Introduction
One of the largest tyrosine kinase families,
erythropoietin-producing hepatocyte receptors (Ephs) and their
corresponding ligands, ephrins, are expressed in neighboring cells
with reciprocal pattern (1). The
interaction between receptors and ligands plays an important role
in different kinds of diseases (2–8).
Interactions between Eph receptors and NMDA receptors and/or
metabotropic glutamate receptors have been reported to cause
excitotoxic neuronal death in central nervous system (CNS)
(8–13). Eph receptors have also shown to be
important in both experimental glaucomatous animals and spontaneous
glaucomatous DBA/2J mice (14,15).
Our previous study has shown that interaction between ephrinB/EphB
signaling and AMPA receptor subunits GluA2 contributes to retinal
ganglion cells (RGCs) apoptosis in a rat chronic ocular
hypertension (COH) model (16).
Since Eph receptor signaling casts a wide net on cellular behavior,
there are probably other pathways involved in RGCs injury induced
by ephrinB/EphB signaling in COH model. The study on the optic
nerve head (ONH) of the DBA/2J mice showed that EphB2-Fc increased
intracellular Ca2+ concentrations in single RGCs axons
(14). Although different kinds of
Ca2+ permeable receptors which mediated Ca2+
overload have been indicated in the RGCs apoptosis in retina
(17–19), elevated levels of Ca2+
concentrations through voltage-gated Ca2+ channels have
been associated with diminished vision in healthy aging (20). Also, according to study on glaucoma
prevalence, calcium oral intake without calcium channel antagonist
can increase the risk for glaucoma (21,22).
Based on these evidences, the aim of the present study was to
investigate the role of calcium channels in a rat COH model and
whether these channels can be regulated by ephrinB/EphB
signaling.
Materials and methods
Animals and rat COH model
Male Sprague Dawley rats, that were 3 to 4 weeks of
age and weighted 100–250 g, were obtained from Shanghai Laboratory
Animal Center Laboratory Animal Co., Ltd. All the animals were
housed in an environment with temperature of 22±1°C, relative
humidity of 50±1% and a light/dark cycle of 12/12 h. Both food
and water were provided ad libitum. All experimental
procedures described were in accordance with the National
Institutes of Health guidelines for the Care and Use of Laboratory
Animals and the Guidelines of the Yangtze University on the Ethical
Use of Animals. Care and use of animals were also approved by the
ethics committee of First Affiliated Hospital of Yangtze University
(Jingzhou, China).
COH rat model was established and validated
following the previously described approach (16,23,24).
Briefly, rats were anesthetized with a mixture of ketamine (25
mg/kg, im) and xylazine (10 mg/kg, im); eyes were locally
anesthetized with topical 0.4% oxybuprocaine hydrochloride drops
(Benoxil; Santen Pharmaceutical, Co., Ltd., Ishikawa, Japan). Three
episcleral veins in the left eye were carefully separated and
cauterized under an OPMI VISU 140 microscope (Carl Zeiss,
Oberkochen, Germany). A sham operation, following a similar
procedure (except for not occluding the vines), was conventionally
performed on the eyes of other rats. After surgery, eyes were
flushed with antibiotic eye drops and covered with antibiotic
ointment. Intraocular pressure (IOP) was measured using a handheld
digital tonometer (Tonopen XL; Mentor O&O, Norwell, IL, USA)
under general and local anesthesia as described above. The average
value of five consecutive acceptable measurements with a deviation
<5% was recorded. All measurements were performed in the morning
to avoid possible circadian differences. IOPs in both eyes were
measured before surgery (baseline), immediately after surgery (day
0), the first day after surgery (G1 day), the third day after
surgery (G3 days) and weekly thereafter.
Western blotting
Western blot analysis was conducted as previously
described (16,23,24),
with some modifications. Previous studies have demonstrated that
Cav3.1, Cav3.2 and Cav3.3 subunits
of T-type Ca2+ channels and the Cav1.2
subunit of L-type Ca2+ channels are mainly expressed in
rat retinal Müller cells and RGCs layer (25). Since both ganglion cell layer and
Müller cells are vulnerable to IOP, we examined Ca2+
channels subunits change in COH retinas using Western blot. Retinas
were homogenized in RIPA lysis buffer (cat. no. 89900; Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
protease and phosphatase inhibitor cocktail (cat. no.
88661&88662; Roche Applied Science, Mannheim, Germany). The
concentration of total proteins was measured using a standard
bicinchoninic acid assay kit (cat. no. 23227, Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The extracted whole protein
samples (50 µg) were resolved by 8% SDS-PAGE gel and electroblotted
onto PVDF membranes (cat. no. ISEQ0001, Immobilon-P; EMD Millipore,
Billerica, MA, USA) using Mini-PROTEAN 3 Electrophoresis System and
Mini Trans-Blot Electrophoretic Transfer System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). After being blocked in 5%
nonfat milk at room temperature for 1.5 h, the membranes were
incubated at 4°C overnight with the following primary antibodies:
monoclonal mouse anti-β-actin (cat. no. A2228, 1:3,000 dilution;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), polyclonal rat
anti-Cav3.1 (cat. no. ACC-021), anti-Cav3.2
(cat. no. ACC-025), anti-Cav3.3 (cat. no. ACC-009),
anti-Cav1.2 (cat. no. CC-003) (1:200 dilution for all
the three; Alomone Labs, Jerusalem, Israel). After being washed in
Tris-buffered saline-Tween-20 (TBST) for three times (5–10 min per
time), the blots were incubated with horseradish-peroxidase-(HRP-)
conjugated donkey anti-mouse (Cat no. 715-035-151) or donkey
anti-rabbit (cat. no. 715-035-151) secondary antibody (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h at
room temperature. Blots were then washed in TBST for another three
rounds, and incubated with chemofluorescent reagent (cat. no.
34080; Pierce; Thermo Fisher Scientific, Inc.) followed by exposure
to X-ray film in a dark room. Experiments were performed in
triplicate. The protein bands were quantitatively analyzed with NIH
Image Analysis software (Image J, version 1.38x; National
Institutes of Health, Bethesda, MD, USA). Protein levels were
normalized to the corresponding β-actin levels.
Immunofluorescence analysis
As previously described (16,23),
retinal sections were examined by immunofluorescence technique.
Rats were anesthetized with ethyl carbamate (1.25 g/kg, ip;
Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) and
transcardially perfused with 4% paraformaldehyde (PFA; in 0.1 M PB,
pH 7.4). Eyes were post-fixed in 4% PFA for 2–4 h and then
dehydrated with graded sucrose solutions at 4°C (4 h in 20% and
overnight in 30% solutions). Retinas were vertically sectioned (14
µm; Leica Microsystems GmbH, Wetzlar, Germany), and sections were
mounted on chrome-alum-gelatin-coated slides (Thermo Fisher
Scientific, Inc.). Sections were then blocked for 2 h in 6% normal
donkey serum, 1% bovine serum, and 0.2% Triton X-100, and dissolved
in PBS at room temperature. Consequently, sections were incubated
with the following primary antibodies at 4°C for 24 h: polyclonal
rat anti-Cav3.1, anti-Cav3.2, (1:200
dilution; Alomone Labs, Jerusalem, Israel), Goat anti-CTB (cat. no.
703; List Biological Laboratories, Campbell, CA, USA), mouse
anti-glial fibrillary acidic protein (GFAP, cat. no. G3893;
Sigma-Aldrich; Merck KGaA). Binding sites of primary antibodies
were visualized by incubating sections with: 488-conjugated donkey
anti-rabbit secondary antibodies (cat. no. 711-546-152, 1:400;
Jackson ImmunoResearch Laboratories, Inc.) for single
Cav3.2 vision, and cy3-conjugated donkey anti-rabbit
(cat. no. 711-165-152), cyanine conjugated donkey anti-goat (cat.
no. 705-175-147), 488-conjugated donkey anti-mouse (cat. no.
715-545-150) were used to stain the co-localization of
Cav3.2, CTB and GFAP. All the secondary antibodies were
incubated for 2 h at room temperature. Finally, sections were
visualized and photographed with a Leica SP2 confocal
laser-scanning microscope. To avoid reconstruction stacking
artifacts, double labeling was evaluated by sequential scanning on
single-layer optical sections at 1.0 µm intervals.
CTB injection for retrograde labeling
of RGCs
Retrograde labeling of RGCs was previously described
in detail (23). Briefly, after
anesthetizing rats with 40 mg/ml sodium pentobarbital (0.1 ml/100
g), 1% Cholera Toxin B subunit (CTB, cat. no. 104; List Biological
Laboratories) was injected into the superior colliculus (6.0 mm
posterior and 2.0 mm lateral to the bregma, and 4–5 mm deep from
the cortical surface). After a survival period of 5–7 days, RGCs
were clearly labeled for immunofluorescence analysis.
Clustering of EphB2-Fc
The EphB2-Fc (cat. no. 467-B2-200, 2 µg/µl; R&D
Systems, Inc., Minneapolis, MN, USA) was clustered by a 40 min
incubation at 37°C in buffer containing an AffiniPure goat
anti-human IgG, Fc-specific (1:2.5 w/w) (Jackson ImmunoResearch,
Milan, Italy) (26).
Intravitreal injection
Intravitreal injection was performed according to
previously described method (16,24).
When the pupil of the anesthetized eye was dilated with tropicamide
drops, clustered EphB2-Fc (2 µl,), or Mibefradil (no. M5441, 3 mM;
Sigma-Aldrich; Merck KGaA) dispersed in 2 µl of 0.9% saline, was
injected into the vitreous space through a postlimbus spot using
stereoscopic microscope (Carl Zeiss). A 30-gauge needle was then
inserted 2 mm behind the temporal limbus and directed toward the
optic nerve. Eyes of vehicle or negative control (Ctr) group were
injected with saline or clustered human Ig-Fc (2 µg; R&D
Systems, Inc.), respectively.
Cell apoptosis
To investigate cell apoptosis, terminal
deoxynucleotidyl transferase-mediated biotinylated UTP nick end
labeling (TUNEL) assay (16,23)
was performed on whole flat-mounted retinas using the DeadEnd
Fluorometric TUNEL System G3250 kit (Promega Corporation, Madison,
WI, USA), following the manufacturer's instructions. TUNEL
signals were visualized with a laser confocal scanning microscope
through a 20× objective (FluoView 1000; Olympus Corporation, Tokyo,
Japan). The retinas were mounted with the ganglion cell layer
(GCL); a serial deep scanning was performed in the GCL according to
the DAPI staining results. All TUNEL-positive signals that merged
well with DAPI in each retina in GCL, were consequently counted
(comparing with INL and ONL, the cell body in GCL was the
largest).
Statistical analysis
The data derived from the TUNEL assay and the IOP
monitoring were presented as mean ± SEM. For Western blot
experiments, the expression level of a protein was first normalized
to the corresponding β-actin levels. The relative expression levels
were averaged for all samples. The mean values of the data obtained
during different postoperational time periods were normalized
according to the mean value of the control group. The data were
presented as the mean ± SEM. A one-way ANOVA with LSD post hoc test
(for protein analysis and RGCs apoptosis), or t test (paired data
for IOP analysis) were used and P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression change of Ca2+
channels in rat COH model
The rat COH model, with increased IOP levels, was
successfully established and validated according to previously
described method (16). Briefly,
significantly higher IOP was observed in COH rats compared to sham
and unoperated group (COH: 25.0±0.7 to 28.2±1.2 mmHg, n=10–74;
sham: 19.0±0.6 to 19.7±0.9 mmHg, n=10–74; unoperated eyes: 19.8±1.0
mmHg, n=9, all P<0.001).
As the summary data showed, the average intensity of
Cav3.1 increased from G1 day until G3 weeks while that
of Cav3.2 increased later from G1 week until G4 weeks
(Fig. 1B and C). The protein level
of Cav3.1 on G1 day was increased to 123.9±3.6% of
control (n=4, P=0.006 and further increased to 208.0±4.3,
241.3±9.5, 224.4±10.6 and 257.7±13.5% of control at G3 days, G1
week, G2 weeks and G3 weeks respectively (all n=4, P<0.001) and
then unexpectedly returned to the control level at G4 weeks
(113.1±6.3% of control, n=4, P=0.109) (Fig. 1B). As for Cav3.2, the
protein level didn't change much in the initial period of time (G1
day and G3 days) and started increasing in G1 week (164.7±3.4% of
control, n=4, P=0.002) and remained at stable higher level
thereafter, which were 175.7±3.2% of control at G2 weeks (n=4,
P=0.001), 228.5±15.6% of control at G3 weeks (n=4, P<0.001), and
210.6±15.9% of control at G4 weeks (n=4, P<0.001). In contrast,
the average intensity of Cav3.3 subunit of T-type
Ca2+ channels and Cav1.2 subunit of L-type
Ca2+ channels didn't change during the whole
postoperational period.
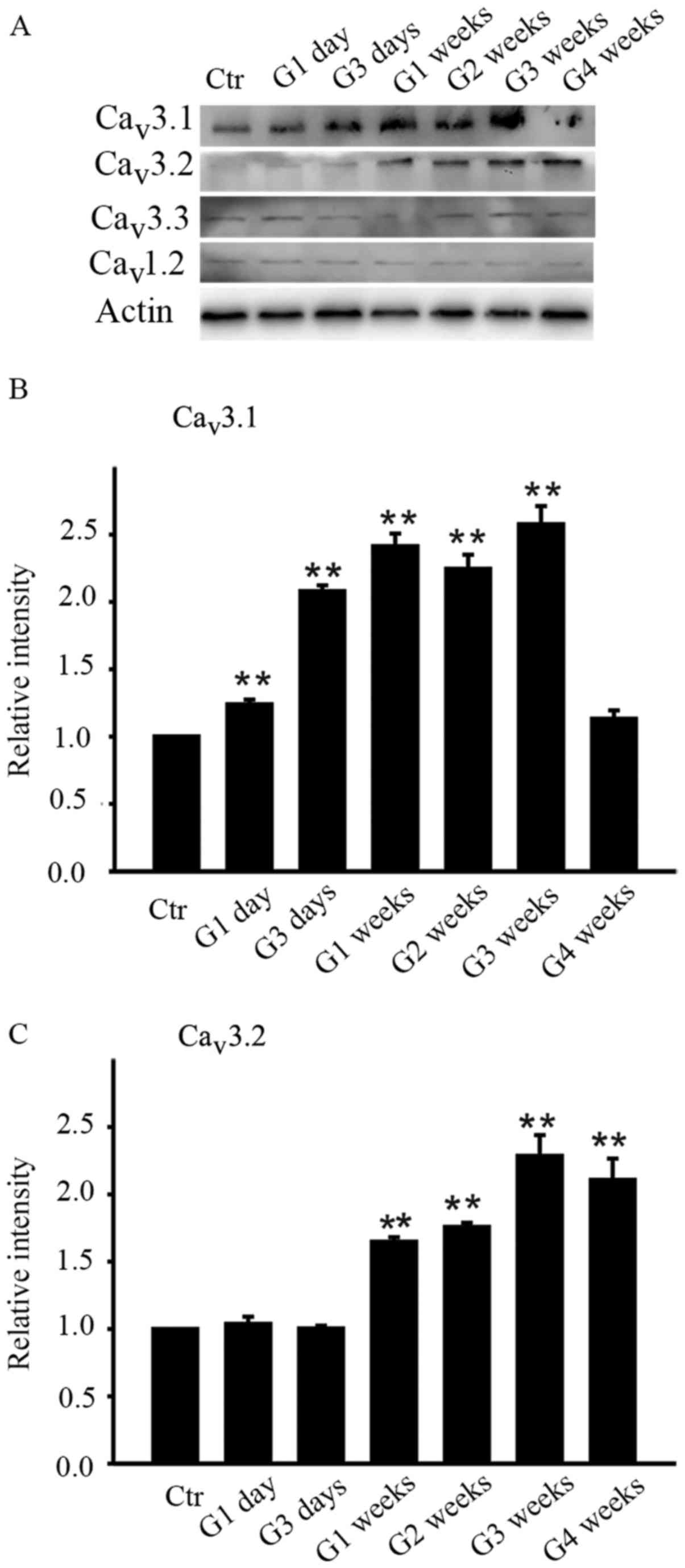 | Figure 1.Differences in the protein levels of
Cav3.1, Cav3.2, Cav3.3 and
Cav1.2 in the retinas of sham and COH rats. (A)
Cav3.1, Cav3.2, Cav3.3 and
Cav1.2 expression in sham-operated and COH retinal
extracts at different time points (G1 day, G3 days, G1 week, G2
weeks, G3 weeks and G4 weeks). Bar charts summarizing the average
densitometric quantification of immunoreactive bands of (B)
Cav3.1 and (C) Cav3.2 expression during
different time points. All data were normalized to control and are
presented as the mean ± standard error of the mean. **P<0.01 vs.
Ctr. Ctr, sham-operated control; G1 day, 1 day following surgery;
G3 days, 3 days following surgery; G1-4 weeks, 1–4 weeks following
surgery; Cav, calcium channel; COH, chronic ocular
hypertension. |
Immunofluorescence analysis further demonstrated
that Cav3.1 and Cav3.2 subunit of T-type
Ca2+ channels were expressed in different locations.
Cav3.1 was mainly expressed in the outer plexiform layer
(OPL) and cell membranes in inter nuclear layer (INL) (Fig. 2); during G1 day and G3 days, most
of the Cav3.1 signal was located in the OPL (Fig. 2Ab and c), while later the signal
was also found in the INL (from G1 week to G3 weeks, Fig. 2Ad-f). Cav3.2 was mainly
located in GCL layer; the signal increased from G1 week through G4
weeks (Fig. 3Ad-g and Cd-g).
Furthermore, all those data were consistent with western blot
results. Moreover, since GCL layer was most vulnerable to IOP, we
then verified whether RGCs and Müller cells were both positive for
Cav3.2 expressions (Fig. 4A
and E), by co-localization with CTB (Fig. 4B) and GFAP (Fig. 4D), respectively.
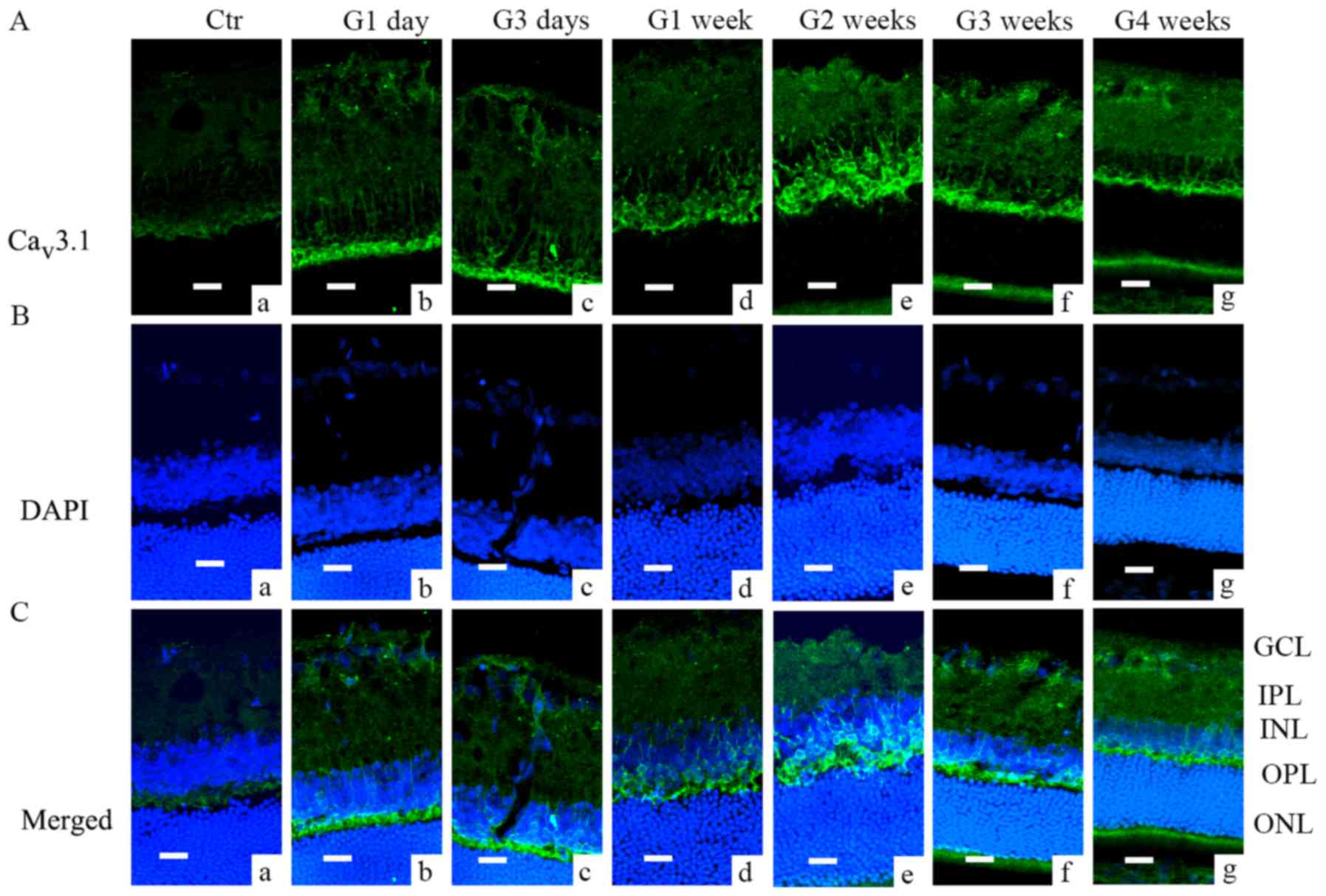 | Figure 2.Cav3.1 expression in COH
rats examined by immunofluorescence. (A) Cav3.1
expression profiles in rat retinal vertical slices taken from
sham-operated retinas (Ctr; image a), and those obtained from COH
rats at different time points (images b-g). (B) DAPI staining in
retinas of control rats (image a), and those obtained from COH rats
at different time points (images b-g). (C) Merged images of
Cav3.1 and DAPI in retinas of control rats (a), and
those obtained from COH rats at different time points (images a-g).
Scale bars=20 µm. GCL, ganglion cells layer; IPL, inner plexiform
layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL,
outer nuclear layer; Ctr, sham-operated control; G1 day, 1 day
following surgery; G3 days, 3 days following surgery; G1-4 weeks,
1–4 weeks following surgery; Cav, calcium channel; COH,
chronic ocular hypertension. |
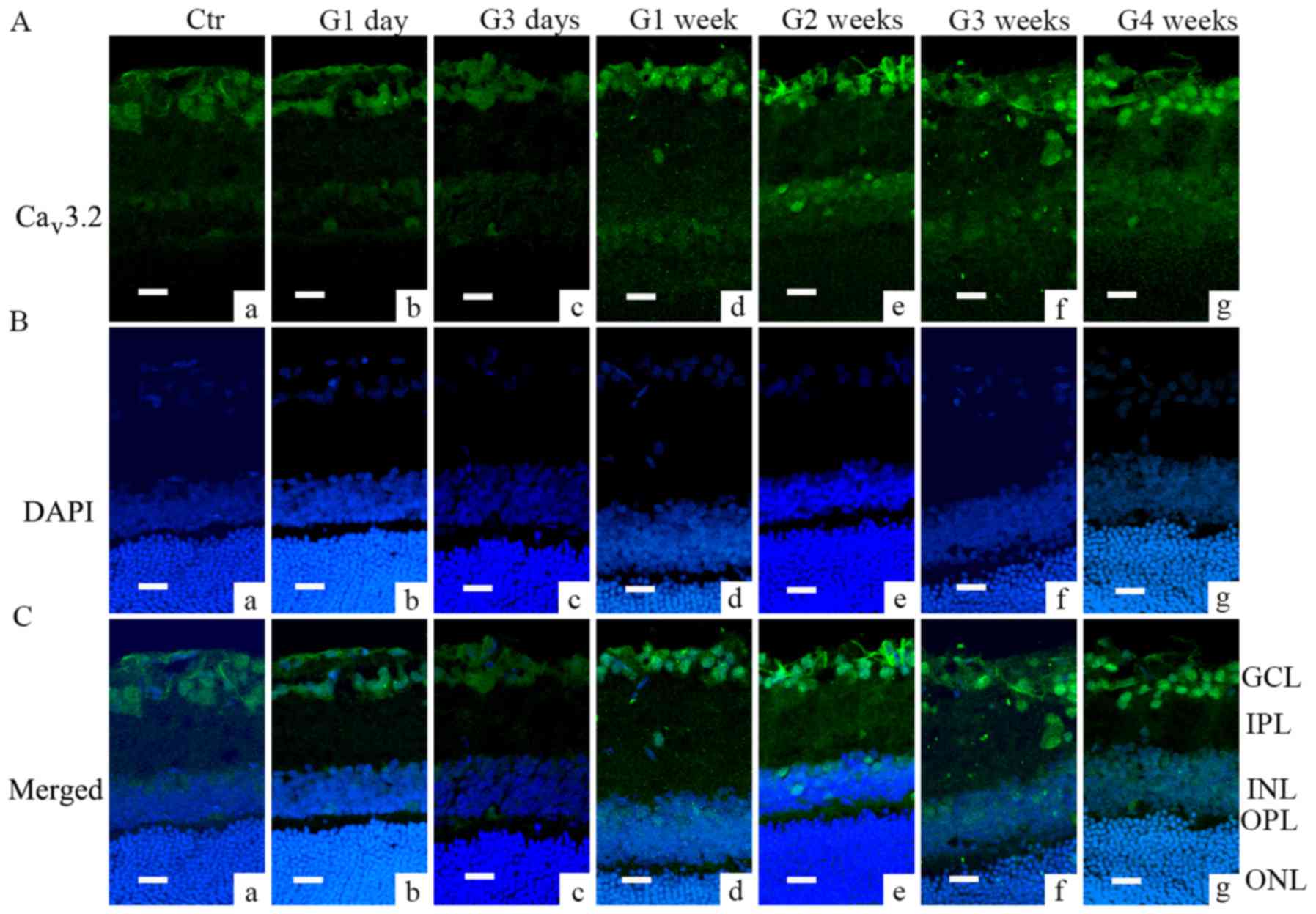 | Figure 3.Cav3.2 expression in COH
rats examined by immunofluorescence. (A) Cav3.2
expression profiles in rat retinal vertical slices taken from
sham-operated retinas (Ctr; image a), and those obtained from COH
rats at different time points (images b-g). (B) DAPI signal in
retinas of control (image a), and those obtained from COH rats at
different time points (images b-g). (C) Merged images of
Cav3.2 and DAPI in retinas of control (image a), and
those obtained from COH rats at different time points (images b-g).
Scale bars=20 µm. GCL, ganglion cells layer; IPL, inner plexiform
layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL,
outer nuclear layer; Ctr, sham-operated control; G1 day, 1 day
following surgery; G3 days, 3 days following surgery; G1-4 weeks,
1–4 weeks following surgery; Cav, calcium channel; COH,
chronic ocular hypertension. |
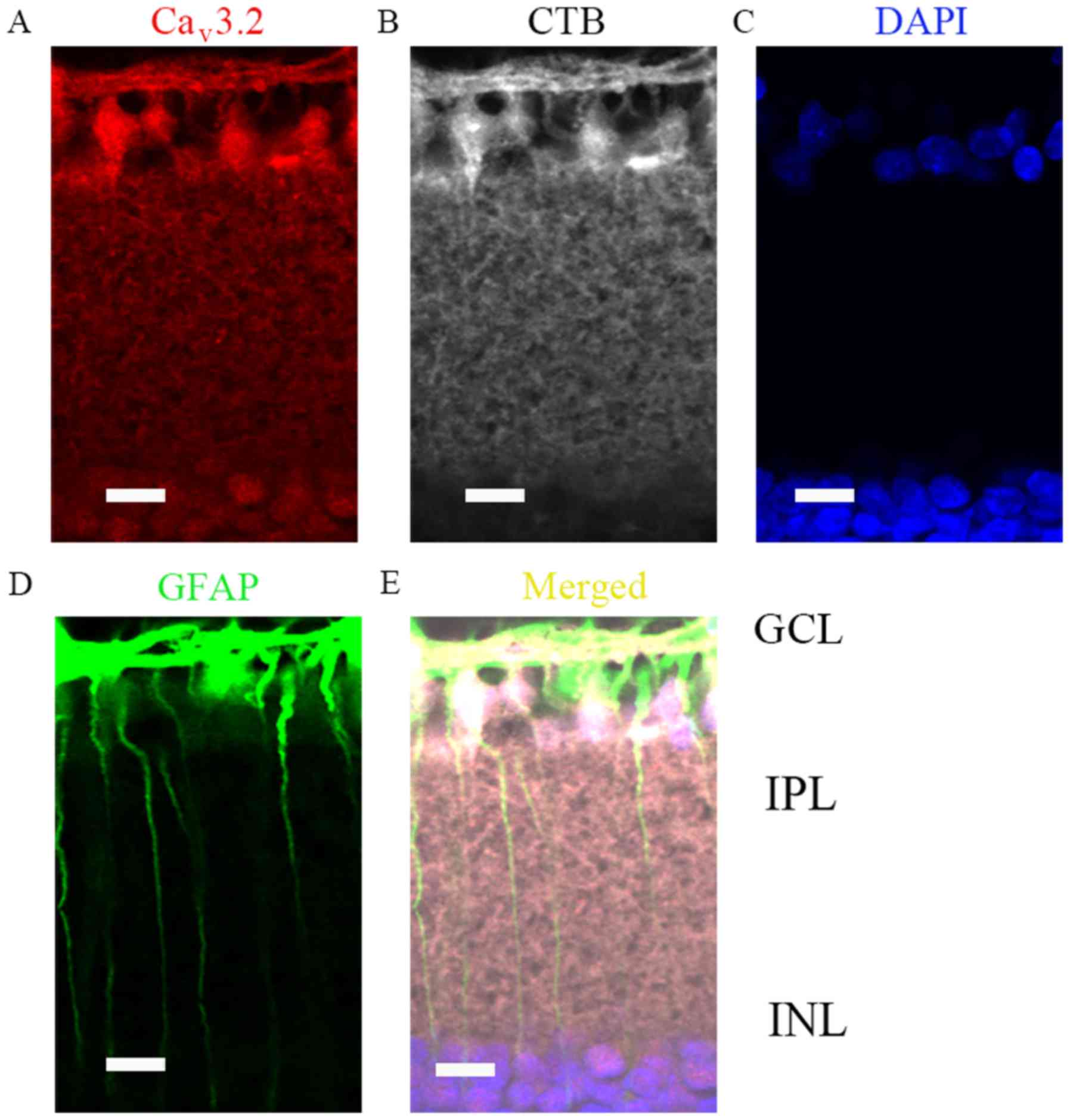 | Figure 4.Cav3.2 expression location
in COH retinas (G2 weeks) by immunofluorescence analysis. (A)
Immunofluorescence labeling for Cav3.2 in G2 week rat
retinal vertical slices following an injection of CTB to the
superior colliculus bilaterally. (B) Immunofluorescence labeling
for CTB (positive for retinal ganglion cells) in the retinal
vertical slices. (C) Immunofluorescence labeling for DAPI in the
retinal vertical slices. (D) Immunofluorescence labeling for GFAP
(positive for Müller cells) in the retinal vertical slices. (E)
Merged images of Cav3.2, CTB, DAPI and GFAP in the
retinal vertical slices. Scale bars=20 µm. Cav, calcium
channel; COH, chronic ocular hypertension; G2 weeks, 2 weeks
following surgery; GCL, ganglion cells layer; IPL, inner plexiform
layer; INL, inner nuclear layer; CTB, Cholera Toxin B subunit;
GFAP, glial fibrillary acidic protein. |
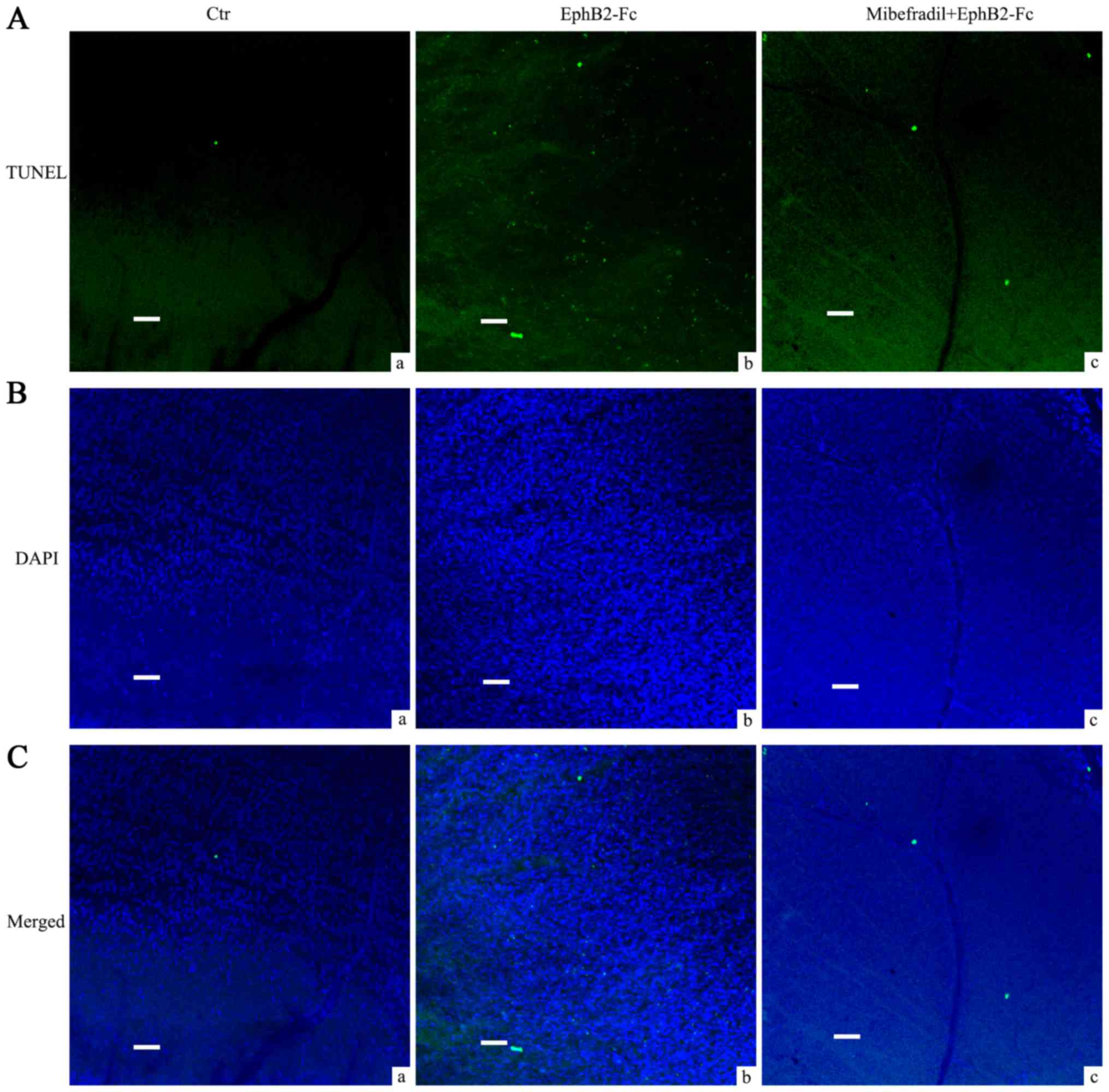
Mibefradil reduced RGCs apoptosis in
EphB2-Fc injected rat retina
In our previous study, we have shown that
intravitreal injection of EphB2-Fc induces apoptosis in RGCs. Since
Cav3.2 was increased in the RGCs and Müller cells in COH
rats, in the present study we used T type Ca2+ channel
blocker Mibefradil to further investigate whether calcium channels
were involved in the process of EphB2-Fc induced apoptosis in RGCs.
As Fig. 5 showed, average TUNEL
signals that matched well with DAPI in GCL by EphB2-Fc injection
were 497.4±25.3, significantly higher than control Fc treatment
(17.2±2.9, n=5, P<0.001). Pretreatment of Mibefradil (2 µl, 3
mM), injected intravitreally 1 day before EphB2-Fc, reduced the
TUNEL signals to 302.4±16.3 (n=5, P<0.001, compared with
EphB2-Fc group) while saline pretreatment had no effect
(505.2±26.1, n=5, P=0.64, compared with EphB2-Fc group).
Discussion
We have previously reported that elevated reverse
EphB/ephrinB signaling contributes to RGCs apoptosis by interacting
with GluR2 subunit of AMPA receptors in a COH rat model (16). By using the same ocular
hypertension rat model, we found that Cav3.1 and
Cav3.2 protein expression increased in retinas, but at
different locations and at different timepoints. It is necessary to
emphasize that Cav3.2 increased both in RGCs and Müller
cells in COH retinas, which was similar to the expression pattern
of ephrinB2, with the exception that ephrinB2 starts to increase
from second week (16) while
increment of Cav3.2 started in G1 week. Consequently,
RGCs apoptosis caused by intravitreal injection of clustered
EphB2-Fc could be partly relieved by Mibefradil, a T type
Ca2+ channel blocker. Numerous studies have shown that
disturbance of calcium homeostasis which occurs through several
pathways contributes to neuronal damage in many neurodegenerative
diseases (27–32). Moreover, the deregulation of
Ca2+ channel activities, which is one of the pathways
causing Ca2+ homeostasis disturbance, has very important
role in neurodegenerative disease like ALS (33) although related reports are
relatively rare and controversial (34). In glaucoma, like in many other
neurodegenerative diseases, Ca2+ channel blockers have
shown to be beneficial (reviewed by Chihiro Mayama in reference)
(35). The most often tested
Ca2+ channel blockers in experimental models are L type
Ca2+ channel blockers, nifedipine and nimodipine. Their
benefits include lowered IOP, improved blood flow to RGCs and
neuron protection due to less Ca2+ influx. In the
present study, we didn't test L type Ca2+ channel
blockers' effect on the EphB2-Fc induced RGCs apoptosis, since no
changes in L type Ca2+ channel expression were observed
in COH retinas. Nonetheless, we didn't totally rule out the
possibility that L type Ca2+ channel could play a role,
for in our other separated preliminary experiments (data not shown
here), clustered EphB2-Fc increased mixed Ca2+ currents
in isolated Müller cells. If this was the same as with RGCs or
whether it was related with Mibefradil's protection on RGCs needs
to be further examined. Our results suggest that elevated
EphB/ephrinB signaling regulates RGCs apoptosis under COH through
more than one pathway, while calcium channels, i.e., T type calcium
channel, might be a target for EphB/ephrinB signaling. Another
important point that needs to be addressed is the way EphB/ephrinB
signaling regulates calcium channels in rat retinas. It is well
known that phosphorylation of calcium channels is important for the
function. Previous studies have shown that L type calcium channel
could be regulated by tyrosine kinase src (36–38).
According to our previous findings, EphB/ephrinB signaling in
retina could interact with src (16). Src kinase acts as a downstream of
Ca2+/calmodulin-dependent protein kinase II (CaMK II) in
intracellular free [Ca2+]i glomerular
mesangial cells (39), while in
striatum basal level of autophosphorylation of CaMK II it requires
T type, but not L or N type calcium channels (40). Although, using high resolution MS
approach several threonine and serine residues with no tyrosine
phosphorylation have been detected in normal rat brain tissue
(41), the situation in retina,
especially under higher IOP remains an open question.
Acknowledgements
This study was supported by the Natural Science
Foundation of Hubei Province (no. 2014CFB213), the Health and Birth
Control Department of Hubei Province (no. 81000380/H1204) and the
Jingzhou Science and Technology funding (2014AC47B). We thank the
grants that supported our study and MedSci (www.MedSci.com) for its linguistic assistance during
the preparation of this manuscript.
References
|
1
|
Pasquale EB: Eph receptor signaling casts
a wide net on cell behavior. Nat Rev Mol Cell Biol. 6:462–475.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamaguchi Y and Pasquale EB: Eph receptors
in the adult brain. Curr Opin Neurobiol. 14:288–296. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egea J and Klein R: Bidirectional
Eph-ephrin signaling during axon guidance. Trends Cell Biol.
17:230–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Himanen JP, Saha N and Nikolov DB:
Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell
Biol. 19:534–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klein R: Bidirectional modulation of
synaptic functions by Eph/ephrin signaling. Nat Neurosci. 12:15–20.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Fu AK and Ip NY: Eph receptors at
synapses: Implications in neurodegenerative diseases. Cell Signal.
24:606–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai KO and Ip NY: Synapse development and
plasticity: Roles of ephrin/Eph receptor signaling. Curr Opin
Neurobiol. 19:275–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu
L, Gale NW and Greenberg ME: EphB receptors interact with NMDA
receptors and regulate excitatory synapse formation. Cell.
103:945–956. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takasu MA, Dalva MB, Zigmond RE and
Greenberg ME: Modulation of NMDA receptor-dependent calcium influx
and gene expression through EphB receptors. Science. 295:491–495.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grunwald IC, Korte M, Adelmann G, Plueck
A, Kullander K, Adams RH, Frotscher M, Bonhoeffer T and Klein R:
Hippocampal plasticity requires postsynaptic ephrinBs. Nat
Neurosci. 7:33–40. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calò L, Spillantini M, Nicoletti F and
Allen ND: Nurr1 co-localizes with EphB1 receptors in the developing
ventral midbrain, and its expression is enhanced by the EphB1
ligand, ephrinB2. J Neurochem. 92:235–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calò L, Cinque C, Patanè M, Schillaci D,
Battaglia G, Melchiorri D, Nicoletti F and Bruno V: Interaction
between ephrins/Eph receptors and excitatory amino acid receptors:
Possible relevance in the regulation of synaptic plasticity and in
the pathophysiology of neuronal degeneration. J Neurochem. 98:1–10.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du J, Tran T, Fu C and Sretavan DW:
Upregulation of EphB2 and ephrin-B2 at the optic nerve head of
DBA/2J glaucomatous mice coincides with axon loss. Invest
Ophthalmol Vis Sci. 48:5567–5581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu CT, Tran T and Sretavan D: Axonal/glial
upregulation of EphB/ephrin-B signaling in mouse experimental
ocular hypertension. Invest Ophthalmol Vis Sci. 51:991–1001. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong LD, Gao F, Wang XH, Miao Y, Wang SY,
Wu Y, Li F, Wu J, Cheng XL, Sun XH, et al: GluA2 trafficking is
involved in apoptosis of retinal ganglion cells induced by
activation of EphB/EphrinB reverse signaling in a rat chronic
ocular hypertension model. J Neurosci. 35:5409–5421. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sappington RM, Sidorova T, Long DJ and
Calkins DJ: TRPV1: Contribution to retinal ganglion cell apoptosis
and increased intracellular Ca2+ with exposure to
hydrostatic pressure. Invest Ophthalmol Vis Sci. 50:717–728. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryskamp DA, Witkovsky P, Barabas P, Huang
W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Rentería RC, et
al: The polymodal ion channel transient receptor potential
vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of
mouse retinal ganglion cells. J Neurosci. 31:7089–7101. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poornima V, Madhupriya M, Kootar S,
Sujatha G, Kumar A and Bera AK: P2×7 receptor-pannexin 1
hemichannel association: Effect of extracellular calcium on
membrane permeabilization. J Mol Neurosci. 46:585–594. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bissig D, Goebel D and Berkowitz BA:
Diminished vision in healthy aging is associated with increased
retinal L-type voltage gated calcium channel ion influx. PLoS One.
8:e563402013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomita G: The optic nerve head in
normal-tension glaucoma. Curr Opin Ophthalmol. 11:116–120. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang SY, Singh K and Lin SC: The
association between glaucoma prevalence and supplementation with
the oxidants calcium and iron. Invest Ophthalmol Vis Sci.
53:725–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Miao Y, Wang XH and Wang Z:
Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal
ganglion cell apoptosis in a rat experimental glaucoma model.
Neurobiol Dis. 43:455–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji M, Miao Y, Dong LD, Chen J, Mo XF,
Jiang SX, Sun XH, Yang XL and Wang Z: Group I mGluR-mediated
inhibition of Kir channels contributes to retinal Müller cell
gliosis in a rat chronic ocular hypertension model. J Neurosci.
32:12744–12755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang W, Li Q, Wang SY, Gao F, Qian WJ, Li
F, Ji M, Sun XH, Miao Y and Wang Z: Cannabinoid receptor agonists
modulate calcium channels in rat retinal Müller cells.
Neuroscience. 313:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calò L, Bruno V, Spinsanti P, Molinari G,
Korkhov V, Esposito Z, Patanè M, Melchiorri D, Freissmuth M and
Nicoletti F: Interactions between ephrin-B and metabotropic
glutamate 1 receptors in brain tissue and cultured neurons. J
Neurosci. 25:2245–2254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hallett PJ and Standaert DG: Rationale for
and use of NMDA receptor antagonists in Parkinson's disease.
Pharmacol Ther. 102:155–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Surmeier DJ: Calcium ageing, and neuronal
vulnerability in Parkinson's disease. Lancet Neurol. 6:933–938.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan MM and Raymond LA:
N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in
Huntington's disease. Prog Neurobiol. 81:272–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bezprozvanny I: Inositol
1,4,5-tripshosphate receptor, calcium signalling and Huntington's
disease. Subcell Biochem. 45:323–335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bezprozvanny I and Mattson MP: Neuronal
calcium mishandling and the pathogenesis of Alzheimer's disease.
Trends Neurosci. 31:454–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Green KN and LaFerla FM: Linking calcium
to Abeta and Alzheimer's disease. Neuron. 59:190–194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Delbono O, Garcia J, Appel SH and Stefani
E: IgG from amyotrophic lateral sclerosis affects tubular calcium
channels of skeletal muscle. Am J Physiol. 260:C1347–C1351.
1991.PubMed/NCBI
|
|
34
|
Arsac C, Raymond C, Martin-Moutot N,
Dargent B, Couraud F, Pouget J and Seagar M: Immunoassays fail to
detect antibodies against neuronal calcium channels in amyotrophic
lateral sclerosis serum. Ann Neurol. 40:695–700. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mayama C: Calcium channels and their
blockers in intraocular pressure and glaucoma. Eur J Pharmacol.
739:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu XQ, Singh N, Mukhopadhyay D and
Akbarali HI: Modulation of voltage-dependent Ca2+
channels in rabbit colonic smooth muscle cells by c-Src and focal
adhesion kinase. J Biol Chem. 273:5337–5342. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bence-Hanulec KK, Marshall J and Blair LA:
Potentiation of neuronal L calcium channels by IGF-1 requires
phosphorylation of the alpha1 subunit on a specific tyrosine
residue. Neuron. 27:121–131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bogdelis A, Treinys R, Stankevičius E,
Jurevičius J and Skeberdis VA: Src family protein tyrosine kinases
modulate L-type calcium current in human atrial myocytes. Biochem
Biophys Res Commun. 413:116–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Mishra R and Simonson MS:
Ca2+/calmodulin-dependent protein kinase II stimulates
c-fos transcription and DNA synthesis by a Src-based mechanism in
glomerular mesangial cells. J Am Soc Nephrol. 14:28–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pasek JG, Wang X and Colbran RJ:
Differential CaMKII regulation by voltage-gated calcium channels in
the striatum. Mol Cell Neurosci. 68:234–243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blesneac I, Chemin J, Bidaud I, Huc-Brandt
S, Vandermoere F and Lory P: Phosphorylation of the Cav3.2 T-type
calcium channel directly regulates its gating properties. Proc Natl
Acad Sci USA. 112:pp. 13705–13710. 2015; View Article : Google Scholar : PubMed/NCBI
|