Introduction
Osteoarthritis (OA) is a chronic and degenerative
joint disease that occurs frequently in elderly individuals
(1). The disability rate of OA
among the older population ranks only second to cardiovascular
diseases (2). Articular cartilage
damage and osteophyte are the primary pathological features of OA,
which is associated with gender, obesity, trauma, inflammation and
genetic factors; however, ageing is a primary factor (3). External and biological factors lead
to the imbalance of chondrocytes, extracellular matrix and
subchondral bone (4–6). Present studies have primarily focused
on how cartilago articularis maintains a dynamic equilibrium
of cell proliferation and extracellular matrix metabolism.
Silent information regulation of transcription 1
(Sirt1) is the most extensively studied protein of the sirtuin
family. Sirt1 is a type of conservative protein and an
NAD+-dependent histone deacetylase that exists in all
mammalian somatic cells. Sirt1 is involved in diseases, including
neural degenerative disease, diabetes, tumor, inflammation and
senility (7). A previous study
demonstrated that Sirt1 is expressed in human articular cartilage
tissues and cells. However, Sirt1 expression levels are reduced in
the chondrocytes of patients with OA. Upregulating Sirt1 expression
levels promotes the expression levels of cartilage specific genes
and survival of chondrocytes, and inhibits apoptosis of
chondrocytes significantly (8).
Another study revealed that upregulating Sirt1 activity an OA mouse
model reduces the expression levels of inflammatory mediators
including matrix metalloproteinase (MMP) 13 and inducible nitric
oxide synthase (iNOS) in chondrocytes, thus inhibits cartilage
degeneration in mice (9). The
expression levels of Sirt1 have significant inhibitory effects on
the occurrence and development of OA; however, the underlying
mechanisms of action remain unclear. Based on the above
observations, the present study investigated the effects and
underlying mechanisms of action of Sirt1 on apoptosis of
chondrocytes and degradation of the extracellular matrix in
patients with OA.
Materials and methods
Patients
Cartilage tissues were obtained from knee
arthroplasty of 28 patients with OA (age, 56–86 years; mean age, 69
years; males, 12; females, 16) from November 2014 to November 2015.
According to the OA diagnostic criteria developed by the American
Institute of Rheumatoid Arthritis in 2008, the OA patients were
diagnosed by clinical examination and X-ray plain films. Informed
consent was obtained from the patients, and the trial was approved
by the ethics committee of Xinyu City People's Hospital (Xinyu,
China).
Reagents and kits
The following primary antibodies were used: Rabbit
polyclonal anti-Sirt1 (cat. no. bs-2257R; Beijing Boosen Biological
Technology Co., Ltd., Beijing, China); rabbit monoclonal
anti-apoptosis regulator B-cell lymphoma 2 (Bcl-2; cat. no.
1017-1), anti-apoptosis regulator Bcl-2-associated X protein (Bax;
cat. no. 1063-1), anti-extracellular signal regulated kinase 1/2
(ERK1/2; cat. no. 8663-1), anti-phosphorylated (p)-ERK1/2 (cat. no.
1418-1), anti-c-Jun N-terminal kinase (JNK; cat. no. 3496-1),
anti-p-JNK (cat. no. 2155-1), anti-p-p38 mitogen activated protein
kinase (MAPK; cat. no. 5359-1), anti-p38 (cat. no. 2132-1; all
purchased from Epitomics, Burlingame, CA, USA); rabbit polyclonal
anti-MMP1 (cat. no. S1023) and MMP13 (cat. no. 1923-1; Abcam,
Cambridge, UK). Mouse anti-GAPDH (cat. no. AG019) and an Annexin
V-propidium iodide (PI; cat. no. C1063) double staining flow
cytometry detection kit were purchased from Beyotime Institute of
Biotechnology (Haimen, China), resveratrol (Res) was obtained from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany), Sirt1 small
interfering (si)RNA was purchased from Shanghai Pharmaceutical
Group Co., Ltd. (Shanghai, China), and type II collagenase, fetal
bovine serum (FBS) and Dulbecco's modified Eagle's medium/nutrient
mixture F-12 (DMEM/F-12) were obtained from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The XRS ChemiDoc™ gel imaging
system was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA,
USA) and the FACSCalibur™ flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA).
Preparation and grouping of
chondrocytes
Under sterile conditions, the OA cartilage tissue
was washed and cut into 1-mm3 sections using
ophthalmology scissors. The tissues were digested with 0.25%
trypsin for 30 min, following which cells were digested 0.2%
collagenase for 2 h. Once the single cell suspension was obtained,
cells were cultured in DMEM/F-12 supplemented with 10% FBS at 37°C
and 5% CO2. After 4–5 days, cells began to fuse and 2–3
generation cells were used.
Grouping
Chondrocytes at 80% fusion degree were randomly
divided into 3 groups: Control (cultured with DMEM/F-12, without
any external stimulus); Res (10 µM resveratrol treatment) and
Res+siRNA [10 µM resveratrol+siRNA Sirt1, transfected using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)].
All groups were cultured for 4 h.
Expression levels of Sirt1 in OA
chondrocytes by reverse transcription-semiquantitative polymerase
chain reaction (RT-sqPCR) analysis
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The primers used were as follows:
Sirt1 primer: Forward, 5′-TGGACTCCACGACGTACT-3′ and reverse,
5′-TCTCCTGGGAGGCATAGACC-3′ (122 bp) for Sirt1; and forward,
5′-AGCCACATCGCTCAGACA-3′ and reverse, 5′-TCTCCTGGGAGGCATAGACC-3′
(314 bp) for GAPDH. RNA was transcribed into cDNA and amplified by
PCR to obtain 5 µl amplification products using a one-step qPCR kit
(cat. no. DRR064A; Takara Bio, Inc., Otsu, Japan). PCR was
performed at 95°C for 5 min followed by 40 cycles at 95°C for 30
sec, at 55°C for 30 sec, at 72°C for 45 sec, and at 72°C for 10 min
for a final extension. The PCR-amplified products were verified
using a 1.2% agarose gel at 100 V for 20 min and the results were
analyzed by gel imaging and analysis system (WE-9413B; Beijing
Liuyi Instrument Company, Beijing, China).
Cell viability detection by MTT
assay
A total of 20 µl 5 mg/ml MTT (cat. no. KA1606;
Abnova, Taipei, Taiwan) and 150 µl dimethyl sulfoxide was added to
cells for 4 h, following which the optical density (OD) value was
detected at a wavelength of 560 nm using a microplate reader. Cell
viability (%)=(OD value of experimental group/OD value of control
group)x100.
Cell apoptosis analysis by Annexin
V-propidium iodide (PI) double staining flow cytometry
Cells were digested with 0.25% trypsin (no EDTA) and
collected, following which 500 µl Binding Buffer was added. A total
of 5 µl Annexin V-fluorescein isothyanate (FITC) was added to the
cells, following which 5 µl PI was added. Avoidance response was
performed at room temperature for 5–15 min and it was subsequently
detected by flow cytometry after 1 h and analyzed by the software
of CellQuest (BD Biosciences).
Western blotting
Proteins were extracted by centrifugation with
13,400 × g at 4 for 5 min, following which lysis buffer (cat. no.
P0013; Beyotime Institute of Biotechnology) was added to obtain the
total protein. Protein concentration was measured using a
Bicinchoninic Acid assay kit. Equal quantity of protein per lane
(50 µg) was separated by 4% SDS-PAGE gel and subsequently
transferred onto PVDF membranes. Membranes were incubated with
primary antibodies overnight at 4°C. Following washing with PBS,
membranes were incubated with secondary antibodies at room
temperature for 1 to 2 h. Following this, the membrane was removed
and washed and an Enhanced Chemiluminescence reagent (cat. no.
WBKLS0500; Merck Millipore) was added. Densitometry was performed
using Quantity One version 4.62 software (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Every experiment was repeated three times. Independent Student's
t-test was used to determine differences between groups. P<0.05
was considered to indicate a statistically significant difference.
All analyses were performed using SPSS software version 17.0 (SPSS,
Inc., Chicago, IL, USA).
Results
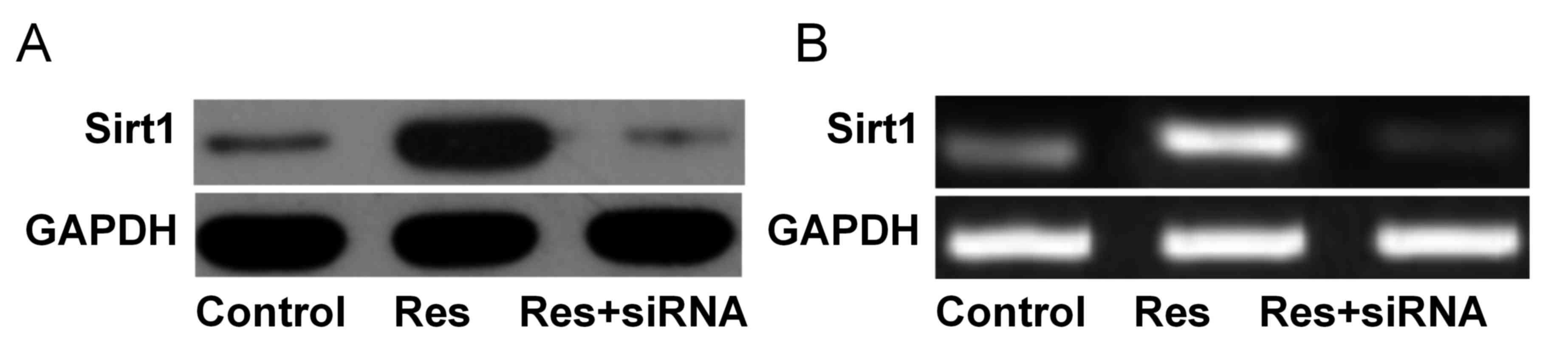
Sirt1 protein and mRNA expression
levels
As presented in Fig.
1, Sirt1 protein expression levels were significantly increased
in the Res group (1.03±0.10) compared with the control (0.22±0.03)
and Res+siRNA groups (0.18±0.01; both P<0.05). Sirt1 mRNA
expression levels were significantly increased in the Res group
(0.98±0.08) compared with the control (0.30±0.03) and Res+siRNA
groups (0.08±0.01; both P<0.05).
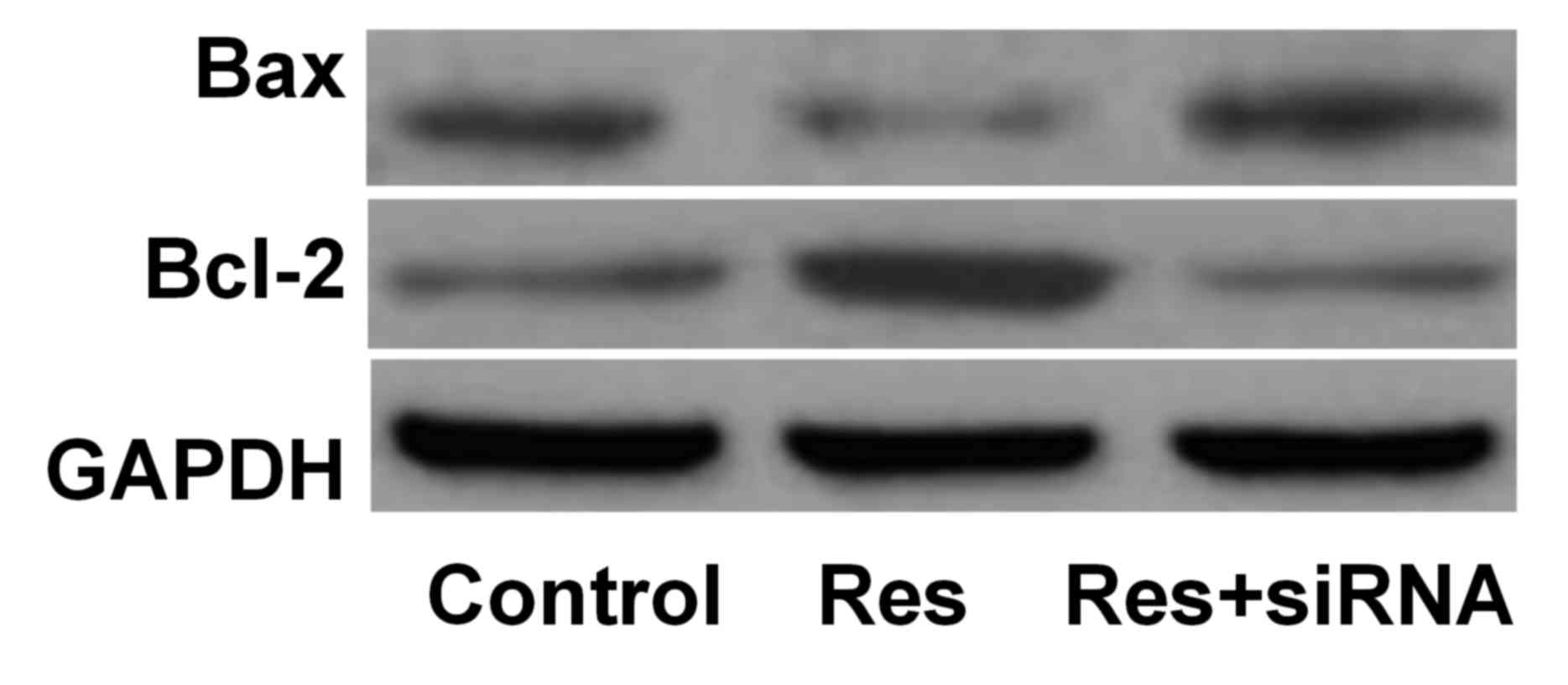
Bax and Bcl-2 protein expression
levels
The protein expression levels of Bax were
downregulated, and Bcl-2 upregulated, in the Res group compared
with the control group. However, Bax and Bcl-2 protein expression
levels increased in the Res+siRNA group compared with the Res group
(Table I; Fig. 2).
 | Table I.Cell viability, apoptosis and protein
expression levels in osteoarthritis chondrocytes. |
Table I.
Cell viability, apoptosis and protein
expression levels in osteoarthritis chondrocytes.
| Parameters | Control | Res | Res+siRNA |
|---|
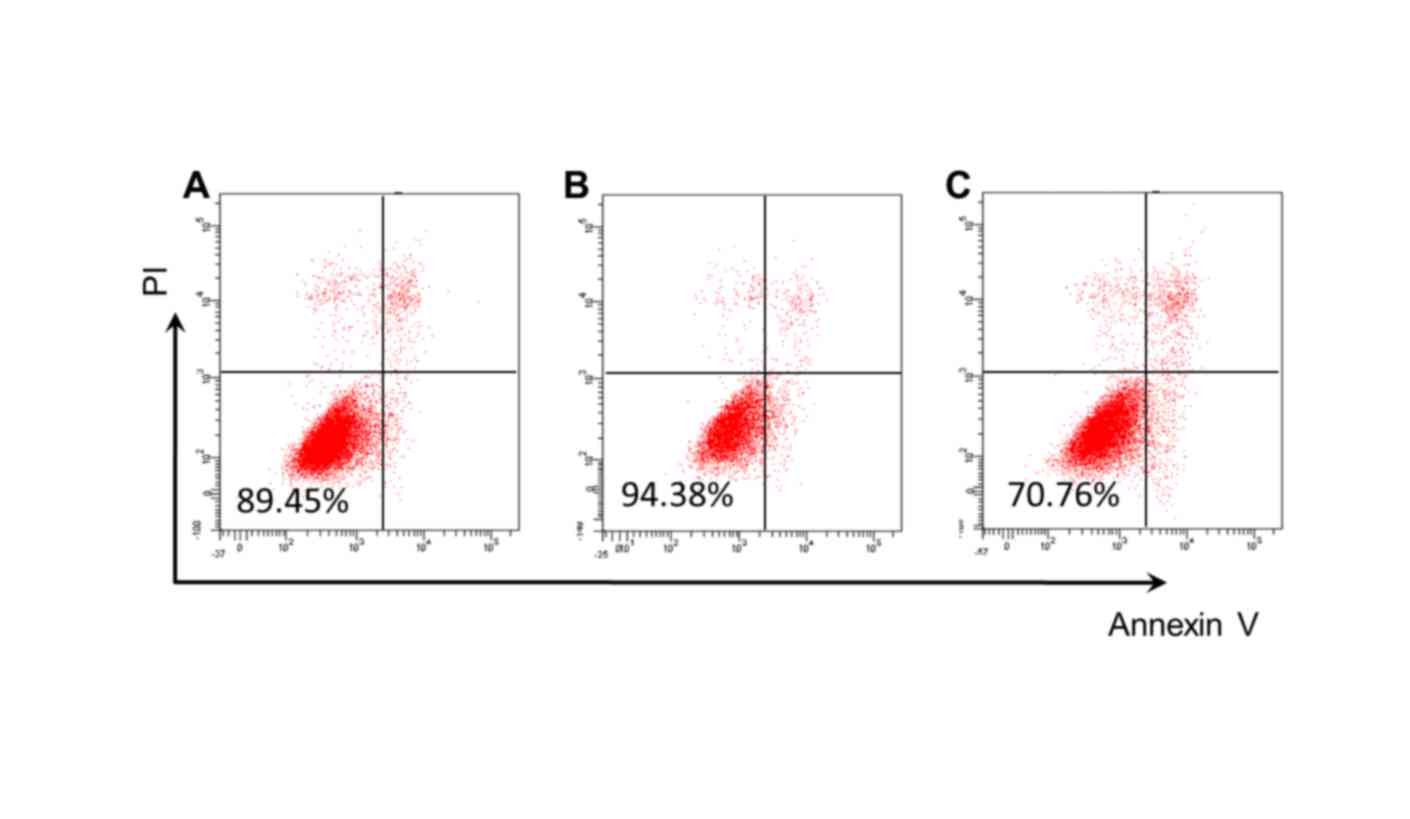
| Cell viability
%a–c | 89.45±8.72 | 94.38±5.06 | 70.76±7.38 |
| Cell apoptosis % |
|
Earlya–c | 2.83±0.22 | 1.70±0.14 | 5.88±0.59 |
|
Latea–c | 4.30±0.41 | 3.10±0.34 | 17.57±1.69 |
|
Bax/GAPDHa,c | 0.45±0.04 | 0.23±0.02 | 0.55±0.04 |
|
Bcl-2/GAPDHa,c | 0.34±0.02 | 1.02±0.10 | 0.22±0.02 |
|
MMP1/GAPDHa,c | 0.48±0.04 | 0.30±0.02 | 0.89±0.08 |
|
MMP13/GAPDHa,c | 0.44±0.04 | 0.29±0.02 | 0.94±0.04 |
|
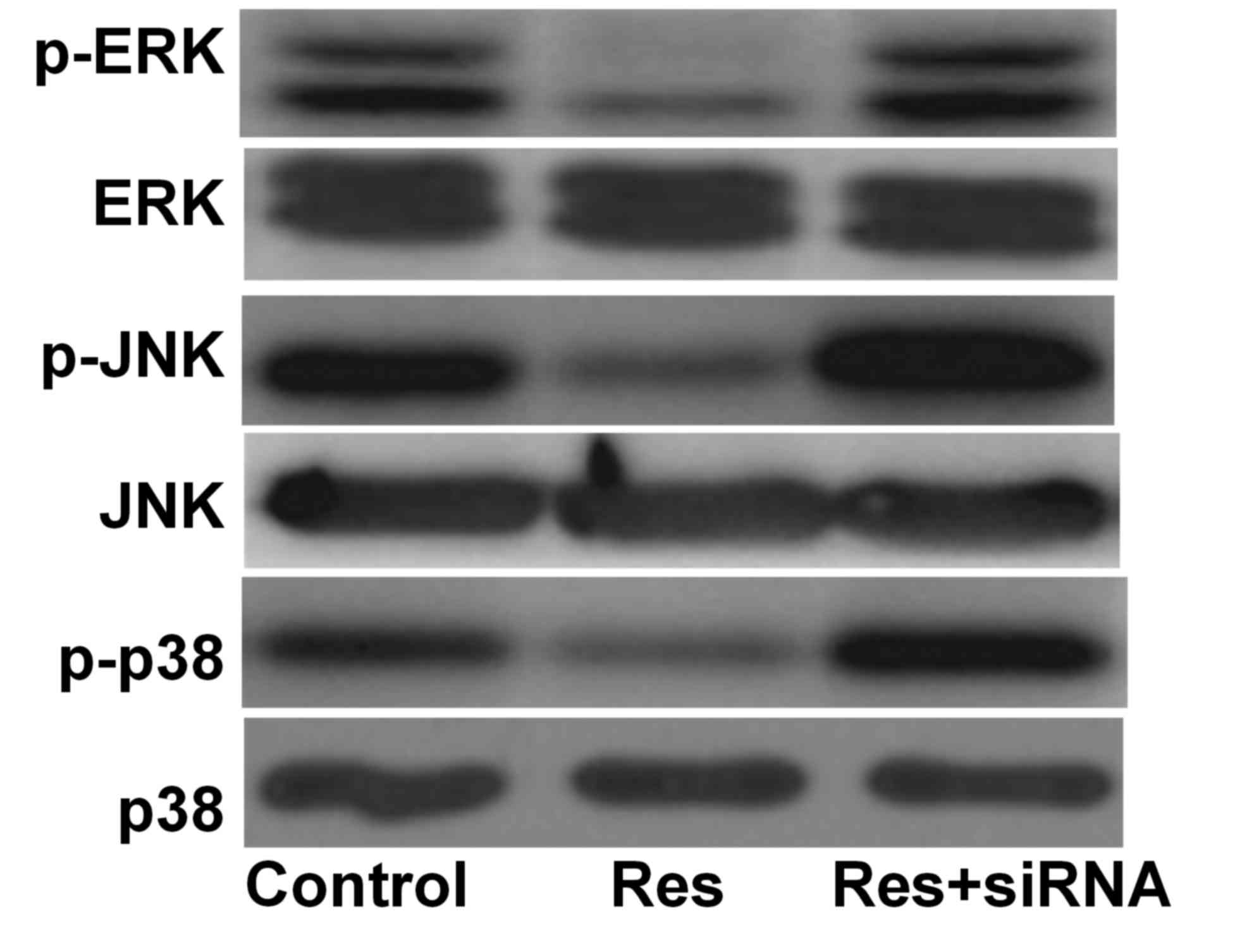
p-ERK/ERKa–c | 0.51±0.04 | 0.14±0.01 | 0.56±0.04 |
|
p-JNK/JNKa–c | 1.03±0.10 | 0.29±0.02 | 1.28±0.13 |
|
p-p38/p38a–c | 0.99±0.08 | 0.28±0.02 | 1.32±0.11 |
MMP1 and MMP13 protein expression
levels
Compared with the control group, the protein
expression levels of MMP1 and MMP13 were downregulated in the Res
group and upregulated in the Res+siRNA group, and were
significantly different between the Res and Res+siRNA groups
(Table I; Fig. 3).
MAPK signal protein expression
Compared with the control group, the phosphorylation
levels of ERK, JNK and p38 were decreased in the Res group and
increased in the Res+siRNA group. They were additionally
significantly different between the Res and Res+siRNA groups
(Table I; Fig. 4).
Detection of cell viability and
apoptosis
Compared with the control group (89.45%; Fig. 5A), cell viability was significantly
increased in the Res group (94.38%; Fig. 5B), and significantly reduced in the
Res+siRNA group (70.76%; Fig. 5C).
Cell apoptosis rates decreased in the Res group, whereas they
increased in the Res+siRNA group (both P<0.05; Table I; Fig.
5).
Discussion
The sirtuin 2 (Sir2) gene family, which exists in
the chromatin of yeast, is widely associated with numerous
physiological and pathological processes. Sirt1, a homologue of
Sir2, is associated with apoptosis, the cell cycle, cell energy
metabolism, lipid accumulation and cell aging (10). Sirt1 has been demonstrated to serve
important roles in extracellular matrix synthesis and cell
survival, and has anti-inflammatory actions in human OA
chondrocytes (11,12). Fujita et al (8) demonstrated that expression levels of
Sirt1 are decreased in OA cartilage cells compared with healthy
articular cartilage cells. Expression levels of transcription
factor Sox9 was significantly promoted in OA chondrocytes
transfected with wild-type Sirt1, and in chondrocytes transfected
with mutant Sirt1, its expression levels reduced. Additionally,
Gagarina et al (13)
reported that Sirt1 may promote OA cartilage-specific gene
expression and slow OA progression. Furthermore, Gabay et al
(14) demonstrated that Sirt1
knockout altered cartilage expression, increased apoptosis and
acceleration cartilage degeneration in mice. Sirt1 is able to block
chondrocyte apoptosis mediated by tumor necrosis factor-α (15). Therefore, chondrocyte apoptosis may
be significantly suppressed by increasing expression levels of
Sirt1, which reduces the degree of cartilage degeneration. The
present study used resveratrol treatment and siRNA interference to
investigate viability and apoptosis of OA cartilage cells. The
results demonstrated that cartilage cell viability was promoted and
apoptosis was reduced significantly on OA chondrocytes in the Res
group compared with the control group. Additionally, cartilage cell
viability was markedly reduced and apoptosis was significantly
increased by siRNA Sirt1 transfection, compared with the control
group. These results illustrated that increased Sirt1 expression
levels serve an inhibitory effect on apoptosis in OA chondrocytes.
Therefore, the present study further investigated the protein
expression levels of Bax and Bcl-2 in each group of chondrocytes.
The results revealed that when Sirt1 expression levels were
increased, Bax expression levels decreased and Bcl-2 expression
levels increased. With the intervention of siRNA Sirt1 and Res
treatment, protein expression levels of Bax increased and Bcl-2
expression levels decreased, consistent with the above results from
cell viability and apoptosis assays. Takayama et al
(16) previously demonstrated
that, by regulating Bax and Bcl-2 levels, Sirt1 resists nitric
oxide-induced chondrocyte apoptosis. This further illustrated that
during OA chondrocyte apoptosis, inhibition of Sirt1 is achieved by
regulating Bax and Bcl-2 expression levels.
Cartilage degeneration is a key pathological feature
of OA, and is mediated by an imbalance of cartilage apoptosis and
extracellular matrix degradation, exacerbating OA progression.
MMPs, a Zn2+ dependent protease superfamily, are the
most important proteolytic enzymes in the extracellular matrix
degrading process. MMPs are present in >25 species, and the most
critical ones in OA are collagenases, including MMP1 and MMP13.
MMP1 and MMP13 may degrade cartilage-specific extracellular matrix
components including collagen type II. MMP1 may degrade
proteoglycans and collagens type I and III. The degradation of
MMP13 was 10 times greater compared with MMP1, which may degrade
the type II collagen triple helix structure, contributing to the
hydrolysis of other proteases. It has previously been reported that
in the process of OA development, the content and activity of MMP1
and MMP13 were increased and enhanced. When their activity or
expression levels were suppressed, collagen synthesis was promoted,
and cartilage degeneration was inhibited (17,18).
Meanwhile, Li et al (9)
demonstrated that resveratrol may inhibit cartilage degeneration in
OA mice by increasing Sirt1 activity and decreasing MMP13 and iNOS
expression levels. Matsuzaki et al (19) demonstrated that Sirt1-conditional
knockout mice were more likely to develop OA compared with 8-week
old wild-type C57BL6/J mice, and exhibited increased expression
levels of collagen X and MMP13. Therefore, based on this, the
present study examined MMP1 and MMP13 expression levels in each
group of cells by western blot analysis. The results revealed that
compared with the control group, upregulation of Sirt1 expression
levels may significantly inhibit MMP1 and MMP13 expression levels
in OA chondrocytes, and with the intervention of siRNA on Sirt1
expression levels, MMP1 and MMP13 expression levels were
significantly downregulated. This indicated that MMP1 and MMP13
expression levels may be significantly inhibited by upregulating
the expression levels of Sirt1 in OA chondrocytes, which may reduce
extracellular matrix degradation and mitigate cartilage
degeneration.
The process of OA development is subject to a
variety of inflammatory cytokines and mechanical stress
stimulation. Stimulation signals are transmitted to various
transcription factors via signal transduction pathways, to regulate
chondrocyte apoptosis and extracellular matrix degradation. MAPK is
the most important signaling pathway in mediating cartilage
degeneration damage (20). The
MAPK signaling pathway regulates cell withered death,
proliferation, hypertrophy, inflammation and other physiological
response in a three-level manner: Inducing MAPKKK phosphorylation,
activating MAPKK and finally phosphorylating MAPK, which enters the
nucleus, mediated by a class of serine/threonine protein kinases
present in eukaryotic cells. There are eight MAPK subfamilies
involved in the pathogenesis of OA: JNK, p38 and ERK. Primarily p38
MAPK mediates inflammatory pathways in OA chondrocytes, inducing
the expression of MMP13 and causing type II collagen degradation
(21,22). Additionally, it has been reported
that the p38 inhibitor may significantly reduce cartilage
degeneration in a rat model of OA, and inhibit the expression
levels of inflammatory factors (23). JNK is involved in the regulation of
MMP3 and MMP13 expression levels by regulating its downstream
target proteins activator protein 1, c-Fos and c-Jun, and is
additionally involved in apoptosis of chondrocytes. Yang et
al (24) demonstrated that the
JNK inhibitor SP600125 significantly inhibits NO-induced
upregulation of MMP13 chondrocytes (21). Yoon et al (25) reported that stimulation of
transforming growth factor-α in chondrocytes leads to increased JNK
activity; JNK is involved in apoptosis and reduces activity of the
apoptosis proteins Bcl-2 and Bcl-2 family apoptosis regulator. The
ERK signaling pathway is primarily associated with chondrocyte
proliferation and hypertrophy; however, research has additionally
reported that ERK inhibitors combined with hyaluronic acid
significantly reduced ERK phosphorylation levels, therefore
reducing the expression levels of MMP13 and delaying hypertrophic
chondrocyte and cartilage degeneration (26). Therefore, by inactivating the MAPK
signaling pathway, apoptosis and degradation of the extracellular
matrix of cartilage cells may be significantly inhibited, relieving
cartilage degeneration. Therefore, the present study examined the
levels of p38, JNK and ERK phosphorylation following Sirt1
overexpression or reduced expression in OA chondrocytes by western
blot. The results indicated Sirt1 overexpression led to reduced
phosphorylation of p38, JNK and ERK in OA chondrocytes, whereas
p38, JNK and ERK phosphorylation levels were increased when OA
chondrocytes were treated with combined Sirt1 siRNA and
resveratrol. Bai et al (27) demonstrated that resveratrol may
inhibit pulmonary vascular endothelial cell apoptosis by
upregulating Sirt1 expression levels and reducing p38 MAPK activity
in burn-induced mice, whereas Sirt1 siRNA promotes apoptosis caused
by burning and increases p38 MAPK activity (27). Becatti et al (28) demonstrated that myocardial
apoptosis injury caused by ischemia-reperfusion, oxidative stress
injury and mitochondrial dysfunction may be inhibited by Sirt1
overexpression, including reduction of p38 and JNK phosphorylation
levels, thus increasing ERK phosphorylation. The results of the
present study illustrated that increased Sirt1 expression levels in
OA chondrocytes may significantly reduce p38, JNK and ERK
phosphorylation levels, thus inhibiting chondrocyte apoptosis and
extracellular matrix degradation. The level of ERK phosphorylation
is inconsistent with the results of Becatti et al (28), potentially due to the fact that at
different time points, Sirt1 promotes cell survival by upregulating
ERK phosphorylation levels and additionally inhibits the secretion
of MMPs into extracellular matrix degradation by decreasing the
level of inhibition of ERK phosphorylation. Therefore, further
examination of phosphorylation levels across time points is
required.
In conclusion, upregulation of Sirt1 expression
levels may inhibit OA chondrocyte apoptosis and extracellular
matrix degradation by increasing of Bcl-2 expression levels and
decreasing Bax, MMP1 and MMP13 expression levels. This may be
achieved by downregulating phosphorylation levels of p38, JNK and
ERK.
References
|
1
|
Musumeci G, Leonardi R, Carnazza ML,
Cardile V, Pichler K, Weinberg AM and Loreto C: Aquaporin 1 (AQP1)
expression in experimentally induced osteoarthritic knee menisci:
An in vivo and in vitro study. Tissue Cell. 45:145–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felson DT and Zhang Y: An update on the
epidemiology of knee and hip osteoarthritis with a view to
prevention. Arthritis Rheum. 41:1343–1355. 1988. View Article : Google Scholar
|
|
3
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musumeci G, Szychlinska MA and Mobasheri
A: Age-related degeneration of articular cartilage in the
pathogenesis of osteoarthritis: Molecular markers of senescent
chondrocytes. Histol Histopathol. 30:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Xu X, Xu T and Qin S:
β-Ecdysterone suppresses interleukin-1β-induced apoptosis and
inflammation in rat chondrocytes via inhibition of NF-κB signaling
pathway. Drug Dev Res. 75:195–201. 2014.PubMed/NCBI
|
|
6
|
Zhang XH, Xu XX and Xu T: Ginsenoside Ro
suppresses interleukin-1beta-induced apoptosis and inflammation in
rat chondrocytes by inhibiting NF-κB. Chin J Nat Med. 13:283–289.
2015.PubMed/NCBI
|
|
7
|
Yu XY, Zhang YL, Cao YJ and Liu CF:
Histone deacetylase SIRT1 and cell autophagy. Chinese J
Pathophysiol. 29:1520–1524. 2013.
|
|
8
|
Fujita N, Matsushita T, Ishida K, Kubo S,
Matsumoto T, Takayama K, Kurosaka M and Kuroda R: Potential
involvement of SIRT1 in the pathogenesis of osteoarthritis through
the modulation of chondrocyte gene expressions. J Orthop Res.
29:511–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Cai L, Zhang Y, Cui L and Shen G:
Intra-articular resveratrol injection prevents osteoarthritis
progression in a mouse model by activating SIRT1 and thereby
silencing HIF-2α. J Orthop Res. 33:1061–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo JS, Moon MH, Jeong JK, Seol JW, Lee
YJ, Park BH and Park SY: SIRT1, a histone deacetylase, regulates
prion protein-induced neuronal cell death. Neurobiol Aging.
33:1110–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dvir-Ginzberg M and Steinmeyer J: Towards
elucidating the role of SirT1 in osteoarthritis. Front Biosci
(Landmark Ed). 18:343–355. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG,
Chun YH, Choi BJ and Kim EC: Cytoprotective and anti-inflammatory
effects of melatonin in hydrogen peroxide-stimulated CHON-001 human
chondrocyte cell line and rabbit model of osteoarthritis via the
SIRT1 pathway. J Pineal Res. 53:225–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gagarina V, Gabay O, Dvir-Ginzberg M, Lee
EJ, Brady JK, Quon MJ and Hall DJ: SirT1 enhances survival of human
osteoarthritic chondrocytes by repressing protein tyrosine
phosphatase 1B and activating the insulin-like growth factor
receptor pathway. Arthritis Rheum. 62:1383–1392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabay O, Zaal KJ, Sanchez C, Dvir-Ginzberg
M, Gagarina V, Song Y, He XH and McBurney MW: Sirt1-deficient mice
exhibit an altered cartilage phenotype. Joint Bone Spine.
80:613–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oppenheimer H, Kumar A, Meir H, Schwartz
I, Zini A, Haze A, Kandel L, Mattan Y, Liebergall M and
Dvir-Ginzberg M: Set7/9 impacts COL2A1 expression through binding
and repression of SirT1 histone deacetylation. J Bone Miner Res.
29:348–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takayama K, Ishida K, Matsushita T, Fujita
N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, et
al: SIRT1 regulation of apoptosis of human chondrocytes. Arthritis
Rheum. 60:2731–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim NH, Meinjohanns E, Meldal M,
Bou-Gharios G and Nagase H: In vivo imaging of MMP-13 activity in
the murine destabilised medial meniscus surgical model of
osteoarthritis. Osteoarthritis Cartilage. 22:862–868. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Du J and Zheng Q: Expression of
MMP-1 in cartilage and synovium of experimentally induced rabbit
ACLT traumatic osteoarthritis: Immunohistochemical study. Rheumatol
Int. 29:31–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzaki T, Matsushita T, Takayama K,
Matsumoto T, Nishida K, Kuroda R and Kurosaka M: Disruption of
Sirt1 in chondrocytes causes accelerated progression of
osteoarthritis under mechanical stress and during ageing in mice.
Ann Rheum Dis. 73:1397–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chowdhury TT, Salter DM, Bader DL and Lee
DA: Signal transduction pathways involving p38 MAPK, JNK, NFkappaB
and AP-1 influences the response of chondrocytes cultured in
agarose constructs to IL-1beta and dynamic compression. Inflamm
Res. 57:306–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim H and Kim HP: Matrix
metalloproteinase-13 expression in IL-1β-treated chondrocytes by
activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch
Pharm Res. 34:109–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei L, Sun XJ, Wang Z and Chen Q:
CD95-induced osteoarthritic chondrocyte apoptosis and necrosis:
Dependency on p38 mitogen-activated protein kinase. Arthritis Res
Ther. 8:R372006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brown KK, Heitmeyer SA, Hookfin EB, Hsieh
L, Buchalova M, Taiwo YO and Janusz MJ: P38 MAP kinase inhibitors
as potential therapeutics for the treatment of joint degeneration
and pain associated with osteoarthritis. J Inflamm (Lond).
5:222008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Guo A and Gu JC: c-Jun N-terminal
kinase and nuclear factor κB mediate nitric oxide-induced
expression of matrix metalloproteinase-13. Int Orthop.
35:1261–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon HS and Kim HA: Prologation of c-Jun
N-terminal kinase is associated with cell death induced by tumor
necrosis factor alpha in human chondrocytes. J Korean Med Sci.
19:567–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prasadam I, Mao X, Shi W, Crawford R and
Xiao Y: Combination of MEK-ERK inhibitor and hyaluronic acid has a
synergistic effect on anti-hypertrophic and pro-chondrogenic
activities in osteoarthritis treatment. J Mol Med (Berl).
91:369–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai X, Fan L, He T, Jia W, Yang L, Zhang
J, Liu Y, Shi J, Su L and Hu D: SIRT1 protects rat lung tissue
against severe burn-induced remote ALI by attenuating the apoptosis
of PMVECs via p38 MAPK signaling. Sci Rep. 5:102772015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Becatti M, Taddei N, Cecchi C, Nassi N,
Nassi PA and Fiorillo C: SIRT1 modulates MAPK pathways in
ischemic-reperfused cardiomyocytes. Cell Mol Life Sci.
69:2245–2260. 2012. View Article : Google Scholar : PubMed/NCBI
|