Introduction
Cerebral atherosclerosis, the primary cause of
ischemic stroke, is divided into extracranial atherosclerosis
(ECAS) and intracranial atherosclerosis (ICAS) (1–3). The
underlying molecular mechanisms of ICAS and ECAS have been
extensively studied; however, remain to be fully elucidated.
Cerebral atherosclerosis is influenced by various risk factors,
including hypertension, hyperlipidemia, obesity, smoking and
diabetes. Previous studies have reported that blood cholesterol
levels and associated lipoprotein levels are associated with the
risk of coronary artery disease; however, this is weakly associated
in ECAS and ICAS, suggesting potential variations in underlying
mechanisms or susceptibility (2,4,5). The
role of hyperlipidemia in ICAS and ECAS remains unclear due to
controversial previously reported findings. A previous study
conducted proteomics analyses of the common carotid artery by
obtaining human carotid atherosclerotic plaques, and demonstrated
certain proteins are in low-abundance, including heat shock protein
27 (HSP27) isoforms, aldehyde dehydrogenase, moesin, protein kinase
C δ-binding protein and inter-α-trypsin inhibitor family heavy
chain-related protein are correlated with biological alterations
associated with atherosclerosis (6). In our previous study, HSP70
expression levels were demonstrated to vary between ICAS and ECAS;
however, the effect of other protein expressions on ICAS and ECAS
remains unclear (7). Investigation
into the impact of hyperlipidemia on ICAS and ECAS have yielded
controversial results, and it is unclear whether any specific
target proteins are involved in the discrepancies between ICAS and
ECAS. A more global analysis of the activities of all relevant
molecules is required, which may provide a complete view of their
interactions. Proteomic analysis has previously been performed to
identify novel therapeutic targets for use in the treatment and
prevention of atherosclerosis (8).
The present study aimed to compare proteomic and biomarker profiles
associated with cerebral ICAS and ECAS. The identification of
protein biomarkers that underlie cerebral atherosclerosis may
provide valuable diagnostic indicators and therapeutic targets for
the treatment of the disease.
Materials and methods
Animals
Male New Zealand White rabbits (n=18; weight,
2.0–2.5 kg) were provided by the Animal Laboratory of Tongji
University (Shanghai, China). They were fed regular rabbit feed for
one week prior to the experiment and were kept in a
temperature-controlled environment (20±1°C; humidity, 55±5%) under
a 12-h light/dark cycle in an air-conditioned room. Rabbits were
randomly divided into two groups: Control (A; n=9) and high-fat (B;
n=9). Rabbits in group A continued to receive regular rabbit feed
and group B were fed a high-fat diet (2% cholesterol, 6% peanut oil
and 92% basic feed) for 12 weeks based on the methods of previous
studies (6,9,10).
Rabbits in groups A and B were fed restricted diets with an equal
amount of food (150–200 g per day) and free access to water. The
present study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Tongji University School of Medicine
(Shanghai, China).
Lipid profile and histopathological
examination
Circulation blood samples were collected from the
rabbits at weeks 0, 4, 8 and 12 on the different diets. The rabbits
in groups A and B were sacrificed at weeks 0, 4, 8 and 12. For
groups A and B, 3 rabbits were sacrificed at each time point (4,8
and 12 weeks). Artery segments [including the common carotid artery
(CCA) and middle cerebral artery (MCA)] were dissected and fixed in
4% paraformaldehyde for histomorphometry observation. Pathological
analysis was performed by hematoxylin and eosin (H&E) staining,
as described previously (7).
Images were observed under a light microscope, following which
intima-media thickness (IMT) was measured using Image Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
The tissues were stored at −80°C for 2-D gel electrophoresis (2-DE)
and proteins expression levels were analyzed by western
blotting.
2-DE and silver staining
CCA and MCA tissues from groups A and B were ground
to a powder using liquid nitrogen, detailed process as follows:
Homogenized in ice-cold homogenizing buffer [9 M urea; 4% w/v
CHAPS; 1% w/v dithiothreitol (DTT); 0.5% chilled acetone (CA) and a
cocktail of protease inhibitors]. The homogenate was centrifuged at
3,500 × g for 30 min at 6°C and the precipitation was collected and
suspended in precooling acetone containing 0.2% DTT, and frozen at
−20°C for 1 h. Precipitated proteins were centrifuged for 30 min at
5,000 × g at 6°C, which was followed by two additions of CA. The
pellets were air dried at room temperature and dissolved overnight
at 4°C in lysis buffer containing 7 M urea, 2 M thiourea, 4% w/v
CHAPS, 1% w/v DTT, 0.5% ampholyte and a cocktail of protease
inhibitors. Tissue proteins were fractionated as described
previously (11–13) and protein concentrations were
determined using the Bradford assay according to methods described
previously (14), and subsequently
stored at −80°C for isoelectric focusing (IEF).
2-DE and silver staining were performed. Protein
sample (200 µg) was mixed with fresh rehydration buffer to a total
of 450 µl, following which IEF was performed using an Immobiline
DryStrip (IDS) gel (GE Healthcare Life Sciences, Chalfont, UK;
length, 24 cm; pH 3–10; non-linear gradient) at room temperature
for 10 min. Following a two-step equilibration, proteins on the IPG
strips were separated by SDS-PAGE using the Ettan-DALTsix
Electrophoresis system (GE Healthcare Life Sciences) at 15°C and
200 V for 6–8 h. The IDS gel was visualized by silver staining
according to the protocol of Shevchenko et al (15). The stained gel was scanned using an
ImageScanner system (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) at a resolution of 300 dots per inch. All gel images were
processed by three steps using PDQuest 2-D Analysis software
version 8.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) as
follows: Spot detection, volumetric quantification and matching.
Differences in protein content between high-fat diet and control
groups were analyzed by Student's t-test (n=3) and calculated as
fold ratio. Fold-change ≥1.5 or ≤0.67 was used to select
differentially expressed protein spots.
Mass spectrometry (MS) analysis
The extracted protein samples were re-suspended with
5 µl 0.1% trifluoroacetic acid (TFA) followed by mixing in a matrix
consisting of a saturated solution of
α-cyano-4-hydroxy-trans-cinnamic acid in 50% acetonitrile and 0.1%
TFA at a 1:1 ratio. Mixture (1 µl) was spotted onto a
stainless-steel sample target plate. Peptide MS and MS/MS were
performed on an AB SCIEX 5800 time-of-flight (TOF)/TOF™ system mass
spectrometer (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). MS and MS/MS datasets were integrated and
processed using GPS Explorer™ software version 3.6 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with default
parameters. Proteins were successfully identified based on 95% or
higher confidence interval of their scores in the Mascot v2.1
search engine (Matrix Science, Ltd., London, UK) using the Mascot
and NCBI protein databases. Peptide mixtures were separated on an
UltiMate™ 3000 Nano Liquid Chromatography system (Dionex; Thermo
Fisher Scientific, Inc.) as described by Moller et al
(11).
Western blotting
Proteins were extracted as described above and
protein lysates (20 µg per lane) were separated by one-dimensional
10% SDS-PAGE and were transferred onto nitrocellulose membranes.
Membranes were blocked with PBS containing 5% nonfat dry milk at
4°C overnight and were subsequently incubated with the following
primary antibodies: Polyclonal mouse anti-α-smooth actin (cat. no.
ab7817, 1:300), mouse anti-HSP70 (cat. no. ab2787, 1:100), rabbit
anti-tropomyosin α-1 chain (cat. no. ab55915, TPM1; 1:500) (all
from Abcam, Cambridge, UK) and mouse GAPDH (cat. no. sc-365062;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight
at 4°C. The membranes were washed three times and incubated with
secondary antibody horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (cat. no. ab97051; 1:5,000) and goat anti-mouse IgG
(cat. no. ab97023; 1:10,000) (both from Abcam) for 2 h at room
temperature. Membranes were washed four times with TBST for 40 min.
Proteins were visualized using Enhanced Chemiluminescence reagents
(GE Healthcare Life Sciences) and densitometry was performed using
Quantity One software 4.2 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Differences between intra- and extracranial cerebral
atherosclerosis were evaluated by Student's t-test for normally
distributed data, or the Mann-Whitney rank sum test for
nonparametric data. Data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Lipid profile
Group B rabbits were fed an atherogenic diet. Mean
cholesterol (CHOL), low density lipoprotein (LDL), high density
lipoprotein (HDL) and triglyceride (TG) levels (mmol/l) were
increased in group B compared with group A at the end of week 12 as
follows: CHOL increased from 1.27±0.01 (Group A) to 41.27±0.93
(Group B), LDL from 0.45±0.12 to 50.80±2.25, HDL from 0.48±0.03 to
10.93±0.13, and TG from 1.54±0.10 to 4.22±2.88. Various lipid
levels in different groups were revealed to increase over time in
group B; in particular, LDL and CHOL levels were markedly
increased. In group A, blood lipids remained within the healthy
range. A statistically significant difference was observed in lipid
levels between the two groups (P<0.05; Table I). These results suggested that an
atherogenic diet resulted in hyperlipidemia.
 | Table I.Blood lipids levels in group A and B
rabbits. |
Table I.
Blood lipids levels in group A and B
rabbits.
| Treatment group | HDL (mmol/l) | LDL (mmol/l) | TG (mmol/l) | CHOL (mmol/l) |
|---|
| Group A |
| Week
0 |
0.42±0.07 |
0.31±0.05 | 0.78±0.09 |
0.97±0.07 |
| Week
4 |
0.56±0.15 |
0.40±0.08 | 0.92±0.16 |
1.15±0.14 |
| Week
8 |
0.49±0.08 |
0.37±0.18 | 0.59±0.24 |
0.81±0.03 |
| Week
12 |
0.48±0.03 |
0.45±0.12 | 1.54±0.10 |
1.27±0.01 |
| Group B |
| Week
0 |
0.41±0.04 |
0.32±0.02 | 0.76±0.08 |
0.59±0.08 |
| Week
4 |
6.65±0.91a |
31.10±12.47a | 0.79±0.36 |
33.48±8.07a |
| Week
8 |
9.00±3.00a |
47.96±10.28a | 1.69±1.37 |
40.26±2.96a |
| Week
12 |
10.93±0.13a |
50.80±2.25a |
4.22±2.88a |
41.27±0.93a |
Pathological sections
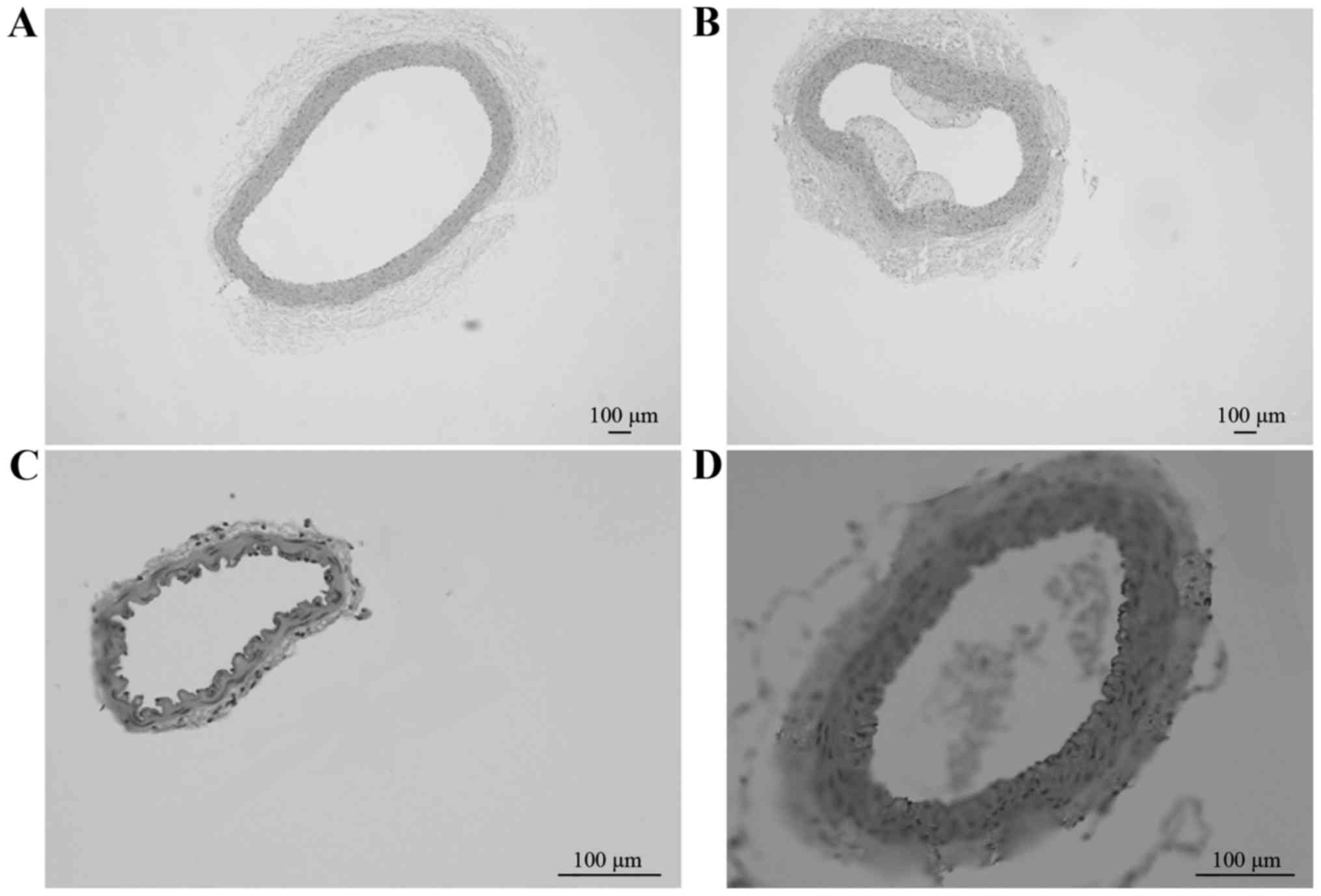
Pathological HE-stained sections of the CCA in
groups A (Fig. 1A; magnification,
×4) and B (Fig. 1B; magnification,
×4), and of the MCA in groups A (Fig.
1C; magnification, ×20) and B (Fig. D; magnification, ×4) revealed that
the degree of AS lesions was increased in the CCA compared with the
MCA between different groups at week 12. Therefore, a simple
high-fat diet may lead to the formation of AS further aggravate the
degree of AS lesions.
Comparison of intra- and extracranial
cerebral artery segments
Previous reports have indicated that IMT
measurements are a representative end point for AS and vascular
disease risk (16). IMT
measurements provide data on the efficacy of novel lipid-modifying
techniques following a high-fat diet. To estimate AS progression
between groups A and B, cross-sectional standardized IMT
measurements were used, and because measurements were standardized,
AS progression estimates were extrapolated from the cross-sectional
data for each group. The IMT of the CCA and MCA were measured in
all animals and combined to a per-subject average. IMT of the CCA
and MCA increased over the 12 weeks in both groups; however, after
4, 8 and 12 weeks of receiving the different diets the CCA and MCA
IMT was significantly increased in group B compared with group A
(P<0.05; Table II). Thus, a
high-fat diet can cause IMT thickness in different cerebral
arteries.
 | Table II.Comparison of cerebral artery
diameters in different groups. |
Table II.
Comparison of cerebral artery
diameters in different groups.
| Treatment
group | Common carotid
artery diameter (µm) | Middle cerebral
artery diameter (µm) |
|---|
| Group A |
| Week
4 | 123.0±5.77 | 31.6±1.3 |
| Week
8 | 129.3±13.4 | 36.1±2.6 |
| Week
12 | 150.2±0.5 | 39.4±4.9 |
| Group B |
| Week
4 |
166.5±5.97a |
58.9±2.04a |
| Week
8 |
176.4±6.6a |
72.2±2.9a |
| Week
12 |
199.9±18.6a |
81.1±2.3a |
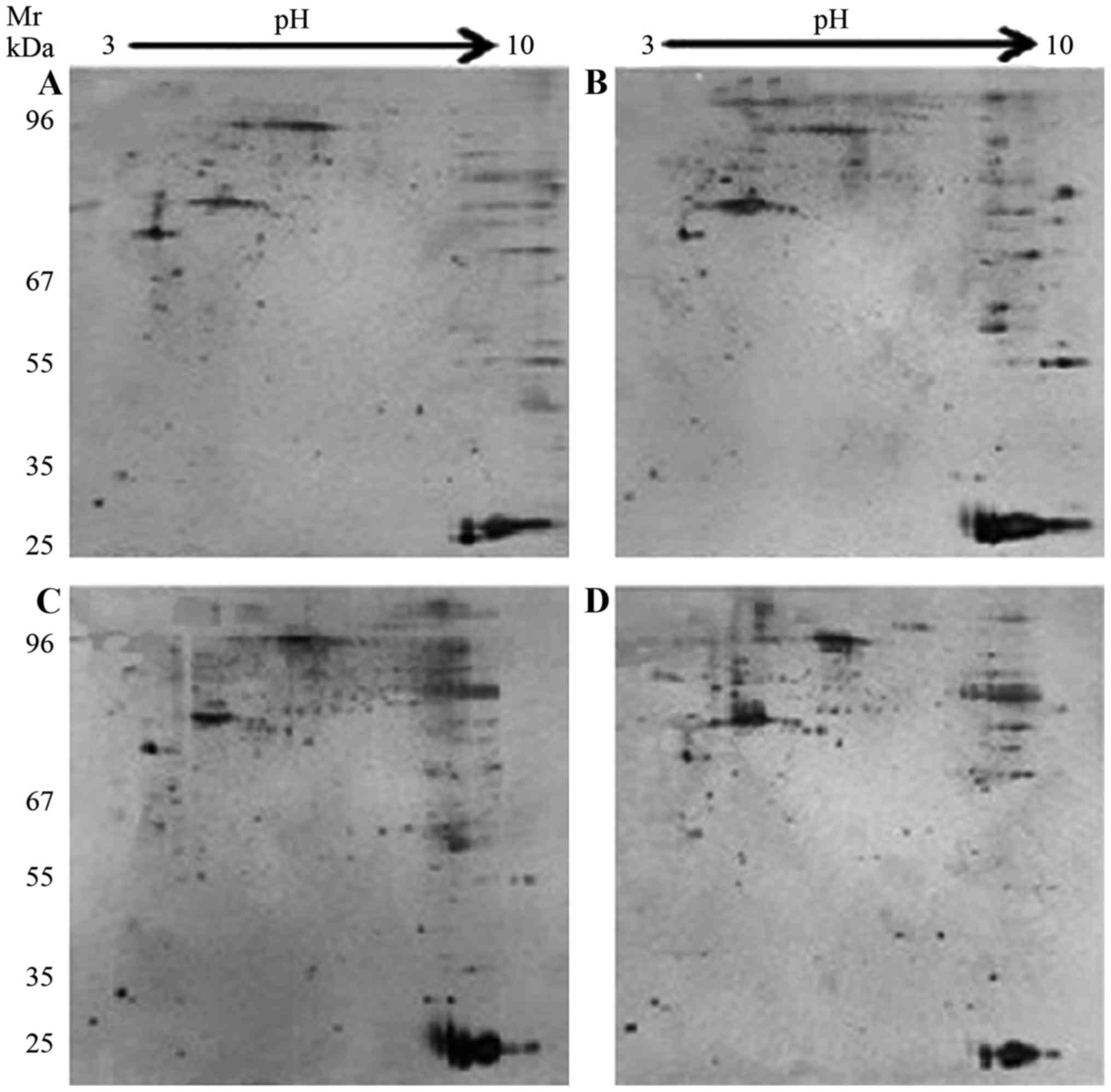
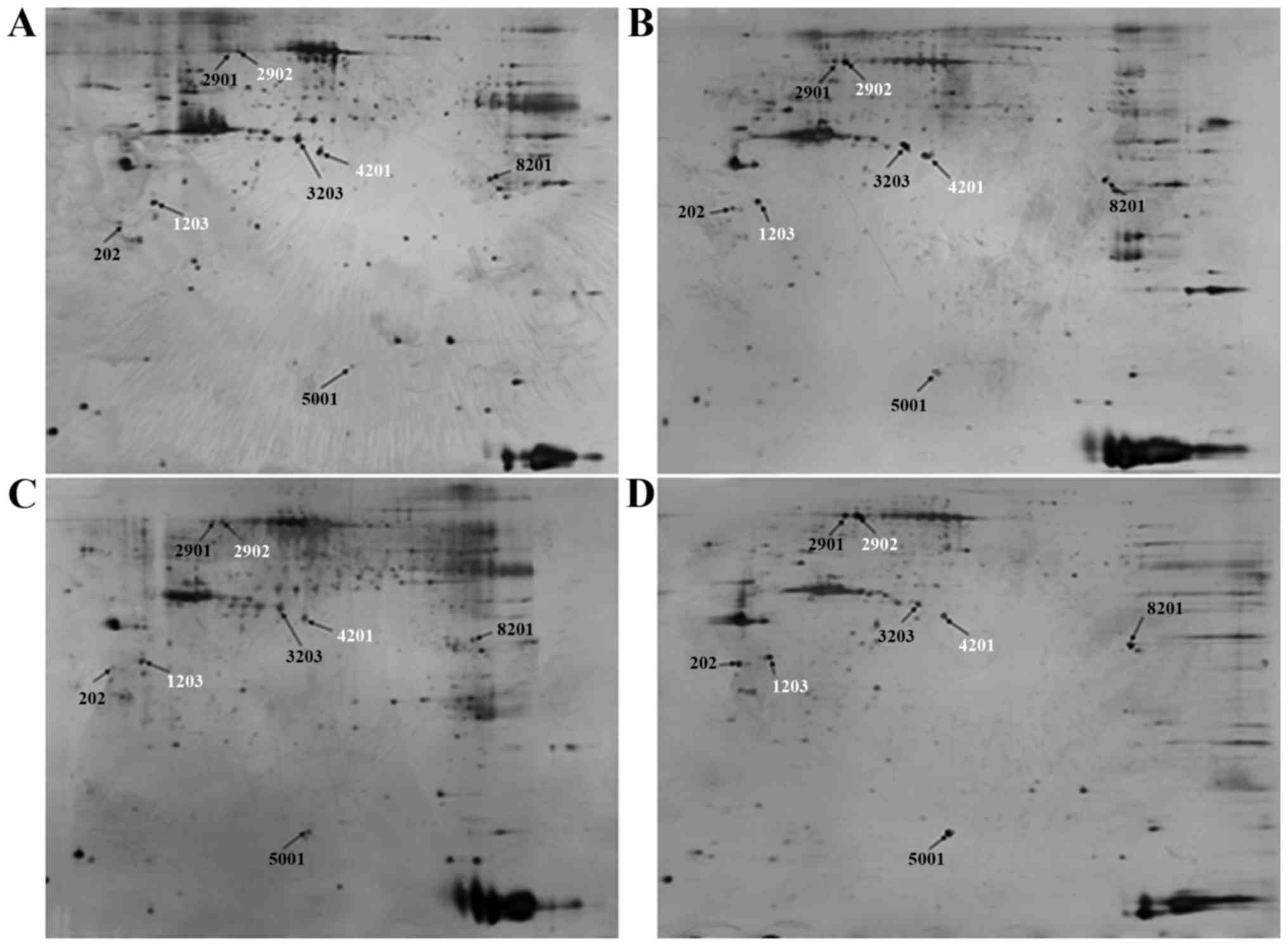
Proteomic analysis
To investigate the key proteins associated with ICAS
and ECAS, 2-DE and silver staining were performed. 2-D gel images
of proteins isolated from the MCA and CCA of groups A and B are
presented in Fig. 2. Total protein
extracts from different groups were separated on 1-D nonlinear IPG
strips (pH 3–10) followed by 2-D SDS-PAGE. Spectrum analysis was
used to identify protein spots, with >2-fold or
<0.5-fold-changes as a standard. Per gel, >439 protein spots
were detected, and well-matched spots and control gels were
analyzed for comparative proteomics. Of the altered proteins, 25
protein expression levels had significant alterations, and were
quantitatively increased enough to be identified by matrix-assisted
laser desorption/ionization (MALDI)-TOF/TOF. MS data were analyzed
using the Mascot search engine. All 25 spots were identified with
high confidence by comparing >70% different degree groups. The
present study identified that protein expression levels of 25
proteins were significantly increased in the MCA and CCA of group B
compared with group A using Mascot retrieval software. A total of 8
differential proteins were observed: Precursor protein (albumin A
chain), TPM1, HSP70, α-smooth muscle actin, β-galactose binding
agglutinin, TMP4 isoform 2, cell keratin 9 and single octylic acid
glyceride-β (Fig. 3; Table III).
 | Table III.Differentially expressed proteins in
intra- and extracranial cerebral arteries. |
Table III.
Differentially expressed proteins in
intra- and extracranial cerebral arteries.
| Spot
no.a | Accession no. | Protein
IDb | kDa | Mascot score |
|---|
| 3203 | gi126723746 | Precursor
protein | 70.861 | 60 |
| 2902 | gi148594078 | Heat shock protein
70 | 71.424 | 105 |
| 4201 | gil49864 | α-smooth muscle
actin | 38.016 | 107 |
| 5001 | gil291414651 | β-galactose binding
agglutinin | 15.170 | 118 |
| 202 | gil4507651 | Tropomyosin α −4
chain isoform 2 | 28.619 | 91 |
| 2901 | gil435476 | Cell keratin 9 | 62.320 | 113 |
| 1203 | gi112448 | Tropomyosin α-1
chain | 32.233 | 49 |
| 8201 | gil395805236 | Singleoctylic acid
glyceride β2 | 69.636 | 53 |
Western blot analysis
Based on the fold-change results and the protein
biological functions, the following proteins were selected for
further evaluation by western blotting: TPM1, HSP70 and α-smooth
muscle actin. GAPDH served as an internal control. Western blot
images of protein expression levels of TPM1, α-smooth muscle actin
and HSP70 from MCA and CCA in groups A and B are presented in
Fig. 4A. Protein expression was
semi-quantitatively evaluated using densitometry (Fig. 4B). The results revealed that the
expression of α-smooth muscle actin and TPM1 chain from CCA in
group B are increased marginally compared with from MCA in group B,
however, the expression of HSP70 from MCA in group B is
significantly increased compared with from CCA in group B. It was
consistent with the results of mass spectrum identification.
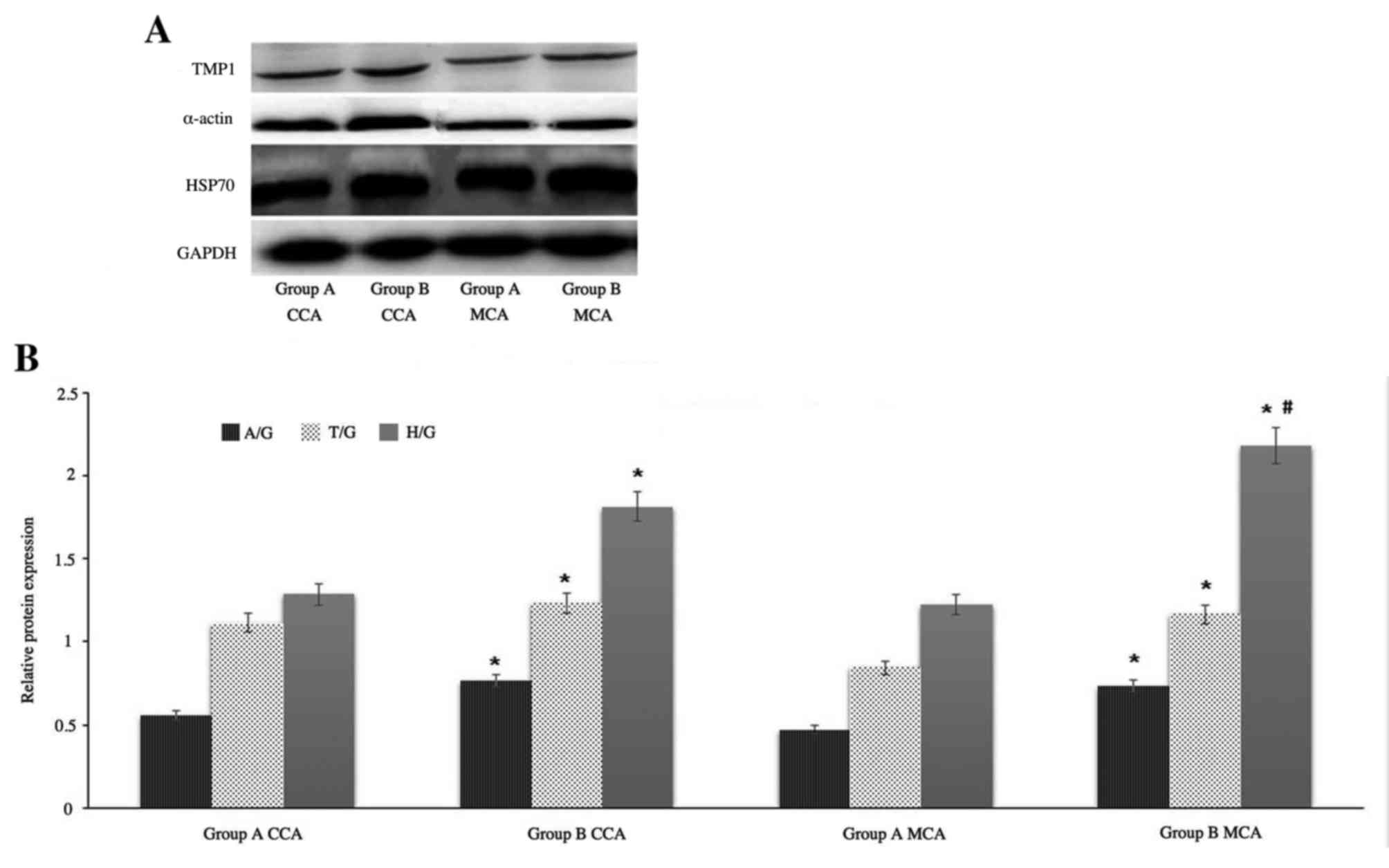 | Figure 4.Western blot analysis. (A)
Representative western blot images and (B) quantification of TPM1,
α-smooth muscle actin and HSP70 protein expression levels in the
CCA and MCA of groups A and B. GAPDH served as an internal control.
Data are expressed as the mean ± standard deviation from two
experiments. *P<0.05 vs. group A CCA and #P<0.05
vs. group B CCA. TPM1, tropomyosin α-1 chain; HSP70, heat shock
protein 70; α-actin, α-smooth muscle actin; CCA, common carotid
artery; MCA, middle cerebral artery; group A, control group; group
B, high-fat diet group; A/G, α-smooth muscle actin/GAPDH; T/G,
TPM1/GAPDH; H/G, HSP70/GAPDH. |
Discussion
Biomarkers including C-reactive protein,
B-typenatriuretic peptides and cardiac troponins have previously
been used by clinicians as predictors of future cardiovascular
events (17,18). However, highly reliable biomarkers
of cerebrovascular events remain to be identified. Existing
proteomic studies on nervous system disease have primarily focused
on penumbral phenomena, movement disorders and neurodegenerative
disease (19–23). In the present study, a proteomics
approach was used to identify if protein expression levels were
altered in response to ICAS and ECAS based on the controversy
between hyperlipidemia and cerebral atherosclerosis. Previous
studies have investigated the differences in risk factors and
stroke mechanisms between ICAS and ECAS, and demonstrated that
hyperlipidemia is more closely associated with ECAS (2), whereas hyperlipidemia was more
closely associated with MCA stenosis (5). The differences of protein expressions
in high-fat-fed rabbits between ICAS and ECAS are still unclear. In
order to provide information about the possible mechanisms of
atherosclerosis progression and identify potential therapeutic
target molecules for ICAS and ECAS, a global proteomic analysis of
different cerebral arteries was performed in the present study.
The present study demonstrated that mean CHOL, LDL,
HDL and TG levels significantly increased by week 12 following a
high-fat diet compared with rabbits that had received a normal
diet. Lipid levels were increased over time in group B compared
with group A; in particular, LDL and CHOL levels were markedly
increased. It has previously been reported that IMT measurements
are a representative end point for AS and vascular disease
(16). To estimate AS progression
following a high-fat diet, IMT of the CCA and MCA were measured in
all rabbits. At week 12, the IMT and AS degree of CCAs and MCAs was
significantly increased in group B compared with group A. These
data indicated the successful establishment of a cerebral AS model
of rabbits with a high-fat diet, and that hyperlipidemia is
positively associated with intra- and extracranial cerebral AS.
Proteomic analysis and comparison of intra- and extracranial
cerebral AS responses to hyperlipidemia in rabbit was performed by
2-DE and MS analyses. The present study identified an average of
439 different proteins in the analyzed samples; 25 proteins were
differentially expressed in group A compared with group B, and 8
spots were significantly different; these 8 spots were
quantitatively increased enough to be identified by MALDI-TOF/TOF
analysis. Only three of the significantly expressed proteins (TPM1,
HSP70 and α-smooth muscle actin) were examined by western blot
analysis due to limited antibody sources.
α-smooth muscle actin is a component of the
contractile apparatus in muscle cells, and is an important marker
of AS plaque formation. Loss of α-smooth muscle actin reduces the
contractile ability of the cell, increases smooth muscle actin
proliferation and leads to excessive neointimal formation with
vascular injury (24,25). The majority of upregulated α-smooth
muscle actin expression may be involved in early remodeling that
occurs prior to marked adaptive alterations (26). The present study demonstrated the
upregulation of α-smooth muscle actin expression in ICAS and ECAS
in rabbits that received a high-fat diet compared with rabbits on
normal diet, and the expression of α-smooth muscle actin from
extracranial cerebral arteries was significantly increased compared
with intracranial cerebral arteries. Increased expression levels of
α-smooth muscle actin following a high-fat diet may enhance
inflammation of injured vessels, leading to proliferation of vessel
smooth muscle actin. The increase of vessel smooth muscle actin and
intimal hyperplasia of arteries is the primary pathological event
leading to stenosis.
Simoneau et al (27) demonstrated that TPM1 may regulate
endothelial barrier integrity in response to oxidative stress
conditions, and phosphorylation of TPM1 at Ser283 regulates
endothelial permeability and transendothelial migration of cancer
cells. The primary finding of the present study was that TPM1
expression levels were abundantly increased in CCAs and MCAs by a
high-fat diet. TPM1 expression was semi-quantitatively evaluated
using densitometry, and the results demonstrated that the
expression of TPM1 in CCA was increased marginally compared with
MCA in group B; the average intensity of TPM1/GAPDH in CCA was
1.232 and the average intensity of TPM1/GAPDH in MCA was 1.164,
which indicates that extracranial cerebral arteries are more
susceptible to endothelial damage in AS than intracranial cerebral
arteries.
HSP70 has been demonstrated to suppress inflammation
and tissue damage via an underlying mechanism that involves an
enhanced regulatory response mediated by antigen-specific
interleukin-10 production (28).
Overexpression of HSP70 serves an important role in cell death and
has neuroprotective effects via an anti-inflammatory underlying
mechanism, and has been demonstrated to be positively associated
with ICAS and ECAS (29,30). However, whether ICAS or ECAS is
more closely associated with altered expression levels of HSP70
remains to be elucidated. Furthermore, expression levels of HSP70
from intracranial cerebral arteries were significantly increased
compared with extracranial cerebral arteries in this study, in
contrast to the expression patterns of TPM1 and α-smooth muscle
actin. Therefore, HSP70 may have AS-protective properties via
anti-inflammatory underlying mechanisms in ICAS compared with ECAS.
This finding is consistent with a previous study, which examined
the proteomic analysis of stable and unstable carotid
atherosclerotic plaques (6). The
findings of the present study may partly explain previous
contradictory observations on the differences in cerebral AS
mechanisms.
The present study demonstrated that hyperlipidemia
exerts varying influences on intra- and extracranial cerebral
arteries. Proteomic analysis revealed that 8 proteins exhibited
varying degrees of differential expression in intra- and
extracranial cerebral arteries, which are involved in oxidative
stress, tumor metastasis, inflammation, cholesterol transport, cell
apoptosis and adhesion. Alterations in protein expression levels of
α-smooth muscle actin, TPM1 and HSP70 may be involved in the
differences between ICAS and ECAS. The underlying mechanisms of
these proteins on the formation of AS require clarification.
Further investigating the pathogenesis of differential protein
expression levels between intra- and extracranial cerebral arteries
may facilitate the identification of novel biological markers for
the diagnosis and treatment of cerebral arteriosclerosis.
Acknowledgements
The present study was supported by the Science and
Technology Foundation of Shanghai (grant no. 13JC1407103
2013–2016), Foundations for the Young Academic Leader of Tongji
University (grant no. 1500144001), the Health Science and
Technology of Pudong Municipal Commission of Health and Family
Planning of Shanghai (grant no. PW2013D-13) and Puxiu Plan of
Shanghai Pudong Hospital (grant no. PX201501).
References
|
1
|
Kim J, Cha MJ, Lee DH, Lee HS, Nam CM, Nam
HS, Kim YD and Heo JH: The association between cerebral
atherosclerosis and arterial stiffness in acute ischemic stroke.
Atherosclerosis. 219:887–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JS, Nah HW, Park SM, Kim SK, Cho KH,
Lee J, Lee YS, Kim J, Ha SW, Kim EG, et al: Risk factors and stroke
mechanisms in atherosclerotic stroke: Intracranial compared with
extracranial and anterior compared with posterior circulation
disease. Stroke. 43:3313–3318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soloperto G and Casciaro S: Progress in
atherosclerotic plaque imaging. World J Radiol. 4:353–371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prabhakaran S and Romano JG: Current
diagnosis and management of symptomatic intracranial
atherosclerotic disease. Curr Opin Neurol. 25:18–26. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong KS, Ng PW, Tang A, Liu R, Yeung V and
Tomlinson B: Prevalence of asymptomatic intracranial
atherosclerosis in high-risk patients. Neurology. 68:2035–2038.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malaud E, Piquer D, Merle D, Molina L,
Guerrier L, Boschetti E, Saussine M, Marty-Ané C, Albat B and Fareh
J: Carotid atherosclerotic plaques: Proteomics study after a
low-abundance protein enrichment step. Electrophoresis. 33:470–482.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tu Z, Huang D, Yang J, Ojha R, Xiao Y, Liu
R, Du C, Shen N, An H, Yu F, et al: Effect of dyslipidemia on
intima-media thickness of intra- and extracranial atherosclerosis
by regulating the expression of hsp70 in rabbits. Int J Clin Exp
Med. 8:5446–5453. 2015.PubMed/NCBI
|
|
8
|
Barderas MG, Vivanco F and Alvarez-Llamas
G: Vascular proteomics. Methods Mol Biol. 1000:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dol F, Schaeffer P, Lamarche I, Mares AM,
Chatelain P and Herbert JM: Effect of SR 33805 on arterial smooth
muscle cell proliferation and neointima formation following
vascular injury. Eur J Pharmacol. 280:135–142. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richardson M, Hatton MW, Buchanan MR and
Moore S: Wound healing in the media of the normolipemic rabbit
carotid artery injured by air drying or by balloon catheter
de-endothelialization. Am J Pathol. 137:1453–1465. 1990.PubMed/NCBI
|
|
11
|
Moller MJ, Qin Z and Toursarkissian B:
Tissue markers in human atherosclerotic carotid artery plaque. Ann
Vasc Surg. 26:1160–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piubelli C, Carboni L, Becchi S, Mathé AA
and Domenici E: Regulation of cytoskeleton machinery, neurogenesis
and energy metabolism pathways in a rat gene-environment model of
depression revealed by proteomic analysis. Neuroscience.
176:349–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tannu NS, Howell LL and Hemby SE:
Integrative proteomic analysis of the nucleus accumbens in rhesus
monkeys following cocaine self-administration. Mol Psychiatry.
15:185–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bu Q, Yang Y, Yan G, Hu Z, Hu C, Duan J,
Lv L, Zhou J, Zhao J, Shao X, et al: Proteomic analysis of the
nucleus accumbens in rhesus monkeys of morphine dependence and
withdrawal intervention. J Proteomics. 75:1330–1342. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shevchenko A, Wilm M, Vorm O and Mann M:
Mass spectrometric sequencing of proteins silver-stained
polyacrylamide gels. Anal Chem. 68:850–858. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Groot E, Hovingh GK, Wiegman A, Duriez
P, Smit AJ, Fruchart JC and Kastelein JJ: Measurement of arterial
wall thickness as a surrogate marker for atherosclerosis.
Circulation. 109:(23 Suppl 1). III33–III38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Apple FS, Collinson PO, et al: IFCC Task
Force on Clinical Applications of Cardiac Biomarkers. Analytical
characteristics of high-sensitivity cardiac troponin assays. Clin
Chem. 58:54–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Angelantonio E, Chowdhury R, Sarwar N,
Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N and
Danesh J: B-type natriuretic peptides and cardiovascular risk:
Systematic review and meta-analysis of 40 prospective studies.
Circulation. 120:2177–2187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abraham JD, Calvayrac-Pawlowski S, Cobo S,
Salvetat N, Vicat G, Molina L, Touchon J, Michel BF, Molina F,
Verdier JM, et al: Combined measurement of PEDF, haptoglobin and
tau in cerebrospinal fluid improves the diagnostic discrimination
between alzheimer's disease and other dementias. Biomarkers.
16:161–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rashedinia M, Lari P, Abnous K and
Hosseinzadeh H: Proteomic analysis of rat cerebral cortex following
subchronic acrolein toxicity. Toxicol Appl Pharmacol. 272:199–207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zgavc T, Hu TT, Van de Plas B, Vinken M,
Ceulemans AG, Hachimi-Idrissi S, Sarre S, Michotte Y and Arckens L:
Proteomic analysis of global protein expression changes in the
endothelin-1 rat model for cerebral ischemia: Rescue effect of mild
hypothermia. Neurochem Int. 63:379–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu W, Wan X, Liu B, Rong X, Zhu L, Li P,
Li J, Wang L, Cui L and Wang X: Specific changes of serum proteins
in Parkinson's disease patients. PLoS One. 9:e956842014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alberio T, Bucci EM, Natale M, Bonino D,
Di Giovanni M, Bottacchi E and Fasano M: Parkinson's disease plasma
biomarkers: An automated literature analysis followed by
experimental validation. J Proteomics. 90:107–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnoldi R, Hiltbrunner A, Dugina V, Tille
JC and Chaponnier C: Smooth muscle actin isoforms: A tug of war
between contraction and compliance. Eur J Cell Biol. 92:187–200.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papke CL, Cao J, Kwartler CS, Villamizar
C, Byanova KL, Lim SM, Sreenivasappa H, Fischer G, Pham J, Rees M,
et al: Smooth muscle hyperplasia due to loss of smooth muscle
α-actin is driven by activation of focal adhesion kinase, altered
p53 localization and increased levels of platelet-derived growth
factor receptor-β. Hum Mol Genet. 22:3123–3137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kern S, Feng HZ, Wei H, Cala S and Jin JP:
Up-regulation of alpha-smooth muscle actin in cardiomyocytes from
non-hypertrophic and non-failing transgenic mouse hearts expressing
N-terminal truncated cardiac troponin I. FEBS Open Bio. 4:11–17.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simoneau B, Houle F and Huot J: Regulation
of endothelial permeability and transendothelial migration of
cancer cells by tropomyosin-1 phosphorylation. Vasc Cell. 4:182012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wieten L, Berlo SE, Ten Brink CB, van
Kooten PJ, Singh M, van der Zee R, Glant TT, Broere F and van Eden
W: IL-10 is critically involved in mycobacterial HSP70 induced
suppression of proteoglycan-induced arthritis. PLoS One.
4:e41862009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tirapelli DP, Carlotti CG Jr, Leite JP,
Tirapelli LF and Colli BO: Expression of HSP70 in cerebral ischemia
and neuroprotetive action of hypothermia and ketoprofen. Arq
Neuropsiquiatr. 68:592–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verma P, Pfister JA, Mallick S and D'Mello
SR: HSF1 protects neurons through a novel trimerization- and
HSP-independent mechanism. J Neurosci. 34:1599–1612. 2014.
View Article : Google Scholar : PubMed/NCBI
|