Introduction
Lung fibrosis is one the oldest recorded
fibroproliferative disorders, which may ultimately lead to lung
failure and mortality (1,2). Several distinct injury-associated or
inflammatory causes of interstitial lung disease (ILD) can result
in progressive lung scarring (3–7).
ILDs that arise with no obvious cause are referred to as
idiopathic. Despite the use of anti-inflammatory or
immunosuppressive drugs, there is currently no effective treatment
for lung fibrosis. Lung transplantation remains the only viable
intervention for end-stage lung fibrosis.
Fibrosis is characterized by abnormal fibroblast to
myofibroblast differentiation (8–10).
Resident pulmonary fibroblasts respond to various stimuli under
chronic inflammatory conditions during fibrogenesis. Once activated
they differentiate into myofibroblasts, which have a more
contractile, proliferative and secretory-active phenotype, and are
characterized by the increased expression of α-smooth muscle actin
(α-SMA) and extracellular matrix (ECM) components (11,12).
High-mobility group box protein 1 (HMGB1) is a
recognized nuclear protein and architectural chromatin-binding
factor that binds DNA and promotes protein assembly on specific DNA
targets (13,14). However, recent studies have
indicated that HMGB1 can be passively released from necrotic cells
or actively secreted into the extracellular milieu under
appropriate signal stimulation (15,16).
Extracellular HMGB1 is a multifunctional cytokine involved in the
processes underlying infection, inflammation, apoptosis and immune
responses, as well as in tumor development by binding to specific
cell-surface receptors (17–20).
Notably, numerous studies have indicated that HMGB1 may be involved
in fibrotic disorders, including myocardial fibrosis, renal
fibrosis and liver fibrosis (21–23).
Pulmonary HMGB1 is markedly upregulated in patients
with lung fibrosis and experimental lung fibrosis (24–26).
Entezari et al (27)
demonstrated that inhibition of HMGB1 may enhance bacterial
clearance and protect against Pseudomonas aeruginosa
pneumonia in cystic fibrosis. Li et al (28) revealed that HMGB1 mediates the
epithelial-to-mesenchymal transition in pulmonary fibrosis, which
involves the transforming growth factor (TGF)-β1/Smad2/3 signaling
pathway. However, the role of HMGB1 in lung fibroblast to
myofibroblast differentiation remains unclear.
In the present study, the potential role of HMGB1 in
fibroblast to myofibroblast differentiation was explored in
vitro. Firstly, the effects of HMGB1 on α-SMA expression and
ECM production in human lung fibroblasts were detected.
Subsequently, the role of TGF-β1 release in HMGB1-induced α-SMA
expression and ECM production in human lung fibroblasts was
investigated. In addition, the present study explored the potential
signaling pathway underlying the regulation of HMGB1-induced
fibroblast to myofibroblast differentiation.
Materials and methods
Reagents
Monoclonal antibodies against human α-SMA (catalog
no. ab32575) and collagen I (catalog no. ab138492) were purchased
from Abcam (Cambridge, UK). Monoclonal antibodies against human
β-actin (catalog no. 3700), nuclear factor (NF)-κB p65 (catalog no.
6956) and phosphorylated (p) -NF-κB p65 (catalog no. 13346), and
horseradish peroxidase (HRP) -conjugated anti-rabbit immunoglobulin
(Ig) G (catalog no. 7074) and anti-mouse IgG (catalog no. 7076)
secondary antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). The enhanced chemiluminescence (ECL)
detection system was purchased from Tanon Science and Technology
Co., Ltd. (Shanghai, China). Polyvinylidene difluoride (PVDF)
membranes were purchased from EMD Millipore (Billerica, MA, USA).
Ammonium pyrrolidinedithiocarbamate (PDTC) was purchased from
Abcam. TRIzol® reagent was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). A RevertAid™
First Strand cDNA Synthesis kit was obtained from Fermentas; Thermo
Fisher Scientific, Inc. (Pittsburgh, PA, USA). TaqMan®
Fast Advanced Master Mix was purchased from Applied Biosystems;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). A human TGF-β1
ELISA kit (catalog no. EK0513) was purchased from Boster (Wuhan,
China). Monoclonal antibody against human TGF-β1 (TGF-β1
neutralization antibody; catalog no. MAB240) and recombinant human
HMGB1 Protein, were obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). RIPA lysis buffer and a BCA kit were
supplied by Thermo Fisher Scientific.
Cell culture and treatment
The MRC-5 human lung fibroblast cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 50 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). Cells
were incubated at 37°C in an atmosphere containing 5%
CO2 and were routinely passaged upon reaching 80%
confluence, using 0.25% trypsin and a 1:3 cell dilution for each
passage (10,29). MRC-5 cells were incubated with
various doses of HMGB1 (0, 0.01, 0.1, 1, 10 and 100 µg/ml) at 37°C
in an atmosphere containing 5% CO2. The mRNA expression
levels of α-SMA were detected using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), 48
h following HMGB1 stimulation. Additionally, MRC-5 cells were
incubated with 10 µg/ml HMGB1 and the mRNA expression levels of
α-SMA and collagen I were detected using RT-qPCR at 0, 3, 6, 12,
24, 36, 48, 72 and 96 h following HMGB1 stimulation, or the
phosphorylation levels of NF-κB p65 were detected by western
blotting at 0, 20, 40, 60, 80 min following HMGB1 stimulation.
Next, MRC-5 cells were incubated with 10 µg/ml HMGB1 or PBS solvent
control, and the protein expression levels of α-SMA and collagen I
were detected by western blotting at 72 h following HMGB1
stimulation. In order to inhibit the activation of NF-κB p65, the
PDTC (20 µΜ) or DMSO solvent control were added into the medium at
30 min prior to addition of HMGB1. In order to block the function
of TGF-β1, TGF-β1 neutralization antibody (10 µg/ml) or IgG control
(10 µg/ml) were added into the medium with HMGB1.
RNA isolation and RT-qPCR
Total RNA was isolated from the cells using
TRIzol® regent (Thermo Fisher Scientific, Inc.). An
equal amount (2 µg) of total RNA was synthesized as first-strand
cDNA using the RevertAid™ First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The cDNA was amplified using the
TaqMan® Fast Advanced Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) to detect the expression levels of
α-SMA, collagen I and β-actin, according to the manufacturer's
protocol. Each sample was assayed in triplicate. The thermal
cycling conditions were as follows: Initial denaturation at 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 15
sec and annealing at 60°C for 60 sec. The 7500 Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for
all experiments. The relative levels of target gene expression were
obtained by calculating the ratio of cycle numbers of the initial
exponential amplification phase as determined by the sequence
detection system for specific target genes and b-actin using the
following formula: 2−ΔΔCq (30,31).
The sequences of primers used were as follows: α-SMA forward,
5′-ACTGCCGCATCCTCATCCTC-3′, α-SMA reverse,
5′-ATGCCAGCAGACTCCATCCC-3′; α-SMA probe 5′-Fam-CGC TGC CCA GAG ACC
CTG TTC CAG CC-Tamra-3′; collagen I forward,
5′-CCCAGTGTGGCCCAGAAGAA-3′, collagen I reverse,
5′-AGGAAGGTCAGCTGGATGGC-3′; collagen I probe 5′-Fam-TGG CGG CCA GGG
CTC CGA CC-Tamra-3′; β-actin forward, 5′-GGCACTCTTCCAGCCTTCCT-3′,
β-actin reverse, 5′-GCACTGTGTTGGCGTACAGG-3′; β-actin probe
5′-Fam-CGT GGA TGC CAC AGG ACT CCA TGC CCA-Tamra-3′.
Western blot analysis
Cells were lysed using RIPA lysis buffer at 4°C for
30 min and then protein concentration was quantified with a BCA kit
according to the manufacturer's protocol. The proteins (50 µg) were
subjected to 4–20% ExpressPlus™ PAGE Gel electrophoresis
(Genscript, Nanjing, Jiangsu, China) and transferred onto PVDF
membranes using a Mini-Protean® system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were
incubated for 1 h at room temperature in blocking buffer [5% skim
milk in tris-buffered saline plus 0.05% Tween 20 (TBS-T)] prior to
overnight incubation with the appropriate antibodies including
α-SMA (1:2,000), collagen I (1:3,000), NF-κB p65 (1:1,000) and
p-NF-κB p65 (1:1,000) and anti-β-actin (1:1,000) as the internal
standard at 4°C. After washing with TBS-T, the membranes were
incubated with HRP-conjugated anti-rabbit IgG (1:2,000) and
anti-mouse IgG (1:2,000) for 1 h at room temperature. The bands
were visualized using the ECL detection system (Tanon Science and
Technology Co., Ltd.). The radiographic band density was measured
using Quantity One software, version 4.6.2 (Bio-Rad Laboratories,
Inc.).
ELISA
The levels of TGF-β1 in the cell media were
determined using a commercial ELISA kit (Boster Systems, Inc.)
according to the manufacturer's protocol. The optical density at a
wavelength of 450 nm was detected by a microplate reader. TGF-β1
concentration was calculated by the standard curve.
Statistical analysis
All statistical analyses were performed using the
SPSS v.11.5 software (SPSS, Inc., Chicago, IL, USA). The
statistical significance of the groups was evaluated by one-way
analysis of variance; simultaneous multiple comparisons among
groups was conducted using the Bonferroni method. P<0.05 was
considered to indicate a statistically significant difference.
Results
HMGB1 induces fibroblast to
myofibroblast differentiation in human lung fibroblasts
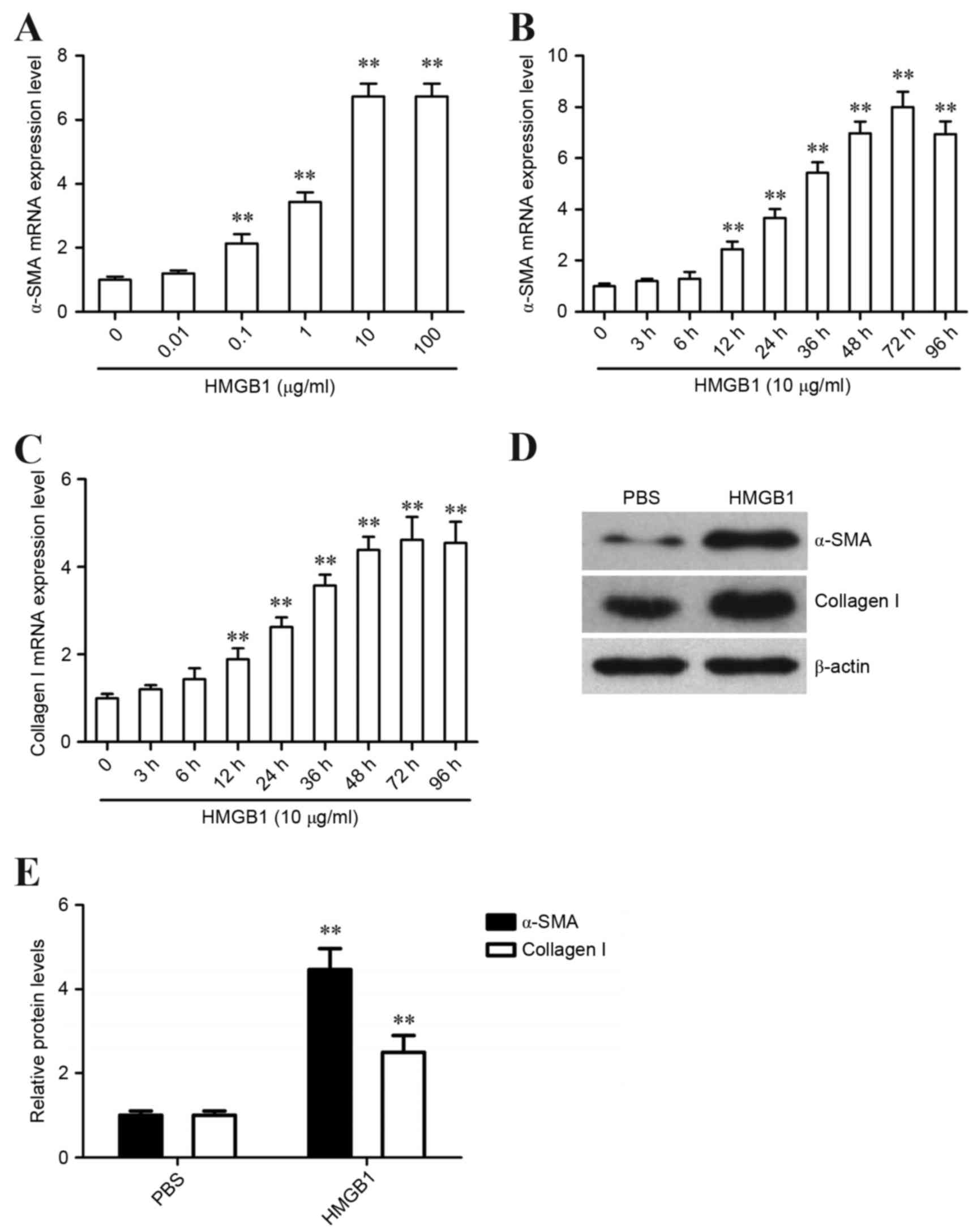
Human lung fibroblasts were initially incubated with
various doses of HMGB1. After HMGB1 stimulation for 48 h, the
expression levels of α-SMA were detected by RT-qPCR. The results
demonstrated that HMGB1 increased α-SMA expression in human lung
fibroblasts in a dose-dependent manner; the maximal effect was
detected following treatment with 10 µg/ml HMGB1 (Fig. 1A). Therefore, 10 µg/ml HMGB1 was
chosen for further studies. Human lung fibroblasts were incubated
with 10 µg/ml HMGB1, and α-SMA expression was detected at 0, 3, 6,
12, 24, 36, 48, 72 and 96 h using RT-qPCR. The results revealed
that α-SMA mRNA expression increased and peaked at 72 h following
HMGB1 stimulation (Fig. 1B). In
addition, the expression of collagen I was examined in human lung
fibroblasts at the same range of time points following HMGB1
stimulation. The results demonstrated that collagen I mRNA
expression increased and peaked at 72 h following HMGB1 stimulation
(Fig. 1C). Further investigations
revealed that the protein expression levels of α-SMA and collagen I
were markedly increased following HMGB1 stimulation for 72 h
(Fig. 1D and E). These results
indicated that HMGB1 may induce fibroblast to myofibroblast
differentiation of human lung fibroblasts.
HMGB1 increases TGF-β1 release from
human lung fibroblasts
Human lung fibroblasts were incubated with 10 µg/ml
HMGB1, and the protein expression levels of TGF-β1 in the media
were detected by ELISA at various time points (0, 3, 6, 12, 24, 36,
48, 72 and 96 h). The results demonstrated that the level of TGF-β1
secretion increased following HMGB1 stimulation, peaked at 6 h and
then gradually decreased up to 96 h (Fig. 2). HMGB1-induced TGF-β1 release
peaked earlier than HMGB1-induced expression of α-SMA and collagen
I in human lung fibroblasts (6 vs. 72 h), thus indicating that
TGF-β1 release may mediate HMGB1-induced fibroblast to
myofibroblast differentiation of human lung fibroblasts.
TGF-β1 release is essential for
HMGB1-induced fibroblast to myofibroblast differentiation of human
lung fibroblasts
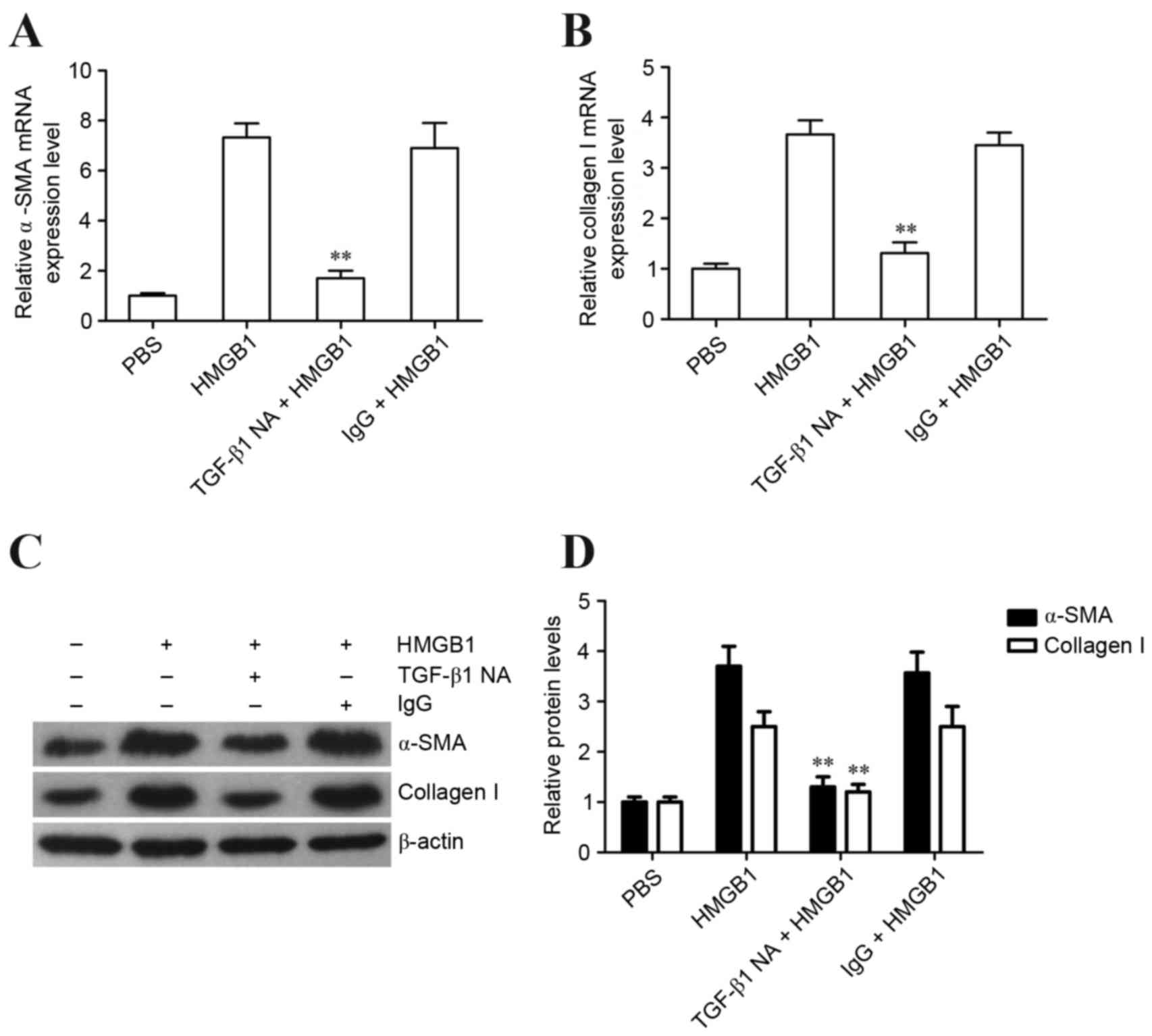
In order to investigate whether TGF-β1 is involved
in HMGB1-induced α-SMA expression in human lung fibroblasts, the
TGF-β1 neutralization antibody was used to block the function of
TGF-β1 during HMGB1 stimulation. Subsequently, the expression
levels of α-SMA and collagen I were detected. The results revealed
that the TGF-β1 neutralization antibody effectively inhibited
HMGB1-induced α-SMA and collagen I expression in human lung
fibroblasts (Fig. 3A-D).
Collectively, these results indicated that HMGB1 may induce
fibroblast to myofibroblast differentiation of human lung
fibroblasts via TGF-β1 release.
NF-κB activation is involved in
HMGB1-induced TGF-β1 release and fibroblast to myofibroblast
differentiation of lung fibroblasts
It has previously been reported that NF-κB
activation may mediate TGF-β1 release (32). Therefore, the present study
initially detected whether HMGB1 could induce NF-κB activation in
lung fibroblasts. The results revealed that HMGB1 significantly
induced NF-κB activation in lung fibroblasts following HMGB1
stimulation for 60 min (Fig. 4A).
Subsequently, the NF-κB inhibitor, PDTC, was used to inhibit NF-κB
activation to determine the role of NF-κB activation in
HMGB1-induced TGF-β1 release, as well as α-SMA and collagen I
expression, in lung fibroblasts. The results demonstrated that the
NF-κB inhibitor markedly reduced HMGB1-induced TGF-β1 release
(Fig. 4B), as well as α-SMA and
collagen I mRNA and protein expression in lung fibroblasts
(Fig. 4C-F). Finally, TGF-β1
suppression did not affect HMGB1-induced NF-κB activation in lung
fibroblasts (Fig. 4G), suggesting
that NF-κB is an upstream regulator of TGF-β1 release in
HMGB1-stimulated lung fibroblasts. Collectively, these results
indicated that HMGB1 may induce fibroblast to myofibroblast
differentiation via NF-κB-mediated TGF-β1 release.
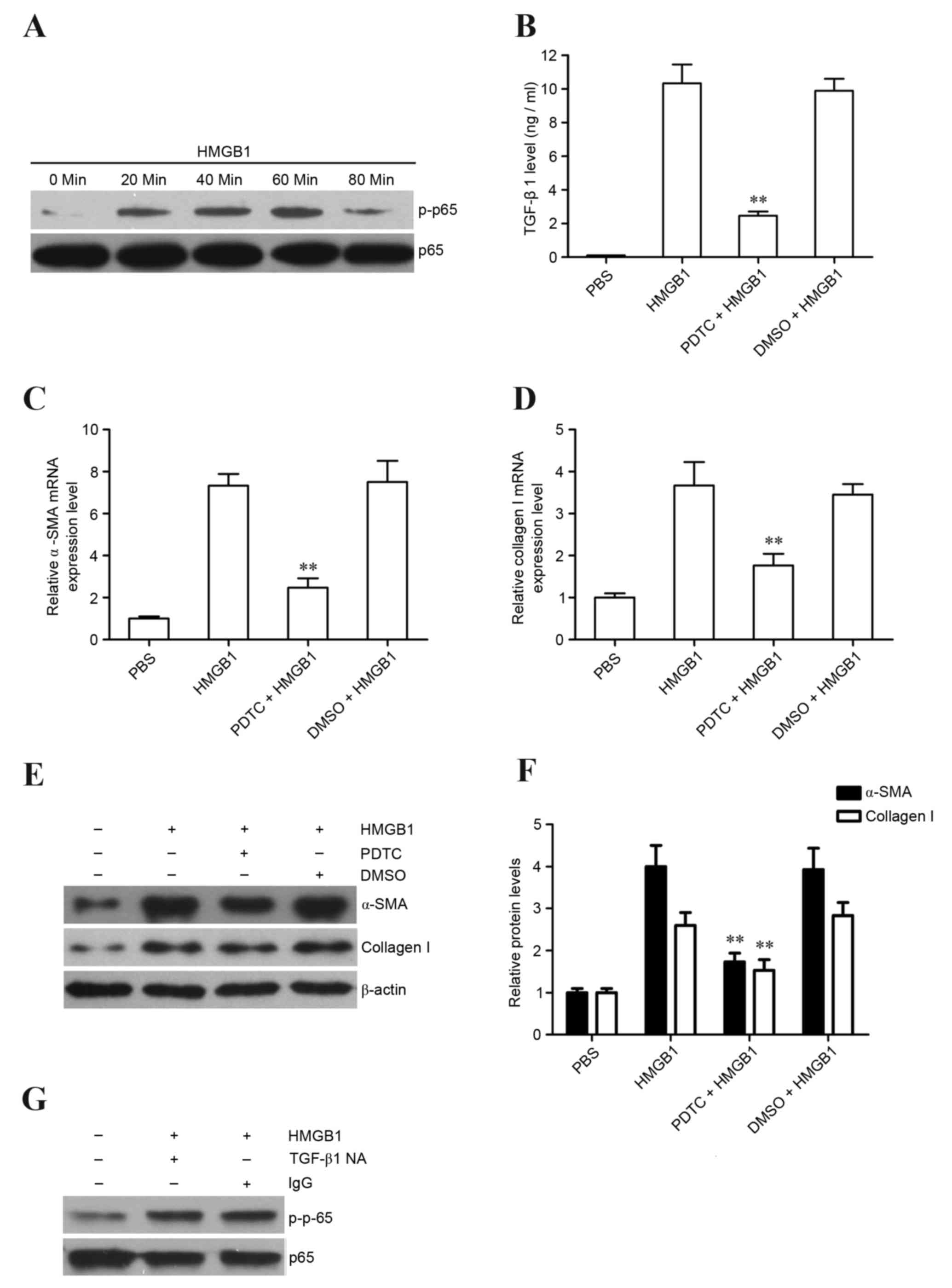 | Figure 4.Role of NF-κB activation in
HMGB1-induced TGF-β1 release and fibroblast to myofibroblast
differentiation in the MRC-5 cell line. (A) Phosphorylation of
NF-κB p65 was detected in MRC-5 cells stimulated with HMGB1 (10
µg/ml) at various time points by western blotting. MRC-5 cells were
treated with PDTC followed by HMGB1 stimulation (10 µg/ml);
subsequently, (B) TGF-β1 release at 6 h was detected by ELISA, and
(C) α-SMA and (D) collagen I expression levels at 72 h were
detected in MRC-5 cells by reverse transcription-quantitative
polymerase chain reaction and (E and F) western blotting. (G)
TGF-β1 NA (10 µg/ml) was used to block the function of TGF-β1
during HMGB1 stimulation, and the phosphorylation of NF-κB p65 was
detected at 60 min following HMGB1 stimulation by western blot
analysis. **P<0.01 vs. HMGB1 and DMSO + HMGB1 groups. The data
are from one experiment, representative of 3 independent
experiments. Results are presented as the mean ± standard deviation
(n=3/group). NF-κB, nuclear factor κB; p, phosphorylated; HMGB1,
high-mobility group box 1; PDTC, ammonium
pyrrolidinedithiocarbamate; TGF-β1, transforming growth factor β1;
α-SMA, α-smooth muscle actin; TGF-β1 NA, TGF-β1 neutralization
antibody; DMSO, dimethyl sulfoxide. |
Discussion
Lung fibrosis is a common fibroproliferative
disorder, which can lead to lung failure and mortality (1,2,33).
Despite the use of anti-inflammatory or immunosuppressive drugs,
there is currently no effective treatment for lung fibrosis.
Previous studies have revealed that fibroblast to myofibroblast
differentiation is pathologically altered during lung fibrosis.
Furthermore, it has been suggested that HMGB1 may be associated
with lung fibrosis (24–28); however, the role of HMGB1 in lung
fibroblast activation and differentiation is unclear. The present
study revealed that HMGB1 increased α-SMA and collagen I expression
in human lung fibroblasts, indicating that HMGB1 may induce
fibroblast to myofibroblast differentiation of lung
fibroblasts.
Since TGF-β1 is a key factor that mediates
fibroblast to myofibroblast differentiation (34,35),
the present study further investigated HMGB1-induced TGF-β1 release
from human lung fibroblasts. The results demonstrated that TGF-β1
secretion was significantly increased following HMGB1 stimulation.
Notably, HMGB1-induced TGF-β1 release occurred earlier than
HMGB1-induced expression of α-SMA and collagen I in human lung
fibroblasts (6 vs. 72 h), indicating that TGF-β1 release may
mediate HMGB1-induced fibroblast to myofibroblast differentiation
of human lung fibroblasts. Further experiments demonstrated that
treatment with the TGF-β1 neutralization antibody effectively
inhibited HMGB1-induced α-SMA and collagen I expression in human
lung fibroblasts. Collectively, these results indicated that HMGB1
may induce fibroblast to myofibroblast differentiation of human
lung fibroblasts via TGF-β1 release.
Previous studies have reported that NF-κB activation
may mediate TGF-β1 release (36,37).
Therefore, the present study determined whether HMGB1 could induce
NF-κB activation in lung fibroblasts. The results revealed that
HMGB1 significantly induced NF-κB activation in lung fibroblasts.
The NF-κB inhibitor, PDTC, was applied to inhibit NF-κB activation
in order to investigate the role of NF-κB activation in
HMGB1-induced TGF-β1 release and fibroblast to myofibroblast
differentiation of lung fibroblasts. The results demonstrated that
the NF-κB inhibitor markedly reduced HMGB1-induced TGF-β1 release,
and α-SMA and collagen I expression in lung fibroblasts.
Collectively, the results indicated that HMGB1 may induce
fibroblast to myofibroblast differentiation of lung fibroblasts via
NF-κB-mediated TGF-β1 release.
It has been reported that HMGB1 accelerates
lipopolysaccharide-induced lung fibroblast proliferation in
vitro via the NF-κB signaling pathway (38). Conversely, the present study
demonstrated that NF-κB activation in lung fibroblasts, induced by
HMGB1, may promote TGF-β1 release and induce fibroblast to
myofibroblast differentiation. Signal transducer and activator of
transcription-3 (STAT-3) has been reported to contribute to lung
fibrosis through epithelial injury and fibroblast to myofibroblast
differentiation (39). In
addition, TGF-β stimulation in vitro leads to
Smad2/Smad3-dependent phosphorylation of STAT-3 in lung fibroblasts
(39). NF-κB and STAT-3 are
involved in fibroblast to myofibroblast differentiation, however,
the associations between these two molecules is unclear. Therefore,
further investigations are required to determine the association
between NF-κB and STAT-3 in the regulation of HMGB1-induced TGF-β1
release and fibroblast to myofibroblast differentiation.
References
|
1
|
Górka J, Szczeklik W, Włudarczyk A, Łoboda
P, Chmura Ł and Musiał J: Rapidly progressive interstitial lung
fibrosis in a patient with amyopathic dermatomyositis and anti-MDA5
antibodies. Pol Arch Med Wewn. 125:685–686. 2015.PubMed/NCBI
|
|
2
|
MacKenzie B, Korfei M, Henneke I, Sibinska
Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, et
al: Increased FGF1-FGFRc expression in idiopathic pulmonary
fibrosis. Respir Res. 16:832015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curtis JR, Sarsour K, Napalkov P, Costa LA
and Schulman KL: Incidence and complications of interstitial lung
disease in users of tocilizumab, rituximab, abatacept and
anti-tumor necrosis factor α agents, a retrospective cohort study.
Arthritis Res Ther. 17:3192015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto Y, Okamoto I, Otsubo K, Iwama E,
Hamada N, Harada T, Takayama K and Nakanishi Y: Severe acute
interstitial lung disease in a patient with anaplastic lymphoma
kinase rearrangement-positive non-small cell lung cancer treated
with alectinib. Invest New Drugs. 33:1148–1150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Y, Zhang X, Qin M and Wang X: Changes
in peripheral CD19(+)Foxp3(+) andCD19(+)TGFβ(+) regulatory B cell
populations in rheumatoid arthritis patients with interstitial lung
disease. J Thorac Dis. 7:471–477. 2015.PubMed/NCBI
|
|
6
|
Kawai T, Watanabe N, Yokoyama M, Nakazawa
Y, Goto F, Uchiyama T, Higuchi M, Maekawa T, Tamura E, Nagasaka S,
et al: Interstitial lung disease with multiple microgranulomas in
chronic granulomatous disease. J Clin Immunol. 34:933–940. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glasser SW, Senft AP, Maxfield MD,
Ruetschilling TL, Baatz JE, Page K and Korfhagen TR: Genetic
replacement of surfactant protein-C reduces respiratory syncytial
virus induced lung injury. Respir Res. 14:192013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin L, Wang Y, Liu W and Huang Y: BAMBI
inhibits skin fibrosis in keloid through suppressing TGF-β1-induced
hypernomic fibroblast cell proliferation and excessive accumulation
of collagen I. Int J Clin Exp Med. 8:13227–13234. 2015.PubMed/NCBI
|
|
9
|
Tsukui T, Ueha S, Shichino S, Inagaki Y
and Matsushima K: Intratracheal cell transfer demonstrates the
profibrotic potential of resident fibroblasts in pulmonary
fibrosis. Am J Pathol. 185:2939–2948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geng J, Huang X, Li Y, Xu X, Li S, Jiang
D, Liang J, Jiang D, Wang C and Dai H: Down-regulation of USP13
mediates phenotype transformation of fibroblasts in idiopathic
pulmonary fibrosis. Respir Res. 16:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma X, Yang F, Yang S, Rasul A, Li T, Liu
L, Kong M, Guo D and Ma T: Number and distribution of
myofibroblasts and α-smooth muscle actin expression levels in fetal
membranes with and without gestational complications. Mol Med Rep.
12:2784–2792. 2015.PubMed/NCBI
|
|
12
|
Jung YS, Liu XW, Chirco R, Warner RB,
Fridman R and Kim HR: TIMP-1 induces an EMT-like phenotypic
conversion in MDCK cells independent of its MMP-inhibitory domain.
PLoS One. 7:e387732012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watson M, Stott K, Fischl H, Cato L and
Thomas JO: Characterization of the interaction between HMGB1 and
H3-a possible means of positioning HMGB1 in chromatin. Nucleic
Acids Res. 42:848–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Little AJ, Corbett E, Ortega F and Schatz
DG: Cooperative recruitment of HMGB1 during V (D)J recombination
through interactions with RAG1 and DNA. Nucleic Acids Res.
41:3289–3301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao T, Ren H, Wang X, Liu P, Yan F, Jiang
W, Li Y, Li J, Gribben JG, Jia L and Hao J: Rituximab-induced HMGB1
release is associated with inhibition of STAT3 activity in human
diffuse large B-cell lymphoma. Oncotarget. 6:27816–27831. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang G, Wang Y, Yun J, Hajrasouliha AR,
Zhao Y, Sun D, Kaplan HJ and Shao H: HMGB1 release triggered by the
interaction of live retinal cells and uveitogenic T cells is
Fas/FasL activation-dependent. J Neuroinflammation. 12:1792015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tabata C, Kanemura S, Tabata R, Masachika
E, Shibata E, Otsuki T, Nishizaki T and Nakano T: Serum HMGB1 as a
diagnostic marker for malignant peritoneal mesothelioma. J Clin
Gastroenterol. 47:684–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laws TR, Clark GC and D'Elia RV: Immune
profiling of the progression of a BALB/c mouse aerosol infection by
Burkholderia pseudomallei and the therapeutic implications of
targeting HMGB1. Int J Infect Dis. 40:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi L, Sun X, Li FE, Zhu BS, Braun FK, Liu
ZQ, Tang JL, Wu C, Xu F, Wang HH, et al: HMGB1 promotes
mitochondrial dysfunction-triggered striatal neurodegeneration via
autophagy and apoptosis activation. PLoS One. 10:e01429012015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parodi M, Pedrazzi M, Cantoni C, Averna M,
Patrone M, Cavaletto M, Spertino S, Pende D, Balsamo M, Pietra G,
et al: Natural killer (NK)/melanoma cell interaction induces
NK-mediated release of chemotactic high mobility group box-1
(HMGB1) capable of amplifying NK cell recruitment. Oncoimmunology.
4:e10523532015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang FP, Li L, Li J, Wang JY, Wang LY and
Jiang W: High mobility group box-1 promotes the proliferation and
migration of hepatic stellate cells via TLR4-dependent signal
pathways of PI3K/Akt and JNK. PLoS One. 8:e643732013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Lavine KJ, Epelman S, Evans SA,
Weinheimer CJ, Barger PM and Mann DL: Necrotic myocardial cells
release damage-associated molecular patterns that provoke
fibroblast activation in vitro and trigger myocardial inflammation
and fibrosis in vivo. J Am Heart Assoc. 4:e0019932015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Guo Y, Fu H, Hu S, Pan J, Wang Y,
Cheng J, Song J, Yu Q, Zhang S, et al: Chop deficiency prevents
UUO-induced renal fibrosis by attenuating fibrotic signals
originated from Hmgb1/TLR4/NFκB/IL-1β signaling. Cell Death Dis.
6:e18472015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ebina M, Taniguchi H, Miyasho T, Yamada S,
Shibata N, Ohta H, Hisata S, Ohkouchi S, Tamada T, Nishimura H, et
al: Gradual increase of high mobility group protein b1 in the lungs
after the onset of acute exacerbation of idiopathic pulmonary
fibrosis. Pulm Med. 2011:9164862011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamada N, Maeyama T, Kawaguchi T, Yoshimi
M, Fukumoto J, Yamada M, Yamada S, Kuwano K and Nakanishi Y: The
role of high mobility group box 1 in pulmonary fibrosis. Am J
Respir Cell Mol Biol. 39:440–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiringer K, Treis A, Kanolzer S, Witt C,
Ghanim B, Gruber S, Schmidthaler K, Renner S, Dehlink E, Nachbaur
E, et al: Differential expression of IL-33 and HMGB1 in the lungs
of stable cystic fibrosis patients. Eur Respir J. 44:802–805. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Entezari M, Weiss DJ, Sitapara R,
Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, et
al: Inhibition of high-mobility group box 1 protein (HMGB1)
enhances bacterial clearance and protects against Pseudomonas
aeruginosa pneumonia in cystic fibrosis. Mol Med. 18:477–485.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li LC, Li DL, Xu L, Mo XT, Cui WH, Zhao P,
Zhou WC, Gao J and Li J: High-mobility group box 1 mediates
epithelial-to-mesenchymal transition in pulmonary fibrosis
involving transforming growth factor-β1/Smad2/3 signaling. J
Pharmacol Exp Ther. 354:302–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Sun Z, Liu H, Ren Y, Shao D, Zhang
W, Lin J, Wolfram J, Wang F and Nie S: Connective tissue growth
factor stimulates the proliferation, migration and differentiation
of lung fibroblasts during paraquat-induced pulmonary fibrosis. Mol
Med Rep. 12:1091–1097. 2015.PubMed/NCBI
|
|
30
|
Hou F, Wang L, Wang H, Gu J, Li M, Zhang
J, Ling X, Gao X and Luo C: Elevated gene expression of S100A12 is
correlated with the predominant clinical inflammatory factors in
patients with bacterial pneumonia. Mol Med Rep. 11:4345–4352.
2015.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di JM, Pang J, Pu XY, Zhang Y, Liu XP,
Fang YQ, Ruan XX and Gao X: Toll-like receptor 9 agonists promote
IL-8 and TGF-beta1 production via activation of nuclear factor
kappaB in PC-3 cells. Cancer Genet Cytogenet. 192:60–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petrosyan F, Culver DA and Reddy AJ: Role
of bronchoalveolar lavage in the diagnosis of acute exacerbations
of idiopathic pulmonary fibrosis: A retrospective study. BMC Pulm
Med. 15:702015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji H, Tang H, Lin H, Mao J, Gao L, Liu J
and Wu T: Rho/Rock cross-talks with transforming growth
factor-β/Smad pathway participates in lung fibroblast-myofibroblast
differentiation. Biomed Rep. 2:787–792. 2014.PubMed/NCBI
|
|
35
|
Sassoli C, Chellini F, Pini A, Tani A,
Nistri S, Nosi D, Zecchi-Orlandini S, Bani D and Formigli L:
Relaxin prevents cardiac fibroblast-myofibroblast transition via
notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS One.
8:e638962013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song J, Zhu Y, Li J, Liu J, Gao Y, Ha T,
Que L, Liu L, Zhu G, Chen Q, et al: Pellino1-mediated TGF-β1
synthesis contributes to mechanical stress induced cardiac
fibroblast activation. J Mol Cell Cardiol. 79:145–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bizzarro V, Fontanella B, Carratù A,
Belvedere R, Marfella R, Parente L and Petrella A: Annexin A1
N-terminal derived peptide Ac2-26 stimulates fibroblast migration
in high glucose conditions. PLoS One. 7:e456392012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li W, Xu Q, Deng Y, Yang Z, Xing S, Zhao
X, Zhu P, Wang X, He Z and Gao Y: High-mobility group box 1
accelerates lipopolysaccharide-induced lung fibroblast
proliferation in vitro: involvement of the NF-κB signaling pathway.
Lab Invest. 95:635–647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pedroza M, Le TT, Lewis K,
Karmouty-Quintana H, To S, George AT, Blackburn MR, Tweardy DJ and
Agarwal SK: STAT-3 contributes to pulmonary fibrosis through
epithelial injury and fibroblast-myofibroblast differentiation.
FASEB J. 30:129–140. 2016. View Article : Google Scholar : PubMed/NCBI
|