Introduction
Colorectal cancer is the third most prevalent cancer
worldwide. Treatment of this disease is challenging due to high
rates of metastasis and recurrence (1,2).
Cell interactions in the tumor microenvironment may promote the
survival, proliferation, malignancy and drug resistance of tumor
cells (3). Accumulating evidence
suggests a need to identify factors associated with this
microenvironment, to develop strategies for the prevention of tumor
progression (4–6).
Seaweeds have been extensively investigated as
sources of natural bioactive compounds. Complex polysaccharides,
including sulfated polysaccharides, from brown, red and green
seaweeds exert a wide range of biological effects (7–9).
Fucoidan, a fucose-rich polysaccharide present in brown seaweed,
has been revealed to exert various biological effects, including
anticancer activities (10).
Fucoidan induces tumor cell injury leading to growth arrest and
tumor suppression via apoptosis (11). Previous studies have demonstrated
that fucoidan is cytotoxic to a number of cancer cell types, and
acts to increase apoptosis, and decrease invasion, metastasis and
angiogenesis (12–15). In particular, apoptosis was induced
in HT-29 colon cancer cells with treatment with a low concentration
(20 µg/ml) fucoidan (16).
A number of studies have been performed using low
molecular weight fucoidan (17,18)
and investigating the synergistic effects of fucoidan with other
components (5), particularly
chemotherapeutic agents. Fucoidan has been revealed to reduce the
toxicity of certain anticancer drugs, and to prolong the survival
of patients with unresectable advanced or recurrent colorectal
cancer (11,19).
Recent studies have indicated the influence of the
microenvironment on tumor progression, including the inflammatory
response (3,20). Cell density is associated with
environmental factors including accumulation of inhibitors,
influence of substrates and exhaustion of essential nutrients or
serum growth factor (21).
Therefore, the aim of the present study was to investigate in
detail the effects of fucoidan treatment on HT-29 colon cancer
cells cultured at a high density.
Materials and methods
Fucoidan
Fucoidan purified from Fucus vesiculosus
(catalog no. F5631) was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Fucodian was dissolved in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
concentrations of 100, 250, 500 or 1,000 µg/ml.
Cell culture
HT-29 human colon adenocarcinoma cells (catalog no.
30038) were purchased from the Korean Cell Line Bank (Seoul,
Korea). The cells were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 100 U/ml penicillin and 100 mg/ml
streptomycin, in an incubator with 5% CO2 at 37°C. HT-29
cells were cultured at 50% growth (4×104 cells/well) at
normal density and 80% growth at high density (1×106
cells/well).
Cell proliferation assay
Cell proliferation was estimated using a Cell Titer
96® Aqueous Non-Radioactive Cell Proliferation assay kit
(catalog no. G5430; Promega Corporation, Madison, WI, USA). Cells
were seeded in 96-well plates at a density of 1×106
cells/well in 100 µl medium and allowed to attach for 24 h.
Attached cells were treated with 100, 250, 500 or 1,000 µg/ml
fucoidan in serum-free medium (SFM) for 24 or 48 h. The cell
proliferation assay solution was added and incubated for 30 min,
and the absorbance of each well was measured at a wavelength of 490
nm using a Benchmark microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Cell cytotoxicity assay
Cell cytotoxicity was estimated using a neutral red
assay (22). Cells were seeded in
96-well plates at 1×106 cells/well in 100 µl medium and
allowed to attach for 48 h. Attached cells were treated with 100,
250, 500 or 1,000 µg/ml fucoidan in SFM for 24 or 48 h.
Subsequently, 10 µg/ml Neutral Red solution and 50 mM sodium
citrate with 50% ethanol (pH 4.2) were added and incubated for 20
min, and the absorbance of each well was measured at a wavelength
of 510 nm using a Benchmark microplate reader (Bio-Rad
Laboratories, Inc.).
Flow cytometric analysis
Cells were harvested and washed once with PBS, fixed
with ice-cold 70% ethanol and stored at 4°C. Prior to analysis, the
cells were washed once again with PBS. The experiments were carried
out using an Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit (BD Biosciences, San Jose, CA, USA). Briefly, cells
were resuspended at 1×106 cells/well in 100 µl Annexin V
binding buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid/NaOH (pH 7.4), 140 mM NaCl and 2.5 mM CaCl]. Annexin V-FITC
and propidium iodide (PI) were subsequently added, according to the
manufacturer's protocol, and cells were incubated on ice for 15 min
in the dark. Cells were acquired using a FACSCalibur flow cytometer
(BD Biosciences).
Cell cycle analysis
Cells were harvested and washed once with PBS, fixed
with ice-cold 70% ethanol and stored at 4°C. Prior to analysis, the
cells were washed once again with PBS, resuspended in 1 ml PI
solution [0.1 mg/ml RNase A, 50 µg/ml PI, 0.1% (w/v) sodium citrate
and 0.1% (v/v) NP-40], and incubated on ice for 30 min in the dark.
Cells were acquired using a flow cytometer (FACSCalibur), and
CellQuest™ analysis program software, version 5.1 (BD Biosciences)
was used to determine the relative DNA content based on the
presence of red fluorescence.
Hoechst 33342 staining
HT-29 cells were cultured for 48 h in SFM containing
fucoidan. Subsequently, cells were washed with PBS and fixed with
10% formaldehyde. Cells were washed once again with PBS, following
which 2 µg/ml Hoechst 33342 solution was added. Cells were
incubated for 30 min at room temperature in the dark, and observed
under a fluorescence microscope.
Western blot analysis
HT-29 cells were cultured with 0, 250, 500 or 1,000
µg/ml fucoidan for 48 h. Subsequently, cells were washed with PBS
and lysed in radioimmunoprecipitation assay lysis buffer (20 mM
Tris, 1 mM EDTA, 150 mM sodium chloride, 1 mM EGTA, 1% Triton
X-100, 2.5 mM sodium pyrophosphate; pH 7.5) containing protease
inhibitors (1 µg/ml leupeptin, 1 mM β-glycerophosphate, 1 mM
phenylmethanesulfonyl fluoride and 1 mM sodium orthovanadate) on
ice for 30 min. The extracts were centrifuged at 9,750 x g for 10
min at 4°C and the supernatant was used for western blot
analysis.
Total protein (40 µg) was electrophoresed on 10–15%
SDS-PAGE gels and transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 1% bovine serum albumin (BSA; GenDepot Inc., Barker,
TX, USA) in TBS (5 mM Tris-HCl, 20 mM sodium chloride; pH 7.4)
containing 0.1% Tween-20 (TBST) and incubated with primary
antibodies diluted 1:1,000 in 1% BSA-TBST with gentle shaking at
4°C overnight. Membranes were washed twice with TBST for 15 min
each time, following which the membranes were incubated with
corresponding horseradish peroxidase-conjugated (HRP) secondary
antibodies (diluted 1:10,000) for 2 h at room temperature.
Following a final wash, protein bands were detected using a
SuperSignal West Dura Extended Duration Substrate kit (Thermo
Fisher Scientific, Inc.) and developed using Kodak X-ray film
(Eastman Kodak Company, Rochester, NY, USA). Densitometry results
were visualized on the Fujifilm Multi Gauge system version 3.0
program (Fujifilm, Tokyo, Japan). The following primary antibodies
were used: anti-FAS (catalog no. se-7886; anti-rabbit),
anti-pro-caspase-8 (catalog no. sc-7890; anti-rabbit),
anti-pro-caspase-9 (catalog no. sc-7885; anti-rabbit),
anti-pro-caspase-7 (catalog no. sc-6138; anti-goat),
anti-pro-caspase-3 (catalog no. sc-7148; anti-rabbit), anti-Bcl-2
(catalog no. sc-492; anti-rabbit), anti-Bcl-xL (catalog no.
sc-7195; anti-rabbit), anti-Bad (catalog no. sc-7869; anti-rabbit),
anti-Bax (catalog no. sc-493; anti-rabbit), anti-cyclin D1 (catalog
no. sc-753; anti-rabbit), anti-phospho-RB (catalog no. sc-21875;
anti-goat), anti-E2F (catalog no. sc-251; anti-mouse) and
anti-β-actin (catalog no. sc-47,778; anti-mouse). These were all
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX USA). The
secondary antibodies used were HRP-conjugated anti-mouse IgG
(catalog no. sc-2031; Santa Cruz Biotechnology, Inc.), anti-rabbit
(catalog no. A-0545; Sigma-Aldrich), and anti-goat (catalog no.
A50-101P; Bethyl Laboratories Inc., Montgomery, TX, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. Significant differences
among multiple mean values were assessed by one-way analysis of
variance followed by the Duncan's multiple range test using PASW
software version 18 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of fucoidan on growth of HT-29
colon cancer cells
Previous studies have demonstrated that low
concentrations (0–20 µg/ml) of fucoidan induce apoptosis (16). The present study aimed to assess
the effect of high concentration (0–1,000 µg/ml) fucoidan in high
density cells (1×106 cells/well). Prior to assessing the
effect of fucoidan on the viability in HT-29 cells cultured at a
high density, the toxicity of fucoidan was evaluated using high
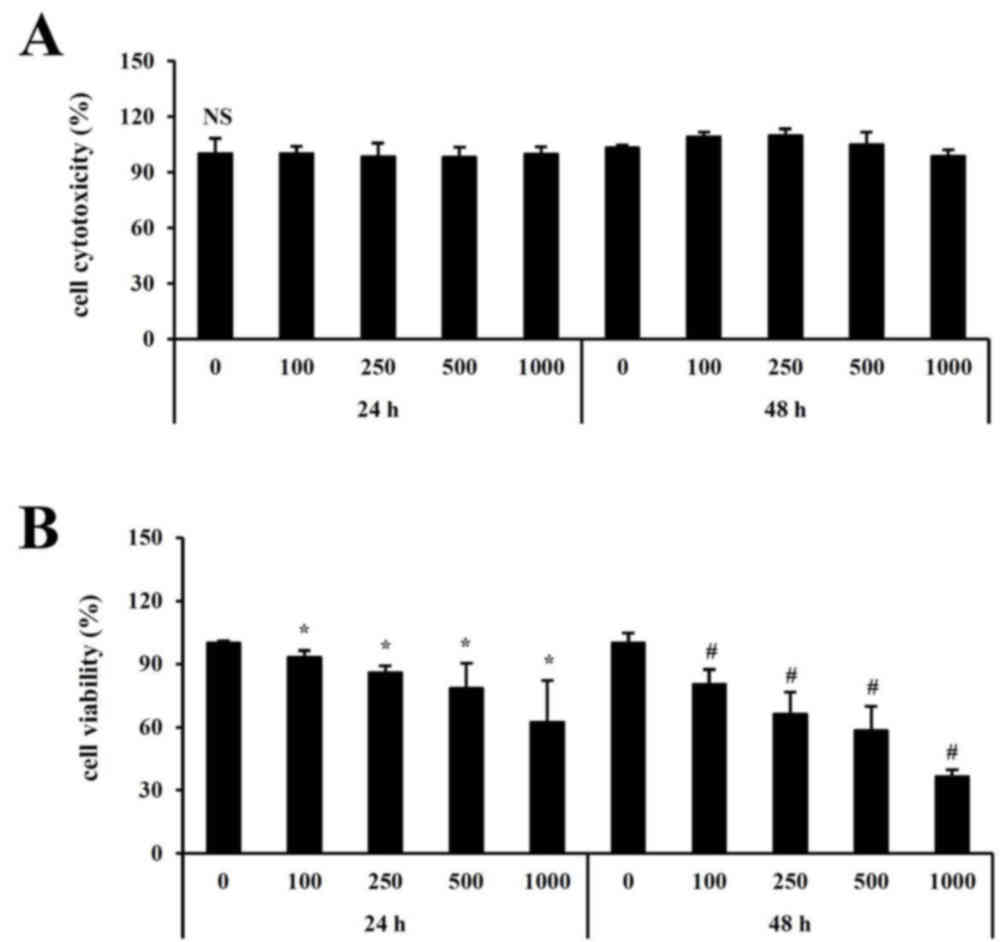
concentrations. As determined by the neutral red assay,
cytotoxicity was not detected at any concentration of fucoidan
assessed (Fig. 1A). To investigate
the effect of fucoidan on cell growth, HT-29 cells were treated
with 0–1,000 µg/ml fucoidan for 24 or 48 h. As presented in
Fig. 1B, cell growth was
significantly inhibited following treatment with 500 µg/ml fucoidan
for 48 h compared with control untreated cells.
Effect of fucoidan on apoptosis of
HT-29 colon cancer cells
Subsequently, it was analyzed whether the growth
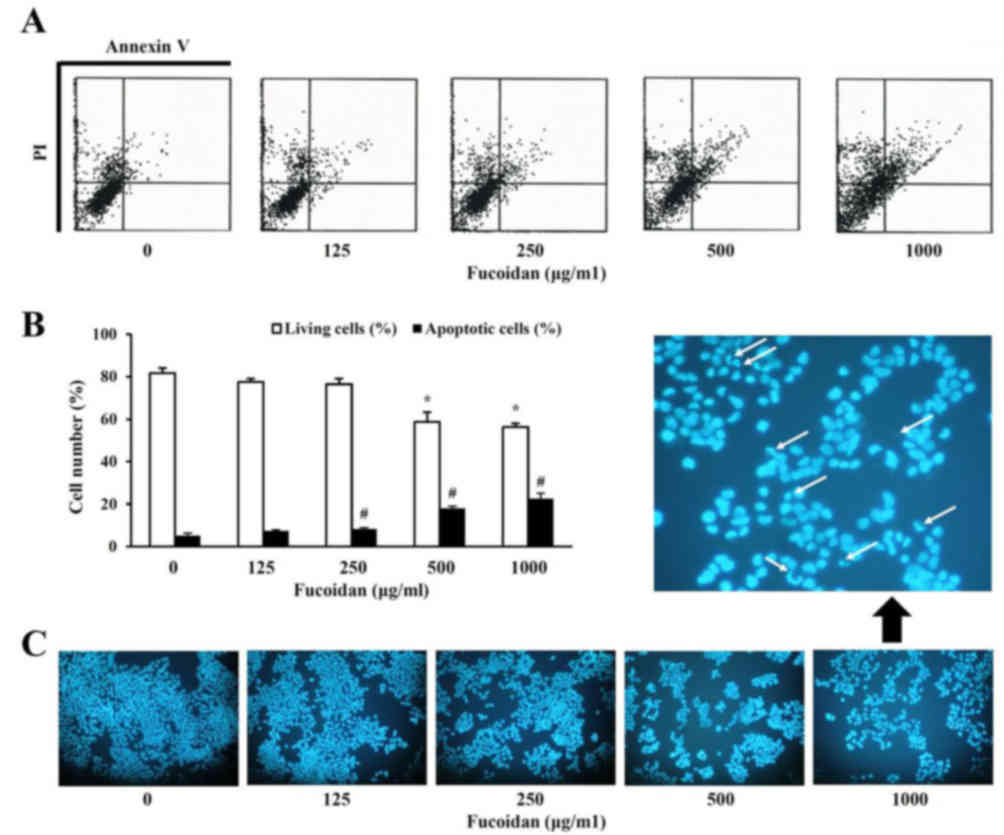
inhibitory effect of fucoidan was due to apoptosis. Apoptosis was
evaluated using two separate assays. Cells double positive for
Annexin V-FITC and PI (late apoptotic or necrotic cells) were
observed 48 h following fucoidan treatment (Fig. 2A) and increased in a dose-dependent
manner (Fig. 2B). In addition, the
effect of fucoidan treatment on the nuclear morphology of HT-29
cells was analyzed to determine whether apoptosis was involved in
fucoidan-induced cell death. Condensed and fragmented nuclei and a
reduced cell volume were evident in 1,000 µg/ml fucoidan-treated
cells, in contrast to untreated cells. These observations indicated
that the decrease in cell viability observed following fucoidan
treatment is associated with the induction of cell death.
Effect of fucoidan on cell
death-associated protein expression levels in HT-29 colon cancer
cells
The effect of fucoidan treatment on the cell death
characteristics of high-density HT-29 cells was assessed. Western
blot analysis revealed that fucoidan had effects on various cell
death-associated proteins, including decreased pro-caspase-9,
pro-caspase-7, pro-caspase-3, Bcl-2, Bcl-xL and increased Fas and
Bax protein expression levels (Fig.
3).
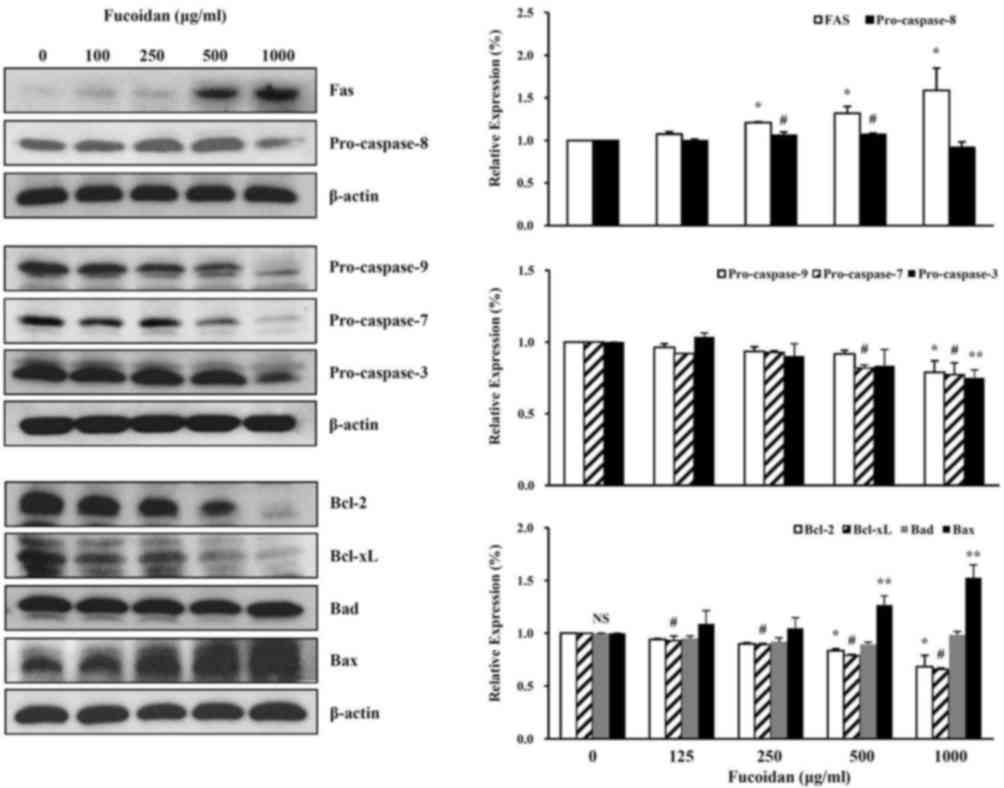 | Figure 3.Effect of fucoidan on cell
death-associated protein expression levels in HT-29 colon cancer
cells. Following fucoidan treatment, western blotting was performed
to detect the cell death-associated proteins Fas, pro-caspase-8,
pro-caspase-9, pro-caspase-7, pro-caspase-3, Bcl-2, Bcl-xL, Bad and
Bax; β-actin served as a loading control. Data was expressed as the
mean ± standard deviation. According to Duncan's multiple range
test, *P<0.05 vs. control group (Fas, pro-caspase-9, Bcl-2);
#P<0.05 vs. control group (pro-caspase-8,
pro-caspase-7, Bcl-xL); **P<0.05 vs. control group
(pro-caspase-3, Bax). NS, not significant. Bcl-2, B-cell lymphoma
2; Bcl-xL, B-cell lymphoma-extralarge; Bad, B-cell lymphoma
2-associated death promoter; Bax, B-cell lymphoma 2-associated X
protein. |
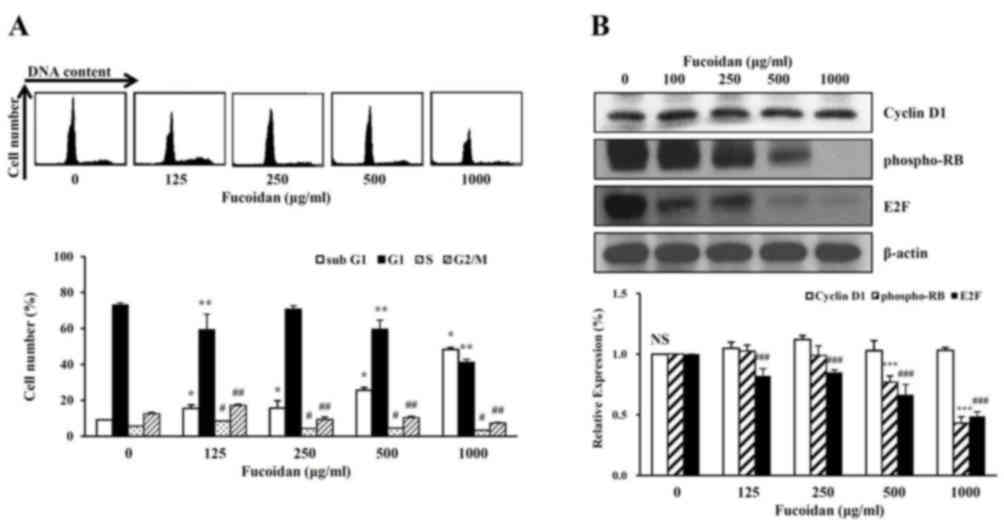
The cell cycle phage distribution of exponentially
growing HT-29 cells was analyzed following fucoidan treatment.
Compared with the untreated control, fucoidan-treated cells
accumulated in the sub-G1 phase of the cell cycle (Fig. 4A). To further investigate the
molecular mechanisms underlying fucoidan-induced sub-G1 arrest, the
HT-29 cells were treated with fucoidan, and western blot analysis
was performed to detect cell cycle progression-associated proteins.
Fucoidan treatment decreased the protein expression levels of
phosphorylated Rb protein and E2F (Fig. 4B). However, fucoidan did not
significantly affect the protein expression levels of cyclin D1.
Therefore, fucoidan treatment may inhibit cell cycle progression in
high-density HT-29 cells, and this may be associated with the
suppression of cancer cell proliferation induced by fucoidan.
Discussion
Cell density is an important factor that affects the
metastatic aggressiveness of cancer cells (4). The generation of cancer cells is due
to accumulated abnormalities in multiple cell regulatory systems
and is reflected in various aspects of cell behavior (4,23).
The metastatic activity of cancer cells may be suppressed by
alterations in the environment, including cell density (4,21).
Cell density may be regulated by the Yes-associated
protein-mediated Hedgehog signaling pathway (24).
There is increased interest in the use of bioactive
natural compounds for the treatment of various diseases, including
cancer. Of various marine resources, algae are particularly
important sources of these compounds.
Fucoidan is a primary sulfated polysaccharide
present in brown seaweed. It has been well characterized and
revealed to have various biological functions (9,10,15).
It has recently been reported that fucoidan may have therapeutic
potential for the treatment of inflammatory diseases (25) and microglia activation-mediated
neurodegenerative diseases (26,27).
Furthermore, fucoidan inhibits cancer cell proliferation and
suppresses cell growth by inducing apoptosis and cell cycle arrest
(12–14). However, this effect is limited in
terms of cancer cell and the molecular mechanisms underlying its
antiproliferative effects in different cellular environments,
including high or low cell densities, remain to be fully
elucidated.
Fucoidan has been widely investigated due to its
anticancer effects and low toxicity. Previous studies have
demonstrated fucoidan to be a potential preventive or therapeutic
agent in cancer (13,20). The antitumor effects of fucoidan
may be associated with the inhibition of tumorigenesis via the
induction of cell death.
Previous studies suggest that tumor progression is
influenced by the microenvironment, including the inflammatory
response (3,20). Cell density has been demonstrated
to be associated with environmental factors including accumulation
of inhibitors, substrates and exhaustion of essential nutrient or
serum growth factor (21).
However, the specific mechanisms underlying the anticancer effects
of fucoidan with regards to the cellular environment remain to be
fully elucidated. The present study aimed to assess the association
between cell density and environmental factors in HT-29 colon
cancer cells. Following this, the effects of fucoidan treatment on
HT-29 colon cancer cells cultured at a high density were
investigated.
Previous studies used cells at a normal density of
4×104 cells/well and low concentrations of 0–20 µg/ml
were used. However, in the present study, a high density of
1×106 cells/well and a low concentration of 0–1,000
µg/ml were used to investigate cell viability and cytotoxicity.
Cytotoxicity was not detected at any concentration
(0–1,000 µg/ml) or time point (24, 48 h) with fucoidan treatment.
Cell viability was significantly inhibited following treatment of
fucoidan compared with untreated cells. In particular, the
viability was reduced to 50% at 500 µg/ml concentration when
treated with fucoidan for 48 h.
Following this, inhibition of cell viability with
treatment of fucoidan was analyzed by flow cytometry using an
Annexin V-FITC apoptosis detection kit. The number of living cells
in the untreated group was 81.6±2.4%, however decreased to
58.7±4.4% with 500 µg/ml fucoidan. In addition, apoptotic cells,
when treated with fucoidan, were increased to 7.43±0.5% at 100
µg/ml and 22.5±2.5% at 1,000 µg/ml compared with untreated cells
(5.17±1.1%).
Typically, morphological alterations, including
destruction of the cell membrane and condensation of the chromosome
and formation of apoptotic bodies, occur in the process of cell
apoptosis. The morphological alterations of nuclei were
investigated in order to provide direct evidence of apoptosis with
treatment of fucoidan. Apoptotic bodies were detected by DNA
fragmentation specific to nuclei. Therefore, inhibition of
viability of HT-29 cells with fucoidan treatment was closely
associated with the induction of apoptosis and DNA synthesis.
The effect of fucoidan treatment on apoptotic
characteristics of high-density HT-29 cells was examined. Western
blot analysis revealed that fucoidan had effects on various
apoptosis-associated and survival proteins.
Receptors including Fas are important in the
physiological regulation of apoptosis. Apoptosis is triggered by
these factors and acts as a defense against diseases that occur in
various tumors or disorders of the immune system. In addition,
apoptosis is an important mechanism that maintains homeostasis
(28,29). The Fas receptor is activated when
the Fas binds to Fas ligand. The death-inducing signaling complex
(DISC) is formed by the binding of the Fas-associated death domain
(FADD) to the death domain of the cytoplasmic tail. Then,
pro-caspase-8 binds to the death effector domain of FADD.
Pro-caspase-8 bound to FADD is activated by autocleavage (30,31).
In present study, the extrinsic pathway was
investigated via the protein expression levels of Fas and
pro-caspase-8 in high density HT-29 cells treated with fucoidan.
Fucoidan treatment increased the expression levels of the Fas
protein in a dose-dependent manner and decreased the expression
levels of pro-caspase-8 protein (Fig.
3). The intrinsic pathway was additionally investigated, with
the protein expression levels of pro-caspase-9, −7 and −3 in high
density of HT-29 cells treated with fucoidan. Treatment with this
reagent decreased the protein expression levels of pro-caspase-9,
−7 and −3 (Fig. 3).
The present study investigated if fucoidan affects
the Bcl-2 family signaling associated with cell survival (Bcl-2 and
Bcl-xL) and cell death (Bad and Bax). Fucoidan treatment increased
the expression level of Bax protein in a concentration-dependent
manner, and decreased the expression levels of Bcl-2, and Bcl-xL
(Fig. 3).
The present study demonstrated that a
high-concentration of fucoidan induced a decrease in cell viability
and morphological changes including nuclear fragmentation.
Therefore, characterization of apoptosis was investigated in the
cell cycle of HT-29 high-density cells treated with fucoidan. The
number of cells in the sub-G1 phase, when treated with fucoidan,
was increased to 15.3±2.0% at 100 µg/ml and 48.3±1.0% at 1,000
µg/ml compared with untreated cells (9.0±0.1%) (Fig. 4A). Conversely, the number of cells
in G1, S, G2/M phase were decreased compared with untreated
cells.
The present study then investigated the expression
levels of proteins that regulate the cell cycle of the G1 phase.
The level of cyclin D1 protein expression was not affected by
treatment with fucoidan (Fig. 4B).
The phosphorylation level of RB proteins that regulate the S phase
transition in G1 were examined. The phosphorylation level of RB was
decreased in a dose-dependent manner with fucoidan treatment. The
E2F protein was inhibited by binding to phosphor-RB in the G1
phase. The expression level of E2F protein decreased in a
dose-dependent manner with fucoidan treatment (Fig 4B). These results suggested that the
decreased DNA synthesis inhibited phosphorylation of the RB protein
by creating a complex between E2F and the RB protein. Therefore,
treatment with a high concentration of fucoidan arrested the G1
phase and inhibited cell growth (cell viability).
The present study was conducted on high-density
cultured HT-29 colon cancer cells to investigate the potential
mechanisms by which fucoidan exerts its effects. The association
between cellular environment and the functional effects of fucoidan
should be considered during future studies and the development of
fucoidan as a potential therapeutic agent for the treatment of
cancer.
Acknowledgements
The present study was supported by Pukyong National
University (grant no. C-D-2015-0953) Busan, Republic of Korea.
References
|
1
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar
|
|
2
|
Jemal A, Center MM, Ward E and Thun MJ:
Cancer occurrence. Methods Mol Biol. 471:3–29. 2009. View Article : Google Scholar
|
|
3
|
Pin AL, Houle F and Huot J: Recent
advances in colorectal cancer research: The microenvironment
impact. Cancer Microenviron. 4:127–131. 2011. View Article : Google Scholar :
|
|
4
|
Kuwano H, Miyazaki T, Tsutsumi S, Hirayama
I, Shimura T, Mochiki E, Nomoto K, Fukuchi M, Kato H and Asao T:
Cell density modulates the metastatic aggressiveness of a mouse
colon cancer cell line, colon 26. Oncology. 67:441–449. 2004.
View Article : Google Scholar
|
|
5
|
Ikeguchi M, Yamamoto M, Arai Y, Maeta Y,
Ashida K, Katano K, Miki Y and Kimura T: Fucoidan reduces the
toxicities of chemotherapy for patients with unresectable advanced
or recurrent colorectal cancer. Oncol Lett. 2:319–322. 2011.
View Article : Google Scholar :
|
|
6
|
Buhrmann C, Kraehe P, Lueders C, Shayan P,
Goel A and Shakibaei M: Curcumin suppresses crosstalk between colon
cancer stem cells and stromal fibroblasts in the tumor
microenvironment: potential role of EMT. PLoS One. 9:e1075142014.
View Article : Google Scholar :
|
|
7
|
Cian RE, Drago SR, de Medina FS and
Martínez-Augustin O: Proteins and carbohydrates from red seaweeds:
evidence for beneficial effects on gut function and microbiota. Mar
Drugs. 13:5358–5383. 2015. View Article : Google Scholar :
|
|
8
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis and the regulation of ErbB signaling by
laminarin in HT-29 human colon cancer cells. Int J Mol Med.
32:291–295. 2013.
|
|
9
|
Min EY, Kim IH, Lee J, Kim EY, Choi YH and
Nam TJ: The effects of fucoidan on senescence are controlled by the
p16INK4a-pRb and P-14Arf-p53 pathways in
hepatocellular carcinoma and hepatic cell lines. Int J Oncol.
45:47–56. 2014.
|
|
10
|
Atashrazm F, Lowenthal RM, Woods GM,
Holloway AF and Dickinson JL: Fucoidan and cancer: a
multifunctional molecule with anti-tumor potential. Mar Drugs.
13:2327–2346. 2015. View Article : Google Scholar :
|
|
11
|
Masahide I, Hiroaki S, Yasunari M and
Takayuki K: Effect of fucoidan dietary supplement on the
chemotherapy treatment of patients with unresectable advanced
gastric cancer. J Cancer Ther. 6:1020–1026. 2015. View Article : Google Scholar
|
|
12
|
Hyun JH, Kim SC, Kang JI, Kim MK, Boo HJ,
Kwon JM, Koh YS, Hyun JW, Park DB, Yoo ES and Kang HK: Apoptosis
inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol
Pharm Bull. 32:1760–1764. 2009. View Article : Google Scholar
|
|
13
|
Boo HJ, Hong JY, Kim SC, Kang JI, Kim MK,
Kim EJ, Hyun JW, Koh YS, Yoo ES, Kwon JM and Kang HK: The
anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar
Drugs. 11:2982–2999. 2013. View Article : Google Scholar :
|
|
14
|
Park HY, Choi IW, Kim GY, Kim BW, Kim WJ
and Choi YH: Fucoidan induces G1 arrest of the cell cycle in EJ
human bladder cancer cells through down-regulation of pRB
phosphorylation. Revista Brasileira de Farmacognosia. 25:246–251.
2015. View Article : Google Scholar
|
|
15
|
Dithmer M, Fuchs S, Shi Y, Schmidt H,
Richert E, Roider J and Klettner A: Fucoidan reduces secretion and
expression of vascular endothelial growth factor in the retinal
pigment epithelium and reduces angiogenesis in vitro. PLoS One.
9:1–10. 2014. View Article : Google Scholar
|
|
16
|
Kim EJ, Park SY, Lee JY and Park JH:
Fucoidan present in brown algae induces apoptosis of human colon
cancer cells. BMC Gastroenterol. 10:962010. View Article : Google Scholar :
|
|
17
|
Xu Y, Zhang Q, Luo D, Wang J and Duan D:
Low molecular weight fucoidan modulates P-selectin and alleviates
diabetic nephropathy. Int J Biol Macromol. 91:233–240. 2016.
View Article : Google Scholar
|
|
18
|
Zhao X, Guo F, Hu J, Zhang L, Xue C, Zhang
Z and Li B: Antithrombotic activity of oral administered low
molecular weight fucoidan from Laminaria Japonica. Thromb Res.
144:46–52. 2016. View Article : Google Scholar
|
|
19
|
Atashrazm F, Lowenthal RM, Dickinson JL,
Holloway AF and Woods GM: Fucoidan enhances the therapeutic
potential of arsenic trioxide and all-trans retinoic acid in acute
promyelocytic leukemia, in vitro and in vivo. Oncotarget.
7:46028–46041. 2016.
|
|
20
|
Gout S and Huot J: Role of cancer
microenvironment in metastasis: focus on colon cancer. Cancer
Microenviron. 1:69–83. 2008. View Article : Google Scholar :
|
|
21
|
Birch JR and Cartwright T: Environmental
factors influencing the growth of animal cells in culture. J Chem
Tech Biotechnol. 32:313–317. 1982. View Article : Google Scholar
|
|
22
|
Wadsworth TL and Koop DR: Effects of the
wine polyphenolics quercetin and resveratrol on pro-inflammatory
cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol.
57:941–949. 1999. View Article : Google Scholar
|
|
23
|
Córdoba-Pedregosa Mdel C, Villalba JM,
González-Aragón D, Bello RI and Alcaín FJ: Cellular density and
cell type are the key factors in growth inhibition induced by
2,5Bis [1-aziridinyl]-1,4 benzoquinone (DZQ). Anticancer Res.
26:3535–3540. 2006.
|
|
24
|
Tariki M, Dhanyamraju PK, Fendrich V,
Borggrefe T, Feldmann G and Lauth M: The Yes-associated protein
controls the cell density regulation of Hedgehog signaling.
Oncogenesis. 3:e1122014. View Article : Google Scholar :
|
|
25
|
Cui YQ, Jia YJ, Zhang T, Zhang QB and Wang
XM: Fucoidan protects against lipopolysaccharide-induced rat
neuronal damage and inhibits the production of proinflammatory
mediators in primary microglia. CNS Neurosci Ther. 18:827–833.
2012. View Article : Google Scholar
|
|
26
|
Park HY, Han MH, Park C, Jin CY, Kim GY,
Choi IW, Kim ND, Nam TJ, Kwon TK and Choi YH: Anti-inflammatory
effects of fucoidan through inhibition of NF-κB, MAPK and Akt
activation in lipopolysaccharide-induced BV2 microglia cells. Food
Chem Toxicol. 49:1745–1752. 2011. View Article : Google Scholar
|
|
27
|
Zhang FL, He Y, Zheng Y, Zhang WJ, Wang Q,
Jia YJ, Song HL, An HT, Zhang HB, Qian YJ, et al: Therapeutic
effects of fucoidan in 6-hydroxydopamin-lesioned rat model of
Parkinson's disease: role of NADPH oxidase-1. CNS Neurosci Ther.
20:1036–1044. 2014. View Article : Google Scholar
|
|
28
|
Siegel RM, Chan FK, Chun HJ and Lenardo
MJ: The multifaceted role of Fas signaling in immune cell
homeostasis and autoimmunity. Nat Immunol. 1:469–474. 2000.
View Article : Google Scholar
|
|
29
|
Wajant H: The Fas signaling pathway: more
than a paradigm. Science. 296:1635–1636. 2002. View Article : Google Scholar
|
|
30
|
Budihardjo I, Oliver H, Letter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar
|
|
31
|
Thornberry N and Lazebnik Y: Caspase:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar
|