Introduction
Hepatocellular carcinoma (HCC) is a life-threatening
type of cancer, which is one of the common tumors in humans. It was
predicted that there were 39,230 new cases of HCC with an
HCC-associated mortality rate of 27,170 in 2016 in the United
States (1). In contrast to the
decreasing trends for other major cancer types, the mortality rate
for HCC has increased. In China, 466,100 new cases of HCC were
predicted and 422,100 patients were predicted to succumb to
HCC-associated mortality in 2015 (2). Although several treatments, including
surgery, radiation and chemotherapy, have been used to treat
patients with HCC, the survival rate has not improved significantly
(3). Therefore, the identification
of novel agents is essential to improve the treatment effects in
patients with HCC.
Rottlerin, also known as mallotoxin, is isolated
from Mallotus phillippinensis (4). It has been reported that rottlerin
inhibits tumorigenesis through the regulation of several mechanisms
involving cells, including cell survival, apoptosis, autophagy and
invasion (4). Rottlerin was
initially identified as a potential protein kinase C (PKC)
inhibitor 20 years ago (5).
Several studies have confirmed that rottlerin inhibits cell
proliferation and induces cell cycle arrest via the inhibition of
protein kinase in several types of tumor in humans (6,7).
Subsequent studies have revealed that rottlerin exerts its tumor
suppressor function via a PKC-independent pathway. For example,
rottlerin was found to sensitize to tumor necrosis-related
apoptosis-inducing ligand (TRAIL) -induced apoptosis through
uncoupling of the mitochondria independently of PKC (8). Similarly, rottlerin was found to
sensitize TRAIL-induced apoptosis via the suppression of cell
division cycle (Cdc)2, survivin and X-linked inhibitor of apoptosis
(XIAP) in glioma cells (9).
Rottlerin has also been shown to suppress nuclear factor-κB and
cyclin D1 in breast cancer cells (10). Studies have also shown that
rottlerin inhibits caspase-2, and induces autophagy and apoptotic
cell death (11,12). These findings indicate that
rottlerin may regulate multiple genes to inhibit tumorigenesis.
The oncoprotein transcriptional co-activator with
PDZ-binding motif (TAZ) has been identified as a key driver in the
Hippo pathway; the Hippo signaling pathway is an essential
regulator of organ size during developmental growth (13). Two transcription factors in the
Hippo pathway, Yes-associated protein (YAP) and TAZ, have shown to
possess oncogenic functions. The overexpression of TAZ was found to
be significantly associated with poor overall survival in HCC and
gastrointestinal cancer (14). The
overexpression of TAZ is also associated with certain
clinicopathologic characteristics, including tumor-node-metastasis
stage, lymph node metastasis and tumor differentiation (14). Higher expression levels of TAZ have
also been reported to indicate a poor prognosis in retinoblastoma
(15). Therefore, TAZ offers
potential as a therapeutic target in human cancer (16). The present study aimed to
investigate whether rottlerin inhibits cell growth, migration and
invasion, and whether it induces cell apoptosis and cell cycle
arrest in HCC cells. In addition, the present study examined
whether rottlerin affects the expression of TAZ in HCC cells. The
results demonstrated that rottlerin suppressed cell growth,
triggered cell apoptosis and induced cell cycle arrest. In
addition, rottlerin inhibited cell migration and invasion of the
HCC cells. Mechanistically, the results showed that rottlerin
exerted its antitumor activity partly through the inhibition of
TAZ. Taken together, these findings indicated that the inhibition
of TAZ by rottlerin may be a useful approach for treating HCC.
Materials and methods
Cell culture and reagents
The human QGY-7703 cell line was purchased from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China), and cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin in a 5% CO2 atmosphere at 37°C.
Anti-TAZ antibody (1:1,000, sc-17130) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Secondary antibodies
(anti-mouse HRP-linked antibody, #7076, 1:4,000; anti-rabbit
HRP-linked antibody, #7074, 1:4,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Monoclonal
anti-tubulin antibody (1:3,000, T-3526), rottlerin (CAS no. R5648;
≥85% rottlerin) and Cell Titer Glo (CTG) were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rottlerin was
dissolved in DMSO to produce a 30 mM stock solution, and was added
directly to the medium at different concentrations. Cells were
treated with 0.1% DMSO as the control group.
Cell viability assay
The cells were seeded at a density of
8×103 cells/well in a 96-well plate for 24 h and treated
with different concentrations (1, 2, 3 and 4 µM) of rottlerin in a
humidified CO2 incubator at 37°C. After 48 and 72 h, 20
µl of CTG (5 mg/ml) solution was added to each well and incubated
for 10 min at 37°C. The reaction mixture was then measured on a
microplate reader at 490 nm (17).
Analysis of cell apoptosis
Cells (3×105 cells/well) were cultured in
a 6-well plate overnight and treated with the various
concentrations of rottlerin for 48 h. Following treatment, the
cells were harvested and washed with PBS, resuspended
(1×105 cells) in 500 µl binding buffer with 5 µl
propidium iodide (PI) and 5 µl FITC-conjugated anti-Annexin V
antibody. Apoptosis was analyzed on a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) as described previously
(18).
Cell cycle analysis
Exponentially growing cells (2×105
cells/well) were seeded in a 6-well plate overnight and then
treated with the different concentrations of rottlerin for 48 h.
After 48 h, the cells were collected and washed with cold PBS. The
cells were suspended with 70% cold alcohol and were maintained at
4°C overnight. Prior to analysis, the cells were washed with cold
PBS, and resuspended at a density of 1×106 cells/ml in
PBS. The cells were incubated with 0.1 mg/ml RNase I and 50 mg/ml
PI at 37°C for 30 min. Cell cycle was analyzed on a flow cytometer
(BD Biosciences) as described previously (18).
Western blot analysis
The harvested cells were washed in PBS and lysed
with protein lysis buffer containing 50 mmol/l Tris (pH 7.5), 100
mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton X-100, 2.5
mmol/l sodium orthovanadate, 10 µl/ml protease inhibitor cocktail
and 1 mmol/l PMSF. The concentrations of proteins were measured
using a Bicinchoninic Acid Protein Assay kit (Thermo Fisher
Scientific, Inc.). Equal quantities of protein samples (30 µg) were
separated by electrophoresis on a 10% sodium dodecyl
sulphate-polyacrylamide gel and then transferred onto a
polyvinylidene difluoride membrane. The membrane was then incubated
with the primary antibodies at 4°C overnight. Following incubation,
the membrane was washed with TBST three times and then incubated
with the secondary antibody at room temperature for 1 h. The
expression of protein was detected using an
electrochemiluminescence assay and were analyzed using ImageJ
version 1.46r (National Institutes of Health, Bethesda, MD,
USA).
Cell invasion analysis
A Transwell invasion assay was used to measure the
invasive capacity of the HCC cells according to the manufacturer's
protocol. HCC cells in serum-free medium containing rottlerin (1, 2
or 3 µM) were seeded onto inserts in the upper chamber
(1×104 cells/well) in a 24-well plate. The lower wells
were filled with complete medium with the same concentration of
rottlerin. Following incubation for 20 h in a humidified
CO2 incubator at 37°C, the cells in the upper chambers
were removed using cotton buds. The cells on the lower surface of
the chambers were stained with 4 µg/ml Calcein AM in PBS at 37°C
for 1 h. Images of these fluorescently labeled invasive cells were
captured under a fluorescent microscope. The invaded cells on the
membrane were stained with Wright's-Giemsa and images were captured
(17).
Cell transfection
The cells were seeded into a 6-well plate and
transfected with TAZ small interfering (si)RNA or control siRNA
(A06001; Shanghai GenePharma Co., Ltd., Shanghai, China) using
Lipofectamine 2000 according to the manufacturer's protocol. The
TAZ siRNA sequences were as follows: Sense,
5′-GCAUCUUCGACAGUCUUCUTT-3′ and antisense,
5′-AGAAGACUGUCGAAGAUGCTT-3′. Following transfection, the cells were
subjected to the analyses described above.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 (Graph Pad Software, Inc., La Jolla, CA, USA).
Student's t-test was performed to evaluate statistical
significance. The results are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Rottlerin inhibits cell
proliferation
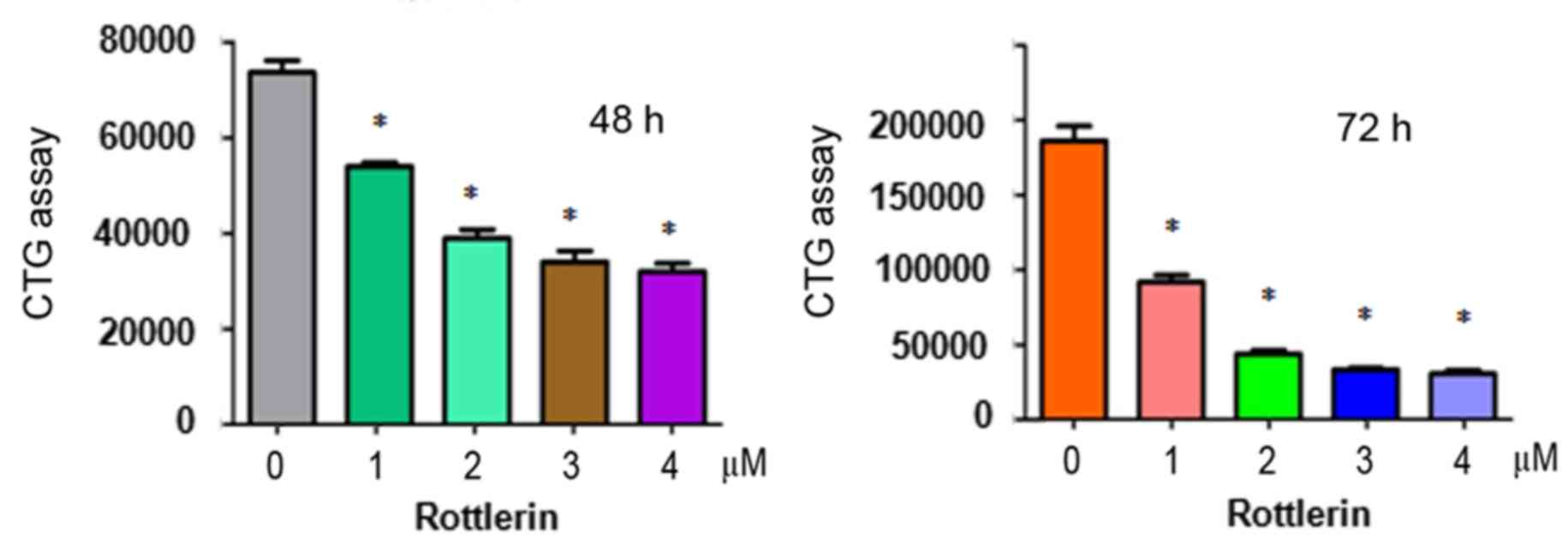
To determine whether rottlerin suppresses the
proliferation of HCC cells, a CTG assay was performed to examine
the viability of QGY-7703 cells treated with different
concentrations of rottlerin for 48 and 72 h. The results showed
that rottlerin significantly inhibited cell proliferation in the
two HCC cell lines (Fig. 1). The
half maximal inhibitory concentration (IC50), which is
the concentration leading to 50% cell growth inhibition, was ~1 µM
at 72 h for the QGY-7703 cells. These results suggested that the
QGY-7703 cells were sensitive to rottlerin treatment. Therefore, a
1 µM concentration of rottlerin was used for QGY-7703 cells in the
subsequent experiments.
Rottlerin induces apoptosis
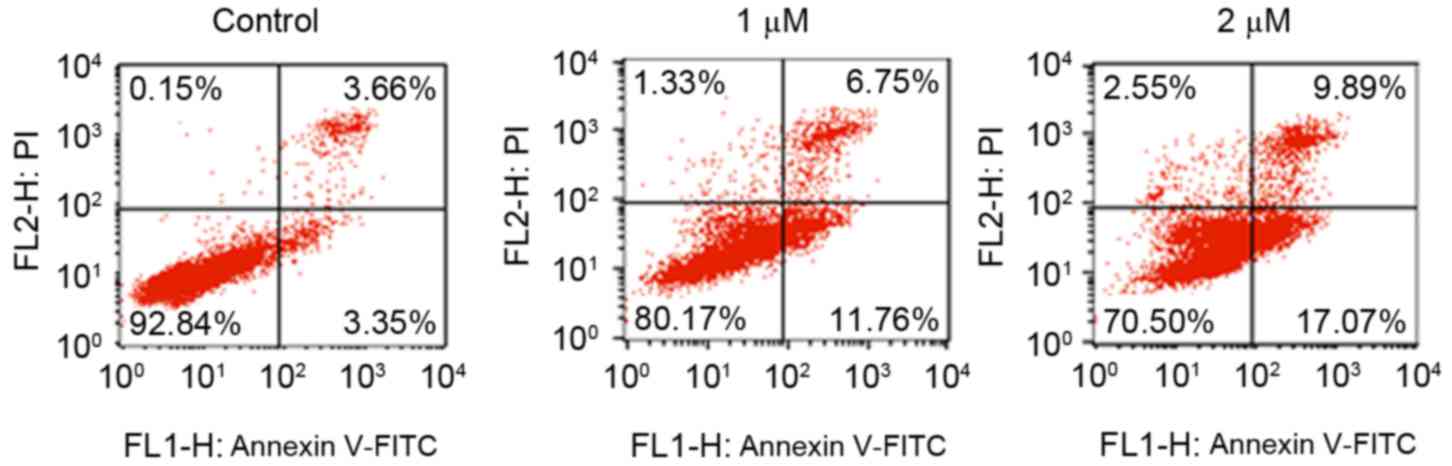
The present study also aimed to determine whether
rottlerin enhances the apoptosis of HCC cells. A PI-FITC-Annexin
assay was performed to measure the rates of apoptotic death of HCC
cells following treatment with rottlerin for 48 h. It was found
that rottlerin triggered cell apoptosis in the HCC cells (Fig. 2). Specifically, cell apoptosis was
increased from 7.0% in the control group to 18.5 and 26.96% in the
1 and 2 µM rottlerin-treated QGY-7703 cell groups, respectively
(Fig. 2). These findings indicated
that rottlerin stimulated the apoptosis of HCC cells.
Rottlerin induces cell cycle
arrest
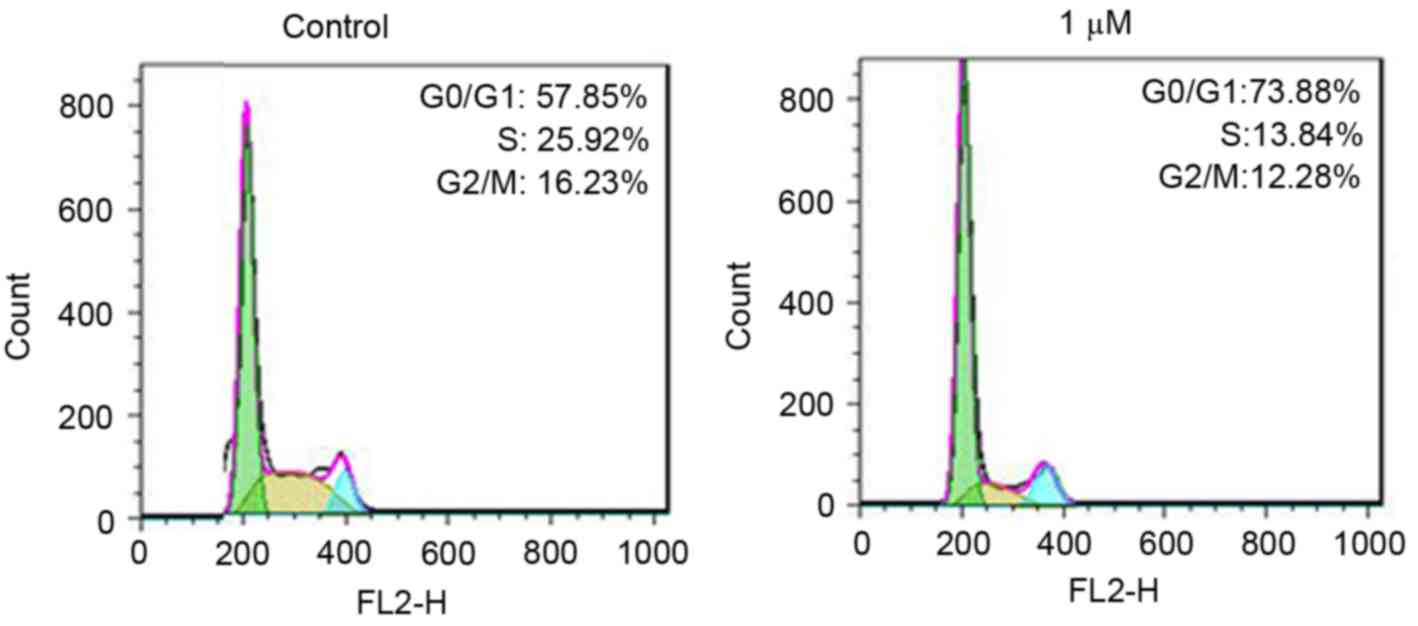
To further define the role of rottlerin in cell
growth inhibition, the present study measured cell cycle in the HCC
cells following rottlerin treatment. Cell cycle analysis using PI
staining and flow cytometry was performed in the HCC cells. The
results revealed that rottlerin induced G0/G1
arrest in the QGY-7703 cells (Fig.
3). Treatment with 1 µM rottlerin led to an increase in the
percentage of G0/G1 cells from 57.85 to
73.88% in the QGY-7703 cells. These results demonstrated that
rottlerin induced cell cycle G0/G1
arrest.
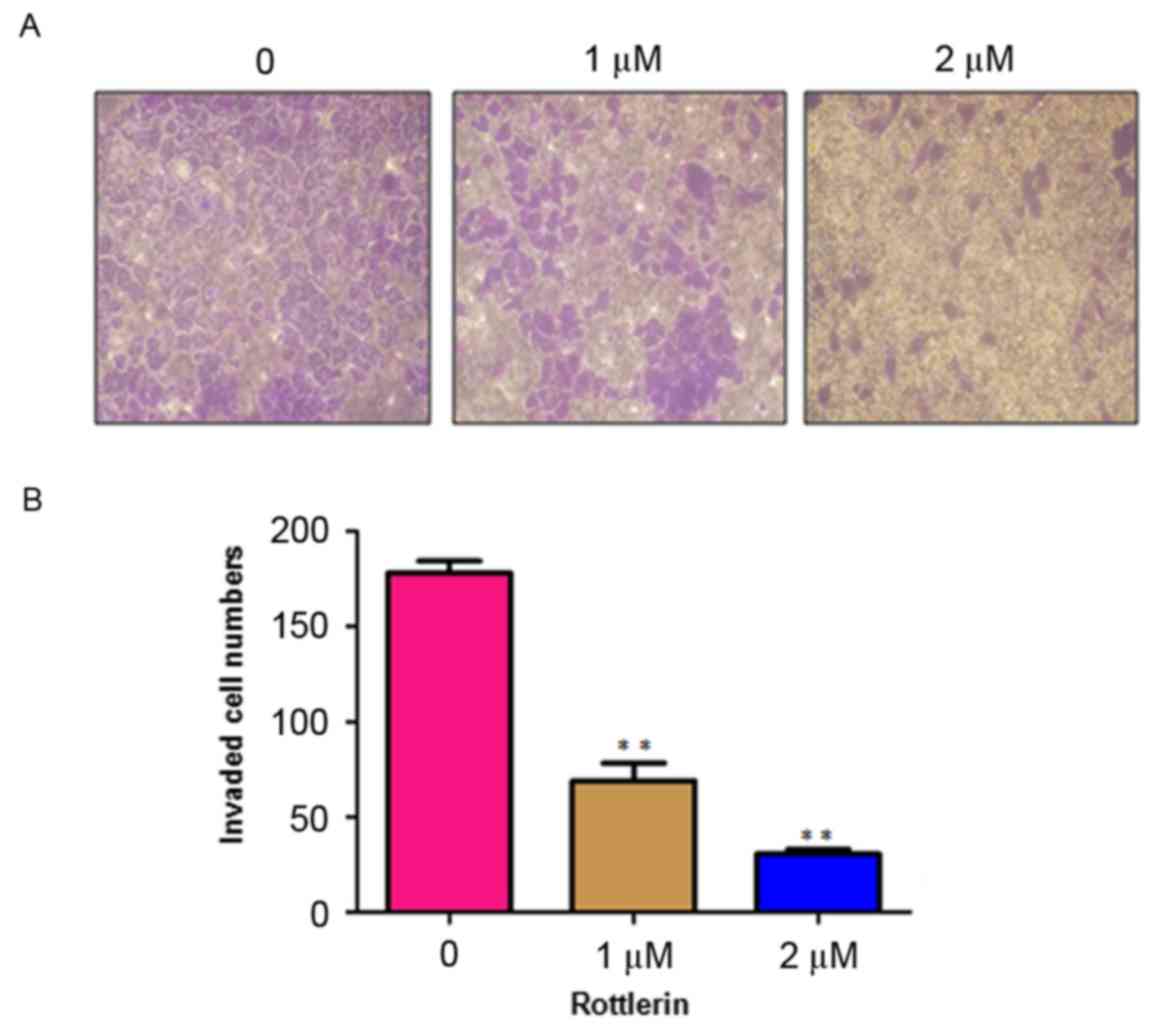
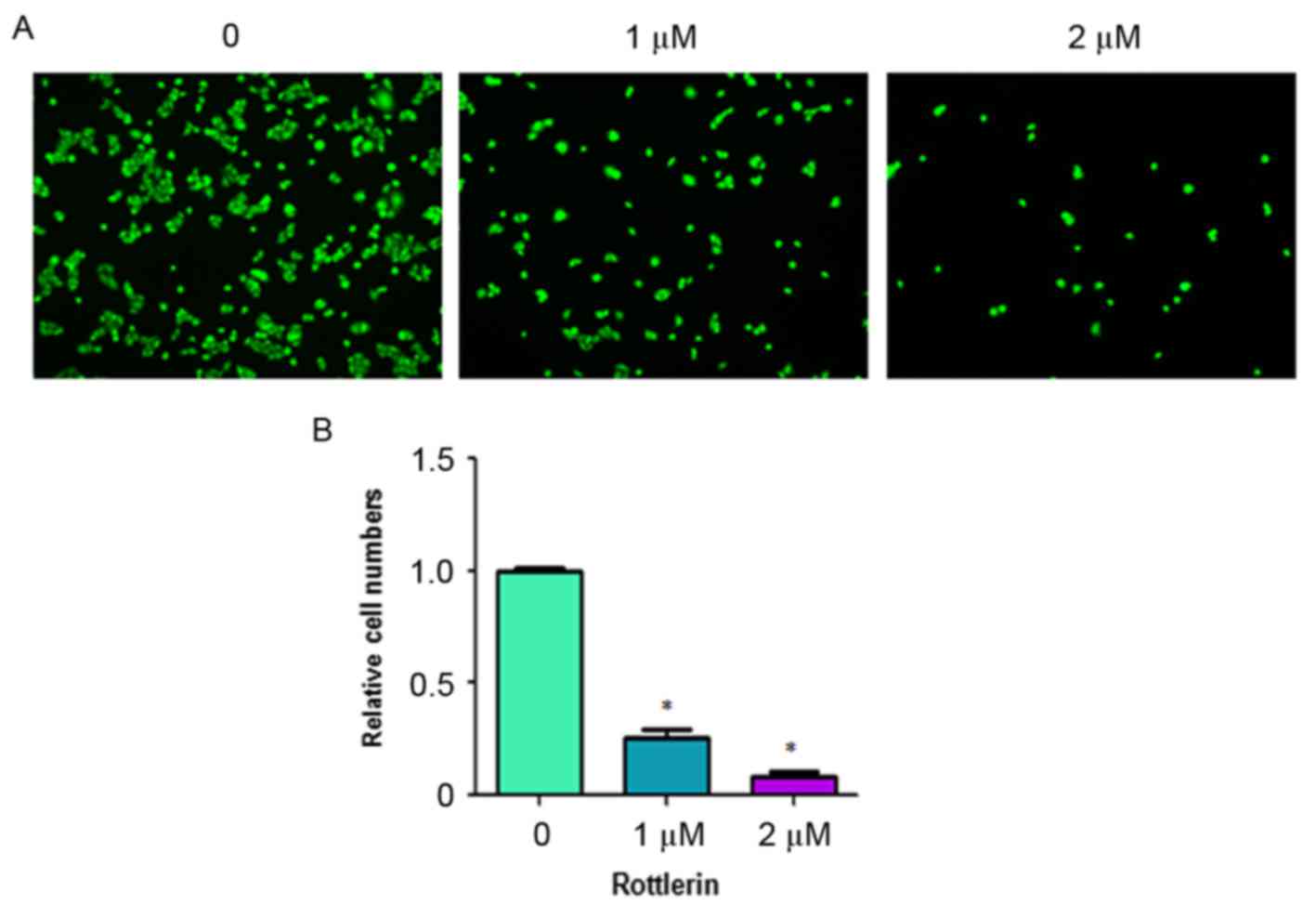
Rottlerin inhibits cell invasion
Rottlerin has been reported to inhibit cell invasion
in pancreatic cancer cells (17).
To analyze whether rottlerin can inhibit the cell motility of HCC
cells, the present study performed an invasion assay using
Matrigel-coated membranes. The results showed that rottlerin
treatment led to decreased penetration of the HCC cells through the
Matrigel-coated membrane, compared with that of the control cells
(Fig. 4). In addition, the results
showed that the numbers of fluorescently-labeled invasive cells
were significantly reduced by rottlerin treatment in the HCC cells
(Fig. 5). These results suggested
that rottlerin inhibited cell invasion of the HCC cells.
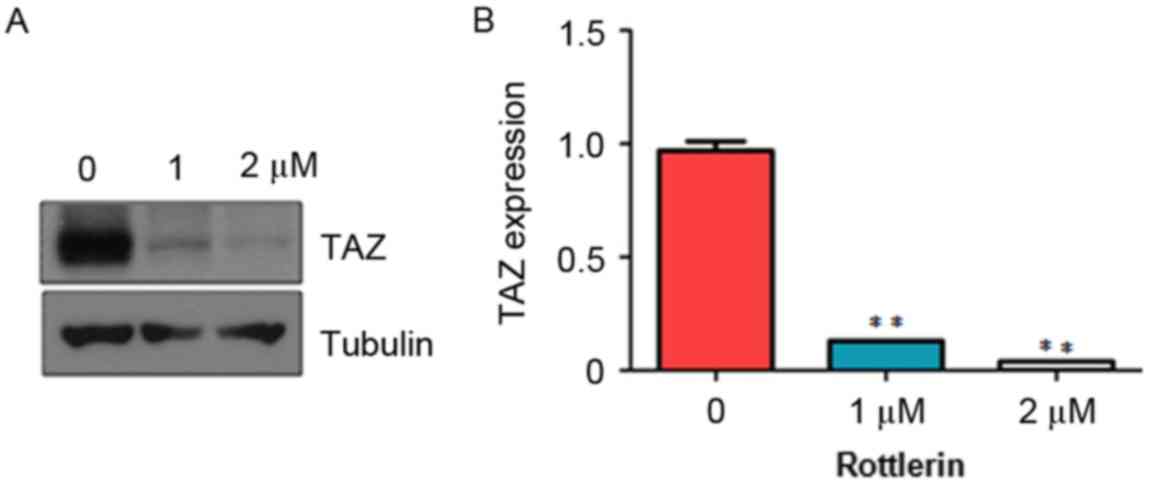
Rottlerin decreases the expression of
TAZ
It has been shown that TAZ exerts oncogenic
functions in tumorigenesis (19).
To determine whether rottlerin mediates antitumor activity via
regulating the expression of TAZ in HCC cells, the present study
measured the expression of TAZ in HCC cells treated with rottlerin
using western blot analysis. The results from the western blot
analysis showed that rottlerin significantly downregulated the
expression of TAZ in the QGY-7703 cells (Fig. 6). This finding indicated that
rottlerin exhibited anticancer activity at least partly due to the
inhibition of TAZ in HCC cells.
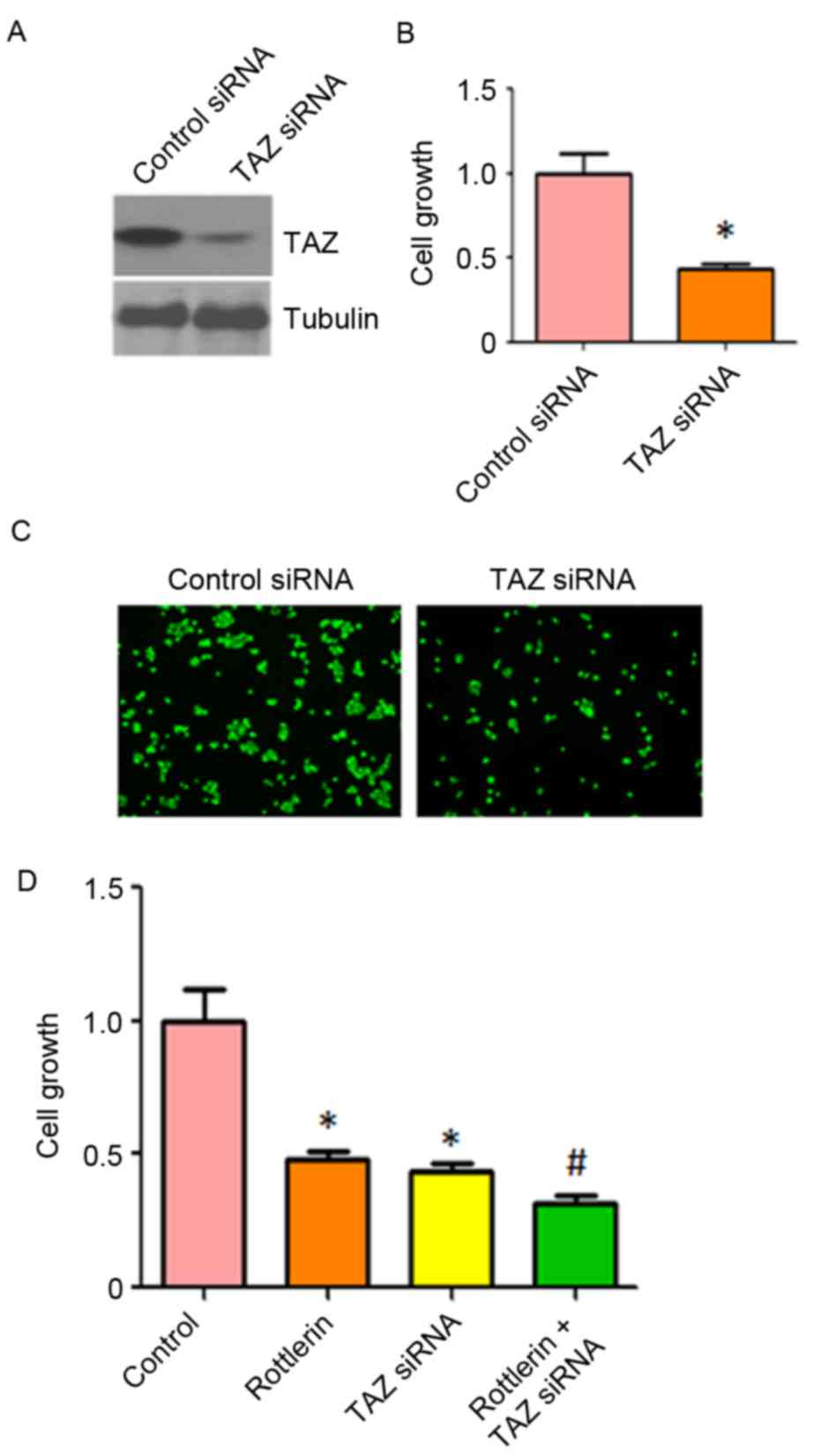
Down-regulation of TAZ enhances
rottlerin-induced inhibition of cell growth
To further confirm the role of TAZ in
rottlerin-induced cell growth inhibition, the HCC cells were
transfected with TAZ siRNA. It was found that TAZ siRNA decreased
the expression of TAZ in QGY-7703 cells (Fig. 7A). The results of the CTG assay
showed that the depletion of TAZ inhibited cell growth (Fig. 7B). It was also observed that the
downregulation of TAZ inhibited cell invasion (Fig. 7C). Of note, cell growth was
significantly inhibited by rottlerin combined with TAZ siRNA
transfection (Fig. 7D). These
results suggested that rottlerin suppressed cell growth partly due
to the inhibition of TAZ in HCC cells.
Discussion
There is increasing evidence supporting the
potential antitumor activity of rottlerin in human cancer. For
example, rottlerin has been shown to induce Wnt co-receptor LRP6
degradation, and inhibit Wnt/β-catenin and mammalian target of
rapamycin (mTOR) C1 signaling in prostate and breast cancer cells
(20). Kumar et al
(21) reported that rottlerin
induced autophagy and apoptosis via regulation of the
phosphoinositide 3-kinase (PI3K)/Akt/mTOR signaling pathway in
prostate and pancreatic cancer stem cells (22). Lim et al (23) found that rottlerin induced
apoptosis via regulating extracellular signal regulated kinase
(ERK) and p38 mitogen-activated protein kinase (MAPK) in colon
carcinoma cells. Another study showed that rottlerin inhibited cell
growth and invasion through the downregulation of Cdc20 in glioma
cells (24). Rottlerin also
exhibited antitumor effect via the inactivation of S phase
kinase-associated protein 2 in pancreatic cancer cells and breast
cancer cells (17,18). These reports identified the
mechanisms underlying rottlerin-mediated antitumor function. In the
present study, it was found that rottlerin inhibited the expression
of TAZ in HCC cells, suggesting that targeting TAZ by rottlerin is
a potential approach for the treatment of HCC.
Previous studies have revealed that the
overexpression of YAP/TAZ promotes the proliferation of cancer
cells. Wang et al (25)
reported that TAZ promoted cell growth and inhibited
celastrol-induced cell apoptosis. In addition, the overexpression
of TAZ was shown to enhance cell proliferation, migration and
epithelial-mesenchymal transition (EMT) in ovarian cancer (26). In support of these findings, the
present study observed that the overexpression of TAZ promoted cell
growth and invasion, whereas the depletion of TAZ inhibited cell
growth and invasion. TAZ has also been shown to promote EMT and
support pancreatic cancer progression (27). In accordance, the knockdown of TAZ
modifies cell sensitivity to EGFR inhibitors in triple-negative
breast cancer cells (28). TAZ and
YAP regulate pancreatic cancer initiation in mice via the direct
upregulation of JAK-STAT3 signaling (29). Further investigation is required to
examine the mechanism underlying TAZ-induced ovarian
tumorigenesis.
As TAZ is a key oncoprotein, it is important to
identify its inhibitors. Several groups have identified multiple
inhibitors of TAZ. It has been reported that statins attenuate cell
proliferative ability and induce apoptosis through TAZ in HCC cells
(30), and statins have been shown
to improve the prognosis of patients with HCC (30). Curcumin has been reported to
downregulate the expression of TAZ in pancreatic cancer cells
(31). In accordance, curcumin
promotes the degradation of Krueppel-like factor 5 via the
downregulation of YAP/TAZ in bladder cancer cells (32). Dasatinib, statins and pazopanid
have been shown to inhibit the nuclear localization of TAZ via
inducting the phosphorylation of TAZ (33). Pazopanib has also been shown to
induce the proteasomal degradation of YAP/TAZ (33). The present study identified
rottlerin as a potential inhibitor of TAZ in HCC. The present study
confirmed that the rottlerin-induced inhibition of cell
proliferation, induction of cell apoptosis and cell cycle arrest,
and suppression of cell invasion and migration in HCC occurred
partly through the downregulation of TAZ. Therefore, the inhibition
of TAZ by rottlerin may be an effective approach for the treatment
of HCC. Further investigations are required to investigate the
functions of rottlerin in additional HCC cell lines and in HCC
in vivo models.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kudo M, Trevisani F, Abou-Alfa GK and
Rimassa L: Hepatocellular carcinoma: Therapeutic guidelines and
medical treatment. Liver Cancer. 6:16–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maioli E, Torricelli C and Valacchi G:
Rottlerin and cancer: Novel evidence and mechanisms. Scientific
World Journal. 2012:3508262012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gschwendt M, Müller HJ, Kielbassa K, Zang
R, Kittstein W, Rincke G and Marks F: Rottlerin, a novel protein
kinase inhibitor. Biochem Biophys Res Commun. 199:93–98. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parmer TG, Ward MD and Hait WN: Effects of
rottlerin, an inhibitor of calmodulin-dependent protein kinase III,
on cellular proliferation, viability, and cell cycle distribution
in malignant glioma cells. Cell Growth Differ. 8:327–334.
1997.PubMed/NCBI
|
|
7
|
Ni H, Ergin M, Tibudan SS, Denning MF,
Izban KF and Alkan S: Protein kinase C-delta is commonly expressed
in multiple myeloma cells and its downregulation by rottlerin
causes apoptosis. Br J Haematol. 121:849–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tillman DM, Izeradjene K, Szucs KS,
Douglas L and Houghton JA: Rottlerin sensitizes colon carcinoma
cells to tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis via uncoupling of the mitochondria
independent of protein kinase C. Cancer Res. 63:5118–5125.
2003.PubMed/NCBI
|
|
9
|
Kim EH, Kim SU and Choi KS: Rottlerin
sensitizes glioma cells to TRAIL-induced apoptosis by inhibition of
Cdc2 and the subsequent downregulation of survivin and XIAP.
Oncogene. 24:838–849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torricelli C, Fortino V, Capurro E,
Valacchi G, Pacini A, Muscettola M, Soucek K and Maioli E:
Rottlerin inhibits the nuclear factor kappaB/cyclin-D1 cascade in
MCF-7 breast cancer cells. Life Sci. 82:638–643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basu A, Adkins B and Basu C:
Down-regulation of caspase-2 by rottlerin via protein kinase
C-delta-independent pathway. Cancer Res. 68:2795–2802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song KS, Kim JS, Yun EJ, Kim YR, Seo KS,
Park JH, Jung YJ, Park JI, Kweon GR, Yoon WH, et al: Rottlerin
induces autophagy and apoptotic cell death through a
PKC-delta-independent pathway in HT1080 human fibrosarcoma cells:
The protective role of autophagy in apoptosis. Autophagy.
4:650–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barron DA and Kagey JD: The role of the
Hippo pathway in human disease and tumorigenesis. Clin Transl Med.
3:252014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Ren P, Gou J and Li Z: Prognostic
significance of TAZ expression in various cancers: A meta-analysis.
Onco Targets Ther. 9:5235–5244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Xue C, Cui H and Huang Z: High
expression of TAZ indicates a poor prognosis in retinoblastoma.
Diagn Pathol. 10:1872015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zanconato F, Battilana G, Cordenonsi M and
Piccolo S: YAP/TAZ as therapeutic targets in cancer. Curr Opin
Pharmacol. 29:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X,
Zhou X and Wang Z: Rottlerin exhibits anti-cancer effect through
inactivation of S phase kinase-associated protein 2 in pancreatic
cancer cells. Am J Cancer Res. 6:2178–2191. 2016.PubMed/NCBI
|
|
18
|
Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X,
Zhao Z, Zhou X, Li Y and Wang Z: Rottlerin exerts its anti-tumor
activity through inhibition of Skp2 in breast cancer cells.
Oncotarget. 7:66512–66524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu W, Lin C and Li Y: Rottlerin induces
Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin
and mTORC1 signaling in prostate and breast cancer cells. Cell
Signal. 26:1303–1309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin induces autophagy and apoptosis in prostate cancer stem
cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett.
343:179–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh BN, Kumar D, Shankar S and
Srivastava RK: Rottlerin induces autophagy which leads to apoptotic
cell death through inhibition of PI3K/Akt/mTOR pathway in human
pancreatic cancer stem cells. Biochem Pharmacol. 84:1154–1163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim JH, Woo SM, Min KJ, Park EJ, Jang JH,
Seo BR, Iqbal T, Lee TJ, Kim SH, Choi YH and Kwon TK: Rottlerin
induces apoptosis of HT29 colon carcinoma cells through NAG-1
upregulation via an ERK and p38 MAPK-dependent and PKC
δ-independent mechanism. Chem Biol Interact. 197:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Ye X, Cai X, Su J, Ma R, Yin X,
Zhou X, Li H and Wang Z: Curcumin suppresses cell growth and
invasion and induces apoptosis by down-regulation of Skp2 pathway
in glioma cells. Oncotarget. 6:18027–18037. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Ma K, Chen L, Zhu H, Liang S, Liu
M and Xu N: TAZ promotes cell growth and inhibits Celastrol-induced
cell apoptosis. Biosci Rep. 36:pii: e00386. 2016. View Article : Google Scholar
|
|
26
|
Chen G, Xie J, Huang P and Yang Z:
Overexpression of TAZ promotes cell proliferation, migration and
epithelial-mesenchymal transition in ovarian cancer. Oncol Lett.
12:1821–1825. 2016.PubMed/NCBI
|
|
27
|
Xie D, Cui J, Xia T, Jia Z, Wang L, Wei W,
Zhu A, Gao Y, Xie K and Quan M: Hippo transducer TAZ promotes
epithelial mesenchymal transition and supports pancreatic cancer
progression. Oncotarget. 6:35949–35963. 2015.PubMed/NCBI
|
|
28
|
Guo L, Zheng J, Zhang J, Wang H, Shao G
and Teng L: Knockdown of TAZ modifies triple-negative breast cancer
cell sensitivity to EGFR inhibitors by regulating YAP expression.
Oncol Rep. 36:729–736. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gruber R, Panayiotou R, Nye E,
Spencer-Dene B, Stamp G and Behrens A: YAP1 and TAZ control
pancreatic cancer initiation in mice by direct up-regulation of
JAK-STAT3 signaling. Gastroenterology. 151:526–539. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higashi T, Hayashi H, Kitano Y, Yamamura
K, Kaida T, Arima K, Taki K, Nakagawa S, Okabe H, Nitta H, et al:
Statin attenuates cell proliferative ability via TAZ (WWTR1) in
hepatocellular carcinoma. Med Oncol. 33:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
down-regulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016.PubMed/NCBI
|
|
32
|
Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K,
Wang K, Wang X, Chang LS, He D and Guo P: Curcumin promotes KLF5
proteasome degradation through downregulating YAP/TAZ in bladder
cancer cells. Int J Mol Sci. 15:15173–15187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oku Y, Nishiya N, Shito T, Yamamoto R,
Yamamoto Y, Oyama C and Uehara Y: Small molecules inhibiting the
nuclear localization of YAP/TAZ for chemotherapeutics and
chemosensitizers against breast cancers. FEBS Open Bio. 5:542–549.
2015. View Article : Google Scholar : PubMed/NCBI
|