Introduction
As an important regulator of cell cycle, cyclin D1
is frequently associated with tumor occurrence and development
(1,2). The gene encoding cyclin D1 is an
oncogene that represents the second most frequently amplified locus
in a diverse range of human cancers (3). Overexpression of the cyclin D1
protein has been described in a variety of human cancers (1,4–6).
Previous studies have demonstrated that the expression of cyclin D1
is an important indicator of therapeutic efficacy for a number of
therapeutic agents, including all-trans retinoic acid, which is
closely associated with prognosis (7). Due to its frequent deregulation and
role in cancer development, cyclin D1 has become a potential target
for anti-tumor therapy.
Antibodies have become powerful therapeutic and
diagnostic tools, as they bind target antigens with a high degree
of specificity and affinity. An antibody in a single-chain fragment
variable (scFv) format is constructed by combining the heavy and
light chain variable region via a flexible linker peptide. It has a
variety of applications in biotechnology and clinical medicine,
particularly in the field of oncology, due to its specific binding
affinity to target antigens, its small size and ease of engineering
(8–12). In a previous study, a novel human
scFv antibody (AD5) was prepared, which demonstrated specific
binding affinity to cyclin D1 (13). Intracellular AD5 was observed to
significantly inhibit tumor cell growth and proliferation, which
provided a novel potential tool for targeting cyclin D1 for the
treatment of cancer (14,15). However, the mechanism of action and
biological characteristics of AD5 remain unclear.
Metal ions in the blood and aqueous solution may
affect protein interactions, as metal ions serve a role in protein
folding, assembly, stability, conformation and activity (16,17).
Trisler et al (18)
designed an antibody that forms an irreversible complex with a
protein antigen in a metal-dependent reaction. Such irreversibly
binding antibodies may facilitate the development of next
generation reactive antibody therapeutics and diagnostics. Iverson
et al (19) constructed a
catalytic metalloantibody (QM212) with a coordinate site for metals
in the antigen-binding pocket. They utilized fluorescence
spectroscopy to clarify the binding affinity between the antibody
and different metal ions. Copper II (Cu2+) and iron III
(Fe3+) are important trace elements in the human body,
and affect the structure and function of a variety of proteins
(20). Therefore, to improve the
use of these antibodies in a clinical setting, it is imperative to
investigate the effects of metal ions on the structure and activity
of antibodies.
In the present study, the structure and activity of
AD5 in the presence of Cu2+ or Fe3+ was
investigated by fluorescence spectroscopy and synchronous
fluorescence. The quenching constants were obtained at various
temperatures. The number of binding sites for the metal ions was
determined, as were the binding constants and the effect of
different conditions. In addition, the effects of Cu2+
and Fe3+ on the biological activity of AD5 were
investigated using enzyme-linked immunosorbent assay analysis
(ELISA). The results verified the biochemical and biophysical
characteristics of AD5, and supported the use of an anti-cyclin D1
single chain antibody in future clinical applications.
Materials and methods
Materials
AD5 and cyclin D1 were purified as previously
described (13,21). A spectroscopic sample of AD5 was
prepared in phosphate-buffered solution (PBS) at pH 7.4. The
concentration of purified AD5 and cyclin D1 was determined using a
Bradford assay (Sangon Biotech Co., Ltd., Shanghai, China),
according to the manufacturer's protocol. The anti-V5-tag antibody
(cat. no. M1008-2) was purchased from Hangzhou HuaAn Biotechnology
Co., Ltd. (Hangzhou, China). Horseradish peroxidase
(HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) (cat. no.
SA00001-1) was purchased from the ProteinTech Group, Inc. (Chicago,
IL, USA). Bovine serum albumin (BSA) and o-phenylenediamine (OPD)
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
CuCl2 and FeCl3 were purchased from Beijing
Chemical Works (Beijing, China). All other chemicals were of
analytical grade.
Apparatus
Fluorescence and synchronous fluorescence
measurements were performed on an RF-5301PC Spectrofluorophotometer
(Shimadzu Corporation, Kyoto, Japan) equipped with 1.0 cm quartz
cells. Data were analyzed using Origin software (version 8.0;
OriginLab Corporation, Northampton, MA, USA).
Measurement of fluorescence
spectra
The concentration of AD5 was maintained at a
constant level (2×10-6 M), whereas the concentration of the metal
ions in solution (CuCl2 and FeCl3) was varied
(0.00, 0.33, 0.67, 1.00, 1.33 and 1.67×10-3 M) by adding 0, 2, 4,
6, 8 and 10 µl CuCl2 or FeCl3 (0.1 mM) to 600
µl PBS. In each fluorescence spectrum test, a fixed concentration
of AD5 and a series of solutions were mixed in a 1 ml quartz cell,
and incubated for 10 min at 293, 298 and 303 K. Fluorescence
quenching spectra were recorded with emission wavelengths that
ranged from 290 to 500 nm, and an excitation wavelength of 280 nm.
The excitation and emission slits were set at 5 nm. The absorbance
of the system was not high enough to consider inner filter effects,
which are caused by the absorption of excitation and emission
radiation. Therefore, inner filter effect calculations were not
included in the fluorescence studies.
Measurement of synchronous
fluorescence
Synchronous fluorescence spectra of AD5 were
obtained by simultaneously scanning the excitation and emission
spectra. The wavelength intervals (∆λ) between the emission and
excitation wavelengths were individually fixed at 15 and 60 nm, at
which the spectrum only demonstrated the spectroscopic behavior of
Tyr and Trp residues in AD5, respectively. The concentration of the
metal ions and AD5 were the same as the steady-state fluorescence
measurement.
Formulas
The Stern-Volmer quenching constant (Ksv)
and the bimolecular quenching rate constant (Kq) were
calculated according to the following Stern-Volmer equation:
F0/F=1+Ksv [Q]=1+Kqτ0
[Q] (22), where [Q] is the
quencher concentration, Ksv is the Stern-Volmer
quenching constant, Kq is the bimolecular quenching rate
constant and τ0 is the lifetime of the fluorophore in the absence
of quencher, which is of the order of 10–8 s. For the static
quenching, the binding constant and number of binding sites were
calculated according to the following equation (23):
log[(F0-F)/F]=logKb+nlog [Q], where n is the
number of binding sites for one AD5 molecule, which can be
respectively obtained from the ordinate and slope of the double
logarithmic regression curve of log [(F0- F)/F] vs. log
[Q] based on the equation.
ELISA analysis
The binding activity of AD5 to cyclin D1 was
evaluated using an ELISA, according to a previous study (13). A total of 100 µl purified human
recombinant cyclin D1 (10 µg/ml) was coated onto the surface of
wells in a 96-well microtiter plate (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) overnight at 4°C. Non-specific binding
sites were blocked with 0.05% Tween-20 in PBS (PBST) containing 5%
non-fat milk for 1 h at room temperature. The wells were incubated
with 100 µl AD5 treated with Cu2+ or Fe3+ (at
the same concentration as the above fluorescence spectra analysis)
for 2 h at 37°C. Following 3 washes with PBST, anti-V5-tag antibody
(1:5,000 dilution in PBST containing 3% non-fat milk) were added to
each well and incubated for 2 h at 37°C. The plate was washed with
PBST followed by incubation with HRP-conjugated goat anti-mouse IgG
(1:5,000 dilution in PBST containing 3% non-fat milk) for 1 h at
37°C. The reaction was developed by adding 200 µl OPD substrate (1
mg/ml in citrate-phosphate buffer with 0.02% hydrogen peroxide) and
the absorbance was measured using a microplate reader (Thermo
Labsystems, Santa Rosa, CA, USA) at a wavelength of 492 nm.
Microplates were incubated with the equal volume of BSA instead of
cyclin D1 as a negative control.
Statistical analysis
Experiments were performed in triplicate and the
reported values are representative of three independent
experiments. Data are expressed as the mean ± standard deviation of
three parallel measurements within the same experiment. Comparisons
among multiple groups were analyzed by one-way analysis of variance
with a Bonferroni post hoc test. Statistical analysis was performed
using GraphPad Prism software (version 5.0; GraphPad Software Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cu2+ and Fe3+
induce the fluorescence quenching of AD5
Fluorescence spectroscopy is considered to be a
comprehensive method for determining protein-ligand interactions.
In the present study, fluorescence spectroscopy was utilized to
investigate the interaction between AD5 and Cu2+ or
Fe3+. When small molecules interact with AD5, the
intrinsic fluorescence fluorophores (Trp, Tyr and Phe) may be
altered depending on the impact of such interaction on the protein
conformation (24,25). As demonstrated in Fig. 1, as the metal ion concentration
increased, the fluorescence intensity of AD5 markedly decreased at
temperatures of 293, 298 and 303 K. Therefore, Cu2+ and
Fe3+ quench the fluorescence intensity of AD5 at
different temperatures, and may alter the microenvironment and
conformation of AD5.
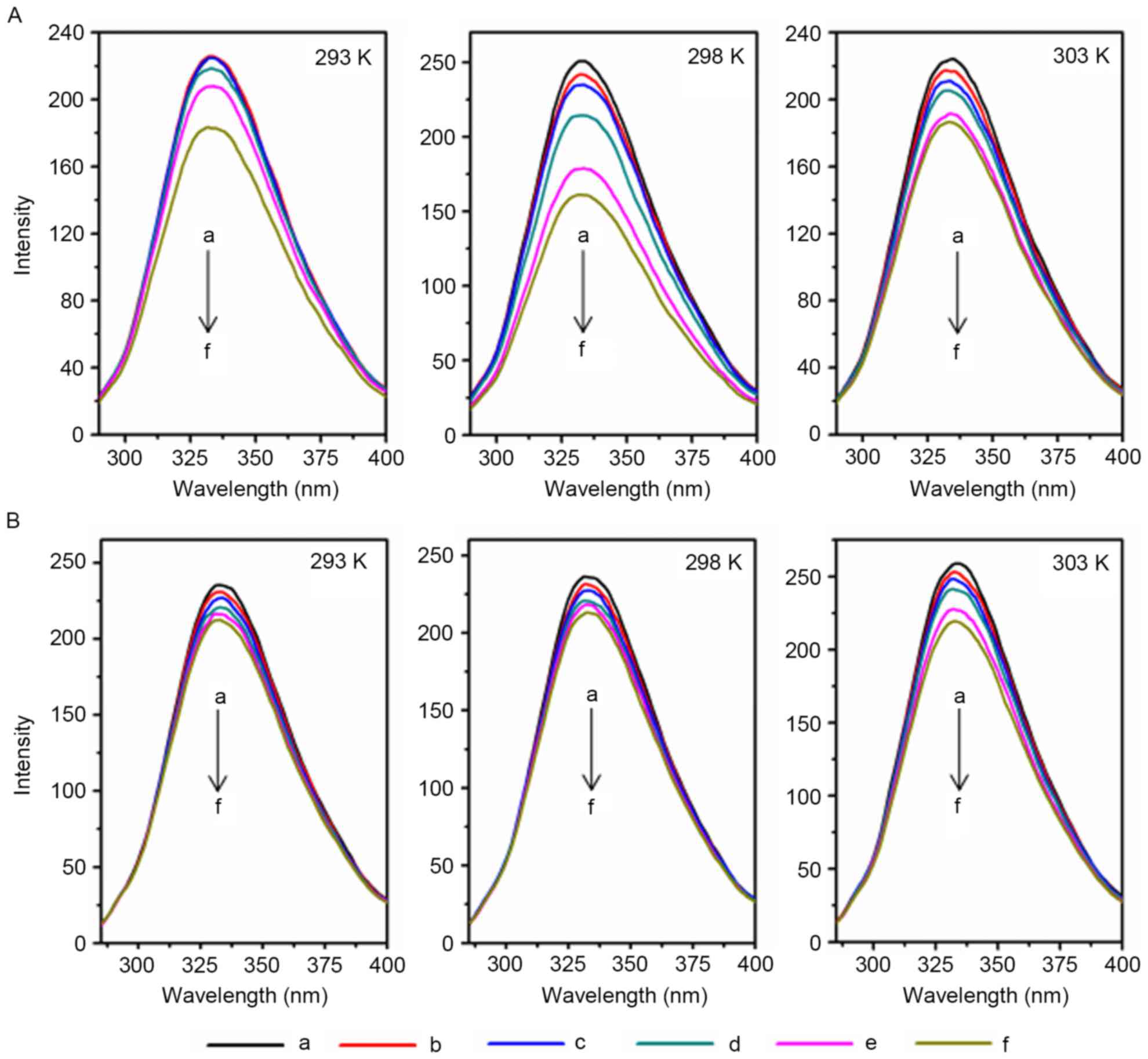 | Figure 1.Fluorescence spectra of AD5 treated
with various concentrations of Cu2+ or Fe3+.
AD5 (2×10−6 M) was treated with various concentrations
of (A) Cu2+ or (B) Fe3+, and the fluorescence
spectra assay was performed. The λex and λem were 280 nm and
290–500 nm, respectively (pH 7.4). a, 0.00×10−3 M; b,
0.33×10−3 M; c, 0.67×10−3 M; d,
1.00×10−3 M; e, 1.33×10−3 M; f,
1.67×10−3 M; AD5, anti-cyclin D1 single-chain variable
fragment; λex, excitation wavelength; λem, emission wavelength. |
Quenching mechanism analysis
The two types of fluorescence quenching mechanisms,
including the dynamic quenching mechanism and the static quenching
mechanism, differ depending on temperature. The dynamic quenching
constant increases as the temperature rises, while the static
quenching constant is reduced accordingly. To verify the quenching
mechanism of Cu2+-AD5 and Fe3+-AD5 complexes,
the fluorescence intensities in the absence or presence of a
quencher (F0/F) was plotted against the concentration of
Cu2+ or Fe3+ (Fig. 2).
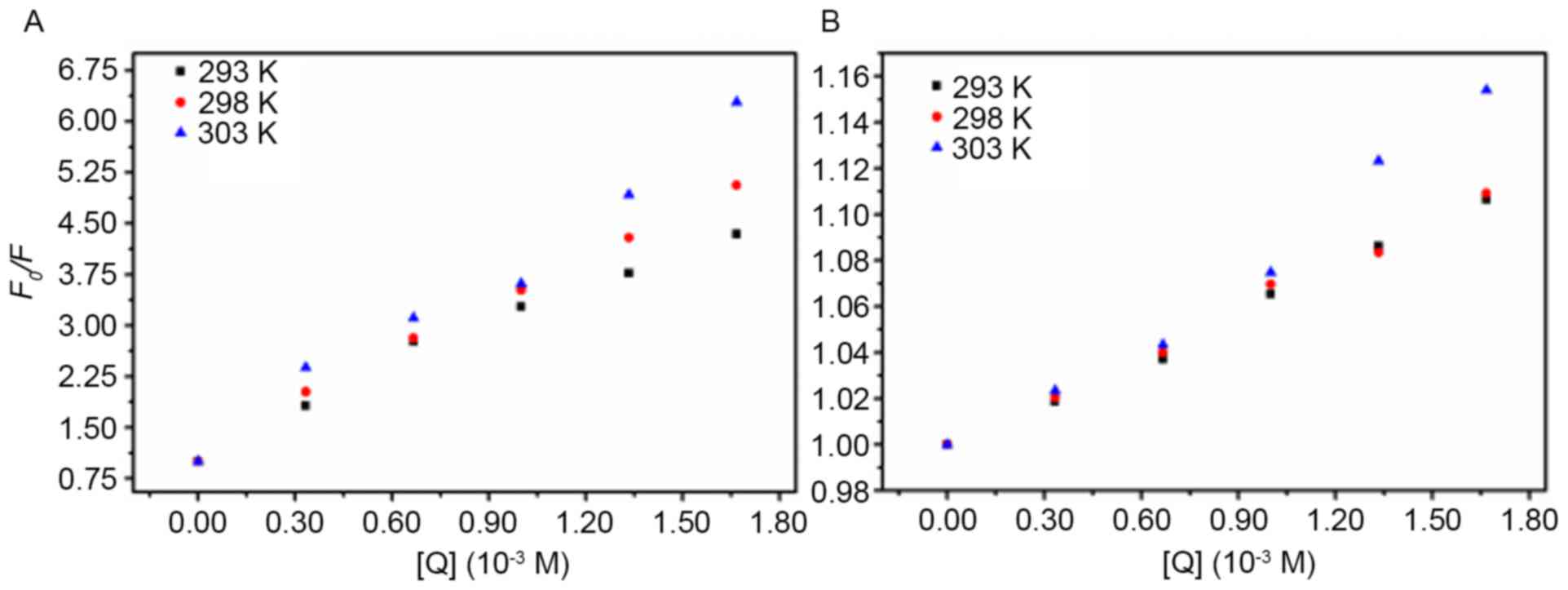 | Figure 2.Stern-Volmer plots of AD5 quenching
by (A) Cu2+ and (B) Fe3+ at temperatures of
293, 298 and 303 K. The concentration of AD5 was 2×10−6
M and the Cu2+ and Fe3+ concentrations were
0.00, 0.33, 0.67, 1.00, 1.33 and 1.67×10−3 M,
respectively. The λex and λem were 280 nm and 290–500 nm,
respectively (pH 7.4). AD5, anti-cyclin D1 single-chain variable
fragment; λex, excitation wavelength; λem, emission wavelength;
F0, fluorescence intensity in the absence of a
quencher; F, fluorescence intensity in the presence of a
quencher; [Q], concentration of quencher. |
The results deduced by the Stern-Volmer equation are
shown in Table I. The standard
deviation provides a measure of how much the observed values differ
from the values provided by the regression line. A low standard
deviation indicates that the data points were similar to the values
obtained from the regression line. For dynamic quenching, the
maximum Kq of various quenchers with a biopolymer
is 2.00×1010 M−1 s−1 (26). As these Kq values
are markedly greater than the maximum collisional quenching
constant (2.00×1010 M−1 s−1), it
was concluded that the static quenching mechanism served a dominant
role in the Cu2+-AD5 and Fe3+-AD5 interaction
(26).
 | Table I.Stern-Volmer Ksv
and Kq of the Cu2+/Fe3+-AD5
interaction system. |
Table I.
Stern-Volmer Ksv
and Kq of the Cu2+/Fe3+-AD5
interaction system.
| A,
Cu2+-AD5 |
|---|
|
|---|
| T (K) |
Ksv
(M−1) |
Kq (M−1
s−1) | R | SD |
|---|
| 293 |
1.98×103 |
1.98×1011 | 0.978 | 0.133 |
| 298 |
2.38×103 |
2.38×1011 | 0.996 | 0.069 |
| 303 |
2.96×103 |
2.96×1011 | 0.973 | 0.218 |
|
| B,
Fe3+-AD5 |
|
| T (K) |
Ksv
(M−1) |
Kq (M−1
s−1) | R | SD |
|
| 293 |
6.54×105 |
6.54×1013 | 0.996 | 0.196 |
| 298 |
6.56×105 |
6.56×1013 | 0.993 | 0.240 |
| 303 |
9.44×105 |
9.43×1013 | 0.973 | 0.697 |
Evaluation of the binding constant
(Kb) and the number of binding sites
For the static quenching, the number of binding
sites for one AD5 molecule can be respectively obtained from the
ordinate and slope of the double logarithmic regression curve
(Fig. 3) of log
[(F0-F)/F] vs. log [Q] based on the
equation. The number of binding sites and Kb values were
calculated and presented in Table
II. The number of binding sites (n) was approximately 1,
indicating that there was one binding site in AD5 for
Cu2+ or Fe3+ (Table II). In addition, Kb
values calculated for the Fe3+-AD5 and
Cu2+-AD5 system suggested that the Fe3+-AD5
system binding affinity increased with increasing temperature,
whereas Cu2+-AD5 system binding affinity was greatest at
298 K (Table II).
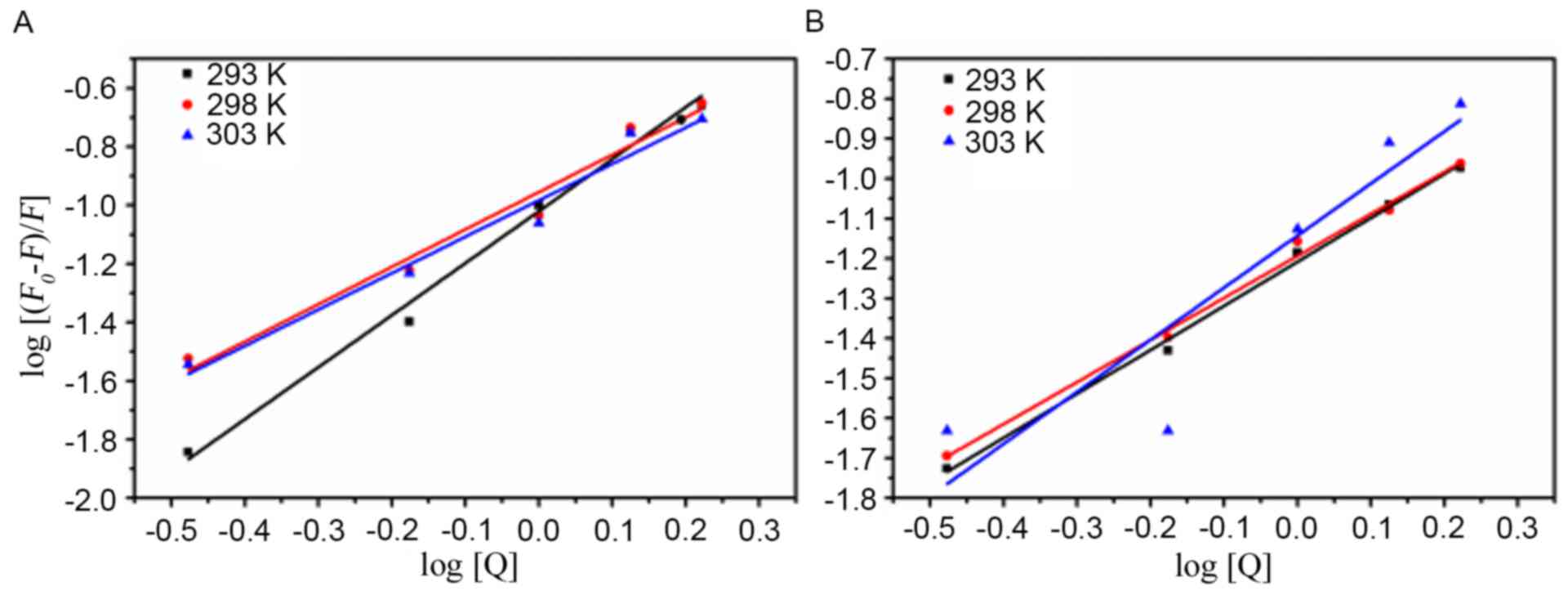 | Figure 3.Quenching effects of Cu2+
or Fe3+ on AD5 fluorescence at different temperatures.
(A) Cu2+-AD5 system and (B) Fe3+-AD5 system
at temperatures of 293, 298 and 303 K. The concentration of AD5 was
2×10−6 M, and the Cu2+ and Fe3+
concentrations were 0.00, 0.33, 0.67, 1.00, 1.33 and
1.67×10−3 M. The λex and λem were 280 nm and 290–500 nm,
respectively (pH 7.4). AD5, anti-cyclin D1 single-chain variable
fragment; λex, excitation wavelength; λem, emission wavelength;
F0, fluorescence intensity in the absence of a
quencher; F, fluorescence intensity in the presence of a
quencher; [Q], concentration of quencher. |
 | Table II.Kb and the number
of binding sites of the Cu2+-AD5 or Fe3+-AD5
interaction at different temperatures. |
Table II.
Kb and the number
of binding sites of the Cu2+-AD5 or Fe3+-AD5
interaction at different temperatures.
|
|---|
| A,
Cu2+-AD5 |
|---|
|
|---|
| T (K) |
Kb (M−1) | n | R | SD |
|---|
| 293 | 95.504 | 1.776 | 0.988 | 0.025 |
| 298 | 110.943 | 1.276 | 0.964 | 0.031 |
| 303 | 104.002 | 1.243 | 0.963 | 0.031 |
|
| B,
Fe3+-AD5 |
|
| T (K) |
Kb (M−1) | n | R | SD |
|
| 293 | 61.916 | 1.102 | 0.995 | 0.010 |
| 298 | 64.055 | 1.053 | 0.992 | 0.012 |
| 303 | 72.033 | 1.304 | 0.800 | 0.081 |
Analysis of AD5 conformational
alterations
Synchronous fluorescence spectroscopy is used to
analyze the microenvironment of amino acid residues and to evaluate
protein conformation by measurement of the emission wavelength
shift, as the maximum emission wavelength of Trp or Tyr residues is
associated with the polarity of their environment (27–29).
The synchronous fluorescence spectra exhibit the spectral character
of Tyr and Trp residues when the ∆λ is 15 and 60 nm, respectively
(27).
The synchronous fluorescence spectra of AD5 upon the
addition of Cu2+ or Fe3+ at ∆λ=60 nm and
∆λ=15 nm are indicated in Fig. 4.
The quenching of the fluorescence intensity with Cu2+
was stronger when compared with Fe3+, suggesting that
Cu2+ contributes to the quenching of the intrinsic
fluorescence of AD5 to a greater extent than Fe3+.
Effect of Cu2+ and
Fe3+ on the biological activity of AD5
To verify the effects of metal ions on the
biological activity of AD5, ELISA assays were performed with
various concentrations of Cu2+ or Fe3+ at
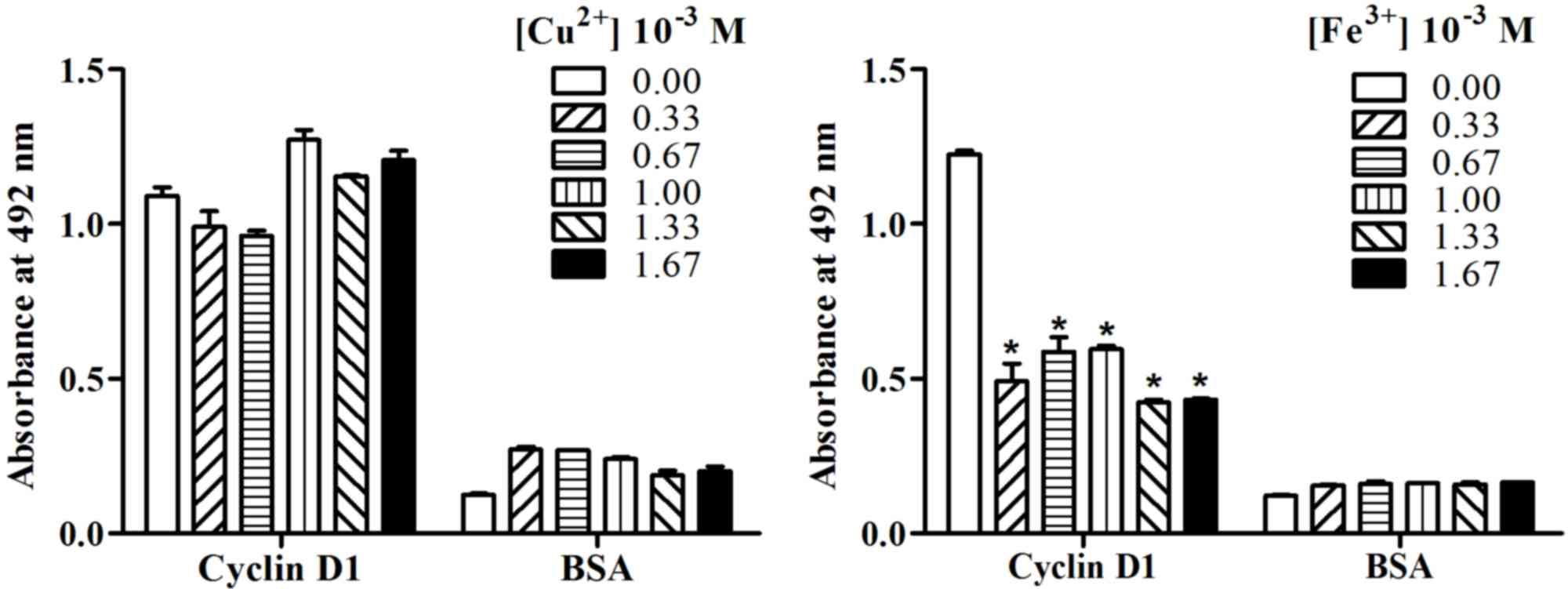
room temperature (298 K). As demonstrated in Fig. 5, the effects of Cu2+ on
the binding activity of AD5 to cyclin D1 was different from that of
Fe3+. Although low concentrations of Cu2+
reduced the binding activity of AD5 to cyclin D1 and relatively
high concentrations of Cu2+ enhanced the binding
activity of AD5 to cyclin D1, the results did not reach statistical
significance when compared with 0.00 M Cu2+ treatment
(Fig. 5). However, the absorbance
at 492 nm was significantly reduced following Fe3+
treatment when compared with the 0.00 M Fe3+-treated
control group (P<0.05; Fig. 5).
This suggested that Fe3+ reduced the binding activity of
AD5 to cyclin D1 and inhibited the biological activity of AD5.
Discussion
Cyclin D1 is the regulatory subunit of a holoenzyme
that phosphorylates and inactivates the retinoblastoma protein and
promotes progression through the G1-S phase of the cell
cycle (1,2). Amplification or overexpression of
cyclin D1 serves a role in the development of a subset of human
cancers, including melanoma and breast, colon and prostate cancer
(30). Overexpression of cyclin D1
may lead to aberrant cell growth, proliferation and tumorigenesis
(31). Cyclin D1 has become a
potential prognostic marker and a therapeutic target for cancer
(4,32,33).
The scFv may be useful for cancer prevention, diagnosis and therapy
due to its specific binding affinity to its antigens, its small
size, and ease of engineering to include modifications, such as the
production of intrabodies (9,10,12,34).
In a previous study, a novel human AD5 scFv with specific binding
affinity to cyclin D1 was designed (13), and an intracellular anti-cyclin D1
scFv suppressed the growth and proliferation of HeLa and MCF-7
cells (14,15). Investigations using intracellular
AD5 have suggested that scFv may be a powerful tool to inhibit the
function of cyclin D1 in cancer cells (14,15).
However, the mechanism of action and biological characteristics of
AD5 remain to be understood. In the present study, the effects of
metal ions on the structure and activity of AD5 were investigated
by spectroscopy analysis and ELISA. The results revealed that the
fluorescence of AD5 may be quenched by Cu2+ and
Fe3+. Quenching mechanism analysis demonstrated that
static quenching was dominant in the AD5-metal ion system. In
addition, the effects of Fe3+ on AD5 activity were
stronger than that of Cu2+. The ELISA results revealed
that Cu2+ and Fe3+ demonstrated different
effects on the biological activity of AD5, where Cu2+
had few effects on AD5 activity, whereas Fe3+
significantly reduced the biological activity of AD5 binding to
cyclin D1. These results may facilitate an improved understanding
of the characteristics of AD5.
Spectroscopy is the favored method in the study of
functional protein structure, including scFvs. Jäger and Plückthun
(35) compared the equilibrium
denaturation and unfolding kinetics of the variable domain light
chain (VL) and variable domain heavy chains (VH) with those of the
fragment variable (Fv) and scFv of an engineered variant of the
McPC603 antibody, in the presence and absence of the
phosphorylcholine antigen by fluorescence spectroscopy. The results
demonstrated that scFv fragment is significantly more stable than
the isolated constitutive domains. Paoletti et al (36) revealed that, in response to an
excitation wavelength of 295 nm, the maximum fluorescence of native
anti-nerve growth factor precursor scFv was 334 nm, whereas in
denaturing conditions, the fluorescence maximum was shifted towards
higher wavelengths of 353 nm (36). In the present study, the structural
alterations of AD5 in the presence of metal ions were investigated
by spectroscopy. The results demonstrated that AD5 fluorescence was
quenched by Cu2+ or Fe3+. Synchronous
fluorescence revealed that the AD5 chromophore environment was
altered slightly in the presence of Cu2+ or
Fe3+, leading to polarity, hydrophobicity and a minor
alteration of AD5 conformation. The findings of the present study
suggested that each AD5 molecule possessed ~1 Cu2+ or
Fe3+ binding site. This may provide additional
information regarding the spectroscopy characteristics of scFv.
The binding activity of an antibody to its antigen
is critical in determining its function. In a previous study, the
active anti-cyclin D1 single chain antibody, AD5, was designed.
This antibody binds specifically to human recombinant cyclin D1
with a moderate affinity constant (13). However, increasing evidence
suggests that antibodies with moderate affinity
(10−7-10−9 M) may facilitate effective
penetration into tumors and to enhance anti-tumor activities in
cancer cells (37–39). In addition, low binding activity
may result in inefficient therapy (8). The results of the present study
revealed that Fe3+ disturbed the binding between AD5 and
cyclin D1. The biological activity of AD5 was significantly reduced
by Fe3+. However, Cu2+ had few effects on the
binding activity of AD5 to cyclin D1. These results may facilitate
the identification of optimal working conditions of AD5 for tumor
therapy or cyclin D1 detection. The specific association between
antibodies and antigens is primarily based on the interactions
between the epitope of the antigen and complementary determinant
regions in the VH/VL domain of the antibody molecule (40,41).
Therefore, a strong antigen-antibody interaction depends on a close
structural fit between antigen and antibody. Therefore, these
interactions may occur if the antigen and antibody have precise
conformations and are in close proximity (41,42).
The results of the present study suggested that Cu2+ and
Fe3+ demonstrate similar effects on AD5 conformation, as
Cu2+ or Fe3+ induced slight AD5 conformation
alterations. Therefore, the underlying reason why Fe3+
significantly inhibited the biological activity of AD5 is not
explained by the Fe3+-induced conformational
alterations. However, it is likely that Fe3+ inhibits
the interaction between AD5 and cyclin D1 via association with key
amino acids of AD5 involved in binding to cyclin D1. Further
investigation to elucidate the underlying mechanisms for the
stronger effects of Fe3+ on AD5 activity than
Cu2+ is warranted.
In conclusion, the results of the present study
provide important information regarding the structure and
biological activity of AD5 and how it is affected by metal ions.
This may provide a foundation for elucidating the mechanism of
action of AD5 and its potential clinical applications.
Acknowledgements
The present study was partially supported by the
National Natural Science Foundation of China (grant nos. 31170882
and 31570934), the S&T Development Planning Program of Jilin
Province (grant nos. 20111806, 20150414027GH and 20160101213JC) and
the Fundamental Research Funds for the Central Universities (grant
nos. 451160306023 and JCKY-QKJC-01).
References
|
1
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclin D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi YJ, Li X, Hydbring P, Sanda T,
Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer
H and Sicinski P: The requirement for cyclin D function in tumor
maintenance. Cancer Cell. 22:438–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beroukhim R, Mermel CH, Porter D, Wei G,
Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J,
Urashima M, et al: The landscape of somatic copy-number alteration
across human cancers. Nature. 463:899–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casimiro MC, Crosariol M, Loro E, Li Z and
Pestell RG: Cyclins and cell cycle control in cancer and disease.
Genes Cancer. 3:649–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy PG, Pratt N, Purdie CA, Baker L,
Ashfield A, Quinlan P and Thompson AM: High CCND1 amplification
identifies a group of poor prognosis women with estrogen receptor
positive breast cancer. Int J Cancer. 127:355–360. 2010.PubMed/NCBI
|
|
7
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen
NB and Hamid M: scFv antibody: Principles and clinical application.
Clin Dev Immunol. 2012:9802502012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scott AM, Wolchok JD and Old LJ: Antibody
therapy of cancer. Nat Rev Cancer. 12:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elvin JG, Couston RG and van der Walle CF:
Therapeutic antibodies: Market considerations, disease targets and
bioprocessing. Int J Pharm. 440:83–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holliger P and Hudson PJ: Engineered
antibody fragments and the rise of single domains. Nat Biotechnol.
23:1126–1136. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weisser NE and Hall JC: Applications of
single-chain variable fragment antibodies in therapeutics and
diagnostics. Biotechnol Adv. 27:502–520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Zou D, Cao Y, Yao N, Wang J, Wang W,
Jiang H and Li GY: Expression and purification of a human
anti-cyclin D1 single-chain variable fragment antibody AD5 and its
characterization. Int J Mol Med. 32:1451–1457. 2013.PubMed/NCBI
|
|
14
|
Zhou LH, Zhu X, Cao YH, Wang L, Chen Y, Du
Br and Li GY: Construction of expression vector for anti-cyclin D1
intrabody AD5N and its inhibitory effects on cell proliferation of
breast cancer. Chin J Immunol. 24:703–706. 2008.
|
|
15
|
Zhou LH, Zhu X, Cao YH, Chen Y, Tian Y,
Wang L and Li GY: Effects of anti-cyclin D1 intrabody AD5N on HeLa
cells of uterine cervix cancer. Chin J Clin Oncol. 35:942–944.
2008.
|
|
16
|
Avanti C, Oktaviani NA, Hinrichs WL,
Frijlink HW and Mulder FA: Aspartate buffer and divalent metal ions
affect oxytocin in aqueous solution and protect it from
degradation. Int J Pharm. 444:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tainer JA, Roberts VA and Getzoff ED:
Protein metal-binding sites. Curr Opin Biotechnol. 3:378–387. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trisler K, Looger LL, Sharma V, Baker M,
Benson DE, Trauger S, Schultz PG and Smider VV: A metalloantibody
that irreversibly binds a protein antigen. J Biol Chem.
282:26344–26353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iverson BL, Iverson SA, Roberts VA,
Getzoff ED, Tainer JA, Benkovic SJ and Lerner RA:
Metalloantibodies. Science. 249:659–662. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YP, Shi SY, Huang KL, Chen XQ and
Peng MJ: Effect of Cu2+ and Fe3+ for drug
delivery: Decreased binding affinity of ilaprazole to bovine serum
albumin. J Lumin. 131:1927–1931. 2011. View Article : Google Scholar
|
|
21
|
Li GY, Zou DS and Zhou LH: Expression and
purification of recombinant human cyclin D1 in E. coli BL21. J
Jilin Univ. 44:839–843. 2006.
|
|
22
|
Lakowicz JR: Principles of Fluorescence
Spectroscopy. Plenum Press; New York, NY: pp. 260–266. 1983
|
|
23
|
Bian H, Li M, Yu Q, Chen Z, Tian J and
Liang Q: Study of the interaction of artemisinin with bovine serum
albumin. Int J Biol Macromol. 39:291–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Chen M, Bian G, Liu J and Song L:
Spectroscopic investigation of the interaction of the toxicant,
2-naphthylamine, with bovine serum albumin. J Biochem Mol Toxicol.
25:362–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X, Sheng F, Zheng J and Liu R:
Composition and stability of anthocyanins from purple solanum
tuberosum and their protective influence on Cr(VI) targeted to
bovine serum albumin. J Agric Food Chem. 59:7902–7909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu D, Zhao X, Zhao Y, Zhang B, Zhang B,
Geng M and Liu R: Binding of Sudan II and Sudan IV to bovine serum
albumin: Comparison studies. Food Chem Toxicol. 49:3158–3164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller JN: Recent advances in molecular
luminescence analysis. Proc Anal Div Chem Soc. 16:203–208.
1979.
|
|
28
|
Ye ZW, Ying Y, Yang XL, Zheng ZQ, Shi JN,
Sun YF and Huang P: A spectroscopic study on the interaction
between the anticancer drug erlotinib and human serum albumin. J
Incl Phenom Macrocycl Chem. 78:405–413. 2014. View Article : Google Scholar
|
|
29
|
Cao X, Dong D, Liu J, Jia C, Liu W and
Yang W: Studies on the interaction between triphenyltin and bovine
serum albumin by fluorescence and CD spectroscopy. Chemosphere.
26–Jan;2013.[Epub ahead of Print]. View Article : Google Scholar
|
|
30
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: Normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neumeister P, Pixley FJ, Xiong Y, Xie H,
Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER and Pestell
RG: Cyclin D1 governs adhesion and motility of macrophages. Mol
Biol Cell. 14:2005–2015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malumbres M: Cell cycle-based therapies
move forward. Cancer Cell. 22:419–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang
J, Perry SR, Labrot ES, Wu X, Lis R, et al: SMAD4-dependent barrier
constrains prostate cancer growth and metastatic progression.
Nature. 470:269–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai H, Gao H, Zhao X, Dai L, Zhang X, Xiao
N, Zhao R and Hemmingsen SM: Construction and characterization of a
novel recombinant single-chain variable fragment antibody against
white spot syndrome virus from shrimp. J Immunol Methods.
279:267–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jäger M and Plückthun A: Domain
interactions in antibody Fv and scFv fragments: Effects on
unfolding kinetics and equilibria. FEBS Lett. 462:307–312. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paoletti F, Malerba F, Konarev PV,
Visintin M, Scardigli R, Fasulo L, Lamba D, Svergun DI and Cattaneo
A: Direct intracellular selection and biochemical characterization
of a recombinant anti-proNGF single chain antibody fragment. Arch
Biochem Biophys. 522:26–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adams GP and Schier R: Generating improved
single-chain Fv molecules for tumor targeting. J Immunol Methods.
231:249–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adams GP, Schier R, Marshall K, Wolf EJ,
McCall AM, Marks JD and Weiner LM: Increased affinity leads to
improved selective tumor delivery of single-chain Fv antibodies.
Cancer Res. 58:485–490. 1998.PubMed/NCBI
|
|
39
|
Adams GP, Schier R, McCall AM, Simmons H,
Horak E, Marks JD and Weiner LM: What are the determinants of
antibody-based targeting? Proc Amer Assoc Cancer Res.
39:4361998.
|
|
40
|
Sela-Culang I, Kunik V and Ofran Y: The
structural basis of antibody-antigen recognition. Front Immunol.
4:3022013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goldsby RA, Kindt TJ, Kuby J and Osborne
BA: Immunology. 5th. W. H. Freeman and Company Publishers; New
York: 2003
|
|
42
|
Burkovitz A, Leiderman O, Sela-Culang I,
Byk G and Ofran Y: Computational identification of antigen-binding
antibody fragments. J Immunol. 190:2327–2334. 2013. View Article : Google Scholar : PubMed/NCBI
|