Introduction
Myocardial infarction (MI) is a major cause of
mortality and disability worldwide (1). Although there have been recent
advances in the treatment of MI, the general prognosis is
unsatisfactory (2–4). Restoration of blood supply, termed
reperfusion, has been used to treat ischemic myocardium and prevent
further tissue damage. However, reperfusion following a period of
prolonged ischemia can often cause myocardial ischemia-reperfusion
(I/R) injury, leading to damage of cardiac tissues (5). The underlying mechanisms behind
myocardial I/R injury are associated with a number of factors,
including substantial free radical production, intracellular
calcium overload, increased inflammation, myocardial necrosis and
apoptosis (6). Thus, inhibition of
oxidative stress and myocardial apoptosis is beneficial in the
treatment of myocardial I/R injury.
Syringic acid (SA), a naturally occurring
O-methylated trihydroxybenzoic acid monomer extracted from
Dendrobium nobile Lindl., exhibits a variety of biological
actions including anti-inflammatory, anti-tumor and anti-oxidant
properties (7–9). A recent study demonstrated that SA
prevented oxidative stress in l-arginine-induced acute pancreatitis
(9). In addition, SA was revealed
to protect against I/R injury. Tokmak et al (10) reported that SA pretreatment in
spinal cord I/R reduced oxidative stress and neuronal degeneration.
SA also attenuated renal I/R injury (11). However, the role of SA in
myocardial I/R injury remains to be elucidated. The present study
aimed to clarify the cardioprotective effect of SA from myocardial
I/R injury in vitro, and the potential molecular mechanisms
were also explored. The results demonstrated that SA inhibited
apoptosis signaling in H9c2 cardiomyocytes via downregulation of
p38 mitogen-activated protein kinase (p38MAPK) and c-Jun N-terminal
kinase (JNK) signaling pathways following hypoxia/reoxygenation
(H/R) injury.
Materials and methods
Cell culture and treatment
H9c2 rat cardiomyocyte cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA.). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with 10% (v/v) heat
inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in a
humidified incubator with 5% CO2.
For treatment, H9c2 cells at a density of
1×104 cells/well were pretreated with various
concentrations of SA (0.1, 1 and 10 µM; Sigma-Aldrich; Merck KGaA)
for 24 h. Then, the cultures were introduced into a humidified
N2 hypoxic chamber (2% O2) at 37°C for 6 h
and then reoxygenated 5 h at 37°C in 5% CO2 (95%
O2). Normoxic control cells were incubated at 37°C in 5%
CO2.
Cell viability assay
Cell viability was detected using a Cell Counting
kit-8 (CCK-8) assay. In brief, following treatment, the medium was
removed and replaced with fresh DMEM (100 µl/well). Then, 10 µl
CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added to each well, and the microplates were incubated
at 37°C for 2 h. The absorbance was measured at 490 nm using a
microplate reader (Benchmark; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Cytotoxicity assay
Cell cytotoxicity was measured by a lactate
dehydrogenase (LDH) assay using the Cytotoxicity Detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Briefly, H9c2 cells were seeded in
96-well plates at a density of 1×104 cells/well. Following
treatment, the medium was collected to measure the LDH activity
according to the manufacturer's protocol. The colorimetric compound
was measured at 530 nm using a microplate reader (Benchmark;
Bio-Rad Laboratories, Inc.).
Measurement of cellular levels of
superoxide dismutase (SOD) and malondialdehyde (MDA)
H9c2 cells were seeded in 96-well plates at a
density of 1×104 cells/well. Following treatment, the activity of
SOD in media was analyzed using a SOD kit from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). The MDA level was
measured with an MDA kit (Beyotime Institute of Biotechnology,
Jiangsu, China). For colorimetric analysis, the absorbance at 532
nm was recorded using a microplate reader (Spectra Max 190;
Molecular Devices, LLC, Sunnyvale, CA, USA).
Western blot analysis
The proteins were extracted from H9c2 cells using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology) and the protein concentration was determined by a
BCA protein assay kit (Invitrogen; Thermo Fisher Scientific, Inc.).
A total of 30 µg protein was separated by 10% SDS-PAGE
electrophoresis followed by electroblotting onto a nitrocellulose
membrane (GE Healthcare Life Sciences, Little Chalfont, UK).
Following blocking with 5% nonfat dry milk in PBS for 4 h, the
membrane was incubated overnight at 4°C with primary rabbit
anti-mouse antibodies (dilution, 1:1,000) targeting B-cell lymphoma
2 (Bcl-2; cat. no. sc-783), Bcl-2-like protein 4 (Bax; cat. no.
sc-6236), cleaved caspase-3 (cat. no. sc-98785), phospho
(p)-p38MAPK (cat. no. sc-101759), total p38MAPK (cat. no. sc-535),
p-JNK (cat. no. sc-135642), total JNK (cat. no. sc-572) and GAPDH
(cat. no. sc-25778; all from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Subsequently, the membrane was washed and incubated
with goat anti-rabbit peroxidase-conjugated immunoglobulin G (cat.
no. sc-516087; Santa Cruz Biotechnology, Inc.) diluted 1:3,000 in
the blocking buffer for 1 h. Then the blot was washed in TBST
buffer (TBS containing 0.1% Tween-20) and positive bands were
visualized using enhanced chemiluminescence reagents (Bio-Rad
Laboratories, Inc.). Densitometry was performed using Gel-Pro
Analyzer software version 4.0 (Media Cybernetics, Inc., Rockville,
MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA). Statistical analysis was
carried out using a one-way analysis of variance followed by a
Bonferroni post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
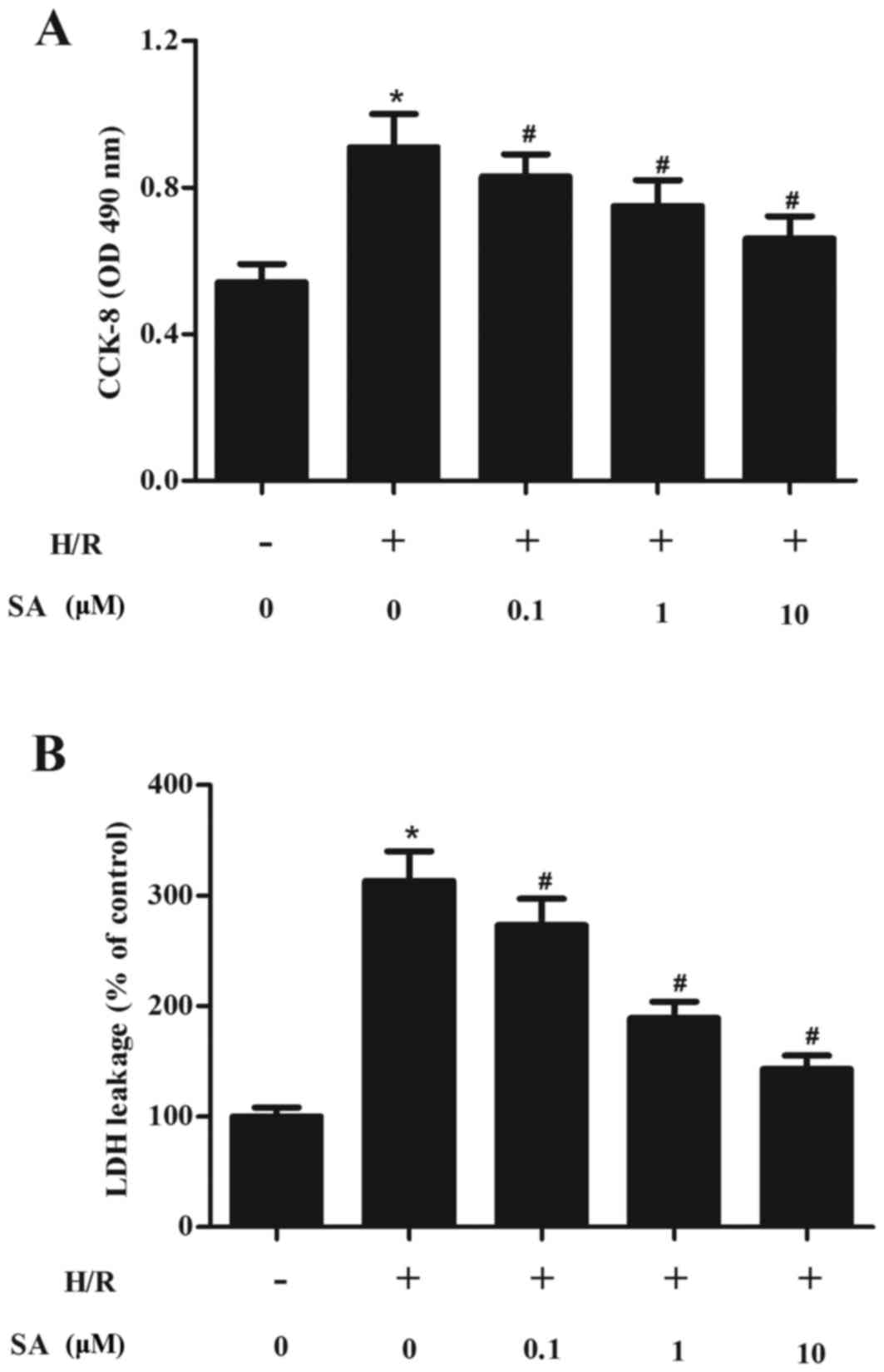
SA protects H/R-induced H9c2 cell
injury
The protective effects of SA on H9c2 cell
cytotoxicity caused by H/R were investigated. The results of the
CCK-8 assay demonstrated that, compared with the normoxia group,
cell viability was significantly reduced following H/R treatment.
Pretreatment with SA for 24 h before H9c2 cells suffered with 6/5 h
H/R injury markedly inhibited H9c2 cell injury, compared with the
H/R group (Fig. 1A).
It was further analyzed whether SA pretreatment
influences the cell death of H9c2 cells. As demonstrated in
Fig. 1B, H9c2 cells subjected to
6/5 h H/R injury caused an marked increase of LDH release. By
contrast, pretreatment of cells with SA for 24 h downregulated LDH
release compared with the H/R group.
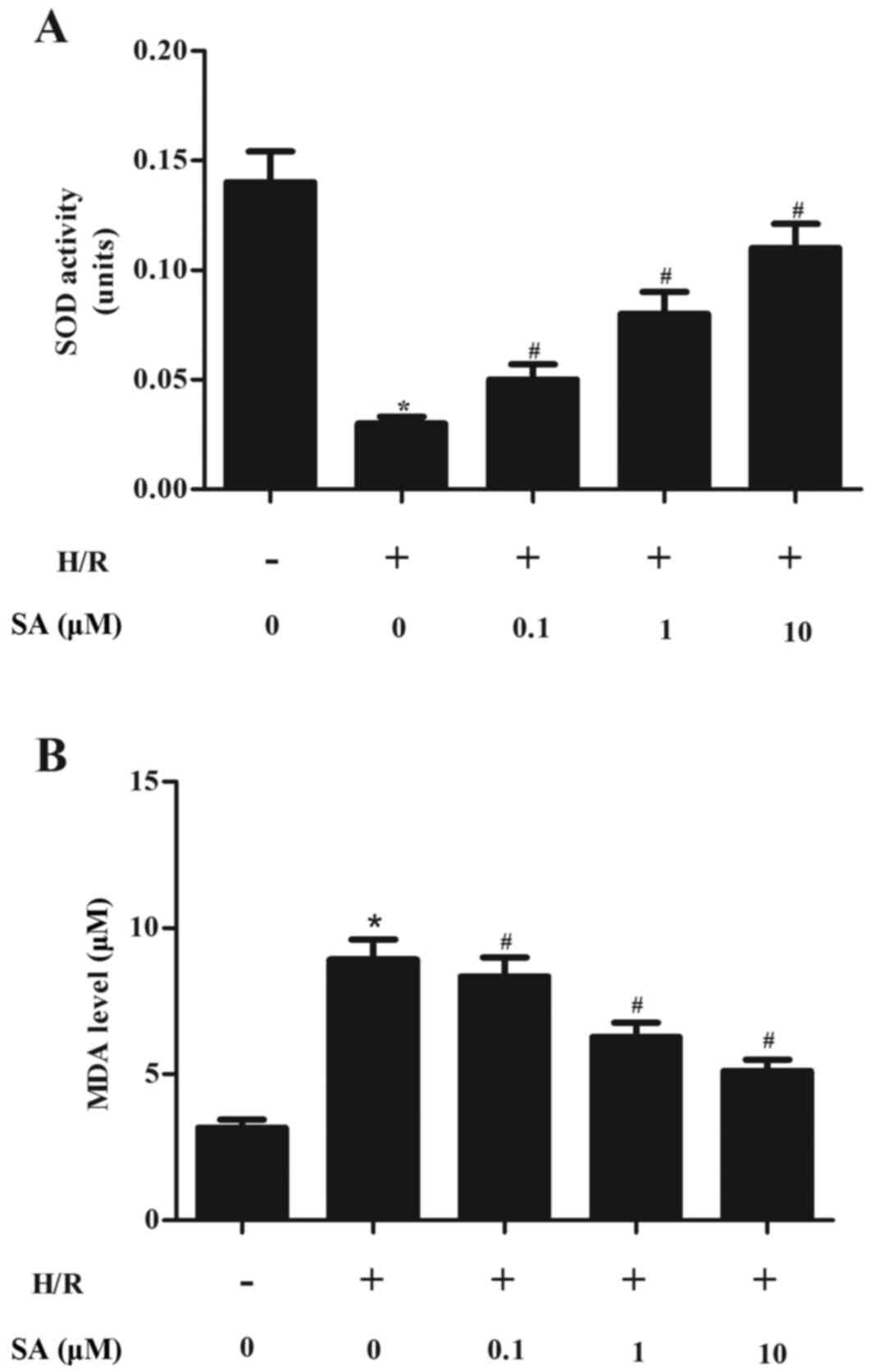
SA inhibits oxidant stress in H/R
induced H9c2 cells
Oxidative stress is widely reported to serve a
critical role in mediating myocardial I/R injury and thus the
effect of SA on oxidant stress in H9c2 cells in response to H/R
injury was investigated. As demonstrated in Fig. 2A, H/R treatment significantly
decreased SOD level in H9c2 cells compared with the normoxia group
and this parameter was significantly increased by SA pretreatment.
By contrast, pretreatment with SA significantly reduced MDA levels
induced by H/R in H9c2 cells (Fig.
2B).
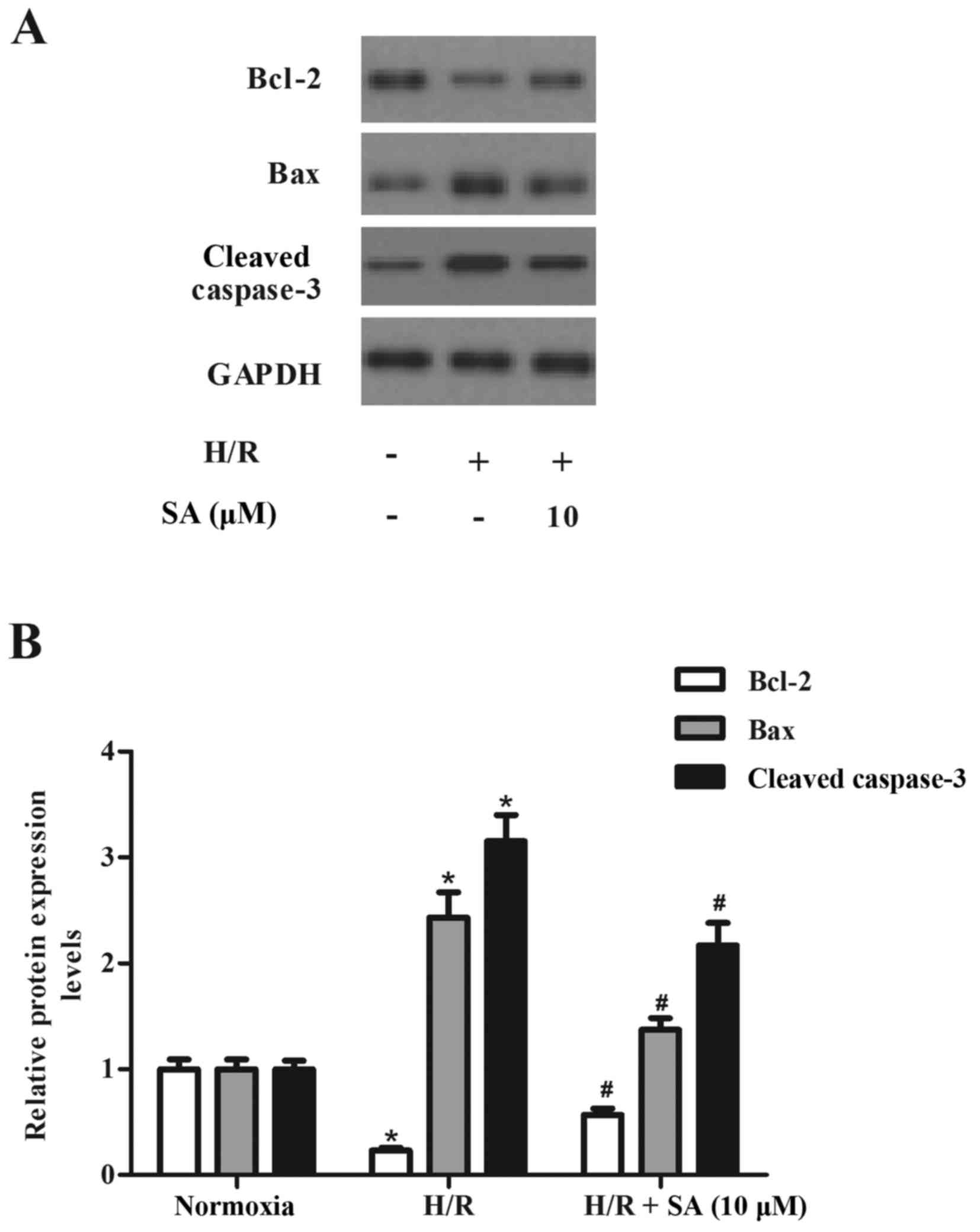
SA attenuates H/R induced H9c2 cell
apoptosis
Oxidative stress is one of the major stimuli
involved in the myocardial apoptosis observed during I/R. Thus, the
effect of SA on H9c2 cell apoptosis in response to H/R injury was
investigated. As demonstrated in Fig.
3, H9c2 cells exposed to 6/5 h H/R injury caused a marked
increase of Bax expression. By contrast, pretreatment of cells with
SA for 24 h markedly decreased the H/R-induced Bax expression. SA
pretreatment significantly increased the expression of Bcl-2 in
H9c2 cells and significantly decreased the level of cleaved
caspase-3 following H/R.
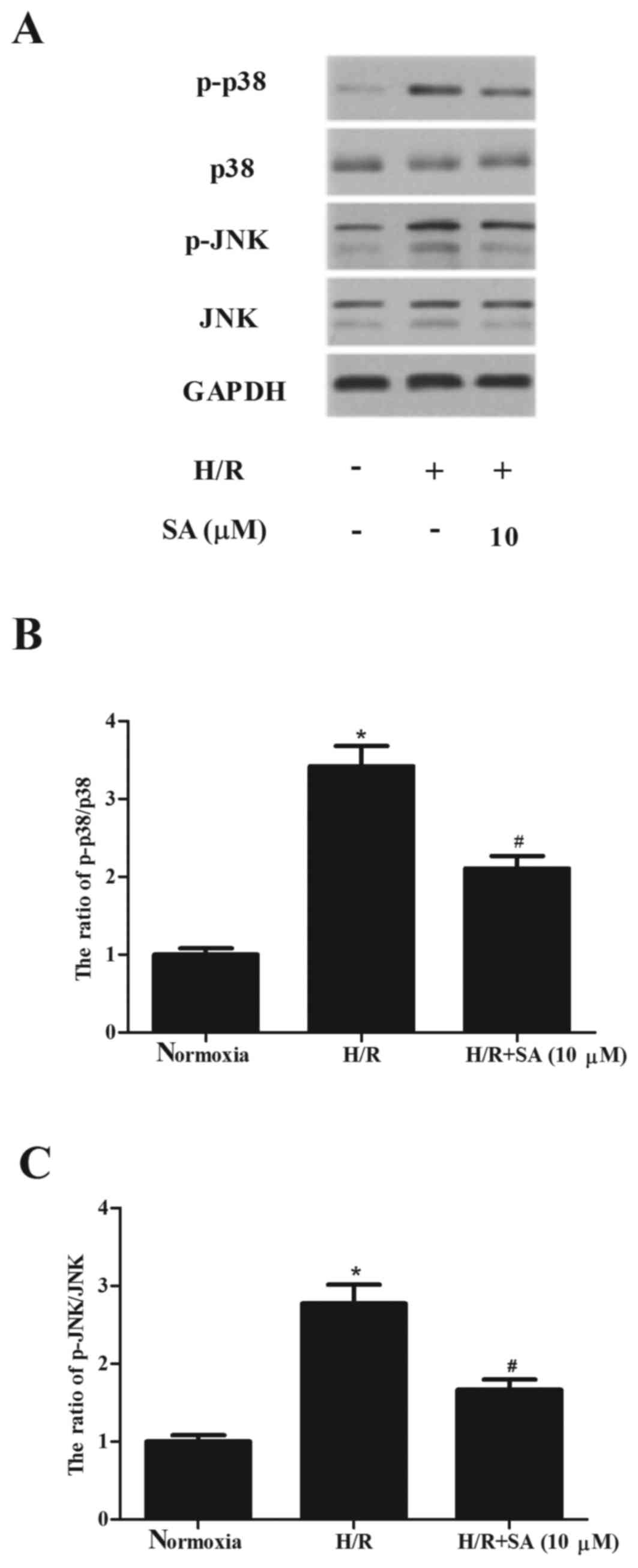
SA inhibits the activation of p38MAPK
and JNK signaling pathways in H9c2 cells
Since p38MAPK and JNK are involved in myocardial
injury caused by I/R, western blot analysis was performed to
investigate whether SA inhibited the activation of p38MAPK and JNK
in H9c2 cells. As demonstrated in Fig.
4, H/R treatment significantly induced the phosphorylation of
p38MAPK and JNK, compared with the normoxia group. However, SA
significantly alleviated H/R-induced phosphorylation of p38MAPK and
JNK in H9c2 cells.
Discussion
The present study demonstrated that pretreatment
with SA increased the viability and inhibited oxidant stress in
H9c2 cardiomyocytes following H/R. SA also markedly upregulated
Bcl-2 expression and inhibited increases in Bax and cleaved
caspase-3 in H9c2 cardiomyocytes induced by H/R. In addition, SA
significantly alleviated the H/R-induced phosphorylation of p38MAPK
and JNK in H9c2 cardiomyocytes.
A growing body of evidence indicates that oxidative
stress contributes to the development of myocardial I/R injury
(12–14). The endogenous defense system,
primarily the antioxidant enzyme system (involving SOD and
glutathione peroxidase), is critical for attenuating the injury
induced by I/R (15). It has been
reported that the activity of SOD is considerably reduced following
myocardial I/R injury. MDA is the end-product of lipid
peroxidization and is increased in myocardial tissue following
myocardial I/R injury (16). The
present study observed that SA inhibited oxidant stress in H9c2
cardiomyocytes following H/R. Thus, the antioxidant activity of SA
may contribute towards a beneficial effect against myocardial I/R
injury.
Apoptosis is an active gene-directed cell death
process which serves a key role in myocardial reperfusion injury
(17,18). Bcl-2 forms a heterodimer with Bax,
thereby preventing Bax homodimerization and the activation of
caspase-3 (19). Caspase-3, a
cysteine protease, also serves a critical role in apoptosis
(20). H/R has been demonstrated
to elicit cardiomyocyte apoptosis in conjunction with the
activation of caspases and an imbalance of pro-/anti-apoptotic
proteins (21). Consistent with
the previous studies, the present study noted that H/R markedly
upregulated expression of Bax and downregulated expression of Bcl-2
in H9c2 cells. However, pretreatment of cells with SA for 24 h
markedly decreased Bax expression and increased Bcl-2 expression in
H9c2 cells induced by H/R. SA pretreatment significantly decreased
the level of cleaved caspase-3 induced by H/R. Thus, the
anti-apoptosis activity of SA may contribute towards a beneficial
influence against myocardial I/R injury.
Previous studies have demonstrated that myocardial
I/R is associated with MAPK activation (22–24).
Cook et al (25) reported
enhanced activation of JNK and p38MAPK in heart tissue from
patients with heart failure caused by ischemic disease. Studies
have demonstrated that the targeted inhibition of p38MAPK and JNK
reduced cardiomyocyte apoptosis and improved cardiac performance
following I/R injury (26–28). Engelbrecht et al (29) reported that pre-treatment with
SB203580, a p38 inhibitor, produced a significant increase in cell
viability and attenuation of the apoptotic index in neonatal
cardiomyocytes during simulated I/R, while SP600125, a specific JNK
inhibitor, caused a significant increase in caspase-3 activation
and apoptotic index. Another study confirmed that treatment with
the novel JNK inhibitor AS601245 during myocardial ischemia and
reperfusion significantly reduced myocardial apoptosis in
anaesthetized rats (23). The
present study demonstrated that H/R treatment significantly induced
the phosphorylation of p38MAPK and JNK. However, SA significantly
alleviated H/R-induced phosphorylation of p38MAPK and JNK in H9c2
cells. These results imply that SA can induce cardio-protection via
inhibition of p38MAPK and JNK signaling pathways in H9c2 cells.
In conclusion, the present study demonstrated that
SA inhibited apoptosis via downregulation of p38MAPK and JNK
signaling pathways in H9c2 cardiomyocytes following H/R injury.
These results support the therapeutic use of SA in the treatment of
MI.
Acknowledgements
The present study was supported by a project of the
Bureau of Public Health of Henan Province (grant no.
201403189).
References
|
1
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fintel DJ, Subacius H and Goldberger J:
Effect of metoprolol versus carvedilol on survival after myocardial
infarction. J Am Coll Cardiol. 67:20912016. View Article : Google Scholar
|
|
3
|
Erlinge D, Götberg M, Noc M, Lang I,
Holzer M, Clemmensen P, Jensen U, Metzler B, James S and Bøtker HE:
Therapeutic hypothermia for the treatment of acute myocardial
infarction-combined analysis of the RAPID MI-ICE and the CHILL-MI
Trials. Ther Hypothermia Temp Manag. 5:77–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henri O, Houssari M, Pouehe C, Galas L,
Nicol L, Edwards-Lévy F, Henry JP, Dumesnil A, Schapman D and
Thuillez C: Therapeutic lymphangiogenesis improves diastolic
function by limiting cardiac edema and fibrosis after myocardial
infarction. Circulation. 132:A12624. 2015.
|
|
5
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. New Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ham JR, Lee HI, Choi RY, Sim MO, Seo KI
and Lee MK: Anti-steatotic and anti-inflammatory roles of syringic
acid in high-fat diet-induced obese mice. Food Funct. 7:689–697.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abaza MS, Al-Attiyah R, Bhardwaj R, Abbadi
G, Koyippally M and Afzal M: Syringic acid from Tamarix aucheriana
possesses antimitogenic and chemo-sensitizing activities in human
colorectal cancer cells. Pharm Biol. 51:1110–1124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cikman O, Soylemez O, Ozkan OF, Kiraz HA,
Sayar I, Ademoglu S, Taysi S and Karaayvaz M: Antioxidant activity
of syringic acid prevents oxidative stress in l-arginine-induced
acute pancreatitis: An experimental study on rats. Int Surg.
100:891–896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tokmak M, Yuksel Y, Sehitoglu MH, Guven M,
Akman T, Aras AB, Cosar M and Abbed KM: The neuroprotective effect
of syringic acid on spinal cord ischemia/reperfusion injury in
rats. Inflammation. 38:1969–1978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sancak EB, Akbas A, Silan C, Cakir DU,
Turkon H and Ozkanli SS: Protective effect of syringic acid on
kidney ischemia-reperfusion injury. Ren Fail. 38:629–635. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanno S, Lee PC, Zhang Y, Ho C, Griffith
BP, Shears LL II and Billiar TR: Attenuation of myocardial
ischemia/reperfusion injury by superinduction of inducible nitric
oxide synthase. Circulation. 101:2742–2748. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao L, Gao E, Jiao X, Yuan Y, Li S,
Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ and Ma XL:
Adiponectin cardioprotection after myocardial ischemia/reperfusion
involves the reduction of oxidative/nitrative stress. Circulation.
115:1408–1416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lefer DJ and Granger DN: Oxidative stress
and cardiac disease. Am J Med. 109:315–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaeschke H and Woolbright BL: Current
strategies to minimize hepatic ischemia-reperfusion injury by
targeting reactive oxygen species. Transplant Rev (Orlando).
26:103–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuzzocrea S, Costantino G, Mazzon E,
Micali A, De Sarro A and Caputi AP: Beneficial effects of melatonin
in a rat model of splanchnic artery occlusion and reperfusion. J
Pineal Res. 28:52–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips DJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HC, Zhang HF, Guo WY, Su H, Zhang KR,
Li QX, Yan W, Ma XL, Lopez BL and Christopher TA: Hypoxic
postconditioning enhances the survival and inhibits apoptosis of
cardiomyocytes following reoxygenation: Role of peroxynitrite
formation. Apoptosis. 11:1453–1460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fülöp N, Zhang Z, Marchase RB and Chatham
JC: Glucosamine cardioprotection in perfused rat hearts associated
with increased O-linked N-acetylglucosamine protein modification
and altered p38 activation. Am J Physiol Heart Circ Physiol.
292:H2227–H2236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrandi C, Ballerio R, Gaillard P,
Giachetti C, Carboni S, Vitte PA, Gotteland JP and Cirillo R:
Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte
apoptosis and infarct size after myocardial ischemia and
reperfusion in anaesthetized rats. Brit J Pharmacol. 142:953–960.
2004. View Article : Google Scholar
|
|
24
|
Ma XL, Kumar S, Gao F, Louden CS, Lopez
BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ and Yue TL:
Inhibition of p38 mitogen-activated protein kinase decreases
cardiomyocyte apoptosis and improves cardiac function after
myocardial ischemia and reperfusion. Circulation. 99:1685–1691.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cook SA, Sugden PH and Clerk A: Activation
of c-Jun N-terminal kinases and p38-mitogen-activated protein
kinases in human heart failure secondary to ischaemic heart
disease. J Mol Cell Cardiol. 31:1429–1434. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao XF, Zhou Y, Wang DY, Lew KS, Richards
AM and Wang P: Urocortin-2 suppression of p38-MAPK signaling as an
additional mechanism for ischemic cardioprotection. Mol Cell
Biochem. 398:135–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou L, Zhao D, An H, Zhang H, Jiang C and
Yang B: Melatonin prevents lung injury induced by hepatic
ischemia-reperfusion through anti-inflammatory and anti-apoptosis
effects. Int Immunopharmacol. 29:462–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomas CJ, Ng DC, Patsikatheodorou N,
Limengka Y, Lee MW, Darby IA, Woodman OL and May CN:
Cardioprotection from ischaemia-reperfusion injury by a novel
flavonol that reduces activation of p38 MAPK. Eur J Pharmacol.
658:160–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engelbrecht AM, Niesler C, Page C and
Lochner A: p38 and JNK have distinct regulatory functions on the
development of apoptosis during simulated ischaemia and reperfusion
in neonatal cardiomyocytes. Basic Res Cardiol. 99:338–350. 2004.
View Article : Google Scholar : PubMed/NCBI
|