Introduction
Ultraviolet (UV) radiation leads to DNA damage, cell
senescence and apoptosis. It also results in the formation of the
two most common DNA lesions, cyclobutane pyrimidine dimers and 6–4
photoproducts. These lesions impede DNA replication and
transcription, and are repaired by nucleotide excision repair (NER)
(1,2). NER is critical in the repair of
UV-induced DNA lesions and its actions involve the endonucleolytic
cleavage of two phosphodiester bonds followed by excision of the
damaged DNA. The excised oligonucleotide is replaced by DNA repair
synthesis, and the continuity of the DNA strand is re-established
by DNA ligase (3).
The circadian rhythm is responsible for regulating
various physiological processes, including hormone production,
temperature and sleep pattern. It is found in the majority of
living organisms, including animals, plants and fungi. The
mammalian circadian clock system is organized as a central
oscillator. This system is controlled by the suprachiasmatic
nucleus (SCN) and the peripheral oscillators (4–6). The
core clock oscillator includes the two heterodimeric
transcriptional factors, CLOCK and brain and muscle aryl
hydrocarbon receptor nuclear translocator (ARNT)-like protein-1
(BMAL1), which activates the transcription of their transcriptional
repressors, Period (Per) and Cryptochrome (Cry)
(5,6).
The DNA damage response pathways include DNA repair,
damage checkpoints and cell cycle arrest. Current evidence
indicates that these pathways are associated with the circadian
clock (2,7,8).
Furthermore, there is evidence that the normal circadian rhythm and
gene expression levels in the skin are suppressed by UVB radiation,
as has been demonstrated for the gene expression levels of
Bmal1, Per and Clock in human keratinocytes (9,10).
MicroRNAs (miRNAs) are small non-coding RNA
molecules, which are involved in the regulation of gene expression
by binding to the 3′-untranslated regions (UTRs) of target mRNAs
and thus interrupting protein translation (11–13).
Numerous studies have shown that miRNAs are important in biological
processes, including cell proliferation, apoptosis, development,
metabolism and differentiation (14–17).
Previous studies have implicated miRNAs in the modulation of the
circadian clock, revealing that miRNA (miR)-219 regulates the
length of the circadian period, whereas miR-132 modulates
Per gene transcription and protein stability (18,19).
Although miR-142-3p is present in other cells, it is involved in
the post-translational modulation of BMAL1 in mouse SCN, NIH3T3 and
human 293ET cells (20,21); however, the molecular mechanisms by
which miR-142-3p mediates the post-transcriptional regulation of
BMAL1 in skin cells remain to be elucidated. Several studies have
reported an association between UV radiation exposure and
alterations in miRNA expression in keratinocytes (22,23).
UVB radiation-exposed keratinocytes exhibit several specific miRNA
response patterns (24).
Trichosanthes kirilowii is a traditional
medicine used in East Asia for treating patients with diabetes,
cancer-associated symptoms, coughing and breast abscesses (25,26).
However, the effects of T. kirilowii on skin cells and its
effect on DNA damage repair remain to be fully elucidated.
Therefore, the aim of the present study was to investigate the
effects of T. kirilowii extract (TKE) against UVB-induced
DNA damage in skin cells. In addition, the involvement of
miR-142-3p and BMAL1 in the TKE-mediated repair of UVB-induced DNA
damage was investigated.
Materials and methods
Preparation of TKE
The T. kirilowii plant material used in the
present study was purchased from Jeong-woo-dang Oriental Medicine
Market (Seoul, Korea). To prepare the TKE, the powdered T.
kirilowii (50 g) was extracted for 3 h with 70% ethanol (1
liter) at room temperature (20–25°C) while stirring. The
supernatant was collected by filtration, and the ethanol was
removed using a rotary vacuum evaporator.
Cell culture conditions
The HaCaT cells were obtained from Cell Line Service
GmbH (Eppelheim, Germany) and were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and
1% penicillin/streptomycin (all HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) in a humidified atmosphere of 5%
CO2 at 37°C.
UVB exposure and subsequent TKE
treatment
The HaCaT cells were incubated in serum-free medium
for 24 h in culture plates, washed twice in phosphate-buffered
saline (PBS), exposed to the desired doses (12.5 mJ/cm2)
of UVB radiation under a thin layer of PBS, and then immediately
incubated with serum-free medium containing various concentrations
of TKE (25, 50, 100 and 200 µg/ml) for 24 h in in a humidified
atmosphere of 5% CO2 at 37°C.
Measurement of cell viability using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The HaCaT cells were cultured in 96-well plates
(1×104 cells/well) for 24 h in complete and serum-free
media, sequentially. The cells were exposed to UVB radiation and
then incubated with serum-free medium containing various
concentrations (25–200 µg/ml) of TKE. Following culture for 24 h,
the cell viability was determined using the MTT assay. Briefly, to
each well, 20 µl of MTT solution (5 mg/ml) was added and the plate
was incubated for an additional 4 h at 37°C. The formazan product,
which formed was solubilized in 100 µl of isopropanol, and the
absorbance was measured at 560 nm.
Western blot analysis
The cells were rinsed twice with ice-cold PBS,
following which cell lysates were prepared using
radioimmunoprecipitation assay lysis buffer, comprising 150 mM
sodium chloride, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1%
sodium dodecyl sulfate (SDS), and 50 mM Tris-hydrochloride (pH 8.0)
containing a protease inhibitor cocktail. The lysate protein
concentrations were determined using bicinchoninic acid reagent.
The cell lysate samples (40 µg) were then separated using 8%
SDS-polyacrylamide gel electrophoresis and blotted onto
polyvinylidene difluoride membranes. The membranes were blocked
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in 1X TBS
Tween-20 buffer for 20 min at room temperature. The membranes were
incubated with anti-human BMAL1 (1:3,000; cat. no. SC-8550; Abcam,
Cambridge, MA, USA) or anti-human actin (1:1,000; cat. no.
SC-1615-R; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight, followed by incubation with a horseradish
peroxidase-conjugated secondary antibody (1:10,000; cat. no.
SC-2768; Santa Cruz Biotechnology, Inc.) at room temperature for 1
h. Finally, the protein-antibody conjugates on the membranes were
visualized using enhanced chemiluminescence reagents (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Comet assay (single-cell gel
electrophoresis)
Following UVB radiation, the cells were scraped and
embedded in low melting point agarose on CometSlides™ (Trevigen,
Gaithersburg, MD, USA) at 4°C for 10 min. The slides were then
incubated with lysis buffer at 4°C for 1 h, followed by immersion
in an alkaline unwinding solution for 30 min at room temperature
(20–25°C). The slides were electrophoresed at 50 V, rinsed with
distilled water and 70% ethanol, and then stained with
SYBR®−Green (Trevigen) for 10 min. The DNA damage was
visualized using a fluorescent microscope (IX71; Olympus
Corporation, Tokyo Japan). These data were analyzed with analySIS
LS Starter version 2.2 (Olympus Soft Imaging Solutions GmbH,
Münster, Germany).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The RNA concentration was quantified
using a UV spectrophotometer, and cDNA was synthesized from 1 µg
total RNA. The cDNA (250 ng) was then subjected to a PCR using
EmeraldAmp® GT PCR master mix (Takara Bio, Inc., Otsu,
Japan). The primers (10 pmol) used for PCR analysis were
synthesized by Bioneer Corporation (Daejeon, Korea). The cycling
conditions were as follows: 30 cycles at 94°C for 30 sec, 60°C for
1 min, and 72°C for 30 sec. The primer sequences used were as
follows: BMAL1, forward 5′-AAGGATGGCTGTTCAGCACA-3′ and reverse
5′-CAAAAATCCATGGCTGCCC-3′; and β-actin, forward
5′-ACACTGTGCCCATCTACG-GGGG-3′ and reverse
5′-ATGATGGAGTTGAAGGTAGTTTCGTGGAT-3′. The PCR products were run on
2% agarose gels containing ethidium bromide.
Bioinformatic analysis
The target gene prediction database Targetscan
(version 6.2; targetscan.org) was used to search
for predicted BMAL-1-targeted miRNAs.
RT-quantitative PCR (RT-qPCR)
analysis
The RNA (1 µg) was reverse-transcribed using the
Mir-X™ miRNA First-Strand Synthesis kit (Clontech Laboratories,
Inc., Mountain View, CA, USA) to generate cDNA. The
LightCycler® 480 SYBR-Green I Master (Roche Diagnostics,
Inc., Indianapolis, IN, USA) was used for the RT-qPCR procedure,
according to the manufacturer's protocol to quantify the miRNA
transcript levels. The appropriate quantification cycle (Cq) value
was determined using the automatic baseline determination feature.
The reaction solution contained 10 µl 2X Master Mix, 1 µl
miR-specific primer for human miR-142-3p, 1 µl universal primer,
cDNA (100 ng) and nuclease-free water to a total volume of 20 µl.
The sequence of the hsa-miR-142-3p primer was as follows:
5′-TGTAGTTTCCTACTTTATGGA-3′. U6 was used for miRNA level
normalization. The U6 primer was as follows: Forward,
5′-CCUCGUGCCGUUCCAGGUAGUU-3′ and reverse,
5′-CUACCUGAUGAACGGCAGGUU-3′. DNA was amplified using 45 cycles of
denaturation for 10 sec at 95°C and annealing for 10 sec at 60°C.
Each sample was assessed in triplicate. The relative expression of
the miRNA was quantified using Lightcycler® 480 II
software (Roche Diagnostics Inc., Indianapolis, IN, USA) and the
2−ΔΔCq method (27).
Transient transfection with synthetic
miRNA mimics and inhibitors
The mimic and inhibitor oligonucleotides for
miR-142-3p and the negative controls were synthesized by Genolution
Pharmaceutical (Seoul, Korea). Prior to miRNA transfection, the
HaCaT cell culture medium was replaced with serum-reduced medium of
opti-minimal essential medium (MEM) I (Gibco; Thermo Fisher
Scientific, Inc.). The transfection was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The HaCaT cells were
incubated with the oligonucleotides/lipofectamine mixture for 6 h,
following which the opti-MEM I medium was replaced with the growth
medium. The cells were harvested 24 h following transfection.
Statistical analysis
Statistical analysis was performed using Student's
t-test and was based on at least three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference. The GraphPad Prism 5 program (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to evaluate statistical
significance.
Results
TKE promotes the repair of
UVB-mediated DNA damage in HaCaT cells
DNA damage is caused by UV radiation. Keratinocytes
are affected by UV radiation in the outermost layer of skin. HaCaT
cells are a spontaneously immortal keratinocyte cell line, which
has been widely used in investigations involving the skin. The
present study first examined the effect of TKE at concentrations of
25, 50, 100 and 200 µg/ml on the viability of HaCaT cells treated
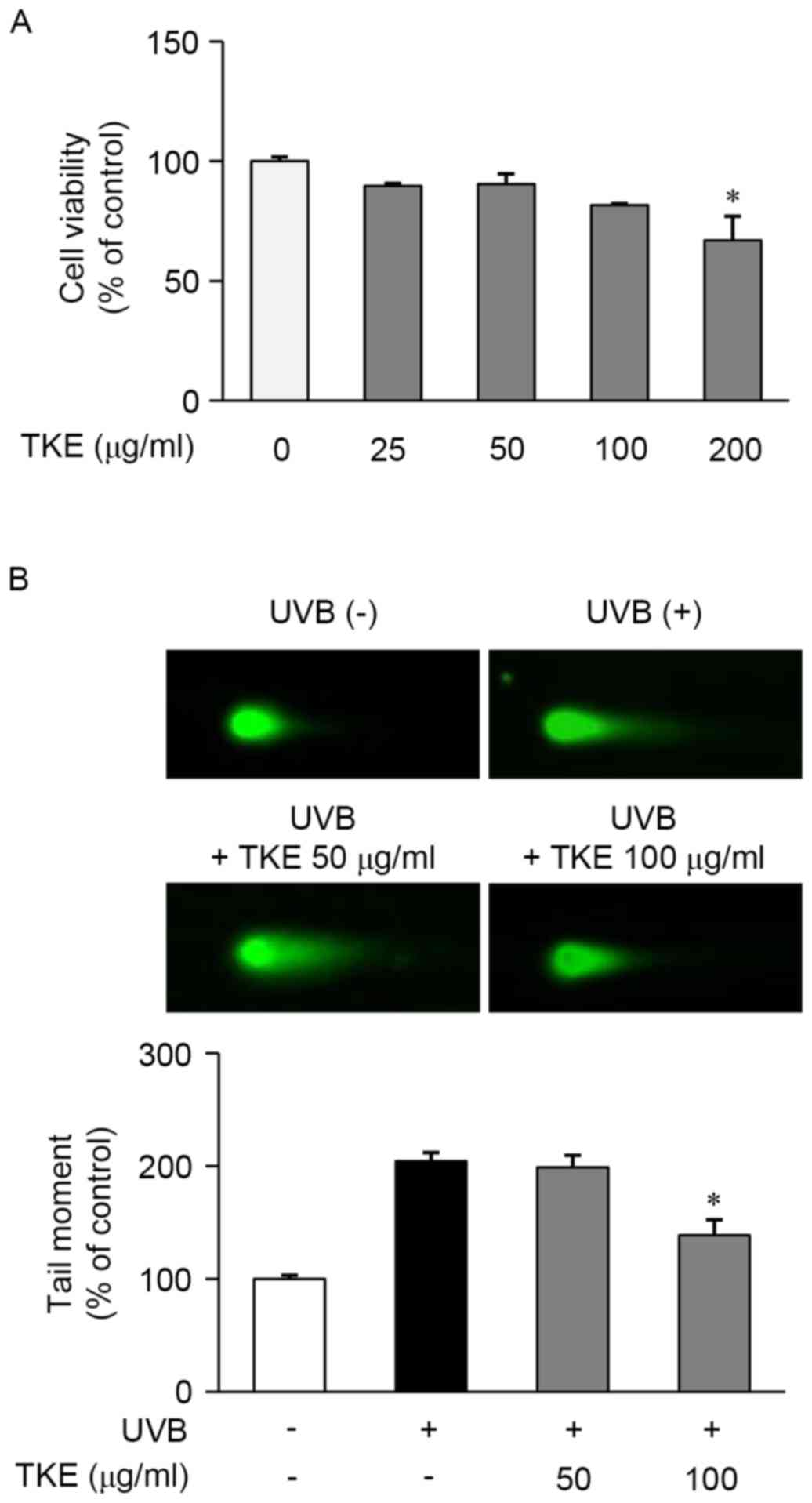
for 24 h using an MTT assay. As shown in Fig. 1A, TKE at a concentration of 200
µg/ml, but not at concentrations of ≤100 µg/ml, was significantly
cytotoxic and, based on these results; concentrations of 50–100
µg/ml were selected for use in the subsequent experiments.
The reparative and protective effects of 8 h
treatment with TKE against DNA damage induced by UVB radiation were
determined in HaCaT cells using a comet assay. The exposure of
HaCaT cells to UVB radiation (12.5 mJ/cm2) induced
extensive DNA damage, as reflected in the difference in tail
lengths between the comets of the cells exposed to UVB radiation
and those not exposed (Fig. 1B).
However, treatment of the UVB-exposed cells with TKE (100 µg/ml)
reduced the DNA damage or fragmentation, compared with that in the
untreated UVB-exposed cells (Fig.
1B). In addition, the preliminary experiments indicated that
TKE protected the HaCaT cells from UVB-induced DNA damage (data not
shown). These findings demonstrated that TKE may be involved in DNA
damage repair and may also protect against UVB-induced DNA
damage.
TKE modulates mRNA and protein
expression levels of BMAL1 in UVB-irradiated HaCaT cells
The results of the present study demonstrated that
TKE exhibited reparative effects on UVB-induced DNA damage
(Fig. 1B). Several reports have
indicated that the NER DNA repair system is dependent on the
circadian rhythm (28–30). To further investigate the molecular
mechanism by which TKE modulates DNA damage repair, the present
study examined changes in the expression of BMAL1, which is
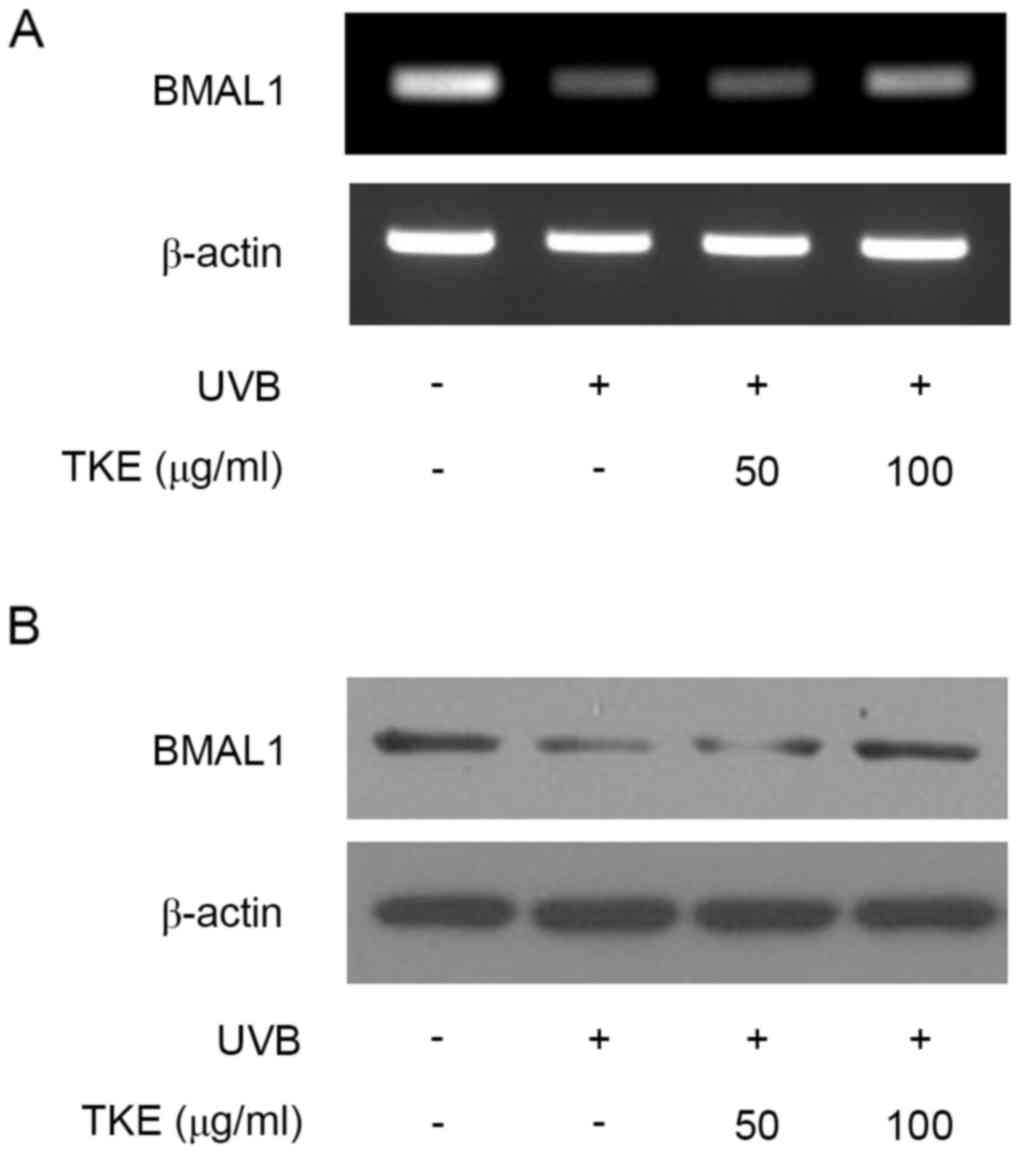
important in the circadian rhythm (9,10).
The UVB radiation decreased the mRNA and protein expression levels
of BMAL1, compared with levels in the unstimulated control cells,
which was consistent with a previous report using normal human
keratinocytes (10). However, TKE
(100 µg/ml) treatment markedly increased mRNA and protein the
expression of BMAL1 (Fig. 2A and
B). Overall, TKE treatment affected the expression levels of
BMAL1, suggesting that specific cellular response mechanisms may be
involved in TKE-mediated DNA damage repair in keratinocytes.
TKE upregulates the expression of
BMAL1 via the inhibition of miR-142-3p
It has been reported that miRNAs are closely
associated with the regulation of DNA damage in addition to
circadian rhythms (31). To
determine the potential regulatory role of BMAL1-targeted specific
miRNAs in target gene transcription, the present study used
TargetScan to predict BMAL1-targeted specific miRNAs in the HaCaT
cells. It was found that miR-142-3p showed a high level of
interaction with the 3′-UTR of BMAL1 and a high probability of
regulating the expression of BMAL1. A previous study demonstrated
that miR-142-3p directly targeted BMAL1 3′-UTRs and regulated the
mRNA and protein levels of BMAL1 in human 293ET cells (21). To investigate whether miR-142-3p is
involved in the regulation of BMAL1 in human keratinocytes, the
HaCaT cells were transfected for 24 h with the miR-142-3p mimic and
a mimic control. The results of the subsequent RT-qPCR analysis
demonstrated that the miR-142-3p mimic significantly increased the
expression of miR-142-3p in the HaCaT cells, compared with that in
cells transfected with the mimic control (Fig. 3A). Furthermore, the results of
western blot analysis demonstrated that the miR-142-3p mimic
markedly inhibited the protein expression of BMAL1 (Fig. 3B). The results of the RT-PCR
analysis also revealed that the mimic suppressed the mRNA
expression levels of BMAL1 (data not shown). Taken together, the
downregulation of the mRNA and protein expression of BMAL1 in HaCaT
cells suggested that miR-142-3p may be involved in the molecular
mechanisms underlying the repair of and protection against
UVB-induced DNA damage.
Based on the above findings, the present study
examined the effects of TKE on miRNA expression levels in the HaCaT
cells. To investigate whether the expression of BMAL1 is regulated
by TKE through the modulation of miR-142-3p, RT-qPCR analysis was
performed in the HaCaT cells treated with or without TKE. In the
HaCaT cells exposed to UVB radiation (12.5 mJ/cm2) for
24 h, the expression levels of miR-142-3p were increased, however,
in UVB-exposed cells treated with TKE (100 µg/ml) for 24 h, a
decrease in the expression of miR-142-3p was observed (Fig. 3C). Taken together, these
observations suggested that TKE-mediated DNA damage repair in HaCaT
cells may be correlated with suppression of the expression of
miR-142-3p.
miR-142-3p suppresses the repair of
UVB-induced DNA damage
To examine the role of miRNAs in the repair of
UVB-induced DNA damage, a comet assay was performed in the HaCaT
cells. The cells were transfected with the miR-142-3p mimic, mimic
control, miR-142-3p inhibitor, or inhibitor control. As shown in
Fig. 4A and B, the miR-142-3p
mimic-transfected cells exhibited increased DNA damage, compared
with the cells not exposed to UVB. However, the mimic negative
control showed no observable effects in the cells.
The present study then determined whether the
miR-142-3p inhibitor affected the repair of UVB-induced DNA damage
in HaCaT cells and found that it significantly decreased the
expression of miR-142-3p in HaCaT cells, compared with control
cells transfected with the inhibitor control (data not shown). The
miR-142-3p inhibitor-transfected cells exhibited reduced DNA
damage, compared with the UVB-exposed cells (Fig. 4C and D). These results indicated
that miR-142-3p inhibited the repair of UVB-induced DNA damage in
HaCaT cells. Taken together, these results suggested that TKE
enhanced the repair of UVB-induced DNA damage by regulating the
expression of miR-142-3p and BMAL1.
Discussion
T. kirilowii has been used in the treatment
of diabetes, respiratory diseases, and cancer-related symptoms
(25,26). The extracts and active components
of T. kirilowii have been reported to exert anticancer
activities (32). However, the
effects of T. kirilowii on skin cells and its effect on DNA
damage repair have not been reported previously. In the present
study, the reparative effect of TKE was shown on UVB-induced DNA
damage in HaCaT keratinocytes, which was likely mediated by
regulation of the circadian clock and miRNA expression.
DNA damage is caused by UV radiation. The DNA damage
response includes the DNA repair system, NER. The mechanism
underlying NER has been shown to involve the circadian rhythm.
Several reports have indicated that the circadian oscillations of
NER activity are associated with that of the protein level of
xeroderma pigmentosum group A (XPA) (2,7,8,28).
XPA is crucial in DNA damage recognition and has a regulatory
function on the circadian clock. XPA is positively regulated by
CLOCK and BMAL1, and negatively regulated by CRY and PER (29). BMAL1 is closely associated with
time-dependent UV sensitivity and the efficiency of DNA repair
(30). These findings are
consistent with a previous report that BMAL1-silenced cells showed
markedly reduced DNA repair responses (33) and another study, which reported
that BMAL1 regulates the proportion of cells in the S-phase of the
cell cycle, which are sensitive to DNA damage (30). In the present study, DNA damage was
measured in human keratinocytes (HaCaT cells) using a comet assay.
The comet tail lengths of cells treated with UVB followed by 100
µg/ml TKE showed a significant decrease compared to that of the
UVB-treated controls. TKE exerted reparative effects against
UVB-induced DNA damage (Fig. 1A).
In addition, TKE upregulated the UVB-reduced expression of BMAL1
(Fig. 2). Therefore, these results
suggested that TKE regulated the expression of BMAL1, which
repaired keratinocytes and protected them from UVB-induced damage.
These events appear to be associated with the BMAL1-modulated DNA
repair system.
The NER system is regulated by miRNA-mediated gene
regulation. Several miRNAs have been implicated in the DNA repair
pathway. The upregulation of miR-192, miR-890 and miR-744-2p
inhibits NER in cancer cells (31). In the present study, the expression
levels of miR-142-3p were significantly decreased in the
TKE-treated HaCaT cells exposed to UVB, compared with the unexposed
cells (Fig. 3C). Several previous
studies have reported that miR-142-3p is expressed in the spleen,
thymus and hematopoietic cells (33–35).
It has also been reported that miR-142-3p functions as a tumor
suppressor by targeting numerous tumor-associated genes (36,37).
miR-142-3p has been shown to be expressed at significantly higher
levels in cells from patients with psoriasis and atopic dermatitis,
compared with normal cells (38,39).
In addition, miR-142-3p was found to be upregulated in patients
with systemic sclerosis (40).
From these previous findings, it was hypothesized that miR-142-3p
may be involved in other skin diseases.
Previous studies have demonstrated that miRNAs are
important regulators of the circadian clock. miR-142-3p directly
targets BMAL1 3′-UTRs and regulates the mRNA and protein levels of
BMAL1 in mouse SCN, NIH3T3 and human 293ET cells (20,21).
In the present study, the miR-142-3p mimic markedly inhibited the
protein expression of BMAL1 in human keratinocytes (Fig. 3B). The miR-142-3p mimic increased
comet tail length, compared with that of control cells without UVB
exposure (Fig. 4A and B). The
miR-142-3p inhibitor suppressed the repair of UVB-induced DNA
damage in the HaCaT cells by its regulation of potential target
genes, including bmal1 (Fig. 4C
and D). These findings provide a novel basis for the
correlation between the regulation of BMAL1 and the suppression of
miR-142-3p by TKE.
Although the present study focused on the repair
mechanism of DNA damage by BMAL1 and miR-142-3p, this may
constitute only one of numerous mechanisms, which may include
regulation by other clock factors within the circadian clock gene
or other miRNAs. Further investigations are necessary to identify
the active component in TKE and to determine the detailed mechanism
involved in the BMAL1-mediated repair of UVB-induced DNA damage. In
conclusion, the results of the present study provided evidence of
the beneficial effects of TKE in the repair of UVB-induced DNA
damage in HaCaT cells. These findings may have important
implications for the treatment of various diseases caused by
UVB-induced photodamage, including photoaging and sunburn.
Glossary
Abbreviations
Abbreviations:
|
TKE
|
Trichosanthes kirilowii
extract
|
|
BMAL1
|
brain and muscle aryl hydrocarbon
receptor nuclear translocator-like protein-1
|
|
UVB
|
ultraviolet B radiation
|
|
Per
|
period
|
|
Cry
|
cryptochrome
|
|
miR-142-3p
|
microRNA 142-3p
|
References
|
1
|
Sinha RP and Häder DP: UV-induced DNA
damage and repair: A review. Photochem Photobiol Sci. 1:225–236.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sancar A, Lindsey-Boltz LA, Kang TH,
Reardon JT, Lee JH and Ozturk N: Circadian clock control of the
cellular response to DNA damage. FEBS Lett. 584:2618–2625. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sancar A: DNA excision repair. Annu Rev
Biochem. 65:43–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsunaga N, Itcho K, Hamamura K, Ikeda E,
Ikeyama H, Furuichi Y, Watanabe M, Koyanagi S and Ohdo S: 24-Hour
rhythm of aquaporin-3 function in the epidermis is regulated by
molecular clocks. J Invest Dermatol. 134:1636–1644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe M, Hida A, Kitamura S, Enomoto M,
Ohsawa Y, Katayose Y, Nozaki K, Moriguchi Y, Aritake S, Higuchi S,
et al: Rhythmic expression of circadian clock genes in human
leukocytes and beard hair follicle cells. Biochem Biophys Res
Commun. 425:902–907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ando N, Nakamura Y, Aoki R, Ishimaru K,
Ogawa H, Okumura K, Shibata S, Shimada S and Nakao A: Circadian
gene clock regulates psoriasis-like skin inflammation in mice. J
Invest Dermatol. 135:3001–3008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desotelle JA, Wilking MJ and Ahmad N: The
circadian control of skin and cutaneous photodamage. Photochem
Photobiol. 88:1037–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang T and Sancar A: Circadian regulation
of DNA excision repair: Implications for chrono-chemotherapy. Cell
Cycle. 8:1665–1667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gery S, Komatsu N, Baldjyan L, Yu A, Koo D
and Koeffler HP: The circadian gene per1 plays an important role in
cell growth and DNA damage control in human cancer cells. Mol Cell.
22:375–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawara S, Mydlarski R, Mamelak AJ, Freed
I, Wang B, Watanabe H, Shivji G, Tavadia SK, Suzuki H, Bjarnason
GA, et al: Low-dose ultraviolet B rays alter the mRNA expression of
the circadian clock genes in cultured human keratinocytes. J Invest
Dermatol. 119:1220–1223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V and Lee RC: Identification of
microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods
Mol Biol. 265:131–158. 2004.PubMed/NCBI
|
|
14
|
Hildebrand J, Rütze M, Walz N, Gallinat S,
Wenck H, Deppert W, Grundhoff A and Knott A: A comprehensive
analysis of microRNA expression during human keratinocyte
differentiation in vitro and in vivo. J Invest Dermatol. 131:20–29.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Wang J, Guo SL, Fan KJ, Li J, Wang
YL, Teng Y and Yang X: miR-21 promotes keratinocyte migration and
re-epithelialization during wound healing. Int J Boil Sci.
7:685–690. 2011. View Article : Google Scholar
|
|
17
|
Xu N, Brodin P, Wei T, Meisgen F, Eidsmo
L, Nagy N, Kemeny L, Ståhle M, Sonkoly E and Pivarcsi A: MiR-125b,
a microRNA downregulated in psoriasis, modulates keratinocyte
proliferation by targeting FGFR2. J Invest Dermatol. 131:1521–1529.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu K and Wang R: MicroRNA-mediated
regulation in the mammalian circadian rhythm. J Theor Biol.
304:103–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng HY, Papp JW, Varlamova O, Dziema H,
Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S
and Obrietan K: microRNA modulation of circadian-clock period and
entrainment. Neuron. 54:813–829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shende VR, Neuendorff N and Earnest DJ:
Role of miR-142-3p in the post-transcriptional regulation of the
clock gene Bmal1 in the mouse SCN. PLoS One. 8:e653002013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan X, Zhang P, Zhou L, Yin B, Pan H and
Peng X: Clock-controlled mir-142-3p can target its activator,
Bmal1. BMC Mol Biol. 13:272012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou BR, Xu Y, Permatasari F, Liu WL, Li
W, Guo XF, Huang QH, Guo Z and Luo D: Characterization of the miRNA
profile in UVB-irradiated normal human keratinocytes. Exp Dermatol.
21:317–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cha HJ, Kim OY, Lee GT, Lee KS, Lee JH,
Park I, Lee SJ, Kim YR, Ahn KJ, An IS, et al: Identification of
ultraviolet B radiation-induced microRNAs in normal human dermal
papilla cells. Mol Med Rep. 10:1663–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pothof J, Verkaik NS, van IJcken W, Wiemer
EA, Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Hoeijmakers
JH and Persengiev SP: MicroRNA-mediated gene silencing modulates
the UV-induced DNA-damage response. EMBO J. 28:2090–2099. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SR, Seo HS, Choi HS, Cho SG, Kim YK,
Hong EH, Shin YC and Ko SG: Trichosanthes kirilowii ethanol extract
and cucurbitacin D inhibit cell growth and induce apoptosis through
inhibition of STAT3 activity in breast cancer cells. Evid Based
Complement Alternat Med. 2013:9753502013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin JW, Son JY, Kang JK, Han SH, Cho CK
and Son CG: Trichosanthes kirilowii tuber extract induces G2/M
phase arrest via inhibition of tubulin polymerization in HepG2
cells. J Ethnopharmacol. 115:209–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Anlysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang TH, Reardon JT, Kemp M and Sancar A:
Circadian oscillation of nucleotide excision repair in mammalian
brain. Proc Natl Acad Sci USA. 106:2864–2867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang TH, Lindsey-Boltz LA, Reardon JT and
Sancar A: Circadian control of XPA and excision repair of
cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase.
Proc Natl Acad Sci USA. 107:4890–4895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon
W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A, et al: Brain and
muscle Arnt-like protein-1 (BMAL1) controls circadian cell
proliferation and susceptibility to UVB-induced DNA damage in the
epidermis. Proc Natl Acad Sci USA. 109:11758–11763. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie QH, He XX, Chang Y, Sun SZ, Jiang X,
Li PY and Lin JS: MiR-192 inhibits nucleotide excision repair by
targeting ERCC3 and ERCC4 in HepG2.2.15 cells. Biochem Biophys Res
Commun. 410:440–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ni L, Zhu X, Gong C, Luo Y, Wang L, Zhou
W, Zhu S and Li Y: Trichosanthes kirilowii fruits inhibit non-small
cell lung cancer cell growth through mitotic cell-cycle arrest. Am
J Chin Med. 43:349–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bee L, Marini S, Pontarin G, Ferraro P,
Costa R, Albrecht U and Celotti L: Nucleotide excision repair
efficiency in quiescent human fibroblasts is modulated by circadian
clock. Nucleic Acids Res. 43:2126–2137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramkissoon SH, Mainwaring LA, Ogasawara Y,
Keyvanfar K, McCoy JP Jr, Sloand EM, Kajigaya S and Young NS:
Hematopoietic-specific microRNA expression in human cells. Leuk
Res. 30:643–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang XS, Gong JN, Yu J, Wang F, Zhang XH,
Yin XL, Tan ZQ, Luo ZM, Yang GH, Shen C and Zhang JW: MicroRNA-29a
and microRNA-142-3p are regulators of myeloid differentiation and
acute myeloid leukemia. Blood. 119:4992–5004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang
H, Hong M, Jiang T, Jiang Q, Lu J, et al: An oncogenic role of
miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by
targeting glucocorticoid receptor-α and cAMP/PKA pathways.
Leukemia. 26:769–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lei Z, Xu G, Wang L, Yang H, Liu X, Zhao J
and Zhang HT: MiR-142-3p represses TGF-β-induced growth inhibition
through repression of TGFβR1 in non-small cell lung cancer. FASEB
J. 28:2696–2704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Joyce CE, Zhou X, Xia J, Ryan C, Thrash B,
Menter A, Zhang W and Bowcock AM: Deep sequencing of small RNAs
from human skin reveals major alterations in the psoriasis
miRNAome. Hum Mol Genet. 20:4025–4040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vennegaard MT, Bonefeld CM, Hagedorn PH,
Bangsgaard N, Løvendorf MB, Odum N, Woetmann A, Geisler C and Skov
L: Allergic contact dermatitis induces upregulation of identical
microRNAs in humans and mice. Contact Dermatitis. 67:298–305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Makino K, Jinnin M, Kajihara I, Honda N,
Sakai K, Masuguchi S, Fukushima S, Inoue Y and Ihn H: Circulating
miR-142-3p levels in patients with systemic sclerosis. Clin Exp
Dermatol. 37:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|