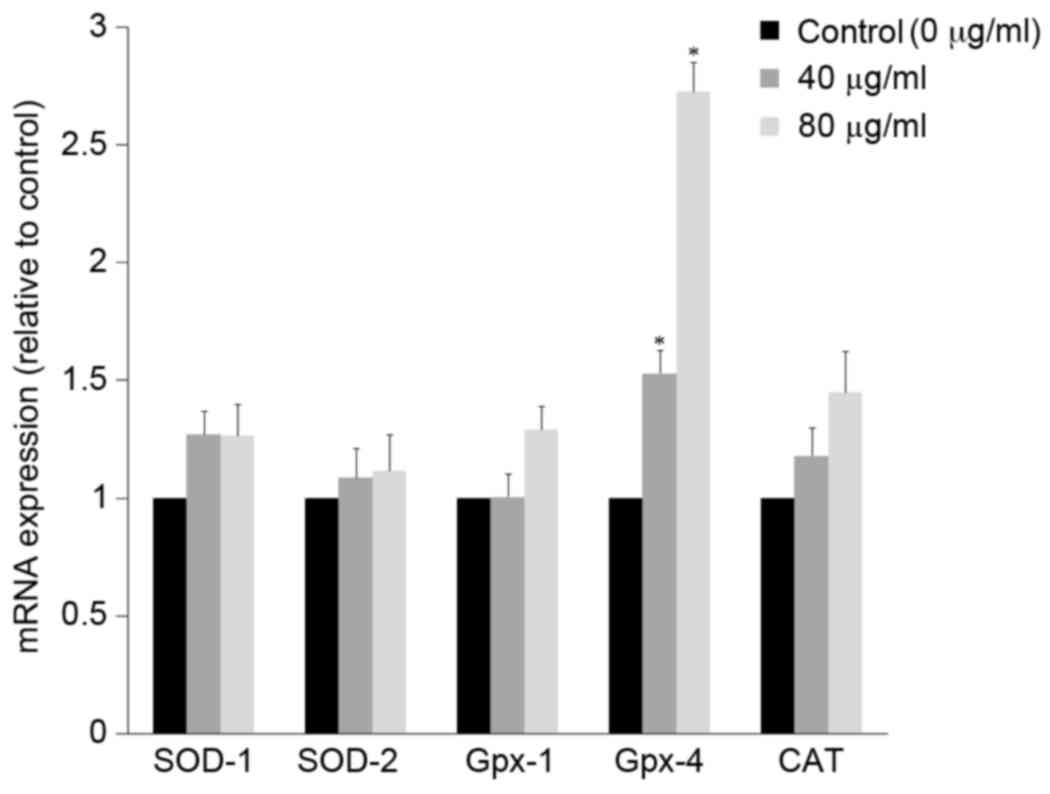
The antioxidant activity of p-cresol on EPCs was
confirmed by RT-qPCR analysis of mRNA expression levels of known
antioxidant markers. The mRNA expression of antioxidant genes
SOD-1, SOD-2, Gpx-1, Gpx-4, and
CAT was analyzed in EPCs treated with 40 or 80 µg/ml
p-cresol (Fig. 3). Gpx-4 mRNA
expression was significantly upregulated by 3-fold with 80 µg/ml
p-cresol after 6 h (P<0.05 compared with control), while
expression of the other genes was not significantly affected
(Fig. 3).
Effects of p-cresol on NOX in human
EPCs
NOX is a major source of ROS in EPCs. The NOX
complex consists of the membrane-associated catalytic CYBB and the
regulatory CYBA subunits, plus cytosolic components, including
neutrophil cytosolic factor 1 (p47phox), neutrophil cytosolic
factor 2 (p67phox), neutrophil cytosolic factor 4 (p40phox), and
the small GTPase Ras-related C3 substrate 1 (Rac1) (13). Since NOX is a major source of ROS,
its subunits were investigated in EPCs treated with p-cresol. The
results demonstrated that incubation of EPCs with p-cresol for 72 h
resulted in a significant downregulation of CYBB and CYBA
expression in a dose-dependent manner (P<0.05 compared with
control; Fig. 4), but it did not
inhibit NOX4 (Fig. 4).
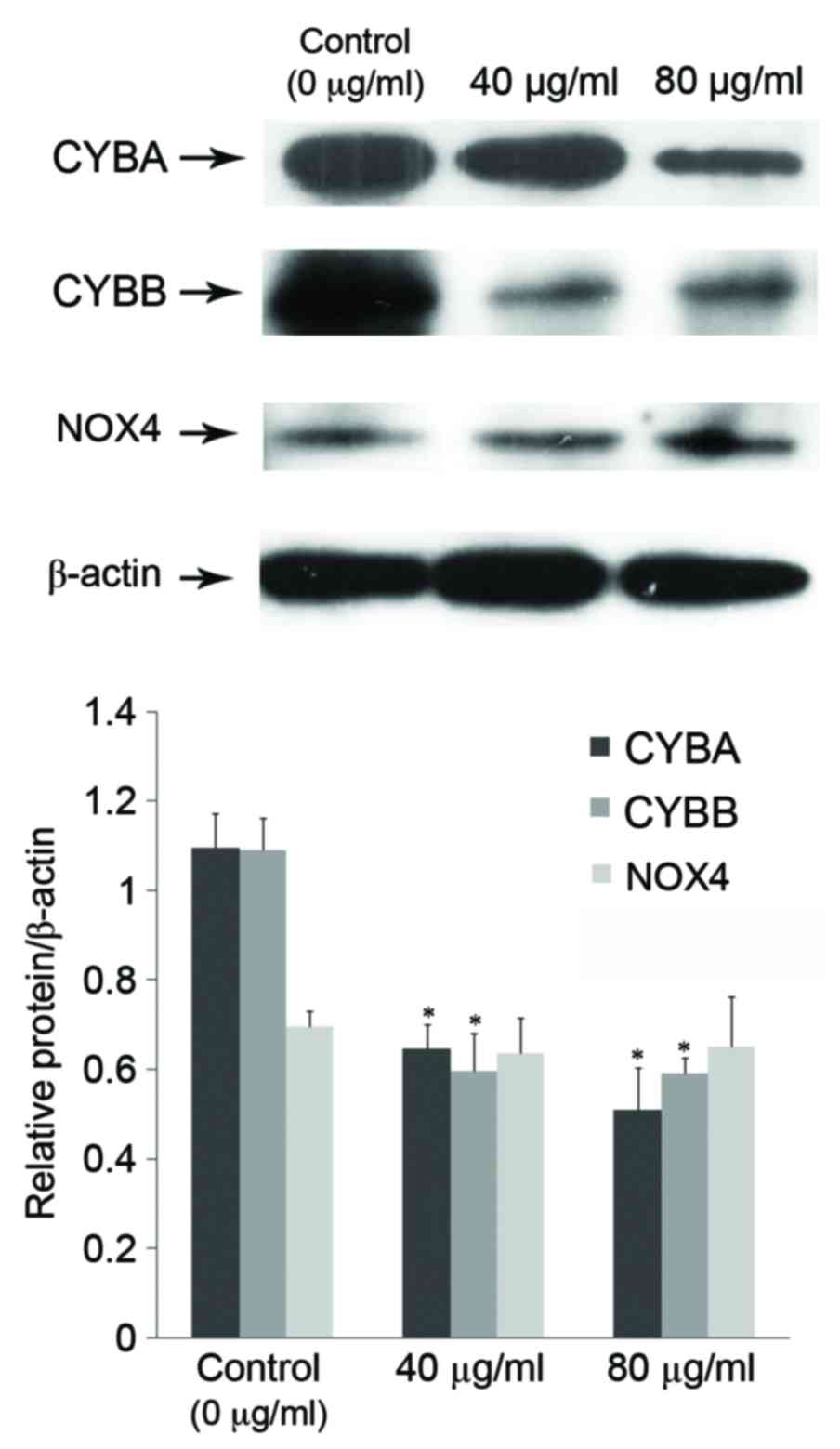 | Figure 4.Effect of p-cresol on protein
expression levels of NADPH oxidase subunits. Following incubation
of EPCs with 0 (control), 40 or 80 µg/ml p-cresol for 72 h, cell
lysates were subjected to western blot analysis to detect protein
expression of CYBA, CYBB and NOX4. β-actin served as a loading
control. Representative blots are depicted in the upper panel,
while a quantification of each protein signal relative to the
β-actin control is depicted in the graph of the lower panel.
*P<0.05 vs. control. p-cresol, para-cresol; NAPDH, nicotinamide
adenine dinucleotide phosphate; EPC, endothelial progenitor cells;
CYBA, cytochrome b-245 alpha chain; CYBB, cytochrome b-245 beta
chain; NOX4, NAPDH oxidase 4. |
Discussion
Protein-bound uremic toxins constitute a
heterogeneous group of compounds that are retained in patients with
CKD. The main common characteristic of this group of toxins is
their difficult removal by dialysis. Vanholder et al termed
them as ‘the forgotten toxins’ (8). According to the European Uremic Toxin
(EUTox) group, 25 protein-bound toxins have been identified to
date, including four phenols (17). In the present study, the
antiproliferation effect of one such phenol, p-cresol, on
EPCs was demonstrated to be related to its antioxidant
activity.
Phenols are a class of chemical compounds consisting
of a hydroxyl group (−OH) bonded directly to an aromatic
hydrocarbon group. They are reactive species toward oxidation, thus
considered to have antioxidant activity. Ujhelyi et al
(18) have previously suggested
that the decreased antioxidant capacity of plasma ultrafiltrate
observed after a hemodialysis session is related to the removal of
several protein-bound uremic toxins, including p-cresol.
This indicates that phenolic uremic toxins, usually retained in
patients with CKD, might have antioxidant activity. Although Chang
et al (12) demonstrated
that p-cresol increases ROS in endothelial and mononuclear
cells, the present study demonstrated that p-cresol inhibits
EPC proliferation via its antioxidant activity, suggesting that the
three remaining phenols identified in the protein-bound uremic
toxins in CKD patients might have similar activity.
There are two different types of EPCs derived from
peripheral blood: The spindle-shaped early EPCs, which have limited
proliferative potential and are unsuitable for long-term culture
under in vitro conditions (19), and the cobblestone-shaped late
EPCs, which are produced by prolonged culture of peripheral
mononuclear cells in the presence of various growth factors and
have potential for rapid growth (20,21).
Previous studies have demonstrated that early and late EPCs make
different contributions to angiogenesis (20); early EPCs contribute to
angiogenesis primarily by secreting angiogenic cytokines that
recruit resident mature endothelial cells and induce their
proliferation and survival, and late EPCs enhance angiogenesis by
providing a sufficient number of endothelial cells by virtue of
their rapid growth. Thus, it is possible that the proliferative
dysfunction of late EPCs induced by p-cresol could directly
contribute to impaired angiogenesis following ischemic insult. An
in vivo study would be required in order to further confirm
this finding.
ROS serve an important role in normal cell growth,
migration, differentiation, apoptosis, and senescence (22,23).
Excess amounts of ROS are toxic and involved in stem/progenitor
cell senescence and apoptosis (24). By contrast, ROS at low levels
function as signaling molecules to regulate EPC proliferation and
EPC-mediated angiogenesis (25).
Signal transduction activated by ROS has been an emerging area of
investigation. NOX is a major source of ROS in EPCs (26). The NOX complex consists of
catalytic subunits (mainly CYBB or NOX4), the regulatory subunit,
CYBA, and the cytosolic subunits p47phox, p67phox, and Rac1
(13). A previous study reported
the role of CYBB-based NOX in the angiogenesis function of EPCs
(27). The present study revealed
that the antiproliferation effect of p-cresol on EPCs was
associated with decreased ROS levels and decreased CYBB and CYBA
expression. In conclusion, the present in vitro study
indicated that a uremic toxin, p-cresol, inhibits EPC
proliferation via its antioxidant activity. The results of the
present study may facilitate understanding of uremic toxins
toxicity on the cardiovascular system.
Acknowledgements
The present study was partly supported by grants
from the Science and Technology Commission of Wenzhou Municipality,
China (grant no. Y20140504).
References
|
1
|
Culleton BF, Larson MG, Wilson PW, Evans
JC, Parfrey PS and Levy D: Cardiovascular disease and mortality in
a community-based cohort with mild renal insufficiency. Kidney Int.
56:2214–2219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu CY: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sezer M, Ozcan M, Okcular I, Elitok A,
Umman S, Umman B, Tayyareci Y, Olcay A, Nisanci Y, Bilge AK and
Meric M: A potential evidence to explain the reason behind the
devastating prognosis of coronary artery disease in uraemic
patients: Renal insufficiency is associated with poor coronary
collateral vessel development. Int J Cardiol. 115:366–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brunet P, Gondouin B, Duval-Sabatier A,
Dou L, Cerini C, Dignat-George F, Jourde-Chiche N, Argiles A and
Burtey S: Does uremia cause vascular dysfunction? Kidney Blood
Press Res. 34:284–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohandas R and Segal MS: Endothelial
progenitor cells and endothelial vesicles-what is the significance
for patients with chronic kidney disease? Blood Purif. 29:158–162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amann K, Wanner C and Ritz E: Cross-talk
between the kidney and the cardiovascular system. J Am Soc Nephrol.
17:2112–2119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schiffrin EL, Lipman ML and Mann JF:
Chronic kidney disease: Effects on the cardiovascular system.
Circulation. 116:85–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vanholder R, De Smet R and Lameire N:
Protein-bound uremic solutes: The forgotten toxins. Kidney Int
Suppl. 78:S266–S270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez AW, Recht NS, Hostetter TH and
Meyer TW: Removal of P-cresol sulfate by hemodialysis. J Am Soc
Nephrol. 16:3430–3436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ying Y, Yang K, Liu Y, Chen QJ, Shen WF,
Lu L and Zhang RY: A uremic solute, P-cresol, inhibits the
proliferation of endothelial progenitor cells via the p38 pathway.
Circ J. 75:2252–2259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andriamihaja M, Lan A, Beaumont M,
Audebert M, Wong X, Yamada K, Yin Y, Tomé D, Carrasco-Pozo C,
Gotteland M, et al: The deleterious metabolic and genotoxic effects
of the bacterial metabolite p-cresol on colonic epithelial cells.
Free Radic Biol Med. 85:219–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang MC, Chang HH, Chan CP, Yeung SY,
Hsien HC, Lin BR, Yeh CY, Tseng WY, Tseng SK and Jeng JH: p-Cresol
affects reactive oxygen species generation, cell cycle arrest,
cytotoxicity and inflammation/atherosclerosis-related modulators
production in endothelial cells and mononuclear cells. PLoS One.
9:e1144462014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ushio-Fukai M and Urao N: Novel role of
NADPH oxidase in angiogenesis and stem/progenitor cell function.
Antioxid Redox Signal. 11:2517–2533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang MC, Wang TM, Yeung SY, Jeng PY, Liao
CH, Lin TY, Lin CC, Lin BR and Jeng JH: Antiplatelet effect by
p-cresol, a uremic and environmental toxicant, is related to
inhibition of reactive oxygen species, ERK/p38 signaling and
thromboxane A2 production. Atherosclerosis. 219:559–565. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piccoli C, D'Aprile A, Ripoli M, Scrima R,
Lecce L, Boffoli D, Tabilio A and Capitanio N: Bone-marrow derived
hematopoietic stem/progenitor cells express multiple isoforms of
NADPH oxidase and produce constitutively reactive oxygen species.
Biochem Biophys Res Commun. 353:965–972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanholder R, De Smet R, Glorieux G,
Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP,
Deppisch R, et al: Review on uremic toxins: Classification,
concentration, and interindividual variability. Kidney Int.
63:1934–1943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ujhelyi L, Balla G, Jeney V, Varga Z, Nagy
E, Vercellotti GM, Agarwal A, Eaton JW and Balla J: Hemodialysis
reduces inhibitory effect of plasma ultrafiltrate on LDL oxidation
and subsequent endothelial reactions. Kidney Int. 69:144–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Y, Weisdorf DJ, Solovey A and Hebbel
RP: Origins of circulating endothelial cells and endothelial
outgrowth from blood. J Clin Invest. 105:71–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ,
Hwang KK, Oh BH, Lee MM and Park YB: Characterization of two types
of endothelial progenitor cells and their different contributions
to neovasculogenesis. Arterioscler Thromb Vasc Biol. 24:288–293.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griendling KK, Sorescu D and Ushio-Fukai
M: NAD(P)H oxidase: Role in cardiovascular biology and disease.
Circ Res. 86:494–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Case J, Ingram DA and Haneline LS:
Oxidative stress impairs endothelial progenitor cell function.
Antioxid Redox Signal. 10:1895–1907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maulik N: Redox signaling of angiogenesis.
Antioxid Redox Signal. 4:805–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ushio-Fukai M: Redox signaling in
angiogenesis: Role of NADPH oxidase. Cardiovasc Res. 71:226–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Urao N, Inomata H, Razvi M, Kim HW, Wary
K, McKinney R, Fukai T and Ushio-Fukai M: Role of nox2-based NADPH
oxidase in bone marrow and progenitor cell function involved in
neovascularization induced by hindlimb ischemia. Circ Res.
103:212–220. 2008. View Article : Google Scholar : PubMed/NCBI
|