Introduction
Odontogenic cysts are the most common lesions in the
jaw, which usually grow large along with resorbing the bone and
expanding into surrounding tissues, leading some vital structures
(i.e., inferior alveolar neurovascular bundle, and maxillary sinus)
damage and causing facial asymmetry, displacement of teeth, and
pathologic fractures (1–3). Removal of the cysts is necessary, but
enucleation of these lesions can bring forth a risk of
complications such as infection, fracture of the jaw, or nerve
injury. Recently, decompression with marsupialization has been
recommended as a more conservative treatment for large odontogenic
cysts. Decompression can mitigate the intramural pressure and
reduce the cytokines production, in favor of maintenance of pulp
vitality, preservation of the inferior alveolar nerve or maxillary
sinus, prevention of fracture of the jaw, and minimization of the
recurrence risk (4–7).
Human bone marrow stromal cells (BMSCs) have been
widely studied and used as a therapeutic tool in numerous studies
and clinical trials due to their unique characteristics, including
ease of isolation, amplification in vitro, immunological
tolerance and multipotent capacity (8,9).
Previous studies have showed that BMSCs from human alveolar/jaw
bone have osteogenic potential to promote regeneration of alveolar
bone (10). Moreover,
differentiation of BMSCs into osteoblasts is regulated by many
factors including growth factors, cytokines, hormones and
mechanical stress. Whilst decompression causes a reduction in the
cyst volume with new bone formation, it is unclear if BMSCs respond
to these changes. In this study, we examined the effects of
decompression on the ‘stemness’ and osteogenic potential of
alveolar BMSCs around the cysts.
Materials and methods
Samples and cell culture
Trabecular bone isolated with a rongeur from five
patients with odontogenic cysts (three women and two men at the age
ranging from 29 to 37 years), who provided informed consent at the
Department of stomatology of the First Hospital of Jiaxing,
Zhejiang, China. The study was carried out under approved
guidelines of the Ethical Committee. Trabecular bone around cysts
was separately collected during the marsupialization surgery and
the second-stage procedure of cysts excision in same individuals.
Nucleated cells isolated from each sample were cultured to
establish primary BMSC using α-modified Eagle's medium (α-MEM;
Gibco-BRL, Carlsbad, CA, USA) supplemented with 20% fetal bovine
serum (FBS) (Equitech-Bio, Kerrville, TX, USA), 100 U/ml
penicillin, 100 mg/ml streptomycin and 2 mM glutamine (Sangon
Biotech Co., Ltd., Shanghai, China), as previously described
(11,12). Cultures were maintained at 37°C in
a humidified atmosphere of 5% CO2 and the culture medium
changed thrice a week. Primary BMSCs from before (pre-BMSCs) and
after (post-BMSCs) marsupialization were further expanded and then
used at passage 2–4 for in vitro experiment.
Cell proliferation assay
BMSCs were seeded into 96-well plates at a density
of 5×103 cells/well and incubated overnight in α-MEM
medium. Then Cell Counting Kit-8 (CCK8; KeyGen Biotech Co., Ltd.,
Nanjing, China) solution was added to each well and incubated at
37°C for 1 h at room temperature. Absorbance at 450 nm was measured
spectrophotometrically with a MRX II absorbance reader (Dynex
Technologies, Inc., Chantilly, VA, USA). The proliferation assay
was performed each day for 18 days consecutively.
Colony forming efficiency assay
For colony forming unit-fibrolast (CFU-F) assays,
BMSCs were cultured in triplicate 25 cm2 plastic culture
flasks at 103, 104 and 105
cells/flask with 6 mls of non-osteogenic growth medium (13). After incubation for 10 days without
medium change, cultures were washed with PBS, fixed with 100%
methanol and dyed bv an aqueous solution of saturated methyl violet
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Aggregates of 50
or more cells were counted as colonies.
Flow cytometric analysis
BMSCs were trypsinized with 0.25% trypsin-EDTA,
centrifuged at 1,500 g for 5 min, resuspended and aliquoted at PBS
containing 3% FBS into a fluorescence-activated cell sorting (FACS)
tube. Each aliquot containing 1×105 cells were incubated
with saturating concentrations of primary antibodies or control IgG
at room temperature for 30 min. The cells were washed twice by PBS
and incubated with a fluorescent conjugated secondary antibody for
30 min at room temperature in dark. The following monoclonal
antibodies (mAbs) were used: CD34-PE, CD90-PE, CD44-FITC, and
CD45-FITC (Becton-Dickinson, Franklin Lakes, NJ, USA). Events were
collected with FACScan (BD Bioscience), and the data were analyzed
with FlowJo software (TreeStar, Ashland, OR, USA).
Immunofluorescence staining
BMSCs were were fixed with 4% paraformaldehyde for
20 min at room temperature and permeabilised with 0.1% Triton X-100
in PBS for 5 min. After rinsing with phosphate-buffered solution
(PBS), the cells were blocked with 5% horse serum in PBS for 1 h at
room temperature and then were incubated with fluorescein
isothiocyanate (FITC)-conjugated STRO-1 (sc-47,733 FITC; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and with phycoerythrin
(PE)-conjugated CD105 (Santa Cruz, sc-18,838 PE) antibodies
antibody (1:100 dilution) for 2 h at room temperature. After
washing three times with PBS, cells were stained with DAPI (100
ng/ml) for 3 min. All of the images were captured with a
fluorescence microscope (BX50 Olympus, Tokyo, Japan).
Osteogenic differentiation
BMSCs were trypsinized, resuspended and seeded in
96-well culture plates in a density of 1×104 cells/well.
The next day, the growth medium was replaced by osteogenic medium
to induce osteogenic differentiation (14). The cells were grown for 28 days and
the medium was refreshed twice a week. Alkaline phosphatase (ALP)
staining to detect osteoblasts was performed with the ALP kit and
protocol (Sigma) on cultures grown in osteogenic medium for 7 days.
All images were captured on a Olympus IX70 microscope (Olympus,
Tokyo, Japan) at the same magnification and light intensity and
analyzed by ImageJ software (NIH, Bethesda, MD, USA). Image
segmentation was used to generate percent stained values for each
field of view. At the 28th day of differentiation, the BMSCs were
washed once with PBS and fixed with 4% paraformaldehyde (Sigma) for
15 min at room temperature. At the end of osteogenic stimulation,
cells were fixed with 4% paraformaldehyde (Sigma) for 15 min,
washed with dH2O, and stained with 1% Alizarin Red S for
30 min to assess the formation of the mineralized matrix. The level
of calcium deposition was quantified by elution of AR-S following
incubation in 10% cetylpyridinium chloride (Sigma-Aldrich) for 1 h
at room temperature. Samples of the resulting solution were
distributed on a 96-well plate and absorbance was read at 570 nm.
All experiments were performed in triplicate with independent
samples.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BMSCs using Trizol
reagenl (Invitrogen, Carlsbad, CA, USA) and then
reverse-transcribed with PrimeScript RT Master mix Kit (Takara,
Otsu, Japan) according to manufacturer's recommended protocol.
Quantitative real-time PCR was performed using an Eppendorf
Mastercycler ep realplex machine (Eppendorf, Germany) and using
SYBR Premix Ex Taq™ II Kit (Takara) according to the
manufacturer's instructions. The primers were present in Table I. Real-time PCR reactions were
carried out with the following conditions: Initial denaturation
step at 95°C for 10 min, followed by 40 cycles of 5 sec at 95°C and
34 sec at 60°C. The specificity of PCR products was checked by
melting curve analysis and gel electrophoresis. Relative mRNA
expression levels were calculated by the 2−ΔΔCt method
after by normalizing to GAPDH as an internal control (15).
 | Table I.The primer sequences for PCR. |
Table I.
The primer sequences for PCR.
| Gene | Forward primers
(5′-3′) | Reverse primers
(3′-5′) | Product size
(bp) |
|---|
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC | 196 |
| ALP |
GGACCATTCCCACGTCTTCAC |
CCTTGTAGCCAGGCCCATTG | 237 |
| RUNX2 |
CCCGTGGCCTTCAAGGT |
CGTTACCCGCCATGACAGTA | 190 |
| OCN |
CCCAGGCGCTACCTGTATCAA |
GGTCAGCCAACTCGTCACAGTC | 224 |
| OPN |
CAGTTGTCCCCACAGTAGACAC |
GTGATGTCCTCGTCTGTAGCATC | 230 |
| Osterix |
GCAGCTAGAAGGGAGTGGTG |
GCAGGCAGGTGAACTCTTC | 218 |
| OCT4 |
GTATTCAGCCAAACGACCATC |
CTGGTTCGCTTTCTCTTTCG | 326 |
| Nanog |
ATTCAGGACAGCCCTGATTCTTC |
TTTTTGCGACACTCTTCTCTGC | 360 |
| SOX2 |
GACTTCACATGTCCCAGCACTA |
CTCTTTTGCACCCCTCCCATT | 298 |
| c-myc |
GCTGCTTAGACGCTGGATTT |
TAACGTTGAGGGGCATCG | 252 |
Western blotting
The BMSCs were collected and lysed in cell lysis
buffer containing protease inhibitors at 15 min (Sangon Biotech
Co., Ltd.). Then, total protein concentration in every lysate was
calculated by a bicinchoninic acid (BCA) protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equivalent amounts of protein
samples (100 µg) were electrophoresed through 10–15% gels by
SDS-PAGE and subsequently transferred to PVDF membranes. The
membranes were then blocked for 1 h in 10% skimmed milk in
Tris-buffered saline Tween (TBST) at room temperature and then
incubated overnight at 4°C with primary antibodies:
Anti-osteopontin (OPN; ab8448, 1:1,000), anti-runt-related
transcription factor 2 (Runx2; ab54868, 1:1,000), anti-osterix
(ab94744, 1:500), anti-ALP (ab83259, 1:500), and anti-osteocalcin
(OCN; ab76690, 1:2,000) (all from Abcam, Cambridge, MA, USA),
extracellular signal-regulated kinase1/2 (ERK1/2; no. 9102,
1;1,000), phosphorylated ERK1/2 (p-ERK1/2; no. 4370, 1;1,000),
c-Jun N-terminal kinase (JNK; no. 9,252, 1:1,000), phosphorylated
JNK (p-JNK; no. 9,251, 1:1,000), anti-p38 mitogen-activated protein
kinase (p38 MAPK; no. 9,212, 1:1,000), phosphorylated-p38 MAPK
(p-p38 MAPK; no. 9,215, 1:1,000), and anti-glyceraldehyde-phosphate
dehydrogenase (GAPDH) (all from Cell Signaling Technology, Inc.,
Danvers, MA, USA), used at dilutions recommended by the
manufacturer. Following three times washing with TBST, membranes
were incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. Relative protein bands intensities were quantified
using RapidStep™ ECL reagent (EMD Millipore, Billerica, MA,
USA).
Statistical analysis
Results are showed as the mean ± standard deviation
(SD) and all experiments were separately repeated at least three
times. Statistical analysis was performed using the GraphPad Prism
statistical package (version 5.0) and the Student's t-test was
performed to assess differences between experimental groups.
Differences were considered statistically significant at
P<0.05.
Results
Isolation and identification of BMSCs
in culture
BMSCs grew as adherent monolayers with a tendency to
grow in clusters under microscope, and showed fibroblast-like
morphology (Fig. 1A). The surface
markers of mesenchymal stem cells (MSCs) were identified by flow
cytometry and the results revealed positive staining for MSC
markers (CD44 and CD90), and negative for hemacyte antigen markers
(CD34 and CD45). Moreover, two early MSC markers STRO-1 and CD105,
were also present on both pre- and post-BMSCs by confocal
microscopy (Fig. 1C). All the
results indicated that the isolated BMSCs were of mesenchymal
origin and of high purity (Fig.
1B).
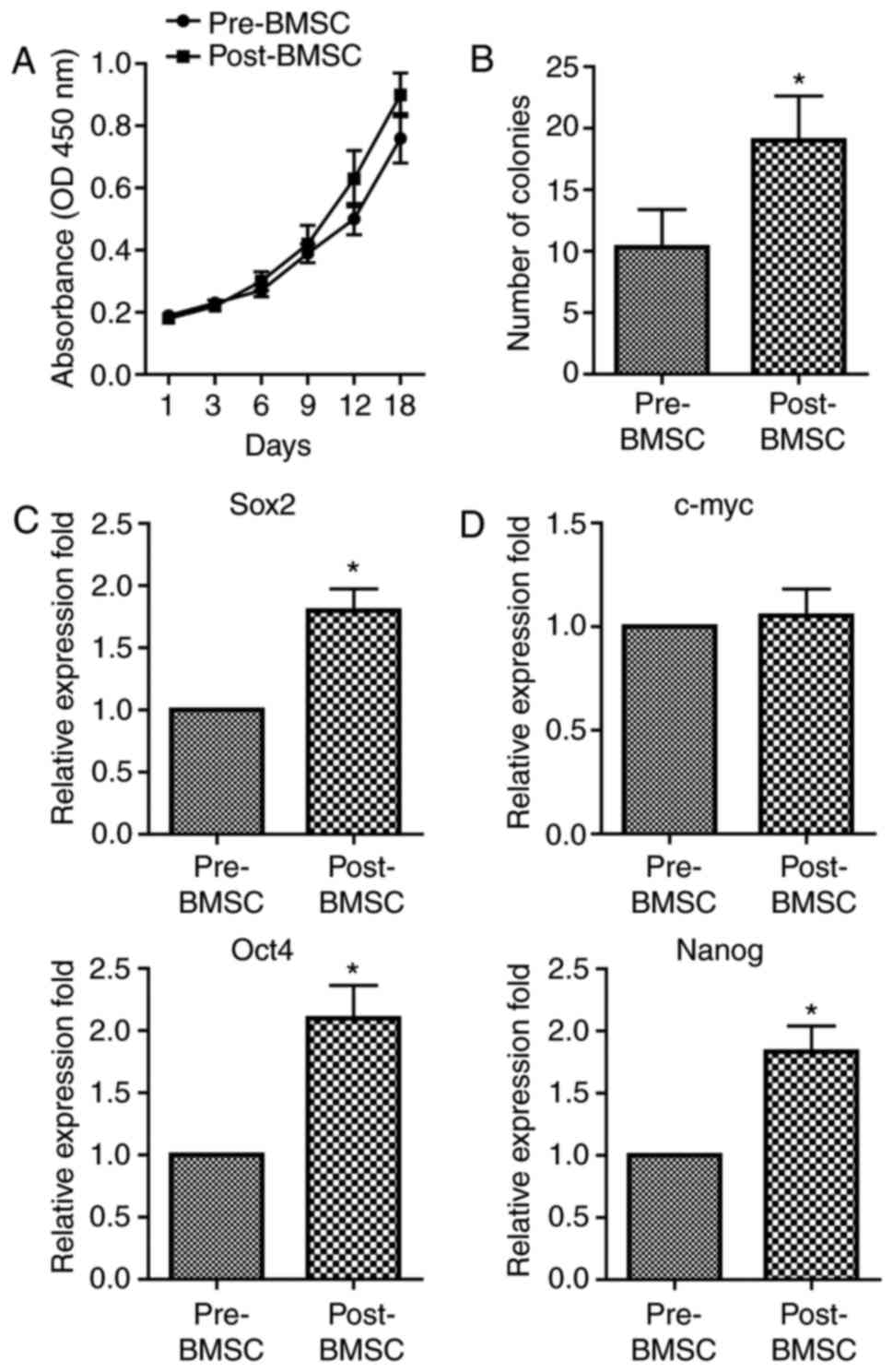
Post-BMSCs proliferate faster and have
an increased number of CFU-F and higher expression of stemness
genes than pre-BMSCs
We first analysed the proliferation rate of
pre-BMSCs and post-BMSC populations. The results showed that the
capacity of proliferation was significantly higher in the
post-BMSCs with respect to pre-BMSCs (Fig. 2A). Then, primary cultures of BMSCs
were established at clonal density in order to obtain discrete
colonies. The CFU-F of post-BMSCs was significant increased when
compared with the pre-BMSCs, indicating that the self-renewal
capability is significantly higher in post-BMSCs (Fig. 2B).
Next, we questioned if the decompression operation
could influence the stemness genes expression in BMSCs. We observed
that both pre-BMSCs and post-BMSCs expressed the transcripts of the
embryonic stem cells including octamer binding protein 4 (OCT4),
Nanog, SRY-related HMG box 2 (SOX2) and c-myc. Importantly, the
mRNA expression of OCT4, Nanog and SOX2 was significantly higher in
the post-BMSCs, whereas no significant difference in c-myc was
detected between the two population (Fig. 3C). Collectively, these results
clearly indicate that decompression surgery can influence several
stem cell properties of BMSCs.
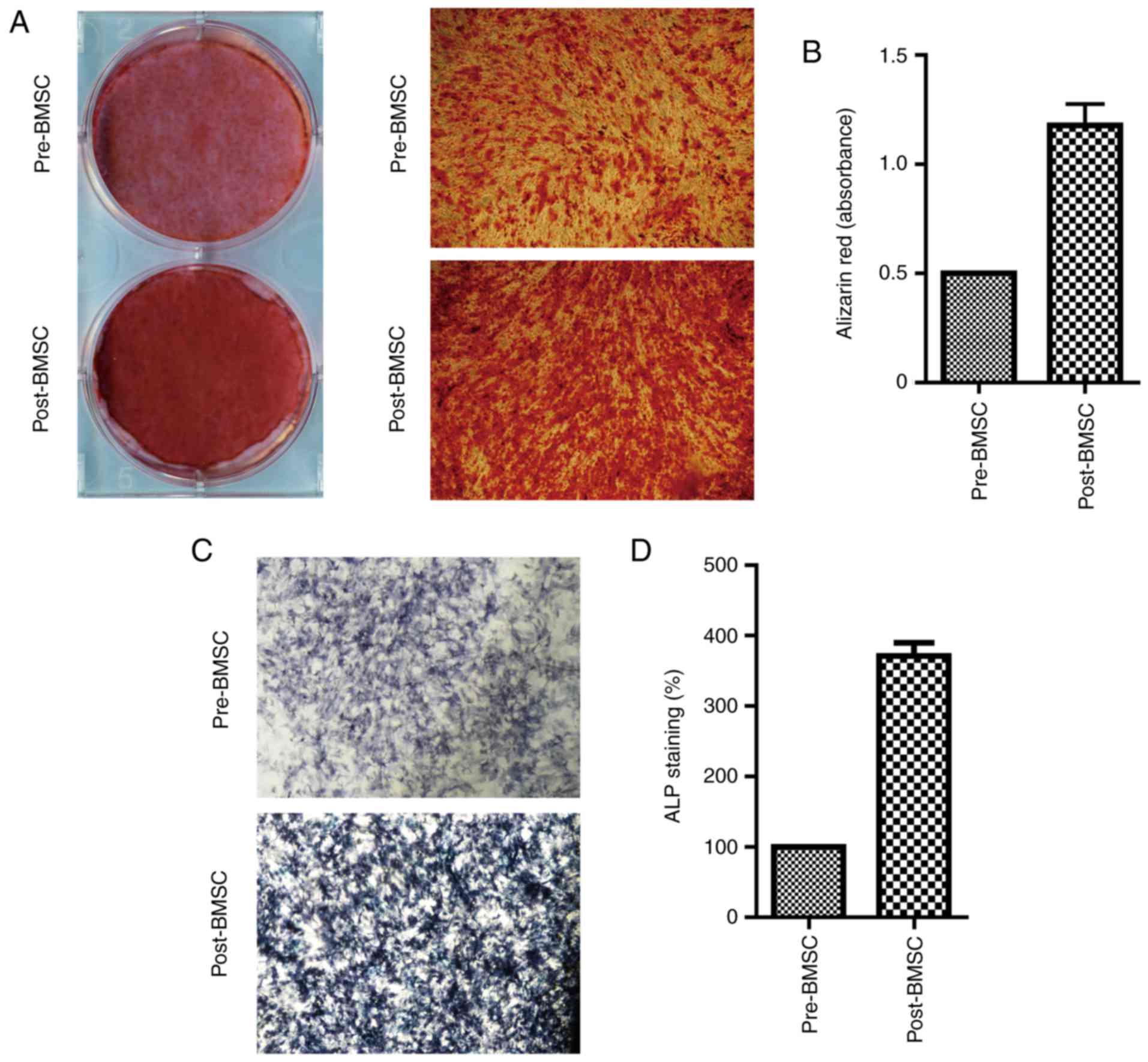
Osteogenic potential is higher in the
post-BMSCs than in pre-BMSCs
Last, to test whether the pre-BMSCs and post-BMSCs
exhibited differences in osteogenic capacity Alizarin Red S
staining was employed to observe the calcium deposition in the
osteogenic differentiation of BMSCs in vitro. The results
showed that decompression operation could enhance the osteogenic
differentiation potential of the BMSCs (Fig. 3A). These results were further
confirmed by spectrophotometric quantification, with significant
more absorbance noted in the post-BMSCs (Fig. 3B). Moreover, both pre- and
post-BMSCs displayed a positive color signal of the ALP staining,
the post-BMSCs showed a more intensive signal compared with the
pre-BMSCs (Fig. 3C).
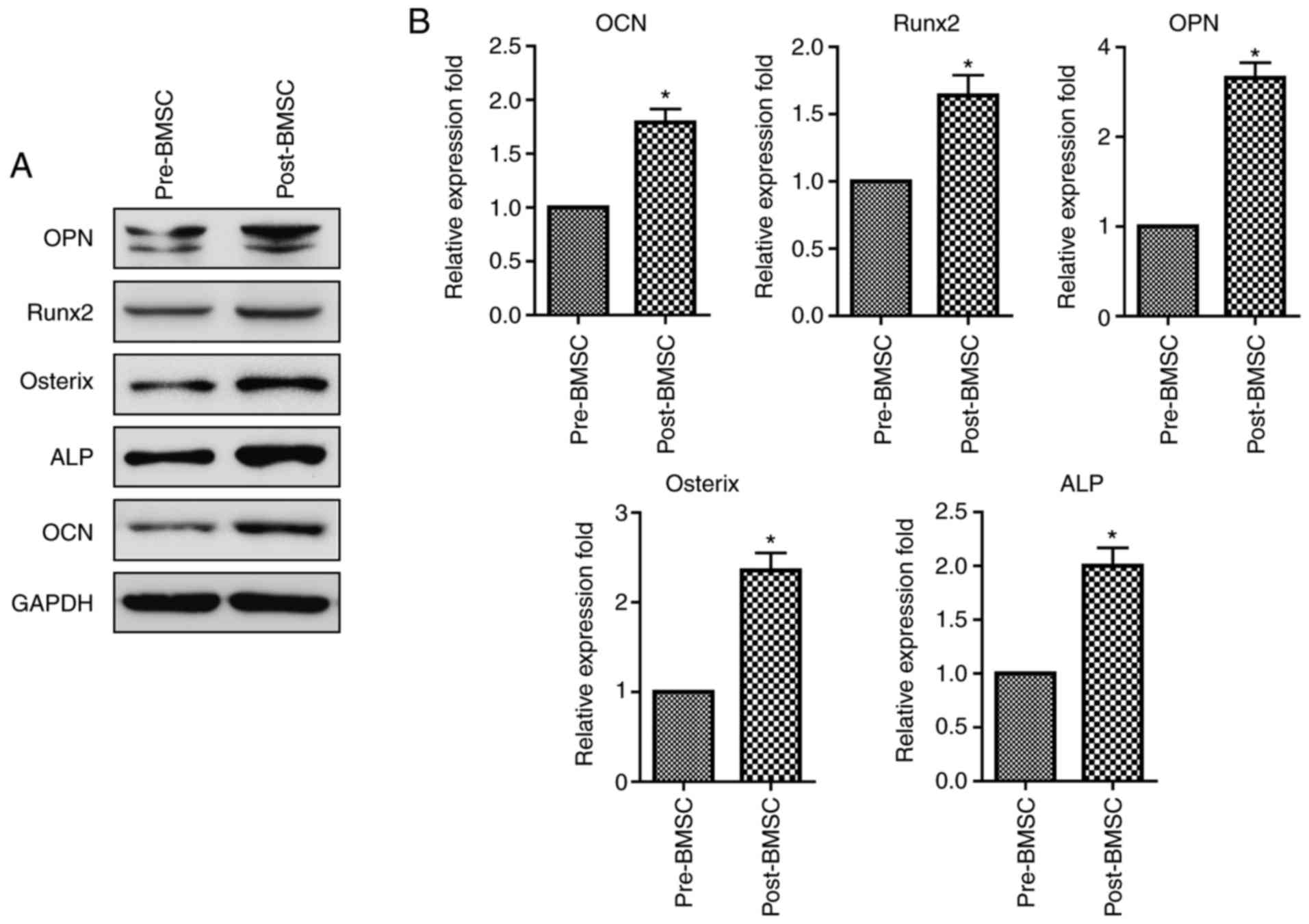
To further investigate the effects of decompression
operation on osteogenic differentiation of BMSCs, the gene and
protein expression levels of OPN, RUNX2, OCN, ALP and osterix were
determined by RT-PCR and Western blotting, respectively. Post-BMSCs
cultured in osteogenic medium showed a significant upregulation of
protein levels in OPN, RUNX2, OCN, ALP and osterix than pre-BMSCs
(Fig. 3A). RT-PCR analyses were
consistent with the protein expression, demonstrating an increase
in the gene levels of OPN, RUNX2, OCN, ALP and osterix in
post-BMSCs with respect to the pre-BMSCs (Fig. 4B).
Decompression operation activates
ERK/JNK signaling pathway
To reveal the underlying mechanisms of decompression
operation on proliferation, stemness and osteogenic differentiation
of BMSCs, the phosphorylation of MAPK signaling pathway was
evaluated in BMSCs. Western blot analysis illustrated
phosphorylated levels of ERK and JNK were obviously enhanced in
post-BMSCs, while no difference in phosphorylated level of p38 MAPK
(Fig. 5), suggesting the
activation of ERK/JNK signaling pathway was an important mechanisms
for decompression operation.
Discussion
BMSCs have the characteristics of self-proliferation
and multi-differentiation (e.g., osteogenic, chondrogenic, and
adipogenic) potential, but marrow aspiration from iliac crest is an
invasive procedure. It has been previously confirmed that orofacial
(maxilla and mandible) BMSCs had the same fibroblastic shape as
that isolated from the iliac crest, and their proliferative and
osteogenic potentials were similar to those of iliac crest derived
BMSCs (10,12). Therefore, orofacial BMSCs may be a
cell source for promoting regeneration and remodeling of orofacial
bone in patients with periodontal disease. However, many factors,
such as growth factors, cytokines, hormones and mechanical stress,
can influence osteogenic differentiation capability of BMSCs. In
the present study, we aimed to explore the role of decompression in
regulating the osteogenic differentiation of the orofacial BMSCs
around the odontogenic cystic lesions.
In this study, we successfully isolated and
characterised orofacial BMSCs from same individuals before and
after marsupialization surgery. We observed that both pre-BMSCs and
post-BMSCs highly expressed the BMSC markers such as CD44 and CD90.
However, the proliferation and CFU-F capacity were significantly
higher in post-BMSCs compared to pre-BMSCs. Importantly, post-BMSCs
expressed a high level of OCT4, Nanog and SOX2, indicating that
marsupialization surgery plays a key role in regulating the
pluripotency of BMSCs around cysts tissues. Furthermore, a
significant difference between the osteogenic potential of
pre-BMSCs and post-BMSCs was detected in our current study. In
fact, the osteogenic differentiation capacity of the post-BMSCs was
significantly higher with respect to the pre-BMSCs. Collectively,
all of these findings suggested that decompression of odontogenic
cysts could promote the stemness and osteogenic potential of
orofacial BMSCs.
There are several reasons may be explained for the
effect of marsupialization on orofacial BMSCs. One reason was that
the intracystic pressure decreased after marsupialization could
promote osteogenesis in BMSCs. It has been shown that the
intracystic fluid pressure in odontogenic jaw cyst could reach up
to 38–47 mmHg (16), and reduced
to 0 mmHg after marsupialization. Yang et al (17) demonstrated that BMSCs showed a
typical appearance of osteoblast after two weeks of induction by
intermittent negative pressure, and the activity of ALP and
expression of OPN increased significantly. Wiesmann et al
(18) also confirmed that
mechanical stimulation could promote the expression of collagen
type I and osteonectin in BMSCs. Another reason was that the
expression of inflammatory factors such as interleukin-1α (IL-1α)
and prostaglandin E2 (PGE2) was decreased by marsupialization could
inhibit osteoclastogenesis in BMSCs (19). It has been showed that IL-1α and
PGE2 evoked an increase in receptor activator of nuclear factor-κB
ligand (RANKL) mRNA, and a decrease in osteoprotegerin (OPG) mRNA
in BMSCs (20,21). All these factors may enhance the
differentiated function of osteoblasts and bone formation. Further
studies by using an in vitro model were needed to confirm
all these potential mechanisms, which might help the application of
marsupialization in patients with odontogenic cyst.
MAPKs, comprised of ERK, P38 MAPK and JNK, are
serine-threonine protein kinases and participate in a mass of
cellular activities, such as proliferation, inflammation, migration
and differentiation (22,23). To elucidate the possible underlying
mechanism, we finally evaluated the phosphorylation of ERK, p38
MAPK and JNK pathways in pre- and post-BMSCs. Results showed that
the phosphorylated levels of ERK and JNK, rather than p38 pathway,
were obviously enhanced in post-BMSCs compared to pre-BMSCs,
suggesting that decompression operation might participate in the
modulation of proliferation, stemness and differentiation through
regulating ERK and JNK pathways in orofacial BMSCs.
In summary, our results show that decompression has
a crucial role in regulating stem cell properties of orofacial
BMSCs. Further understanding of the relation between intracystic
pressure and cytokines changes and osteogenic differentiation
potential of orofacial BMSCs are needed.
Acknowledgements
This study was supported by grants from the
Science and Technology Plan Project of Jiaxing Zhejiang Province,
China (no. 2016BY28004) and Medical and Health Science and
Technology Project of Zhejiang Province, China (no. 2017KY650).
References
|
1
|
Borrás-Ferreres J, Sánchez-Torres A and
Gay-Escoda C: Malignant changes developing from odontogenic cysts:
A systematic review. J Clin Exp Dent. 8:e622–e628. 2016.PubMed/NCBI
|
|
2
|
Sharifian MJ and Khalili M: Odontogenic
cysts: A retrospective study of 1227 cases in an Iranian population
from 1987 to 2007. J Oral Sci. 53:361–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nuñez-Urrutia S, Figueiredo R and
Gay-Escoda C: Retrospective clinicopathological study of 418
odontogenic cysts. Med Oral Patol Oral Cir Bucal. 15:e767–e773.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castro-Núñez J: Decompression of
odontogenic cystic lesions: Past, present and future. J Oral
Maxillofac Surg. 74:e1–e9. 2016. View Article : Google Scholar
|
|
5
|
Allon DM, Allon I, Anavi Y, Kaplan I and
Chaushu G: Decompression as a treatment of odontogenic cystic
lesions in children. J Oral Maxillofac Surg. 73:649–654. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao L, Wang XL, Li SM, Liu CY, Chen C, Li
JW, Yan XJ, Zhang J, Ren WH and Zhi KQ: Decompression as a
treatment for odontogenic cystic lesions of the jaw. J Oral
Maxillofac Surg. 72:327–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pogrel MA: Decompression and
marsupialization as a treatment for the odontogenic keratocyst.
Oral Maxillofac Surg Clin North Am. 15:415–427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egusa H, Sonoyama W, Nishimura M, Atsuta I
and Akiyama K: Stem cells in dentistry-part I: Stem cell sources. J
Prosthodont Res. 56:151–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda MJ, Imaizumi M, Tsuchiya S and
Morsczeck C: Dental follicle stem cells and tissue engineering. J
Oral Sci. 52:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsubara T, Suardita K, Ishii M, Sugiyama
M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K,
et al: Alveolar bone marrow as a cell source for regenerative
medicine: Differences between alveolar and iliac bone marrow
stromal cells. J Bone Miner Res. 20:399–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Damek-Poprawa M, Stefanik D, Levin LM and
Akintoye SO: Human bone marrow stromal cells display variable
anatomic site-dependent response and recovery from irradiation.
Arch Oral Biol. 55:358–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akintoye SO, Lam T, Shi S, Brahim J,
Collins MT and Robey PG: Skeletal site-specific characterization of
orofacial and iliac crest human bone marrow stromal cells in same
individuals. Bone. 38:758–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuznetsov S and Robey Gehron P: Species
differences in growth requirements for bone marrow stromal
fibroblast colony formation In vitro. Calcif Tissue Int.
59:265–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marrelli M, Paduano F and Tatullo M: Cells
isolated from human periapical cysts express mesenchymal stem
cell-like properties. Int J Biol Sci. 9:1070–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skaug N: Intracystic fluid pressure in
non-keratinizing jaw cysts. Int J Oral Surg. 5:59–65. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Liu M, Zhang YG, Guo X and Xu P:
Effects of intermittent negative pressure on osteogenesis in human
bone marrow-derived stroma cells. J Zhejiang Univ Sci B.
10:188–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiesmann A, Buhring HJ, Mentrup C and
Wiesmann HP: Decreased CD90 expression in human mesenchymal stem
cells by applying mechanical stimulation. Head Face Med. 2:82006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ninomiya T, Kubota Y, Koji T and Shirasuna
K: Marsupialization inhibits interleukin-1alpha expression and
epithelial cell proliferation in odontogenic keratocysts. J Oral
Pathol Med. 31:526–533. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh S, Kyung TW and Choi HS: Curcumin
inhibits osteoclastogenesis by decreasing receptor activator of
nuclear factor-kappaB ligand (RANKL) in bone marrow stromal cells.
Mol Cells. 26:486–489. 2008.PubMed/NCBI
|
|
21
|
Brändström H, Jonsson KB, Ohlsson C, Vidal
O, Ljunghall S and Ljunggren O: Regulation of osteoprotegerin mRNA
levels by prostaglandin E2 in human bone marrow stroma cells.
Biochem Biophys Res Commun. 247:338–341. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu L, Tang T, Miao Y, Zhang S, Qu Z and
Dai K: Stimulation of osteogenic differentiation and inhibition of
adipogenic differentiation in bone marrow stromal cells by
alendronate via ERK and JNK activation. Bone. 43:40–47. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Q, Walmsley AD, Cooper PR and Scheven
BA: Ultrasound stimulation of different dental stem cell
populations: role of mitogen-activated protein kinase signaling. J
Endod. 42:425–431. 2016. View Article : Google Scholar : PubMed/NCBI
|