Introduction
Chronic liver injury, playing a key role in the
pathogenesis of cirrhosis, liver failure or hepatocellular
carcinoma can be caused by the infection, metabolism, alcohol and
drugs (1,2). It has been confirmed that the
apoptosis of hepatocyte induced by immune response in chronic liver
injury is one of the main reasons for the onset or development of
liver diseases such as viral hepatitis, autoimmune liver disease,
post-orthotopic liver transplantation, alcoholic liver disease,
drug-induced liver injury and non-alcoholic fatty liver (3). The inhibition of hepatocyte apoptosis
in the process of liver injury becomes an important strategy for
the treatment of liver diseases clinically.
Sphingolipids are enriched in lipid rafts of
cellular membranes and contribute to important physiological
cellular processes including cell proliferation, differentiation
and apoptosis (4). Recently, it
has been confirmed that sphingolipids play roles in regulating
liver injury and regeneration, which may have a great impact on the
development of novel therapeutic modalities for a variety of liver
diseases (5). Our previously
series of studies showed that sphingolipids and their metabolism
played important roles in the development of liver diseases
(6–10). Based on our results, we have found
that the increase of plasma hexosylceramide in chronic hepatitis C
patients significantly correlated with hepatic necroinflammation,
and it showed a close relationship with liver inflammation or
fibrosis. Glycosphingolipids include a family of heterogeneous
lipids which regulate cell death pathways through mitochondria, or
endoplasmic reticulum to mediate apoptosis, endoplasmic reticulum
stress, autophagy, and necroptosis (11). Hexosylceramide is formed by
glycosylation of ceramide caused by glucosylceramide synthase (GCS)
which linked glucose to 1-hydroxy group of ceramide. In the
metabolism process of glycosphingolipids, GCS which is encoded by
the UDP-glucose ceramide glucosyltransferase (UGCG) gene, is a key
enzyme catalyzing glycosylation of ceramide to regulate the
physiological activity of cells by influencing the metabolic
balance of ceramide and glycosphingolipids (12). However the detailed mechanisms of
the glycosphingolipids' metabolism in the proliferation and
apoptosis of liver cells remained poorly understood.
Therefore, on the basis of our previous studies, the
present study speculated that there might be a close relationship
between GCS and the proliferation or apoptosis of liver cells. This
study was aimed to evaluate the role of GCS in the proliferation
and apoptosis of liver cells and explore the underlying
mechanisms.
Materials and methods
Cell culture
Human liver cells line HL-7702 was a persevered cell
line in Artificial Liver Center at Beijing You'an Hospital, Capital
Medical University (Beijing, China). The cells were cultured in
RPMI-1640 medium containing 10% fetal bovine serum (both from
Hyclone, Logan, UT, USA) in 37°C and 5% CO2 cell culture
incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
medium was changed every other day after plating and the cells were
digested with 0.05% trypsin when they were 80–90% confluent.
siRNA transfection
The HL-7702 cells were transfected with siRNA when
the cells were 80% confluent. The siRNA and Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) were diluted
separately and mixed according to the product instructions. The
serum-free medium containing siRNA-Lipofectamin 2000 (the
concentration of siRNA: 0.12 µmol/l) was left to incubate for 25
min at room temperature, and then the siRNA-Lipofectamin 2000 mix
was put into the culture medium of the adherent cells.
Proliferation assay
The cells were added into 96-well plate (Corning
Incorporated, Corning, NY, USA) as 8,000 cells/well with RPMI-1640
medium containing 10% fetal bovine serum (both from Hyclone). After
that the cells were 80% confluent, the medium was without fetal
bovine serum in the blank control group. The cells with
Lipofectamin 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) or
with Lipofectamin 2000-siRNA max were cultured for 24 h, and then
the supernatant was discarded. MTT solution (5 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into the
cells and the cells were incubated for 5 h in 37°C. The wave length
of 490 nm was chosen to determine the optical density value of each
well.
Apoptosis assay
Cells (1×105/well) was added into 96-well
plate. The HL-7702 cells were cultured with siRNA-Lipofectamin 2000
mix for 6 h in 37°C and then the medium was discarded. The cells
subsequently continued to be cultured in the serum-free medium for
24 h. The binding buffer, Annexin V-FITC, or PI (both from Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) was added into each well
and the reaction at room temperature was done for 5 min in dark,
the intracellular fluorescence was observed by fluorescence
microscopy (Nikon Corporation, Tokyo, Japan).
ELISA assay
The HL-7702 cells with a density of
1×106/ml were cultured by RPMI-1640 medium with 10%
fetal bovine serum in culture box with the diameter of 6 cm. The
cells were treated with or without siRNA (0.12 µmol/l). After the
transfection of siRNA into the cells for 24 h, the cell cultural
supernatant was collected and detected by ELISA (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) method according to
instructions. The 450 nm wavelength was employed to detect the
absorbance of each hole and the concentrations of tumor necrosis
factor (TNF) α and cytochrome c were calculated.
RT-qPCR
Cells (1×105/well) were put into 24-well
plate with 1 ml medium containing 10% fetal bovine serum. Total RNA
from hepatic cells were extracted using TRIzol lysate and
chloroform on the ice after siRNA being transfected into the cells
for 24 h. The precipitation was formed at −20°C for 2 h after
centrifugation and UV spectrophotometry (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) using absorbance at 260 and 280 nm
(A260/280) was used to determine the total RNA concentration and
purity. And then the RNA was reversely transcripted into cDNA using
the PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. Real-time PCR
amplification was carried out by the SYBR Green-based PCR Master
Mix (Takara Bio, Inc.), which was used for the detection of mRNA
expression. Gene expression levels were calculated after
normalization to GAPDH. The ΔΔCt was calculated as follows:
ΔCttreated-ΔCtcontrol. And 2−ΔΔCt
was calculated to represent the relative mRNA expression of target
genes. The sequences of primers are presented in Table I.
 | Table I.Primer sequences for qPCR
analysis. |
Table I.
Primer sequences for qPCR
analysis.
| Gene | Primer sequences
(5′→3′) |
|---|
| Human Bcl-2 | Positive sequence:
GTGGCC |
|
| TTCTTTGAGTTCGG |
|
| Reverse sequence:
GGCCGT |
|
| ACAGTTCCACAAAG |
| Human Bax | Positive sequence:
ATGAAG |
|
| ACAGGGGCCCTTTT |
|
| Reverse sequence:
GCAATC |
|
| ATCCTCTGCAGCTC |
| Human caspase-3 | Positive sequence:
ACTGGA |
|
| CTGTGGCATTGAGA |
|
| Reverse sequence:
GCACAA |
|
| AGCGACTGGATGAA |
| Human GAPDH | Positive sequence:
CCAGAA |
|
| CATCATCCCTGCCT |
|
| Reverse sequence:
CCTGCT |
|
| TCACCACCTTCTTG |
| Human UGCG siRNA | Positive sequence:
CGCGAA |
|
| UCCAUGACAAUAUTT |
|
| Reverse sequence:
AUAUUG |
|
| UCAUGGAUUCGCGTT |
| Negative control
siRNA | Positive sequence:
GCGACG |
|
|
AUCUGCCUAAGAUdTdT |
|
| Reverse sequence:
AUCUUA |
|
|
GGCAGAUCGUCGUCGCdTdT |
| Human GCS | Positive sequence:
TTCATG |
|
| TGTCATTGCCTGGC |
|
| Reverse sequence:
AGCGTA |
|
| ATCTGTAGCGACCA |
Western blot analysis
The HL-7702 cells with a density of
3.0–3.5×105/ml were cultured in 6 cm diameter tissue
culture dish (Corning Incorporated) overnight, and the supernatant
was removed. The cells were treated with or without siRNA (0.12
µmol/l), the cells were cultured for 6 h and then were cultured in
serum-free medium for 24 h. The cell suspension was centrifuged by
2,000 rpm for 3–5 min, and were lysed by the RIPA with PMSF
(RIPA:PMSF=100:1) on the ice for 10 min, and then in centrifuge by
12,000 rpm for 3–5 min. Bicinchoninic acid methods was employed for
determination of protein concentration. Equivalent amounts of
protein were subjected to polyacrylamide gel electrophoresis,
followed by electroblotting onto PVDF membrane (Bio-Rad
Laboratories, Inc.). PVDF membrane was incubated with primary
antibody by 1:800 Caspase-3 antibody (Cell Signaling Technology,
Inc., Danvers, MA, USA) at 4°C overnight. Then the PVDF was in
1:2,000 anti-rabbit antibody (Beijing Zhongshanjinqiao Co., Ltd.,
China), incubation for 1 h at room temperature, washed with TBST
for 3 times. The protein on membranes was visualized by ECL western
blotting kit (Thermo Fisher Scientific, Inc.) The densitometric
analyses of images were performed using ImageJ software 1.46
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS software version 19.0 (IBM Corp., Armonk, NY,
USA) was used for data analysis. According to the data distribution
type, the two groups of continuous variables were analyzed by
independent sample t test or Mann-Whitney test. P<0.05 was
statistically significant between the two groups.
Results
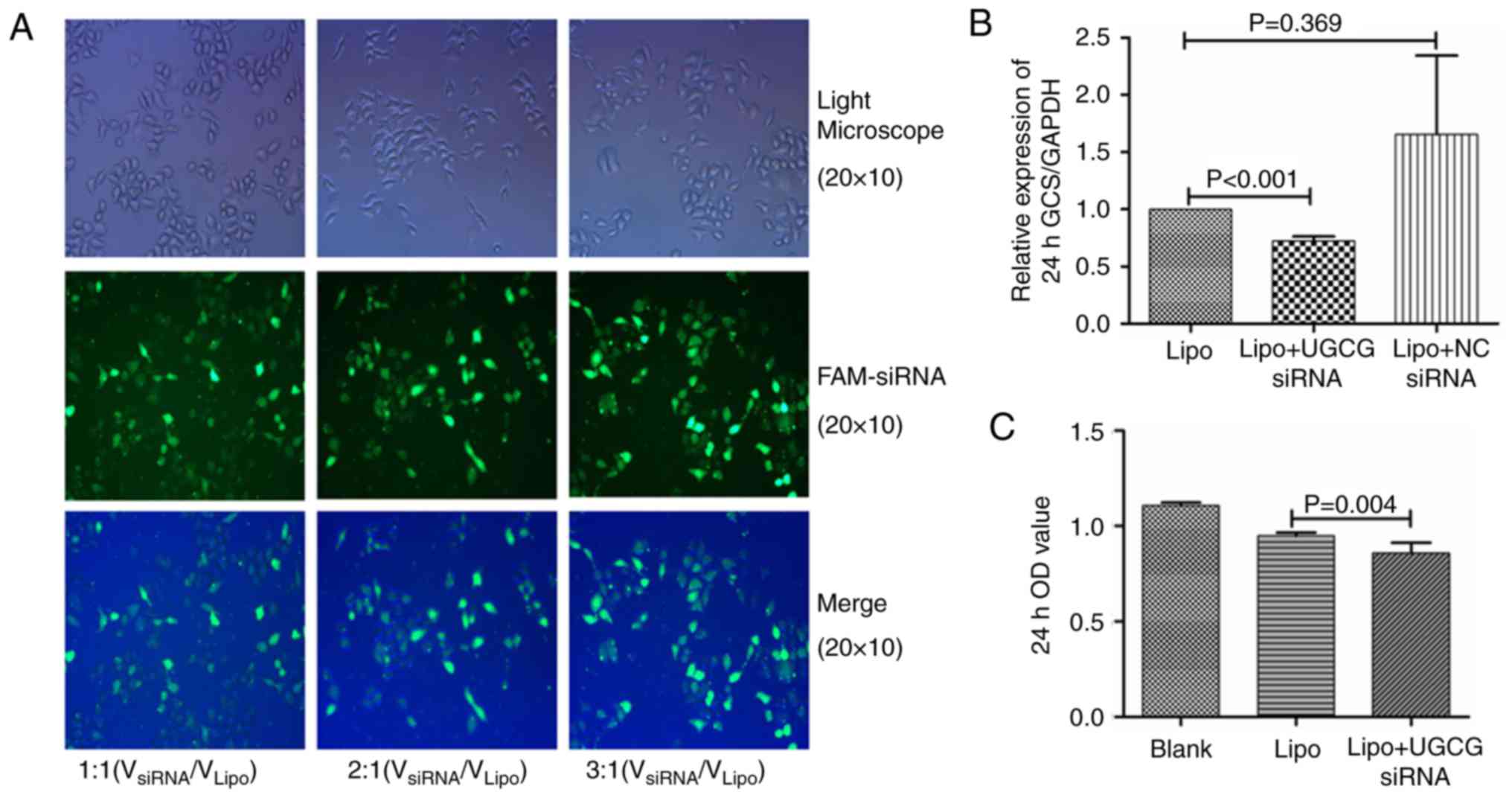
UGCG siRNA inhibits the GCS gene and
the proliferation of hepatic cells
According to our transfection procession, the
optimal concentrations of siRNA was 0.12 µmol/l
(VsiRNA/VLipo=3:1, Fig. 1A), which showed higher transfection
efficiency. HL-7702 cells were transfected by UGCG siRNA and
negative control siRNA for 24 h. The PCR results showed that
compared with the transfection reagent group, the expression of GCS
gene was significantly suppressed by UGCG siRNA (P<0.05), while
the expression of GCS gene did not change significantly in negative
control siRNA group (P>0.05) (Fig.
1B). The results of MTT methods showed the proliferation of
hepatic cells was significantly inhibited after the treatment of
UGCG siRNA (P<0.05) compared with the transfection reagent
(Fig. 1C).
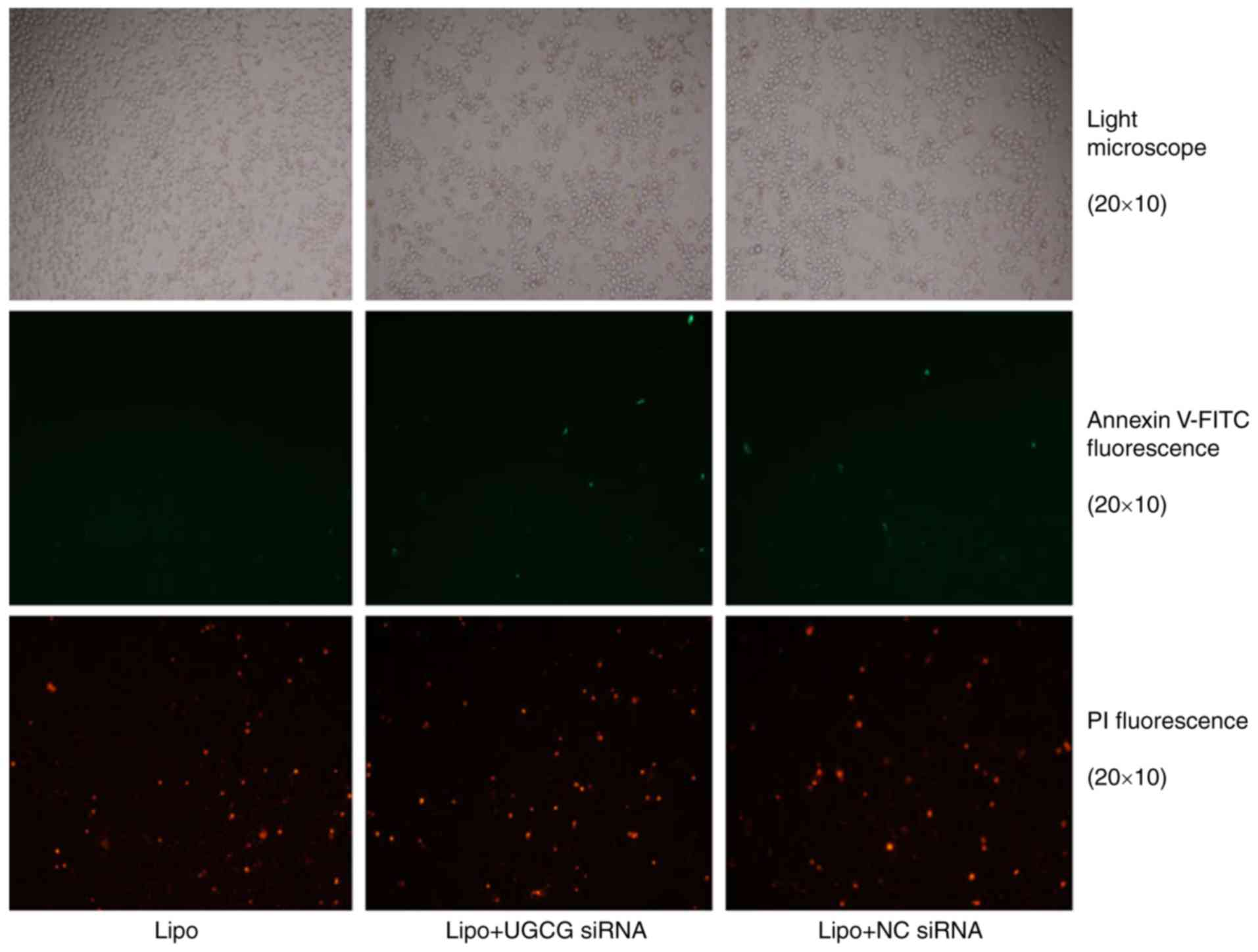
Hepatic cells undergo apoptosis
following UGCG siRNA treatment
The results showed that hepatic cells had apoptosis
phenomenon, especially late apoptosis was obvious, but compared
with the transfection reagent Lipofectamin 2000, the early and late
apoptosis of liver cells differed slightly significantly in UGCG
siRNA transfection group (Fig.
2).
UGCG siRNA exhibits no effect on the
secretion of TNF α and cytochrome c
In order to observe the expression of GCS gene on
the secretion of TNF α and cytochrome c, the concentrations
of TNF α and cytochrome c in cell culture supernatant were
detected by ELISA after the treatment of UGCG siRNA for 24 h. The
results showed the concentrations of TNF α and cytochrome c
did not change significantly between UGCG siRNA group and the
transfection reagent Lipofectamin 2000 (P>0.05) (Fig. 3).
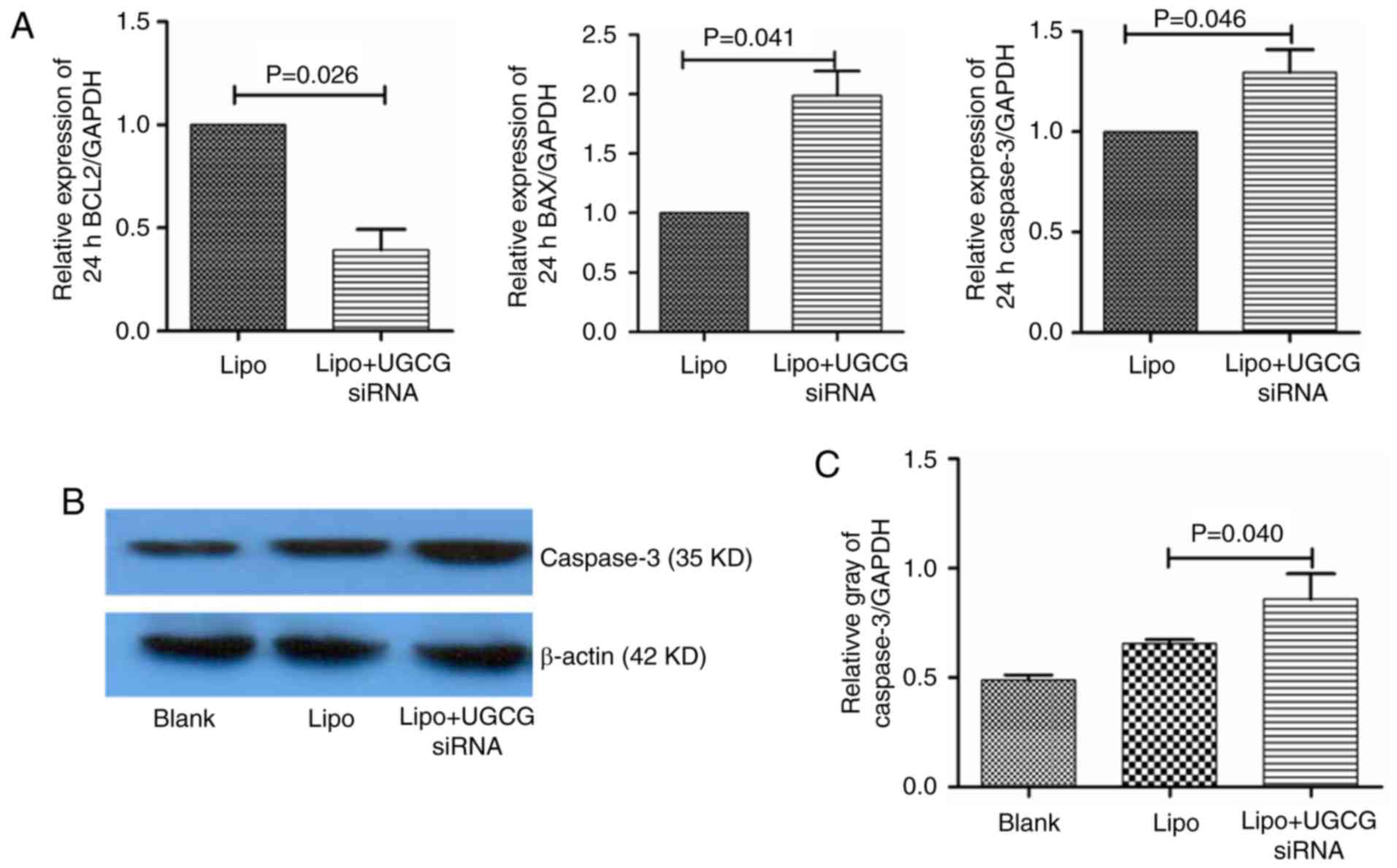
UGCG siRNA regulates the expression of
key apoptosis related-molecules in hepatic cells
In order to observe the effect of GCS on apoptosis
signaling pathway in hepatic cells, we observed the gene expression
of the key apoptosis related-molecules. The results showed that
compared with the transfection reagent Lipofectamin 2000, the
expression of Bcl-2 mRNA decreased (P<0.05), Bax mRNA increased
(P<0.05), the expression of caspase-3 mRNA also increased
(P<0.05) in hepatic cells. As for the protein of caspase-3, we
also observed that after transfection of UGCG siRNA for 24 h,
compared to transfection reagent group, the expression of caspase-3
protein upregulated in hepatic cells (Fig. 4).
Discussion
The present study is the first time to verify that
the inhibition of expression of GCS gene may lead to the apoptosis
of hepatic cells. In the design of this study, we used siRNA
technology to target GCS gene expression, and on the basis of
specific inhibition of GCS expression, our aims were to explore the
effect of GCS on the proliferation and apoptosis of hepatic cells
and the possible mechanisms. Based on the present results, this
study suggested that GCS might be involved in the cell cycle of
hepatic cells.
In sphingolipid metabolism, GCS is the key enzyme
catalyzing the glycosylation of ceramide, by regulating the balance
of ceramide and glycosphingolipid to regulate the physiological
activity of cells. During the glycosylation of ceramide, glucose is
attached to the 1-hydroxyl group of ceramide to produce
glucoseceramide (13,14). GCS, which expresses in the
eukaryotic cell membranes, is an intrinsic membrane protein encoded
by the UGCG gene. The decreased expression of GCS leads to the
decreased glycosylation of ceramide or elevated ceramide levels,
and the elevated ceramide can trigger the endogenous or exogenous
apoptosis. The main pathway for the regulation of cell apoptosis by
ceramide activation is the endoplasmic reticulum and the
mitochondrial pathways (15),
which are involved in the TNF mediated-apoptosis pathway (16). The present study verified the
targeting effect of UGCG siRNA sequence for GCS gene, which could
inhibit the expression of GCS gene. The results showed that the
proliferation of hepatic cells was obviously inhibited, and the
cell activity of the hepatocytes after being transfected with UGCG
siRNA obviously decreased. Followed by AnnexinV-FITC/PI double
staining, the apoptosis of hepatic cells was also affected. Despite
that the difference between the early and late apoptosis of hepatic
cells was not significant, however, the trend of early and late
apoptosis increased after the treatment of UGCG siRNA. This
suggested that the proliferation or apoptosis of hepatic cells
might be related to the inhibition of GCS, and the inhibition of
the enzyme might lead to the increased ceramide levels, which
contributed to the process of decreased activity of
hepatocytes.
In order to study the mechanisms of GCS gene
expression in the apoptosis of liver cells, the present study also
observed the effect of UGCG siRNA on the Bcl-2 apoptosis pathway.
As an anti-apoptotic effector, Bcl-2 could form a two-polymer with
Bax to inhibit apoptosis, while Bax could increase the effect of
Bcl-2 to promote apoptosis (17).
It has been confirmed that the formation of Bax channels promotes
the release of cytochrome c and enter the cytoplasm, which
can make the separation of Bcl-2 and Apaf1, and then activate
caspase to induce apoptosis (18,19).
Our study found that after the inhibition of GCS gene expression,
the upregulation of gene expression of Bcl-2 contributed to the
decrease of Bax gene expression. In addition, caspase-3 is the
downstream of the caspase cascade, and is one of the most important
proteases to perform the key function of apoptosis. The present
study found that after the inhibition of GCS gene expression, the
gene and protein expression of caspase-3 were both significantly
increased. It might be due to the altered expression of
apoptosis-related genes, which resulted in the execution of
apoptosis effect.
TNF is involved in the proliferation and apoptosis
of hepatocytes in the pathological processes of various liver
diseases such as alcoholic liver disease, fulminant hepatitis,
viral hepatitis and fatty hepatitis (20). The recent study showed that
sphingomyelinase in sphingolipid metabolism was crucially involved
in the regulation of TNF-induced liver cells death (21). Our study showed the extracellular
concentration of TNF α did not change significantly after the
treatment of UGCG siRNA. It was speculated that the apoptosis
induced by GCS might not depend on the TNF α. Moreover, it has been
confirmed that the apoptotic pathways are divided into endogenous
and exogenous ways (22). In the
endogenous pathway, the permeabilization of mitochondrial membrane
can lead to the release of cytochrome c into the cytosol,
and then interact with caspase to start a series of early apoptotic
responses, the permeabilization and Bcl-2 are involved in the
mitochondrial membrane contributing to the release of cytochrome
c (23–25). In the present study, no changes of
cytochrome c were found in the groups. This might indirectly
reflect the apoptosis induced by the endogenous expression of GCS
gene had no relevance with cytochrome c-mediated endogenous
apoptosis, however the further study is needed to verify the
detailed mechanisms.
However, there are still issues to be addressed
concerning other mechanisms which are not included in our study.
The limitation of this study is paying no attention to other
apoptotic related-pathways and shows no data about the effect of
GCS on the apoptotic pathways in vivo. This is needed to be
clarified in future.
In conclusion, the present study performed UGCG
siRNA interference in vitro in the hepatic cells, and
initially found that the decreased expression of GCS could
contribute to the inhibition of proliferation and the increased
apoptosis of hepatic cells, which was related to Bcl-2
mediated-apoptosis. Our study provided new clues for the role of
GCS in the pathogenesis of apoptosis of hepatic cells. It will be
needed to focus on the effect of abnormal metabolism of
glycosphingolipid and other sphingolipid related-apoptosis pathway
in the hepatic cells in the further research.
Acknowledgements
This work was supported by the Beijing Municipal
Administration of Hospitals Ascent Plan (DFL20151601), the Fund of
the First Hospital of Lanzhou University (ldyyyn2017-17) and the
National Science and Technology Key Project on ‘Major Infectious
Diseases such as HIV/AIDS, Viral Hepatitis Prevention and
Treatment’ (2017ZX10202203-006, 2017ZX10302201-004,
2017ZX10203201-005, 2017ZX10201201).
References
|
1
|
Luedde T and Schwabe RF: NF-κB in the
liver-linking injury, fibrosis and hepatocellular carcinoma. Nat
Rev Gastroenterol Hepatol. 8:108–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YA, Wallace MC and Friedman SL:
Pathobiology of liver fibrosis: A translational success story. Gut.
64:830–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heymann F and Tacke F: Immunology in the
liver-from homeostasis to disease. Nat Rev Gastroenterol Hepatol.
13:88–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nojima H, Freeman CM, Gulbins E and
Lentsch AB: Sphingolipids in liver injury, repair and regeneration.
Biol Chem. 396:633–643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JF, Qu F, Zheng SJ, Ren JY, Wu HL, Liu
M, Liu H, Ren F, Chen Y, Zhang JL and Duan ZP: Plasma sphingolipids
as potential indicators of hepatic necroinflammation in patients
with chronic hepatitis C and normal alanine aminotransferase level.
PLoS One. 9:e950952014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li JF, Qu F, Zheng SJ, Wu HL, Liu M, Liu
S, Ren Y, Ren F, Chen Y, Duan ZP and Zhang JL: Elevated plasma
sphingomyelin (d18:1/22:0) is closely related to hepatic steatosis
in patients with chronic hepatitis C virus infection. Eur J Clin
Microbiol Infect Dis. 33:1725–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li JF, Qu F, Zheng SJ, Ren F, Wu HL, Liu
M, Ren JY, Chen Y, Duan ZP and Zhang JL: Plasma sphingolipids:
Potential biomarkers for severe hepatic fibrosis in chronic
hepatitis C. Mol Med Rep. 12:323–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng SJ, Qu F, Li JF, Zhao J, Zhang JY,
Liu M, Ren F, Chen Y, Zhang JL and Duan ZP: Serum sphingomyelin has
potential to reflect hepatic injury in chronic hepatitis B virus
infection. Int J Infect Dis. 33:149–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JY, Qu F, Li JF, Liu M, Ren F, Zhang
JY, Bian DD, Chen Y, Duan ZP, Zhang JL and Zheng SJ: Up-regulation
of plasma hexosylceramide (d18:1/18:1) contributes to genotype 2
virus replication in chronic hepatitis C: A 20-year cohort study.
Medicine (Baltimore). 95:e37732016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia-Ruiz C, Morales A and
Fernández-Checa JC: Glycosphingolipids and cell death: One aim,
many ways. Apoptosis. 20:607–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Merrill AH Jr: Sphingolipid and
glycosphingolipid metabolic pathways in the era of
sphingolipidomics. Chem Rev. 111:6387–6422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YY, Hill RA and Li YT: Ceramide
glycosylation catalyzed by glucosylceramide synthase and cancer
drug resistance. Adv Cancer Res. 117:59–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haynes CA, Allegood JC, Park H and
Sullards MC: Sphingolipidomics: Methods for the comprehensive
analysis of sphingolipids. J Chromatogr B Analyt Technol Biomed
Life Sci. 877:2696–2708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morales A, Lee H, Goñi FM, Kolesnick R and
Fernandez-Checa JC: Sphingolipids and cell death. Apoptosis.
12:923–939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verheij M, Bose R, Lin XH, Yao B, Jarvis
WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al:
Requirement for ceramide-initiated SAPK/JNK signalling in
stress-induced apoptosis. Nature. 380:75–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brooks C and Dong Z: Regulation of
mitochondrial morphological dynamics during apoptosis by Bcl-2
family proteins: A key in Bak? Cell Cycle. 6:3043–3047. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan G, O'Rourke K and Dixit VM: Caspase-9,
Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem.
273:5841–5845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mari M and Fernández-Checa JC:
Sphingolipid signalling and liver diseases. Liver Int. 27:440–450.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
García-Ruiz C, Colell A, Marí M, Morales
A, Calvo M, Enrich C and Fernández-Checa JC: Defective
TNF-alpha-mediated hepatocellular apoptosis and liver damage in
acidic sphingomyelinase knockout mice. J Clin Invest. 111:197–208.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armstrong JS: Mitochondrial membrane
permeabilization: The sine qua non for cell death. Bioessays.
28:253–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa T, Shimizu S, Watanabe T,
Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T and Tsujimoto Y:
Cyclophilin D-dependent mitochondrial permeability transition
regulates some necrotic but not apoptotic cell death. Nature.
434:652–658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gogvadze V, Orrenius S and Zhivotovsky B:
Multiple pathways of cytochrome c release from mitochondria in
apoptosis. Biochim Biophys Acta. 1757:639–647. 2006. View Article : Google Scholar : PubMed/NCBI
|