Introduction
Currently, gastric cancer is the 2nd most common
malignant tumour in the world. Tumour resection surgery is the main
clinical treatment for curing gastric cancer. However, most
patients have a less than five-year survival rate and a poor
quality of life after the surgery (1,2).
Therefore, the understanding of the biological mechanism of current
agents and the development of new therapeutic agents for the
clinical management of gastric cancer are still urgent.
Complementary and alternative medicine (CAM) is increasingly
accepted in United States both as treatment for illness and as
self-care to promote health and well-being (3). The use of CAM has increased
substantially in conventional and western medical practices over
the past decade, notably in cancer treatment (4). CAM includes traditional Chinese
medicine (TCM) and acupuncture. TCM has a variety products, such as
Chinese herbal medicines, fungi and insects (5). In addition to common Chinese herbal
medicines, insects also show potential for alternative therapy
(6–8). Periplaneta americana is a
species widely disseminated in China and throughout major
cosmopolitan areas of the world. Additionally, Periplaneta
americana has been exploited as an alternative naturopathic
remedy for ulcers, burns, and heart disease in southwestern China
and northeastern Brazil (4). As an
extract of Periplaneta americana, Kangfuxin (KFX) is a drug
approved by the China Food and Drug Administration (CFDA)
(Z51021834). The therapeutic effects of KFX include gastric ulcers,
tuberculosis, burns and trauma. This study wanted to explore the
effects and the mechanism of KFX on gastric cancer therapy. An
in vitro model of gastric cancer was set up using the
cultured human carcinoma cell lines SGC-7901.
As reported, endoplasmic reticulum (ER) stress is a
new pathway that leads to apoptosis, which followed the discovery
of the death receptor signalling and mitochondrial pathways
(9). Studies reported that stress
signals were relayed from the ER to the mitochondria. ER
stress-induced apoptosis was similar to mitochondrial-mediated
apoptosis (10). ER stress is
provoked by the accumulation of unfolded or misfolded proteins in
the ER lumen (9). However,
prolonged or irreversible ER stress switches the adaptive nature of
the unfolded protein response (UPR) into a cell death program
(11). Moderate ER stress promotes
cancer cell survival; however, excessive ER stress leads to cancer
apoptosis (10). During excessive
ER stress, an apoptotic pathway may be activated via CHOP/GRP78 and
caspase-12. Apoptosis in ER stress conditions facilitates cell
death, which is also associated with the glucose-regulated protein
78 (GRP78), the transcription activation of the C/EBP homologous
transcription factor (CHOP) and the activation of ER-associated
caspase-12 (12). Therefore, the
overload of ER stress or the blocked UPR can explain the
anti-cancer effects of anti-cancer drugs. In addition, autophagy
can be induced by ER stress (13,14).
To alleviate ER stress, the UPR signalling may activate autophagy
to clear the accumulated misfolded proteins from the ER lumen
(15).
In this study, we investigated the inhibition of KFX
on SGC-7901 cells in vitro and demonstrated whether ER
stress and autophagy were involved in its anti-cancer effects. Our
data showed that KFX attenuated the survival rate and significantly
induced apoptosis in all chemicals. Moreover, the signalling
pathways and molecular mechanisms involved in ER stress and
autophagy in KFX treated SGC-7901 cells were elucidated. This study
supports that KFX is a potential therapy for gastric cancer. ER
stress, autophagy and the apoptosis-inducing effects of KFX in
cancer cells may have promising anticancer effects in many other
cancers.
Materials and methods
Reagents
KFX (Good Doctor Pharmaceutical Group, Sichuan,
China) was dissolved in cell culture medium to the required
concentration before use. 4-phenylbutyric acid (4-PBA; Aladdin,
Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) to make
a 2 mmol/l stock solution and was diluted in cell culture medium to
the required concentration before use. A FITC-Annexin V Apoptosis
detection kit (BD Biosciences, San Jose, CA, USA) were prepared for
the apoptosis assay. All chemicals in the present study were used
for experimental research only.
Cell culture
A human gastric cancer cell line, SGC-7901, was
purchased from the Shanghai Institute of Cell Biology (Shanghai,
China). Cells were routinely maintained in RPMI-1640 (31800–022;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS, SV30087.02; HyClone;
GE Healthcare Life Sciences, Logan, UT, USA), penicillin (100 U/ml,
P3032) and streptomycin (100 U/ml, S9137) (both from Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) at 37°C in a humidified 5%
CO2 incubator.
Cytotoxicity
To detect the anticancer activity of different
concentrations of KFX, the SGC-7901 cell viability in different
treatment groups was assessed by using a CCK-8 kit. CCK-8 was used
to measure cell viability and evaluate the survival rate of
SGC-7901 cells by KFX treatment. Cells were seeded in a 96-well
plate at a density of 2×103 cells/well and incubated for
12 h in 0.1 ml RPMI-1640 medium supplemented with 10% FBS at 37°C.
Then, the medium was replaced with fresh medium containing KFX at
various concentrations ranging from 0.1 to 5 µg/ml. The untreated
cells were used as controls. After 24, 48 and 72 h incubation, 10
µl of CCK-8 was added into each well. After 4 h, absorption in each
well was observed by a micro plate reader at 450 nm (Multiskan MK3;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell migration
SGC-7901 cells were seeded into 6-well plates at
3×105 cells/well and were incubated for 12 h. A scratch
was created with a sterile clear tip (200 µl). Then, the cells were
washed twice with PBS to remove the debris and detached cells.
Scratch pictures were recorded immediately using an inverted
fluorescence microscope (Nikon, Tokyo, Japan). Afterwards, the
cells in the wells were cultured with various concentrations of KFX
in medium, separately. The only cells treated with medium were the
control group. After 24 or 48 h of treatment, cell migration was
observed by using an inverted fluorescence microscope.
Cell apoptosis assay
SGC-7901 cells were seeded in 6-well plates at a
density of 3×105 cells/well and incubated overnight at
37°C. Cells were treated with a different concentration of KFX with
the untreated cells as the controls. After 48 h treatment, the
cells were collected and analysed. For quantitative assays, the
cells were harvested by trypsinisation, then centrifuged at 1,000
rpm for 3 min and suspended in binding buffer. After adding 5 µl of
Annexin V and 5 µl propidium iodide (50 µg/ml), the cells were
incubated at room temperature for 5 min in the dark using the FITC
Annexin V Apoptosis detection kit (BD Biosciences). Cell apoptosis
was analysed by flow cytometry (FACSCalibur FCM; Becton-Dickinson,
San Jose, CA, USA).
Western blotting
Cells were washed with PBS and lysed in Lysis-M
reagent supplemented with complete mini-protease inhibitor cocktail
tablets (Roche Diagnostics, Indianapolis, IN, USA). The equivalent
of 60 µg of protein was separated on a 12% gel and then transferred
onto a PVDF membrane (Millipore, Billerica, MA, USA). After
blocking with 5% (w/v) non-fat milk for 2 h, the membranes were
incubated with the following antibodies: Bax, Bcl2, GRP78, Chop,
LC3, Beclin1 (1:000; Abcam, Cambridge, UK) and GAPDH (1:200; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Next,
the membranes were incubated with a goat-anti-rabbit secondary
antibody for 2 h at room temperature. Signals were visualised using
the ChemiDoc™ XRS+ Imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Immunofluorescence assay
SGC-7901 cells were seeded in 6-well plates at
3×105 cells/well and incubated for 12 h. The cells were
treated with various concentrations KFX or without KFX for 48 h.
After treatment, the cells were washed with PBS three times, fixed
with 4% paraformaldehyde in PBS for 20 min, permeabilised with 0.5%
Triton X-100 in PBS for 15 min and incubated with 5% bovine serum
albumin (BSA) in PBS for 1 h to block nonspecific antibody binding
at 37°C. After blocking with 1% BSA for 1 h, the cells were
incubated with anti-CHOP (1:100; Santa Cruz Biotechnology, Inc.)
antibody. Then, the cells were incubated with goat-anti-rabbit
secondary fluorescence antibody for 2 h at room temperature. The
nuclei were stained with DAPI. Fluorescence images were obtained
using a positive position fluorescence microscope or confocal laser
microscope (both from Nikon).
Transmission electron microscopy (TEM)
image
After fixation in 2.5% (w/v) glutaraldehyde
overnight, cells were post-fixed in 2% (v/v) osmium tetroxide and
blocked with 2% (v/v) uranyl acetate. Following dehydration in a
series of acetone washes, the cells were embedded in Araldite.
Semi-thin sectioning and toluidine blue staining were performed to
observe the location. Finally, ultra-thin sections of at least
three blocks per sample were cut and observed using a Hitachi
TEM.
Statistical analyses
Statistical analyses were carried out using SPSS
20.0 statistical software (SPSS, Inc., Chicago, IL, USA). All
values are presented as the means ± standard error of the mean
(SEM). Statistical evaluation of the data was performed using
one-way analysis of variance (ANOVA) and Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
KFX inhibited cell growth and
migration in human gastric cancer cell line SGC-7901
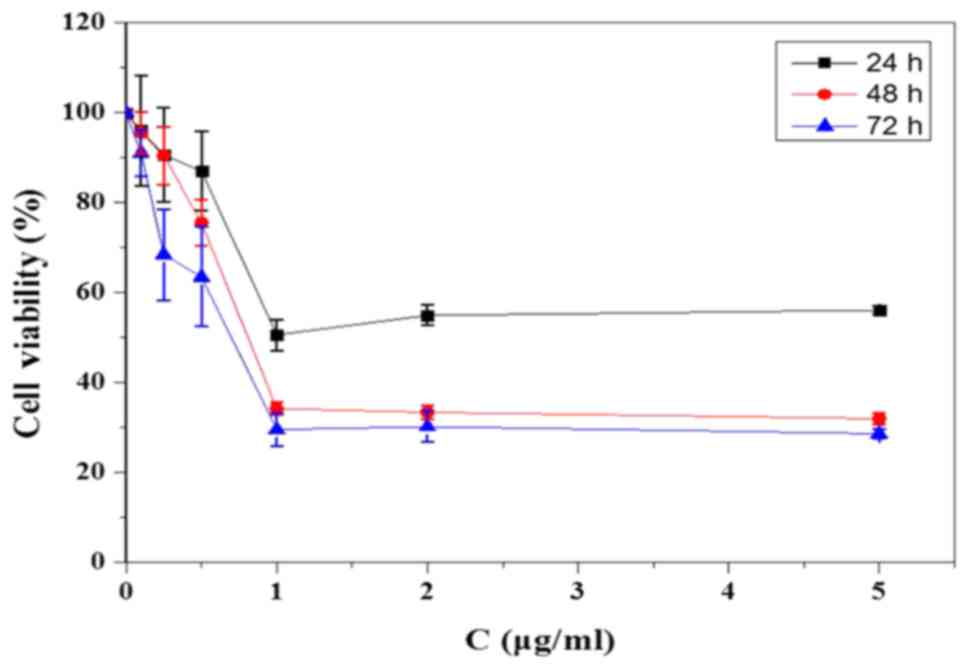
In the CCK-8 assay, the SGC-7901 cells were treated
with KFX for 24, 48 and 72 h at different concentrations (0.1,
0.25, 0.5, 1, 2, 5 µg/ml) to evaluate the optimised KFX treatment
on the inhibition of cell growth. In each group, 1 µg/ml of KFX
showed substantial inhibition in SGC-7901 cells, whereas there was
no concentration dependence over 1 µg/ml (Fig. 1). From the results of the cell
viability test, KFX showed the strongest inhibition on SGC-7901
cell survival at 48 h, while the cancer cell inhibition of KFX
treatment at 24 h was much lower than that at 48 h. However, there
was no difference at 48 and 72 h. Thus, in the CCK-8 analysis, 48 h
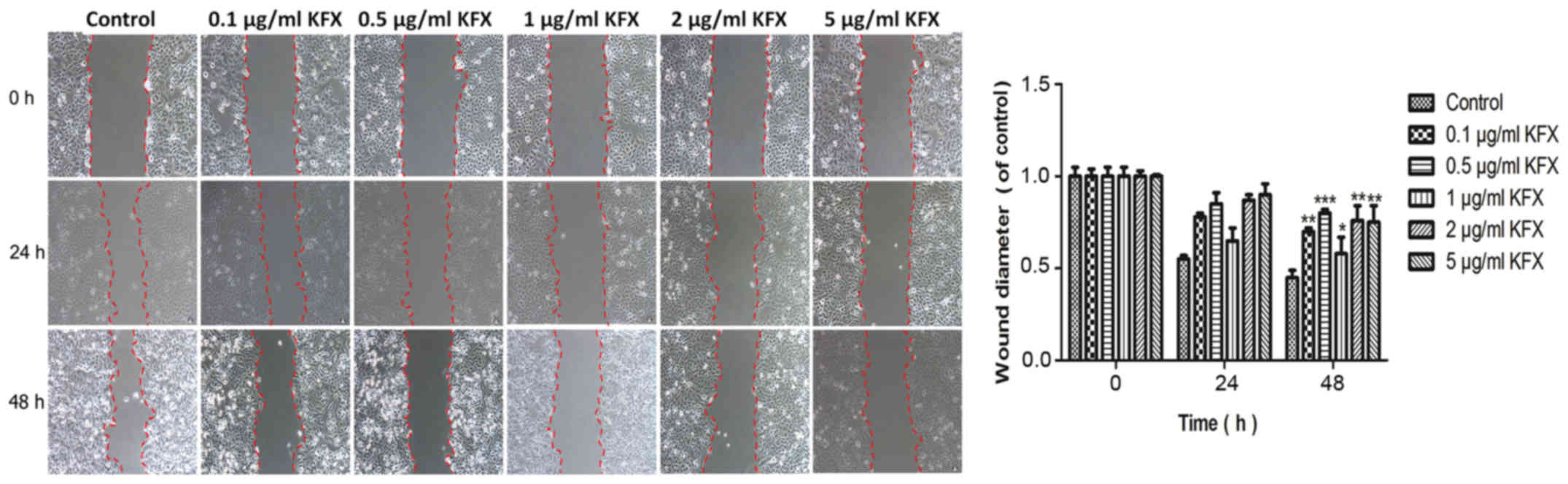
of KFX treatment was the optimal administration time. Consistent
with the CCK-8 results, the scratch assay revealed that KFX could
achieve the strongly inhibition on cell migration at 24 h. However,
the inhibition on cell migration at 48 h was similar or even
stronger than that at 24 h. Considering the optimized drug
bioavailability, the treatment time at 48 h was better than 24 h
(Fig. 2). The semi-quantification
bar chart was show in Fig. 2.
According to those two assays, the optimum treatment concentration
(1 µg/ml) and time (48 h) for the KFX anticancer effect on SGC-7901
cells were used in the following studies.
KFX induced apoptosis in human gastric
cancer cell line SGC-7901
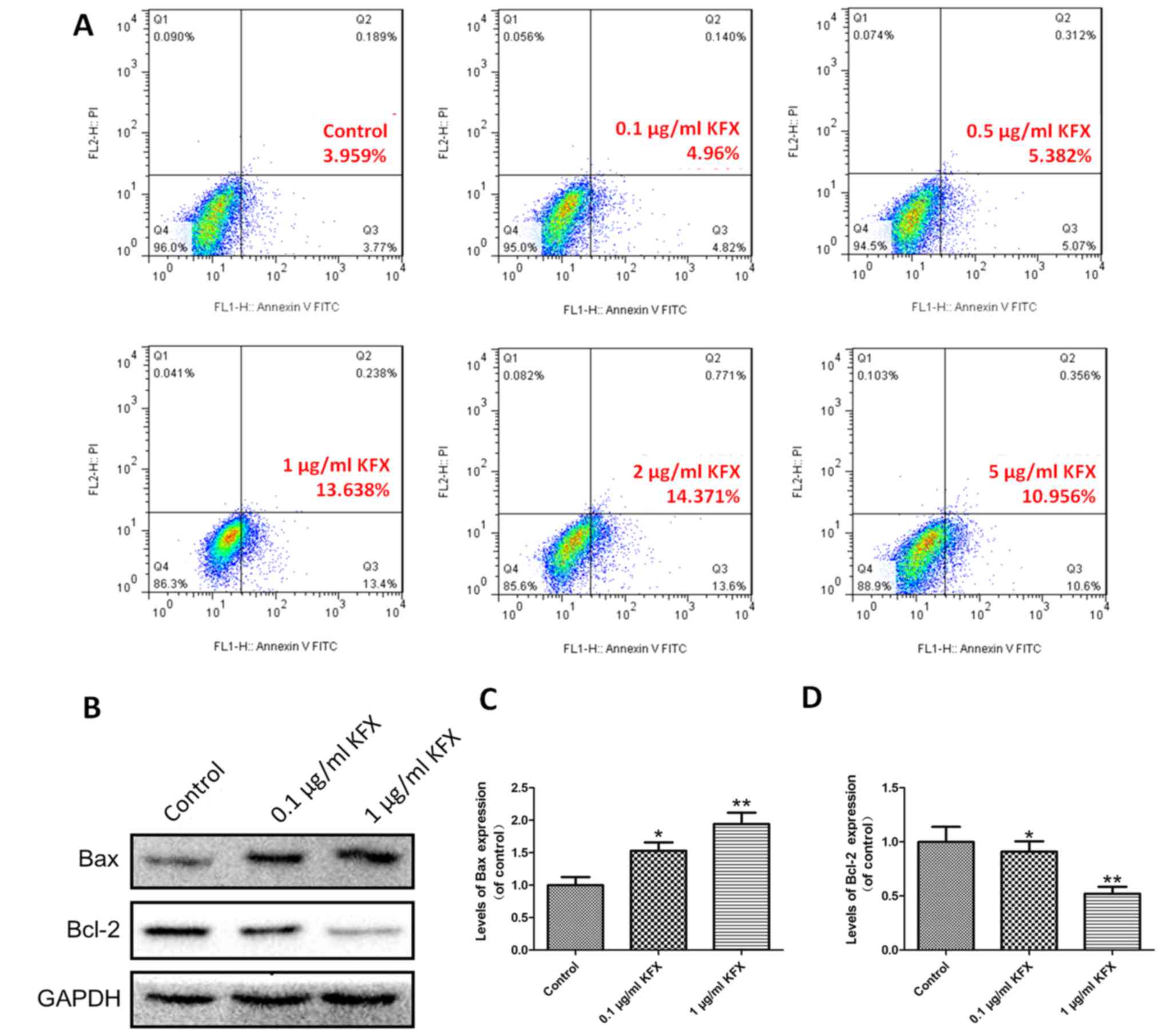
SGC-7901 cells were treated with various
concentrations of KFX and apoptosis was investigated using a FITC
Annexin V apoptosis detection kit using flow cytometry. Compared
with the control, treatment of SGC-7901 cells with different doses
of KFX could induce different stages of apoptosis. Among all the
experimental groups, the cells showed a progressive increase in
apoptosis with increasing amounts of KFX treatment. In detail, the
cells treated with 1 µg/ml of KFX showed nearly 2.53- and 2.75-fold
higher apoptosis than those treated with 0.5 µg/ml KFX and 0.1
µg/ml KFX, respectively, suggesting the strongest and most
effective tumour inhibition at 1 µg/ml of KFX (Fig. 3A). Furthermore, the apoptosis
effect of KFX was validated by western blot analysis. Compared with
the control, both 0.1 and 1 µg/ml of KFX upregulated the expression
of Bax and downregulated the expression of Bcl-2, and the 1 µg/ml
KFX treatment group had the strongest apoptosis effect compared to
the 0.1 µg/ml KFX treatment group (Fig. 3B). The quantitative analysis of the
western blot results are shown in Fig.
3C and D.
KFX promoted SGC-7901 cells apoptosis
through activating ER stress
It is known that the ER can induce ER stress marker
proteins (such as GRP78 and phospho-eIF2a), which upregulates the
expression of CHOP (16–18). CHOP can influence the expression of
Bcl −2 family proteins and inhibit the anti-apoptotic protein
expression of Bcl-2 (19).
Regarding this study, many studies have proven that many TCMs
inhibit cancers via activating ER stress (20–23).
Therefore, we hypothesized that ER stress was involved in the
anti-cancer effect of KFX and whether ER stress was associated with
the mechanism of KFX-induced apoptosis in SGC-7901 cells. We
examined the expression of ER stress proteins in the cells after
treatment with KFX (Fig. 4). The
Western blot results showed that the expressions of CHOP, GRP78 and
caspase-12 were increased with KFX treatment group, and the 1 µg/ml
KFX group was stronger than 0.1 µg/ml KFX group (Fig. 4A). To further confirm that KFX
promoted apoptosis through ER stress, the ER stress inhibitor 4-PBA
(2 mM) was used (24). 4-PBA is a
chemical chaperone that contributes to protein folding and
trafficking within the ER, alleviating ER stress and acting as an
ER stress inhibitor (24). After
exposure to 4-PBA for 6 h, the expressions of CHOP, GRP78 and
caspase-12 were significantly suppressed by 4-PBA, whereas these
protein expressions were significantly restored after KFX treatment
(Fig. 4C). Quantitative analysis
of the western blot analysis is shown in Fig. 4B and D. In the immunofluorescence
results, the CHOP expression in the cells in the 4-PBA+KFX group
was stronger than the 4-PBA group (Fig. 4E). All these results demonstrated
that KFX could reverse the expressions of the 4-PBA-inhibited
ER-related proteins. ER-stress played a critical role in the KFX
anticancer effect.
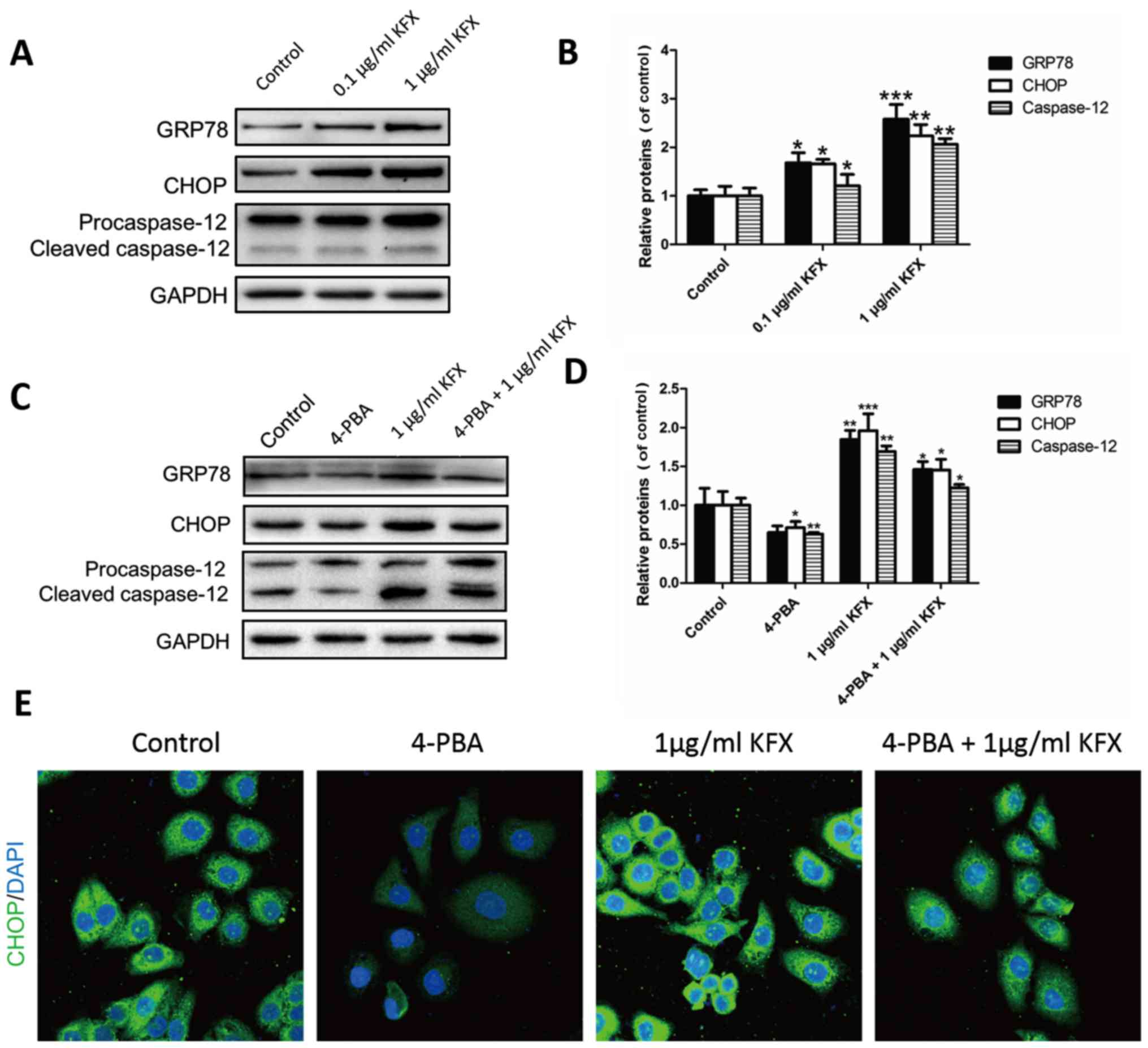 | Figure 4.KFX accelerated the SGC-7901 cell
apoptosis by upregulating ER stress. (A and B) Western blot
analysis of the GRP78, CHOP and caspase-12 protein expressions.
After the KFX treatment, the expressions of GRP78, CHOP and
caspase-12 were significantly upregulated compared with the control
group. GAPDH was used as the loading control and for band density
normalization. (C and D) Western blot analysis of GRP78, CHOP and
caspase-12 protein expression after treatment with the ER stress
inhibitor 4-PBA. We found that expression of GRP78, CHOP and
caspase-12 were significantly upregulated compared with the control
group and, on the basis of giving 1 µg/ml KFX, the protein
expression reversed. (E) Images of CHOP immunofluorescence in
different groups. All experiments were repeated three times. Data
are shown as the means ± SD, n=3. Student's t-test compared to
control, *P<0.05, **P<0.01, ***P<0.001 vs. control.
KFX, Kangfuxin; ER, endoplasmic reticulum; GRP78, glucose-regulated
protein 78; CHOP, C/EBP homologous transcription factor; 4-PBA,
4-phenylbutyric acid. |
KFX activated the autophagy in
SGC-7901 cells
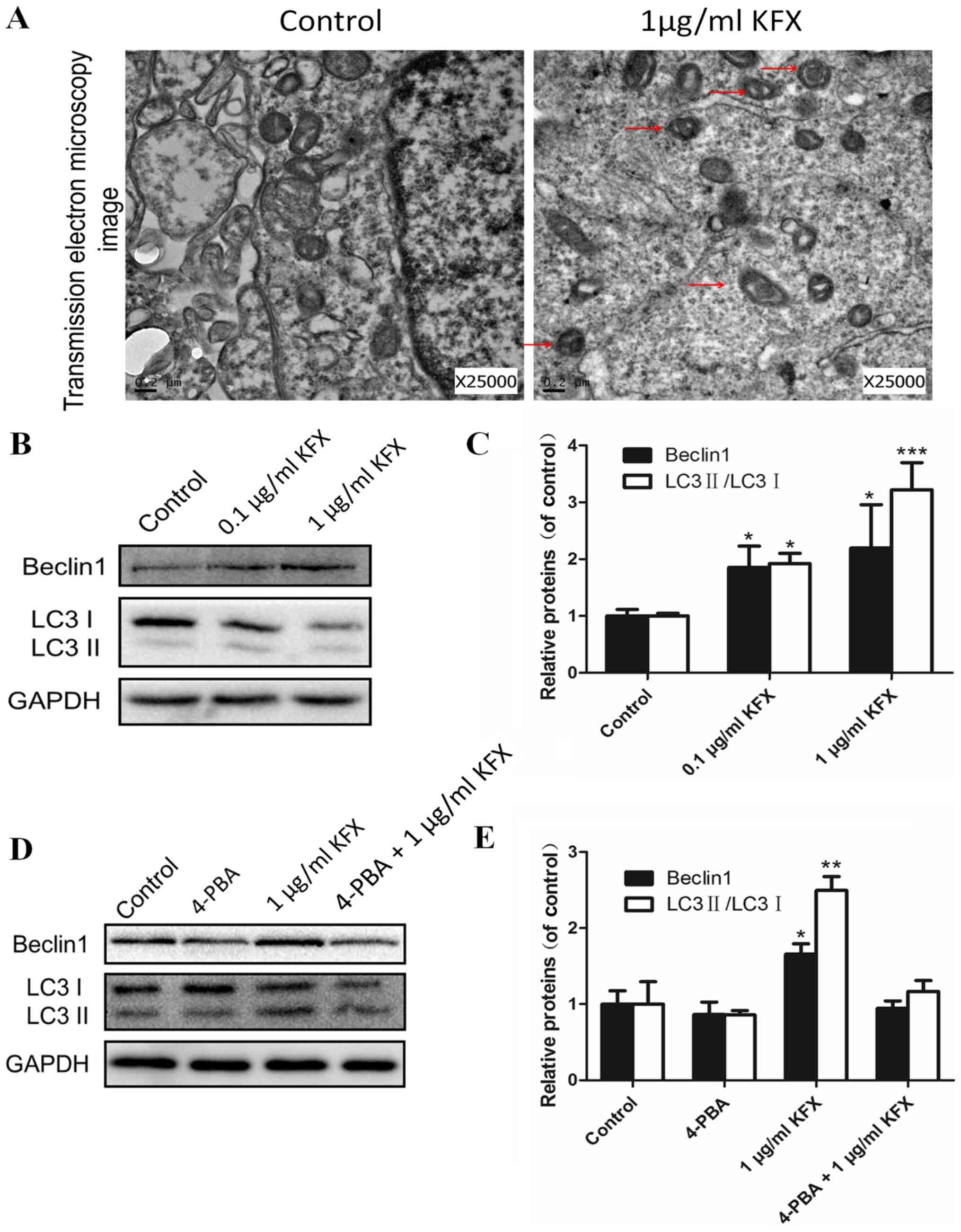
In addition to ER stress, autophagy may also be
involved in SGC-7901 cell apoptosis after KFX treatment. TEM images
verified that KFX activated autophagy. As shown in Fig. 5A, the KFX-treated group had many
autophagosomes compared with the control group. These data revealed
that KFX could activate autophagy in SGC-7901 cells, resulting in
an increase in autophagosomes in cells. In addition, we further
examined the expression of the protein Beclin-1, which is a key
protein in autophagy. SGC-7901 cells were treated with 0.1 and 1
µg/ml KFX for 48 h. As shown in Fig.
5B, the expression of Beclin-1 was significantly upregulated in
the KFX groups compared with the control group. Moreover,
autophagosome formation is mediated by the conjugation systems
composed of ATG proteins, which culminate in the conjugation of
ATG12 to ATG5 and conversion of a soluble form of
microtubule-associated protein 1 light chain 3 (LC3)-I to another
bound form, LC3-II (25). Thus,
the ratio of LC3-II/LC3-I is a marker of autophagy in some studies
(26,27). The ratio of LC3-II/LC3-I in the
KFX-treated groups was significantly higher than the control group,
and the 1 µg/ml KFX group showed the greatest effect (Fig. 5B and C). Furthermore, the autophagy
activation in SGC-7901 cells was suppressed when treated with the
ER stress inhibitor 4-PBA. Interestingly, the expressions of
Beclin-1 and LC3-II/LC3-I were slightly increased in 4-PBA+KFX
group (Fig. 5D). These results
illustrated that KFX induces apoptosis through activation of ER
stress and autophagy. However, the KFX autophagy effect may be
controlled by ER stress activation. Quantitative analysis of the
Western blot is shown in Fig. 5C and
E.
Discussion
Gastric carcinoma causes the second highest number
of cancer-related deaths in the world. Currently, surgical removal
of the stomach is the only curative clinical treatment, but
non-surgical cancer therapies are always the best treatment for
patients. Many studies have worked on agents for inhibiting gastric
cancer in rodents; however, the exact mechanisms of those agents
remain unclear. As a TCM, KFX is the organic extract of
Periplaneta americana and is widely used in the treatment of
gastric ulcer in patients. To our surprise, the present study found
that KFX could promote SGC-7901 cell apoptosis, reflecting the
anti-cancer potential in gastric carcinoma. Based on this
phenomenon, studies of the further mechanisms of KFX in SGC-7901
cells were performed in this study.
Many studies have demonstrated that cancer cell
apoptosis is related to ER stress-mediated autophagy (13,28).
It is known that ER stress induces the misfolding of the ER lumen
as well as unfolded protein aggregation and calcium ion balance
disorder. ER overload of caspase-12 could mediate the apoptosis
signalling pathways (14). Zhang
et al study showed that the targeting of apoptotic
mechanisms had a close correlation with the ER stress pathway
(21). Meanwhile, autophagy is a
membrane process that results in the transport of cellular contents
to lysosomes for degradation (29). A previous study indicated that ER
stress and autophagy were involved in the apoptosis induced by
cisplatin in human lung cancer cells (15). Thus, both ER stress and autophagy
appear to be critical for general cell homeostasis as well as
tumourigenesis and chemo-resistance. Furthermore, studies proved
that the cooperation of autophagy and ER stress could achieve
cancer inhibition in particular environments (30–33).
The process of ER stress has been tightly linked to autophagy.
Therefore, we hypothesized that ER stress and autophagy were
involved in regulating the KFX-induced apoptosis in SGC-7901
cells.
In this study, KFX promoted the apoptosis of
SGC-7901 cells, as characterized by decreased cell activity
accompanied by the inhibition of cell migration. The result
basically confirmed that KFX inhibited cancer cell growth in
vitro and showed the potential anti-cancer effects on gastric
carcinoma. In the underlying molecular mechanisms, KFX accelerated
ER stress as demonstrated by the upregulated expressions of ER
stress-related proteins, such as CHOP, GRP78, and caspase-12
(Fig. 6), which was consistent
with the activated apoptosis effects of KFX in SGC-7901 cells. To
test the critical role of ER stress from KFX, the ER stress
inhibitor 4-PBA was used to suppress the ER stress-related
proteins. In particular, 4-PBA inhibited ER stress-related proteins
were upregulated by treatment with KFX. As a result, the KFX
promoted SGC-7901 cell apoptosis and was closely related to the
activation of ER stress.
Previous studies have reported that autophagy could
induce apoptosis. Apoptosis and autophagy are both tightly
regulated biological processes that play a central role in tissue
homeostasis, development, and disease (34). In addition, many agents aggravate
ER stress, leading to increased autophagic activity (33,35).
Autophagy is an evolutionarily conserved, dynamic and
lysosome-mediated process that involves the sequestration and
delivery of cytoplasmic material to the lysosome, where it is
degraded and recycled (36). In
theory, autophagy may contribute to promoting cell survival, either
by removing the damaged organelles and toxic cell metabolites or
providing the nutrients necessary for cell survival during
nutrient-limiting conditions. Conversely, autophagy may also
promote cell death through excessive self-digestion of cell
components. Despite recent advances in the understanding of its
molecular mechanisms and biological functions, it is still unclear
whether autophagy acts as a cell survival or cell death pathway or
both (31–34). In our study, the treatment of KFX
increased the expression of autophagy-related proteins compared
with the control, which showed the same tendency with the promotion
of apoptosis and the activation of ER-stress by KFX treatment
(Fig. 6). However, KFX could not
preserve the autophagy activation in cells whose ER-stress action
was suppressed by 4-PBA. Therefore, we concluded that KFX promoted
apoptosis through activation of autophagy, whereas KFX-induced
autophagy was followed by the promotion of ER stress.
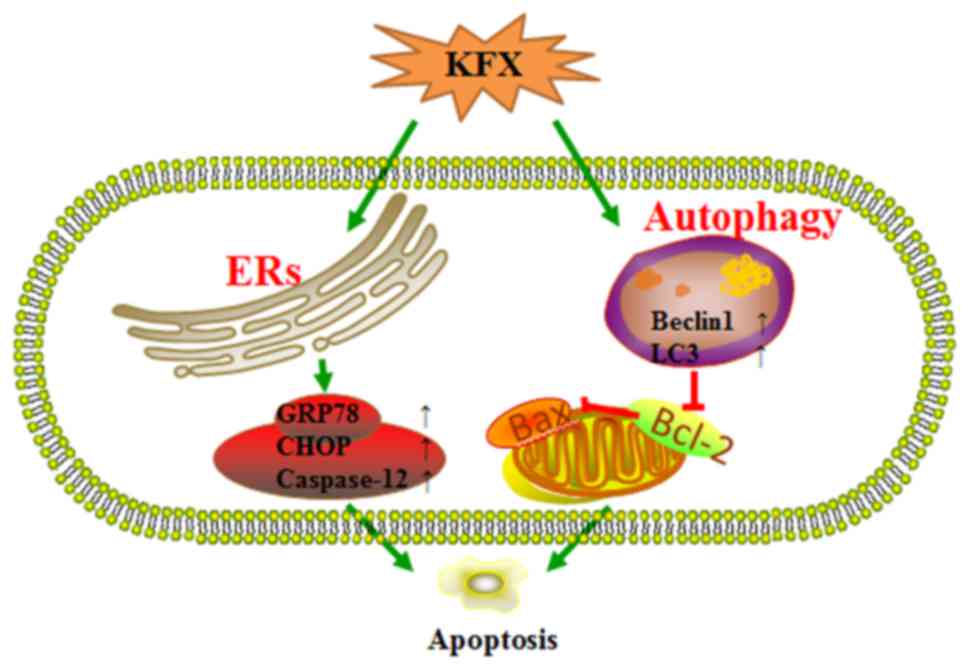
In conclusion, KFX can be a promising and safe
anticancer agent against gastric carcinoma, which can promote
apoptosis through activating ER stress and autophagy (Fig. 6). ER stress may be the critical
mechanism for the anti-cancer effects of KFX on gastric carcinoma
and the secondary effects on autophagy. However, the specific
interaction between these still need to be further explored. In the
present study, we explored the potential anticancer effects of KFX
and its related cancer mechanism. As a promising anti-cancer agent,
further studies will be performed, including its effects on normal
cell lines or other cancerous cell lines and, further, its effects
on lung cancer in vivo. The further roles of KFX will be
learned in our future studies.
Acknowledgements
This study was supported by crosswise tasks from
Good Doctor Pharmaceutical Group (KJHX1505), research fund for the
doctoral program of High Education by Ministry of Education of
China (grant no. 20133321120005, Cui-Tao Lu), Zhejiang Provincial
Program for the Cultivation of High-level Innovative Health Talents
(Ying-Zheng Zhao), 151 Talent Project of Zhejiang province and 551
Talent Project of Wenzhou (Ying-Zheng Zhao), Key Support of High
Level Talent Innovation and Technology Project of Wenzhou (Zhao
Ying-Zheng), Zhejiang Provincial Foundation for Health Department
(grant nos. 2015ZDA023 and 2016KYA136), Medicine Grant from Wenzhou
Bureau of Science and Technology (grant no. Y2014730), School
Talent Start Fund of Wenzhou Medical University (grant no.
QTJ15020).
References
|
1
|
Li Y, Li B, Xiang CP, Zhang Y, Li YY and
Wu XL: Characterization of gastric cancer models from different
cell lines orthotopically constructed using improved implantation
techniques. World J Gastroenterol. 18:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zou P, Xia Y, Chen T, Zhang J, Wang Z,
Chen W, Chen M, Kanchana K, Yang S and Liang G: Selective killing
of gastric cancer cells by a small molecule targeting ROS-mediated
ER stress activation. Mol Carcinog. 55:1073–1086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honda K and Jacobson JS: Use of
complementary and alternative medicine among United States adults:
The influences of personality, coping strategies, and social
support. Prev Med. 40:46–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang XY, He ZC, Song LY, Spencer S, Yang
LX, Peng F, Liu GM, Hu MH, Li HB, Wu XM, et al: Chemotherapeutic
effects of bioassay-guided extracts of the American cockroach,
Periplaneta americana. Integr Cancer Ther. 10:NP12–NP23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alves RR, Lima HN, Tavares MC, Souto WM,
Barboza RR and Vasconcellos A: Animal-based remedies as
complementary medicines in Santa Cruz do Capibaribe, Brazil. BMC
Complement Altern Med. 8:442008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonnemain B: Helix and Drugs: Snails for
Western Health Care From Antiquity to the Present. Evid Based
Complement Alternat Med. 2:25–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castro ML, Vilela WR, Zauli RC, Ikegaki M,
Rehder VL, Foglio MA, de Alencar SM and Rosalen PL: Bioassay guided
purification of the antimicrobial fraction of a Brazilian propolis
from Bahia state. BMC Complement Altern Med. 9:252009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z, Liu Y, Liao J, Gong C, Sun C, Zhou
X, Wei X, Zhang T, Gao Q, Ma D and Chen G: Quercetin induces
endoplasmic reticulum stress to enhance cDDP cytotoxicity in
ovarian cancer: Involvement of STAT3 signaling. FEBS J.
282:1111–1125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaufman RJ: Orchestrating the unfolded
protein response in health and disease. J Clin Invest.
110:1389–1398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu SP, Wang ZG, Zhao YZ, Wu J, Shi HX, Ye
LB, Wu FZ, Cheng Y, Zhang HY, He S, et al: Gelatin nanostructured
lipid carriers incorporating nerve growth factor inhibit
endoplasmic reticulum stress-induced apoptosis and improve recovery
in spinal cord injury. Mol Neurobiol. 53:4375–4386. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogata M, Hino S, Saito A, Morikawa K,
Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K,
et al: Autophagy is activated for cell survival after endoplasmic
reticulum stress. Mol Cell Biol. 26:9220–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang XY, Zhang TT, Song DD, Zhou J, Han
R, Qin ZH and Sheng R: Endoplasmic reticulum chaperone GRP78 is
involved in autophagy activation induced by ischemic
preconditioning in neural cells. Mol Brain. 8:202015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi S, Tan P, Yan B, Gao RI, Zhao J, Wang
J, Guo J, Li N and Ma Z: ER stress and autophagy are involved in
the apoptosis induced by cisplatin in human lung cancer cells.
Oncol Rep. 35:2606–2614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida T, Shiraishi T, Nakata S, Horinaka
M, Wakada M, Mizutani Y, Miki T and Sakai T: Proteasome inhibitor
MG132 induces death receptor 5 through CCAAT/enhancer-binding
protein homologous protein. Cancer Res. 65:5662–5667. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang CC, Chen LH, Gillespie S, Kiejda KA,
Mhaidat N, Wang YF, Thorne R, Zhang XD and Hersey P: Tunicamycin
sensitizes human melanoma cells to tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis by up-regulation of
TRAIL-R2 via the unfolded protein response. Cancer Res.
67:5880–5888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Q, Lee DI, Rong R, Yu M, Luo X, Klein
M, El-Deiry WS, Huang Y, Hussain A and Sheikh MS: Endoplasmic
reticulum calcium pool depletion-induced apoptosis is coupled with
activation of the death receptor 5 pathway. Oncogene. 21:2623–2633.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouman L, Schlierf A, Lutz AK, Shan J,
Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, et
al: Parkin is transcriptionally regulated by ATF4: Evidence for an
interconnection between mitochondrial stress and ER stress. Cell
Death Differ. 18:769–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu J, Chen M, Chen N, Ma A, Zhu C, Zhao
R, Jiang M, Zhou J, Ye L, Fu H and Zhang X: Glycyrrhetinic acid
induces G1-phase cell cycle arrest in human non-small cell lung
cancer cells through endoplasmic reticulum stress pathway. Int J
Oncol. 46:981–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YS, Shen Q and Li J: Traditional
Chinese medicine targeting apoptotic mechanisms for esophageal
cancer therapy. Acta Pharmacol Sin. 37:295–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim B, Kim HS, Jung EJ, Lee JY, K Tsang B,
Lim JM and Song YS: Curcumin induces ER stress-mediated apoptosis
through selective generation of reactive oxygen species in cervical
cancer cells. Mol Carcinog. 55:918–928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HS, Lim JM, Kim JY, Kim Y, Park S and
Sohn J: Panaxydol, a component of Panax ginseng, induces apoptosis
in cancer cells through EGFR activation and ER stress and inhibits
tumor growth in mouse models. Int J Cancer. 138:1432–1441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Chen L, Shen Y and Xu J:
Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via
inhibition of the PERK-ATF4-CHOP pathway. Toxicol In Vitro.
36:186–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tork OM, Khaleel EF and Abdelmaqsoud OM:
Altered cell to cell communication, autophagy and mitochondrial
dysfunction in a model of hepatocellular carcinoma: Potential
protective effects of curcumin and stem cell therapy. Asian Pac J
Cancer Prev. 16:8271–8279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma K, Huang MY, Guo YX and Hu GQ:
Matrine-induced autophagy counteracts cell apoptosis via the ERK
signaling pathway in osteosarcoma cells. Oncol Lett. 12:1854–1860.
2016.PubMed/NCBI
|
|
27
|
Wu L, Maimaitirexiati X, Jiang Y and Liu
L: Parkin regulates mitochondrial autophagy after myocardial
infarction in rats. Med Sci Monit. 22:1553–1559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang C, Li H, Zhou H, Zhang S, Liu Z,
Zhou Q and Sun F: Recombinant Lz-8 from Ganoderma lucidum induces
endoplasmic reticulum stress-mediated autophagic cell death in
SGC-7901 human gastric cancer cells. Oncol Rep. 27:1079–1089. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiong HY, Guo XL, Bu XX, Zhang SS, Ma NN,
Song JR, Hu F, Tao SF, Sun K, Li R, et al: Autophagic cell death
induced by 5-FU in Bax or PUMA deficient human colon cancer cell.
Cancer Lett. 288:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Zhu F, Jiang J, Sun C, Zhong Q, Shen
M, Wang X, Tian R, Shi C, Xu M, et al: Simultaneous inhibition of
the ubiquitin-proteasome system and autophagy enhances apoptosis
induced by ER stress aggravators in human pancreatic cancer cells.
Autophagy. 12:1521–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie WY, Zhou XD, Yang J, Chen LX and Ran
DH: Inhibition of autophagy enhances heat-induced apoptosis in
human non-small cell lung cancer cells through ER stress pathways.
Arch Biochem Biophys. 607:55–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie WY, Zhou XD, Li Q, Chen LX and Ran DH:
Acid-induced autophagy protects human lung cancer cells from
apoptosis by activating ER stress. Exp Cell Res. 339:270–279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma K, Ishaq M, Sharma G, Khan MA,
Dutta RK and Majumdar S: Pentoxifylline triggers autophagy via ER
stress response that interferes with Pentoxifylline induced
apoptosis in human melanoma cells. Biochem Pharmacol. 103:17–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Periyasamy P, Guo ML and Buch S: Cocaine
induces astrocytosis through ER stress-mediated activation of
autophagy. Autophagy. 12:1310–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR- and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|