Introduction
Patients with diabetes exhibit an increased
susceptibility to develop a wide range of macro-vascular
complications, including atherosclerotic cardiovascular and
cerebrovascular disease, which account for the majority of deaths
and disability in diabetes patients (1,2).
Improving macro-vascular outcomes through glucose-lowering
interventions have remained a difficult, complicated, and to date,
largely unsuccessful enterprise. There is an ongoing need for new
therapeutic targets which would slow the accelerated progression of
diabetic atherosclerosis (3).
The elevated blood glucose level (hyperglycemia) is
considered one of the major causes of accelerated atherosclerosis
in diabetes (4,5). Together with endothelial dysfunction,
the proliferation of vascular smooth muscle cells (VSMCs) is one of
the characteristic features of atherosclerosis (6). Under high glucose conditions, human,
porcine and rodent VSMCs proliferate and migrate from the media to
the subendothelial space of the vessel wall where early
atherosclerotic lesions are localized (5,6).
However, the mechanisms underlying VSMCs proliferation are very
complicated and have not yet been completely elucidated.
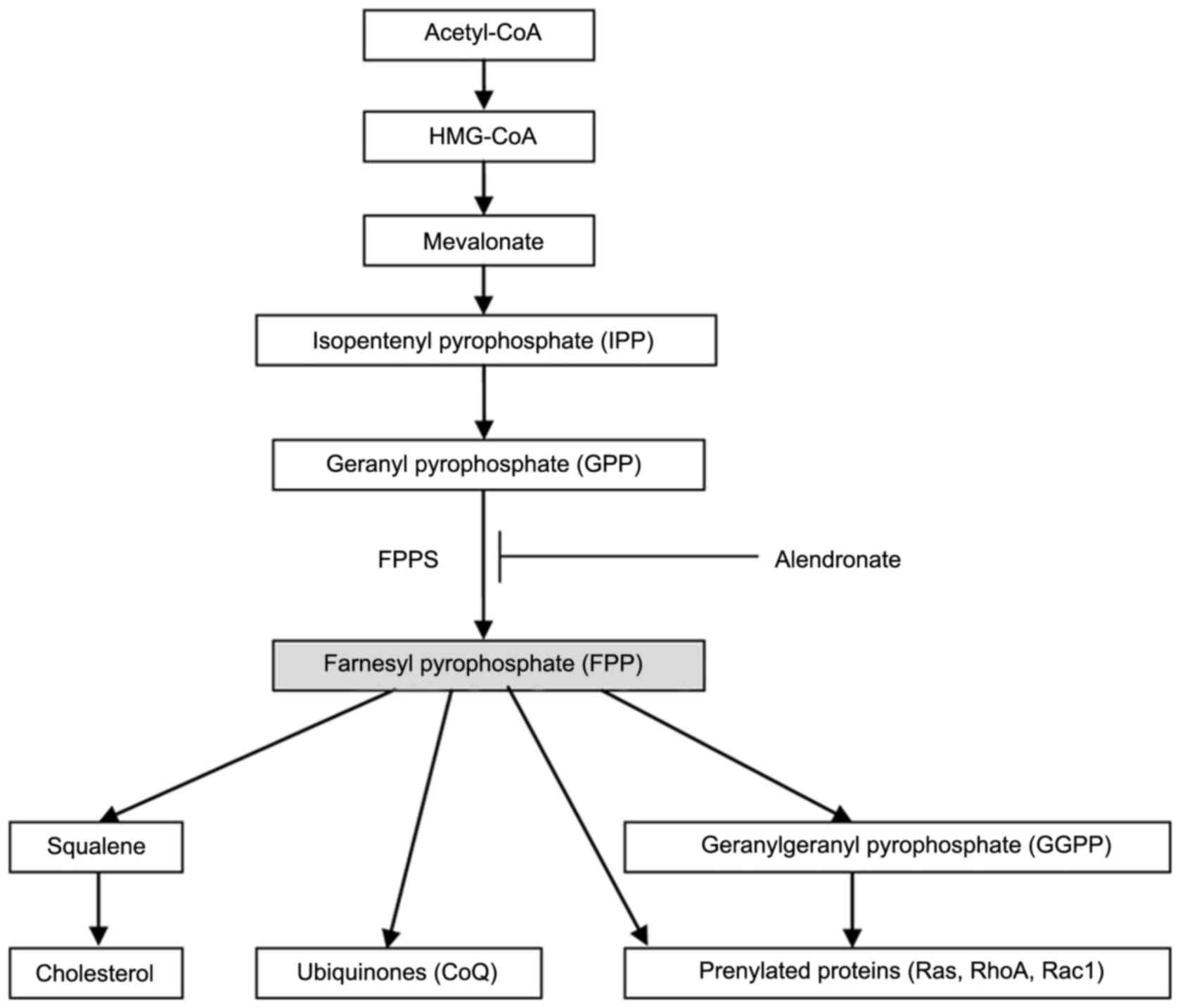
Farnesyl pyrophosphate synthase (FPPS, EC 2.5.1.10),
an essential enzyme in the mevalonate pathway, catalyzes the
synthesis of FPP (7). As seen in
Fig. 1, FPP is a crucial branching
point precursor in the synthesis of several classes of essential
metabolites, including sterols (such as cholesterol), ubiquinones
(also known as coenzyme Q, CoQ) and non-sterols substrates for
prenylation of proteins (especially the small GTPases) (7–9).
These metabolites serve as the basis for the biosynthesis of
molecules used in processes as diverse as terpenoid synthesis,
protein prenylation, cell membrane maintenance, hormones, protein
anchoring, N-glycosylation, and steroid biosynthesis (10). In our previous studies, we found
that the FPPS expression in aorta from streptozotocin (STZ)-induced
diabetic mice was remarkably upregulated along with the accelerated
process of atherosclerosis (11).
Meanwhile, we also observed that high glucose (22.2 mM) induced
both VSMCs proliferation and FPPS upregulation in vitro
(11). Thus, we assumed that
increased expression of aortic FPPS may contribute to the
accelerated atherogenic process in diabetic mice. However, its
exact relationship and mechanisms remain to be explored.
Therefore, the present study was designed to
determine whether the inhibition of FPPS attenuated high
glucose-induced VSMCs proliferation in vivo or in
vitro, and if present, to investigate the underlying
mechanisms. For this purpose, alendronate, an inhibitor of FPPS in
mevalonate pathway (12,13), was used to examine the potentially
critical roles of FPPS in VSMCs proliferation during the
accelerated atherogenic process in diabetes mellitus.
Materials and methods
Animal treatment
Female BALB/c mice (SPF, 20±2.5 g) were purchased
from the Shanghai Laboratory Animal Center (Chinese Academy of
Sciences), and housed in a pathogenfree laboratory at the First
Affiliated Hospital of Zhejiang University. The procedures and
protocols of the study conformed to the Guide for the Care and Use
of Laboratory Animal published by the US National Institutes of
Health (NIH Publication no. 85-23, revised 1996) and the guidelines
of the Animal Care and Use Committee of Zhejiang University. All
chemicals and reagents were purchased from Sigma (S. Louis, MO,
USA), unless otherwise stated. As described in our previous report
(11), diabetic mice were induced
by daily injection of STZ at a dose of 50 mg/kg for 5 days after a
4 h fast. Non-diabetic mice received the vehicle (citrate buffer;
0.05 M, pH 4.5). Twelve diabetic mice were randomly divided into
two groups as follows: i) Diabetic mice treated with distilled
water (control) group (D+C, n=6); ii) diabetic mice treated with
alendronate (15 mg/kg/day) group (D+A, n=6). Twelve age and weight
matched non-diabetes mice were also divided to control and
alendronate group (ND+C and ND+A, n=6). Alendronate was
administrated every day for 16 weeks by the intragastric route.
Glucose and lipid analysis
Fasting blood glucose (FBG) levels were evaluated in
venous blood drawn from the tail by using a CONTOUR glucose meter
(Bayer, Mishawaka, IN, USA). Serum total cholesterol (TC),
high-density lipoprotein cholesterol (HDL-C), low-density
lipoprotein cholesterol (LDL-C), and triglyceride (TG)
concentrations were determined by commercial enzymatic methods
(test kits from Shanghai Rongsheng Biotech, Inc., Shanghai,
China).
Histological analysis
The aorta was dissected in situ from the
ascending aorta to the iliac bifurcation, cleaned of peripheral fat
under a dissecting microscope, and then fixed in 10% neutral
formalin, embedded in paraffin, and sequentially stained with
hematoxylin and eosin. Lesion areas (LA) per section were counted
by taking the average of 6 sections spaced 30 µm apart, beginning
at the base of the aortic root. Media thickness (MT) at 10
different points of the thoracic aorta was measured and calculated.
Morphometric analysis above was performed with Image-Pro Plus
6.0.
Cell culture and treatments
VSMCs were isolated from thoracic aortic explants of
female BALB/c mice as previously described (11,14).
In brief, aortic explants were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco) and maintained at 37°C in
a humidified atmosphere of 5% CO2 and 95% air. After 2
weeks, cells that had migrated onto the tissue culturedish were
collected by trypsinization and subcultured successively. The
identity of the VSMCs was determined by the positive
immunocytochemistry reactivity to smooth muscle specific α-actin.
To ensure the consistency of results, passages 5–12 of VSMCs were
used for experiment. According to our previous report (11), VSMCs proliferation was induced by
culturing cells in diabetic medium containing 22.2 mM glucose.
Mannitol was used as an osmotic control. VSMCs were also cultured
in the presence of various concentrations of alendronate (0, 3, 10,
30 and 100 µM) for 72 h.
Cell proliferation assay
After above treatments, cell proliferation was
assessed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT) assay as described previously (11). Cells were cultured in 96-well
plates (5×103 cells/well). When the VSMCs reached a 60%
confluent state, different treatments as stated above were given.
Then the cells of 96 wells were incubated with 100 µl of 0.5 mg/ml
MTT at 37°C for 4 h, washed with cold PBS, and lysed with 100 µl of
DMSO. After the insoluble crystals were completely dissolved, the
optical density of each well was immediately measured at 570 nm
using an automatic microplate reader (Molecular Devices, Sunnyvale,
CA, USA).
Western blot analysis
Total proteins were isolated from the aortic media
(VSMCs were the only cell type in this layer) or cultured VSMCs,
and the procedure of western blot analysis was performed as
described in our previous reports (11,15).
10 µg protein from each sample was separated on 10% sodium dodecyl
sulphate-polyacrylamide gel, electrophoresed, and transferred onto
nitrocellulose membranes. The membrane was blocked with
Tris-buffered saline (TBS, pH 7.6) containing 5% skim milk and
0.05% Tween-20, and then incubated with specific antibodies for 12
h at 4°C. The expressions of H2S-producing enzyme
[cystathionine γ-lyase (CSE)] and total small GTPases (Ras, RhoA,
and Rac1) were detected using their specific antibodies: mouse
anti-CSE monoclonal antibody (1:1,000, sc-374249; Santa Cruz
Biotechnology Co., Ltd., Japan), rabbit anti-Rac1 polyclonal
antibody (1:1,000, sc-95; Santa Cruz Biotechnology Co., Ltd.),
rabbit anti-RhoA monoclonal antibody (1:2,000, 2117; Cell Signaling
Technology, Inc., Danvers, MA, USA), and rabbit anti-Ras monoclonal
antibody (1:2,000, 3,339; Cell Signaling Technology, Inc.). To
ensure equal protein loading, mouse monoclonal antibody to β-actin
(1:5,000, ab8226; Abcam, Cambridge, UK) was used as an endogenous
control. After the membrane was incubated with goat-anti-rabbit IgG
conjugated to horseradish peroxidase (1:5,000 dilution;
MultiSciences, Hangzhou, China) for 1 h at 37 °C, the immune
complexes were visualized by the enhanced chemiluminescence (ECL)
method. Quantification of the bands was carried out using
densitometric analysis software Quantity One (Bio-Rad, Berkeley,
CA, USA).
Small GTPases activation assay
Small GTPase (Ras, RhoA, or Rac1) activation was
determined from tissue or cell lysates using Ras, RhoA, or Rac1
activation G-LISA kit (BK131, BK124, BK128; Cytoskeleton, Denver,
CO, USA) according to the manufacturer's instruction. The signal
was measured at 490 nm with a microplate spectrophotometer. Results
are expressed as fold increase in activity compared with control
group.
CoQ quantification
Concentrations of total CoQ9 and
CoQ10 were determined by isocratic high-performance
liquid chromatography (HPLC) according to Lang et al
(16) with some modifications as
follows. Cells were cultured in 12-well plates (1×105
cells/well). When the VSMCs reached a 60% confluent state,
different treatments as stated above were given. Cells were
resuspended and lysed in ultrapure water with addition of
1,4-benzoquinone (2 mg/ml in ethanol) to allow a complete oxidation
of ubiquinol (reduced form of CoQ). The mixtures were then
extracted twice by hexane/ethanol (5/2, v/v). Collected organic
layers were evaporated under nitrogen, and the dried compounds were
dissolved in ethanol and injected on the column Agilent TC-C18 (15
cmx0.46 cm, id 5 µm; Agilent Technologies, Inc., Palo Alto, CA,
USA). All steps of sample preparation were carried out rapidly, on
ice, with protection from light. The mobile phase consisted of
methanol/acetonitrile/ethanol (6/2/2, v/v/v), and the isocratic
elution carried out at 1 ml/min. The concentrations of compounds
were detected spectrophotometrically at 275 nm using external
standards CoQ11 and expressed as nmol per gram of
protein (the residue mixtures were also dried under nitrogen and
subjected to the protein analysis).
Measurement of Hydrogen sulfide
(H2S) concentration
H2S concentrations were measured in
cultured medium as described previously (17). Briefly, after different treatments,
cultured medium was collected from VSMCs plate. 75 µl medium was
mixed with 250 µl of 1% (w/v) zinc acetate and 425 µl distilled
water in a glass test tube. Then 20 mM N,
N-dimethyl-p-phenylenediamine sulfate in 7.2 mM HCl (133 µl) and 30
mM FeCl3 in 1.2 mM HCl (133 µl) were also added to the
test tube for 10 min incubation at room temperature. The protein in
the medium was removed by adding 250 µl of 10% trichloroacetic acid
to the reaction mixture and pelleted by centrifugation at 14,000 g
(5 min). The absorbance of the resulting solution at 670 nm was
measured with a spectrophotometer (Molecular Devices) in a 96-well
plate. All samples were assayed in duplicate, and concentration of
H2S in the solution was calculated against a calibration
curve of NaHS (3.125–250 µM).
Statistical analysis
All experiments were performed at least three times,
and the results were expressed as mean ± standard errors of mean
(SEM). All analyses were performed with SPSS (version 13.0; SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA)
followed by Bonferroni post hoc test was used to determine
significant differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Alendronate had no effect on glucose
and lipid levels
As expected the STZ-induced diabetic mice had
extremely higher levels of glucose than age-matched control mice.
16-week treatment with alendronate had no effect on glucose levels
in neither diabetic nor non-diabetic groups (Table I). Then, in all groups, serum lipid
levels (TC, LDL-C, HDL-C, and TG) were comparable (Table I).
 | Table I.Effect of alendronate treatment on
glucose and lipid levels. |
Table I.
Effect of alendronate treatment on
glucose and lipid levels.
| Group | FBG (mM) | TC (mM) | HDL-C (mM) | LDL-C (mM) | TG (mM) |
|---|
| Non-diabetic
mice |
|
|
|
|
|
|
Control | 7.62±0.53 | 1.76±0.07 | 0.87±0.07 | 0.56±0.05 | 1.42±0.07 |
|
Alendronate | 7.68±0.67 | 1.85±0.07 | 0.81±0.06 | 0.54±0.06 | 1.33±0.06 |
| Diabetic mice |
|
|
|
|
|
|
Control |
22.54±1.71a | 1.90±0.09 | 0.90±0.08 | 0.58±0.06 | 1.28±0.06 |
|
Alendronate |
21.20±1.62a | 1.86±0.08 | 0.79±0.08 | 0.63±0.06 | 1.31±0.05 |
Alendronate attenuated diabetic
atherosclerosis development
The morphologic data of lesion area (LA) and media
thickness (MT) were summarized in Fig.
2. In our study, STZ-induced diabetic mice developed moderate
aortic atherosclerotic plaques, with a mean LA of (11.39±0.81) ×
103 µm2 and MT of 91.74±6.52 µm. 16-week
treatment of alendronate attenuated diabetic atherosclerosis
development with a decline of LA (8.32±0.77) × 103
µm2 and MT 82.24±6.39 µm.
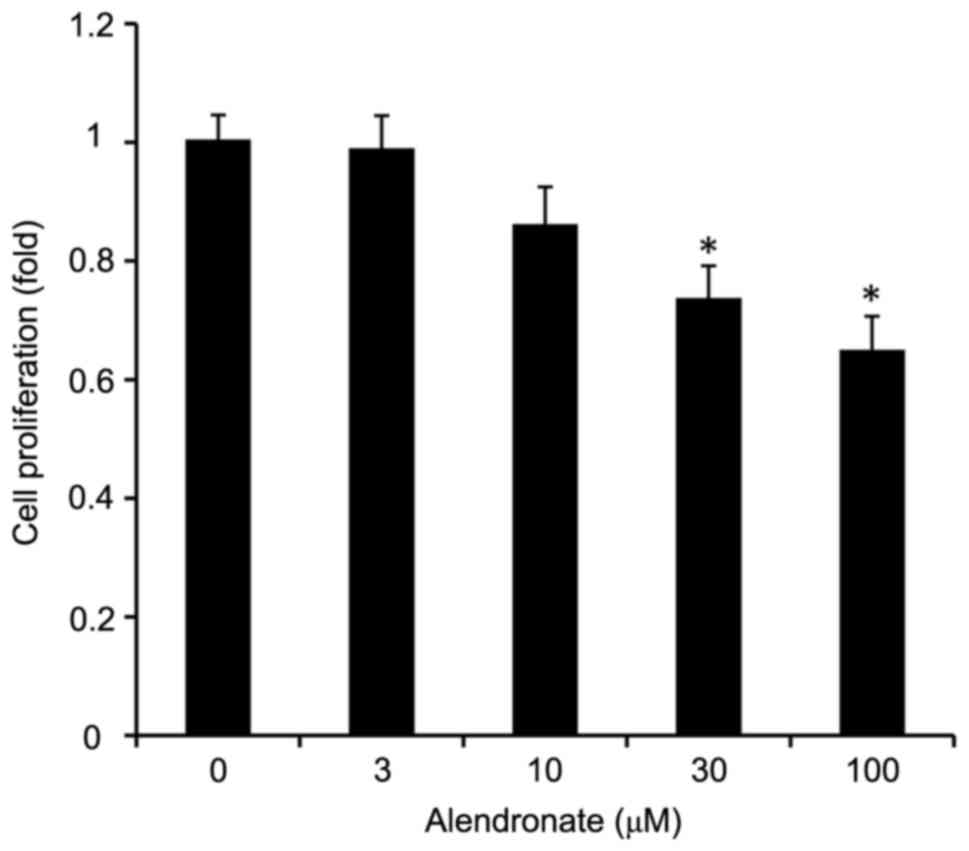
Alendronate inhibited the
proliferation of VSMCs induced by high glucose
As an inhibitor of FPPS, alendronate
dose-dependently inhibited the VSMCs proliferation induced by high
glucose (Fig. 3). Alendronate at
drug concentrations of 30 and 100 µM remarkably inhibited the cell
proliferation in a dose-dependent manner.
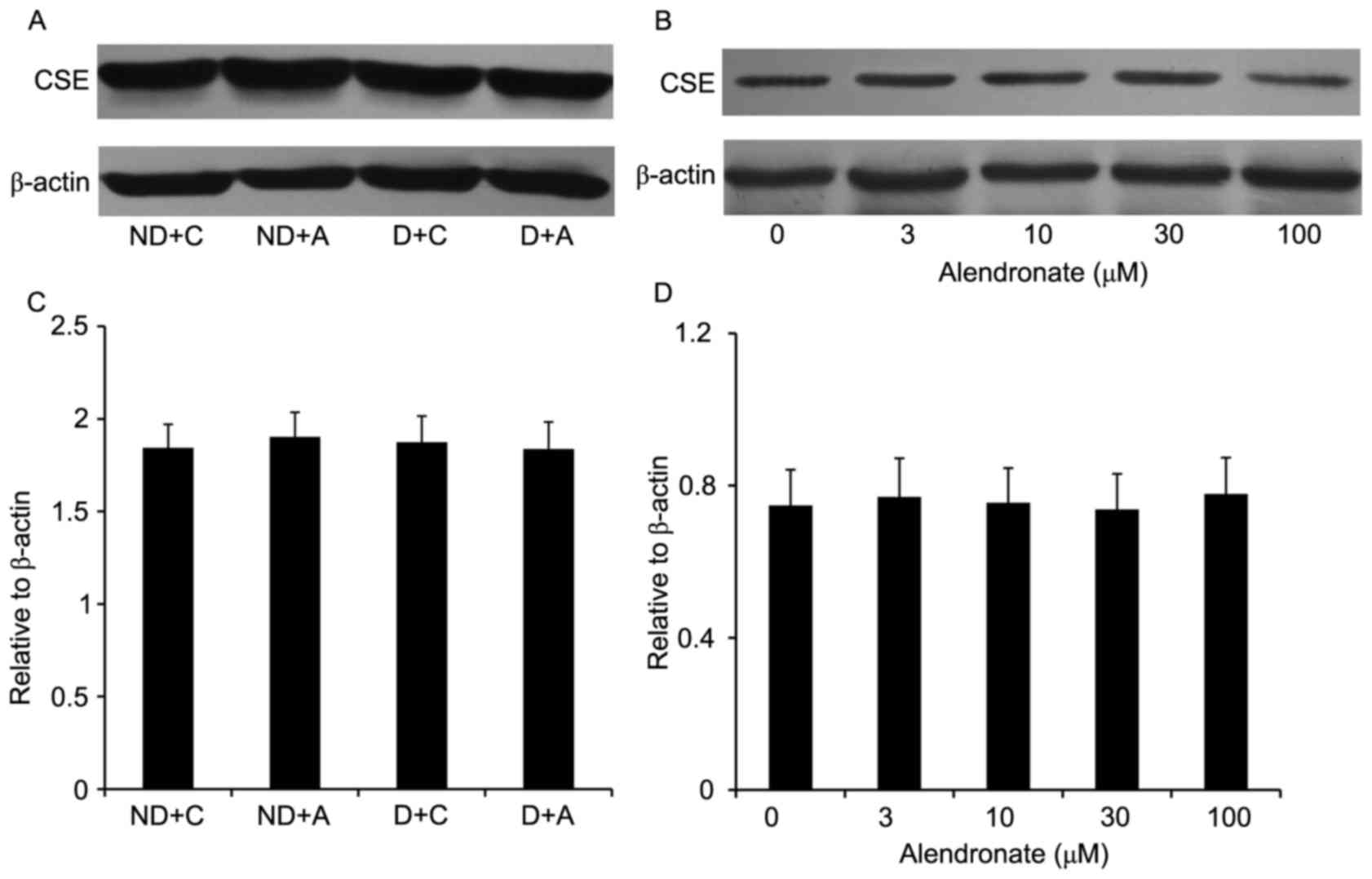
Alendronate had no effect on CSE
expression
16-week treatment with alendronate had no effect on
aortic CSE expression of STZ-induced diabetic mice (Fig. 4). Similarly, in high
glucose-treated VSMCs, alendronate (0–100 µM) didn't change the
expression of CSE expression (Fig.
4).
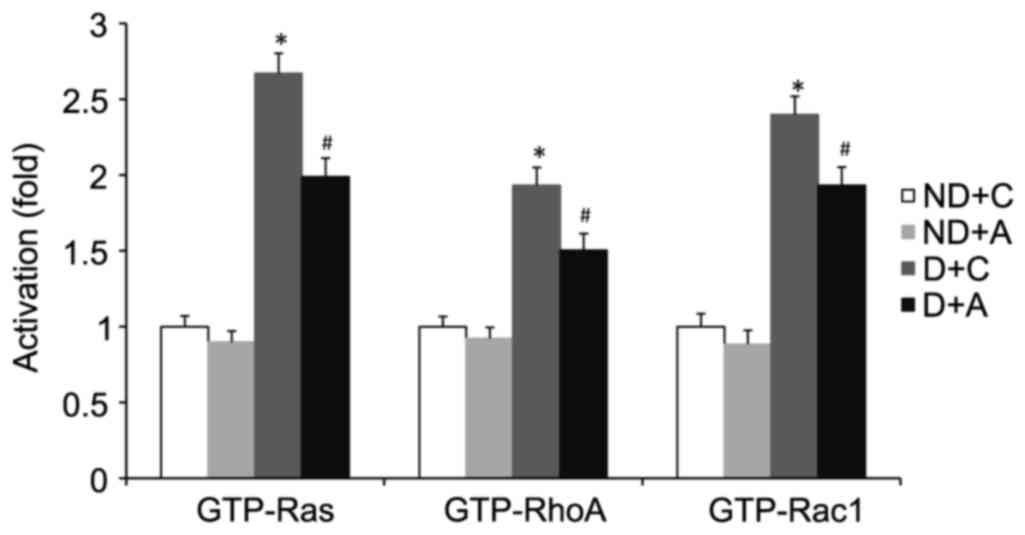
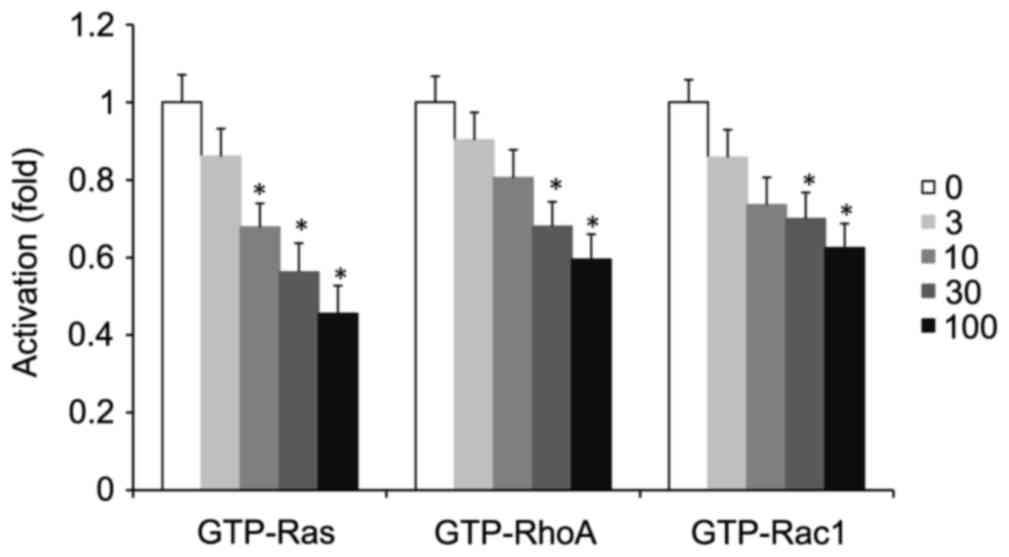
Alendronate suppressed the activation
of small GTPases
Small GTPase (Ras, RhoA, or Rac1) activation depends
on its conversion from the GDP- to the GTP-bound state. The levels
of the GTP-bound active form of small GTPase (Ras, RhoA, or Rac1)
in aorta and cultured VSMCs were determined by G-LISA kits. The
total level of small GTPase (Ras, RhoA, or Rac1) was detected by
western blot using its specific antibody. 16-week treatment with
alendronate had no effect on aortic expression of total Ras, RhoA,
or Rac1 in four groups of mice (data not shown). However, the
GTP-Ras, RhoA, and Rac1 levels were decreased in diabetic
alendronate (D+A) gourp (Fig. 5).
Meanwhile, the same situation occurred in high glucose-treated
VSMCs in vitro. Alendronate (0–100 µM) had no effect on the
expression of total Ras, RhoA, or Rac1 (data not shown). However,
treatment with alendronate decreased GTP-Ras, RhoA, and Rac1 levels
in a dose-dependent manner (Fig.
6).
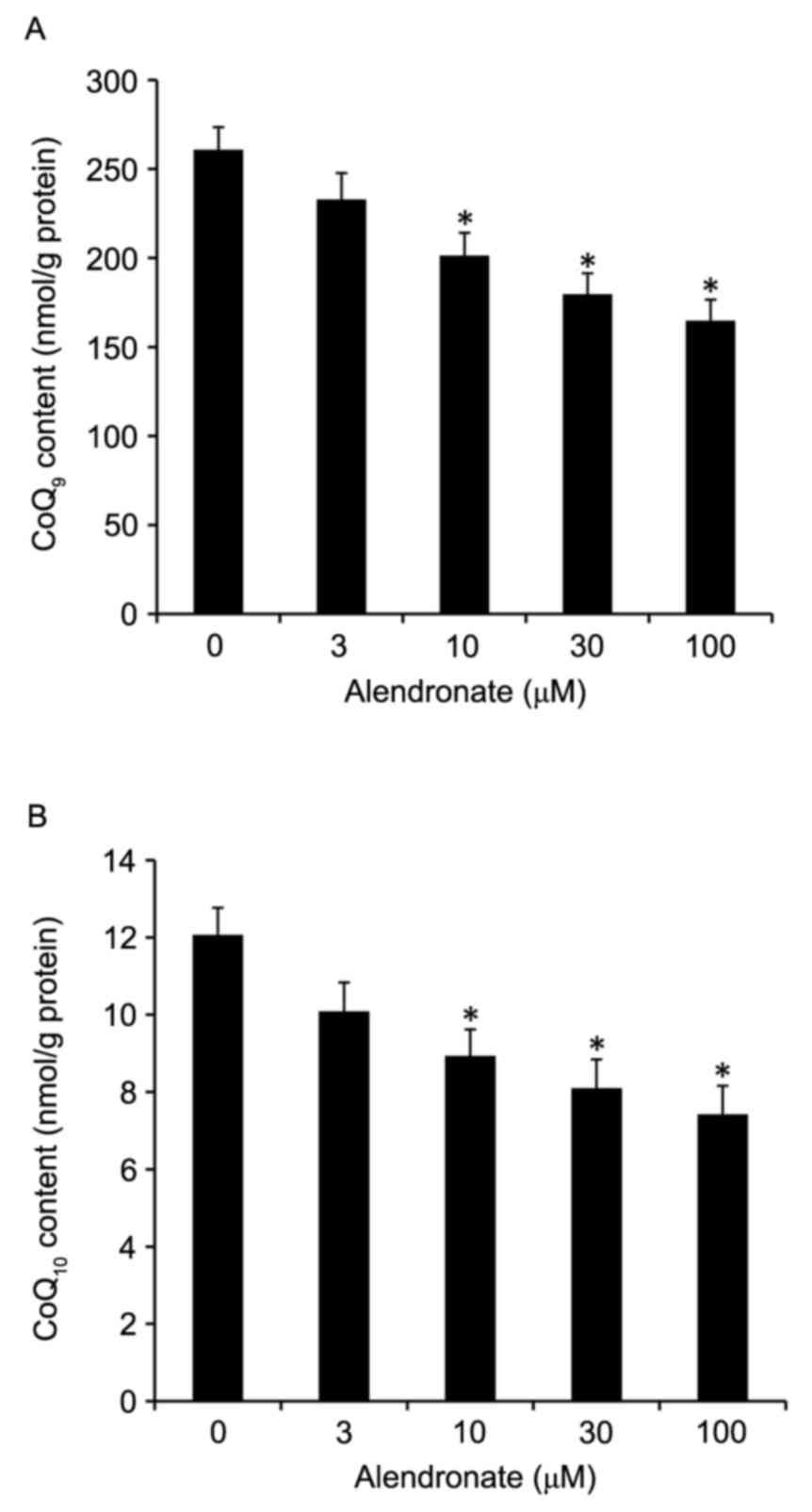
Alendronate decreased the
concentrations of total CoQ9 and CoQ10
In rodents, CoQ exists as two homologues, mainly
CoQ9 with lesser amounts of CoQ10. In our
study, alendronate dose-dependently decreased the total
CoQ9 and CoQ10 concentrations in high
glucose-treated VSMCs (Fig.
7).
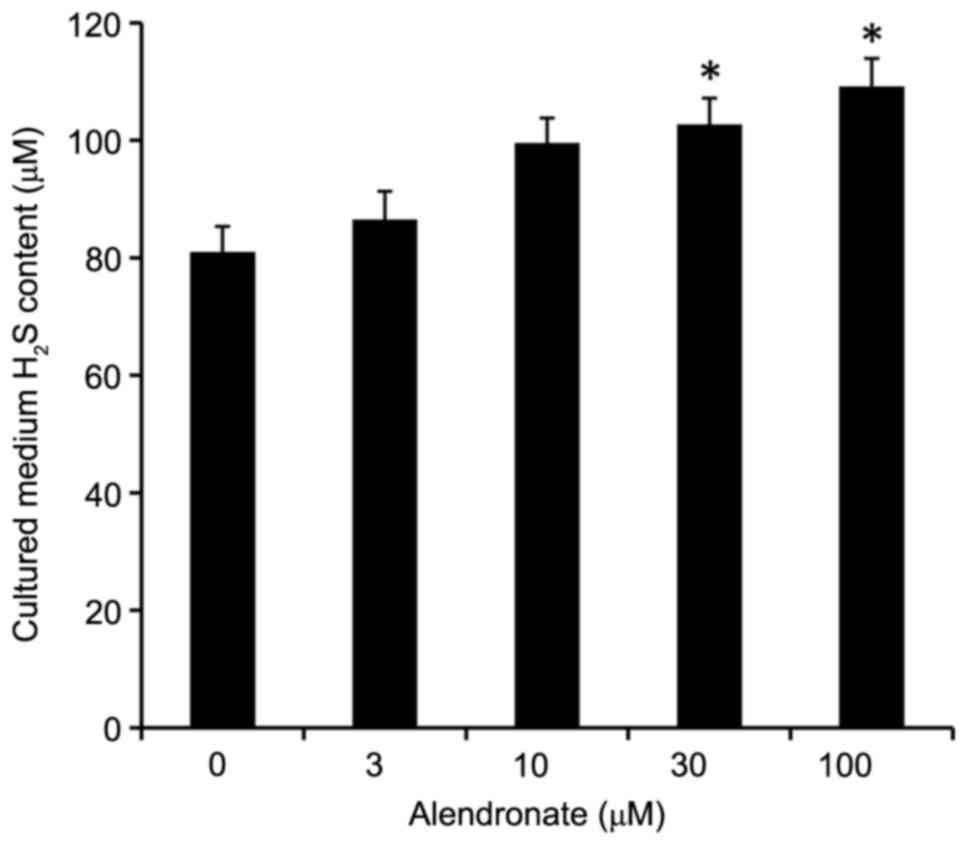
Alendronate increased H2S
levels
Alendronate dose-dependently increased the
H2S levels in cultured medium, in which VSMCs were
treated with high glucose (22.2 mM, Fig. 8).
Discussion
We herein demonstrate that inhibition of FPPS
attenuates high glucose-induced VSMCs proliferation in vivo
and in vitro, and the mechanisms probably involve inhibiting
H2S metabolism and decreasing small GTPases
activation.
It is well established that accelerated
proliferation of VSMCs is the fundamental event in the development
of atherosclerosis in diabetes (4–6). In
our previous study, high glucose (22.2 mM) remarkably induced the
VSMCs proliferation and FPPS upregulation in vitro and in
vivo (11). Then, our present
experiments found that treatment of alendronate attenuated the
diabetic atherosclerosis process in STZ-induced mice, and that
inhibition of FPPS dose-dependently attenuated high glucose-induced
proliferation of VSMCs in vitro. All these findings
suggested the important role of FPPS in VSMCs proliferation during
diabetic accelerated atherosclerosis, but the mechanisms involved
were not clear.
H2S is a member of the endogenous
gasotransmitter family, in addition to nitric oxide and carbon
monoxide, and plays an important role in the cardiovascular and
other systems (18–20). Endogenous H2S is
synthesized from L-cysteine in three different enzymatic reaction
catalyzed by cystathionine β-synthase (CBS), CSE, or the sequential
action of cysteine aminotransferase and 3-mercaptopyruvate
sulfurtransferase (21). Among
them, CSE is the pivotal H2S-producing enzyme in VSMCs,
providing the main source of H2S in cardiovascular
system and suppressing VSMCs proliferation in different ways
(22–24). On the other hand, H2S is
enzymatically scavenged or metabolized in mitochondria with the
participation of CoQ (an important product of FPPS) (25,26).
Thus, endogenous H2S concentration depends on its
dynamic balance between synthesis and metabolism. In our study,
alendronate dose-dependently increased H2S levels, but
decreased total CoQ content in high glucose-treated VSMCs, without
any change of CSE expression. All these data indicated that
inhibition of FPPS decreased the CoQ content, depressed the
H2S metabolism, increased endogenous H2S
concentration, and finally attenuated the high glucose-induced
proliferation of VSMCs.
On the other hand, numerous molecular and cellular
studies have demonstrated that small GTPases, consisting of the
Ras, RhoA and Rac1, participate in the VSMCs proliferation
triggered by hyperglycemia (27–31).
The membrane localization and activation of small GTPase depend on
its conversion from the GDP- to the GTP-bound state via the process
of protein prenylation (32–34).
In our study, G-LISA method could precisely determine the level of
GTP-boud active small GTPase, so as to indicate the level of
protein prenylation. Alendronate, in vivo and in
vitro, inhibited small GTPases (Ras, RhoA, and Rac1)
activation, but had no effect on the expressions of total Ras,
RhoA, and Rac1, demonstrating that FPPS inhibition only affected
the activated process of small GTPases, possibly by inhibition of
their prenylation. Our presumption was that inhibition of FPPS
decreased isoprenoid intermediates content (FPP and GGPP, Fig. 1), depressed small GTPases (Ras,
RhoA, and Rac1) activation, and finally attenuated the high
glucose-induced proliferation of VSMCs.
Furthermore, alendronate, an FPPS inhibitor used in
our study, is one of the bisphosphonates, using widely in
bone-related disorders (35). One
concern is that the half-life of bisphosphonates in the circulation
is short, and they enter rapidly and extensively into bone because
of their high affinity to the calcium and hydroxyapatite crystals
(12). During our in vitro
study, alendronate directly acted on VSMCs and exerted inhibitory
effects, but whether it is still working when the drug is applied
on the whole animals? Fortunately, there are supportive evidences.
Bisphosphonates have also been reported to accumulate markedly in
the aortas of both healthy and atherosclerotic rabbits in
vivo (35) and in human
internal mammary arteries in vitro (36). Thus, this leads to the hypothesis
that bisphosphonates, when used in vivo, may also exert
direct effects on VSMCs, which are the major cellular components of
vascular wall. Indeed, our in vivo study confirmed the
protective effect of FPPS inhibition on diabetic accelerated
atherogenic process.
In conclusion, our study provided the experimental
evidences that FPPS inhibition attenuated the high glucose-induced
proliferation of VSMCs in vivo and in vitro, though
depressing H2S metabolism and suppressing small GTPases
(Ras, RhoA, and Rac1) activation. Although there are many essential
differences between experimental and clinical studies, our finding
represents a potentially promising therapeutic strategy for the
treatment of diabetic macrovascular disease in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81500616), Natural Science
Foundation of Zhejiang Province (no. LQ13H070001 and LQ16H070002),
Medical Science and Technology Project of Zhejiang Province (no.
2013KYA062, 2015KYA076 and 2016KYA012), and Traditional Chinese
Medicine Project of Zhejiang Province (no. 2014ZB006).
References
|
1
|
Nickerson HD and Dutta S: Diabetic
complications: Current challenges and opportunities. J Cardiovasc
Transl Res. 5:375–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmidt AM: Recent highlights of ATVB:
Diabetes mellitus. Arterioscler Thromb Vasc Biol. 34:954–958. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porter KE and Riches K: The vascular
smooth muscle cell: A therapeutic target in type 2 diabetes? Clin
Sci. 125:167–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gray SP and Jandeleit-Dahm K: The
pathobiology of diabetic vascular complications-cardiovascular and
kidney disease. J Mol Med. 92:441–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chait A and Bornfeldt KE: Diabetes and
atherosclerosis: Is there a role for hyperglycemia? J Lipid Res.
50:(Suppl). S335–S339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
7
|
Dhar MK, Koul A and Kaul S: Farnesyl
pyrophosphate synthase: A key enzyme in isoprenoid biosynthetic
pathway and potential molecular target for drug development. N
Biotechnol. 30:114–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun S and McKenna CE: Farnesyl
pyrophosphate synthase modulations: A patent review (2006–2010).
Expert Opin Ther Pat. 21:1433–1451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buhaescu I and Izzedine H: Mevalonate
pathway: A review of clinical and therapeutical implications. Clin
Biochem. 40:575–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen GP, Zhang XQ, Wu T, Li L, Han J and
Du CQ: Alteration of mevalonate pathway in proliferated vascular
smooth muscle from diabetic mice: Possible role in
high-glucose-induced atherogenic process. J diabetes Res.
2015:3792872015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russell RG: Bisphosphonates: The first 40
years. Bone. 49:2–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen GP, Li L, Yang Y, Fu M, Yao L, Wu T,
Zhang XQ and Hu SJ: Chronic inhibition of Farnesyl pyrophosphate
synthase improves endothelial function in spontaneously
hypertensive rats. Biochem Pharmacol. 80:1684–1689. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehrhof FB, Schmidt-Ullrich R, Dietz R and
Scheidereit C: Regulation of vascular smooth muscle cell
proliferation: Role of NF-kappaB revisted. Circ Res. 96:958–964.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen GP, Yao L, Lu X, Li L and Hu SJ:
Tissue-specific effects of atorvastatin on
3-hydroxy-3-methylglutarylcoenzyme A reductase expression and
activity in spontaneously hypertensive rats. Acta Pharmacol Sin.
29:1181–1186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lang JK, Gohil K and Packer L:
Simultaneous determination of tocopherols, ubiquinols, and
ubiquinones in blood, plasma, tissue homogenates, and subcellular
fractions. Anal Biochem. 157:106–116. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC,
Huang SH, Tan CS, Whiteman M, Lu J and Moore PK: Hydrogen sulfide
and its possible roles in myocardial ischemia in experimental rats.
J Appl Physiol (1985). 102:261–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu S, Liu Z and Liu P: Targeting hydrogen
sulfide as a promising therapeutic strategy for atherosclerosis.
Int J Cardiol. 172:313–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vandiver M and Snyder SH: Hydrogen
sulfide: A gasotransmitter of clinical relevance. J Mol Med (Berl).
90:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gadalla MM and Synder SH: Hydrogen sulfide
as a gasotransmitter. J Neurochem. 113:14–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whiteman M, Le Trionnaire S, Chopra M, Fox
B and Whatmore J: Emerging role of hydrogen sulfide in health and
disease: Critical appraisal of biomarkers and pharmacological
tools. Clin Sci. 121:459–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mani S, Untereiner A, Wu L and Wang R:
Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid
Redox Signal. 20:805–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiao W, Chaoshu T, Hongfang J and Junbao
D: Endogenous hydrogen sulfide is involved in the pathogenesis of
atherosclerosis. Biochem Biophys Res Commun. 396:182–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elsey DJ, Fowkes RC and Baxter GF:
Regulation of cardiovascular cell function by hydrogen sulfide
(H(2)S). Cell Biochem Funct. 28:95–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martelli A, Testai L, Marino A, Breschi
MC, Da Settimo F and Calderone V: Hydrogen sulphide:
Biopharmacological roles in the cardiovascular system and
pharmaceutical perspectives. Curr Med Chem. 19:3325–3326. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beltowski J and Jamroz-Wisniewska A:
Modulation of H(2)S metabolism by statins: A new aspect of
cardiovascular pharmacology. Antioxid Redox Signal. 17:81–94. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Dai M and Wang Y: Paeonol inhibits
proliferation of vascular smooth muscle cells stimulated by high
glucose via Ras-Raf-ERK1/2 signaling pathway in coculture model.
Evid Based Complement Alternat Med. 2014:4842692014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Liu J, Li L, Liu L and Wu L: Role
of Ras/PKCzeta/MEK/ERK1/2 signaling pathway in angiotensin
II-induced vascular smooth muscle cell proliferation. Regul Pept.
128:43–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishiko K, Sakoda T, Akagami T, Naka T, Doi
T, Tsujino T, Masuyama T and Ohyanagi M: Hyperglycemia induced cell
growth and gene expression via the serum response element through
RhoA and Rho-kinase in vascular smooth muscle cells. Prep Biochem
Biotechnol. 40:139–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawada N, Li Y and Liao JK: Novel aspects
of the roles of Rac1 GTPase in the cardiovascular system. Curr Opin
Pharmacol. 10:116–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu LH, Wang L, Wang D, Jiang H, Tang QZ,
Yan L, Bian ZY, Wang XA and Li H: Puerarin attenuates high-glucose
and diabetes-induced vascular smooth muscle cell proliferation by
blocking PKCbeta2/Rac1-dependent signaling. Free Radic Biol Med.
48:471–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McTaggart SJ: Isoprenylated proteins. Cell
Mol Life Sci. 63:255–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roskoski R Jr: Protein prenylation: A
pivotal posttranslational process. Biochem Biophys Res Commun.
303:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Casey PJ: Protein lipidation in cell
signaling. Science. 268:221–225. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ylitalo R, Mönkkönen J, Urtti A and
Ylitalo P: Accumulation of bisphosphonates in the aorta and some
other tissues of healthy and atherosclerotic rabbits. J Lab Clin
Med. 127:200–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ylitalo R, Kalliovalkama J, Wu X,
Kankaanranta H, Salenius JP, Sisto T, Lähteenmäki T, Ylitalo P and
Pörsti I: Accumulation of bisphosphonates in human artery and their
effects on human and rat arterial function in vitro. Pharmacol
Toxicol. 83:125–131. 1998. View Article : Google Scholar : PubMed/NCBI
|