Introduction
Doxorubicin (Dox) is one of the most widely used
antineoplastic drugs. Its anticancer effects are believed to occur
through the inhibition of topoisomerase enzyme and subsequent
blockage of DNA resealing during cell replication (1). Despite its highly beneficial effects
against cancer, the clinical use of Dox has been confined by its
drawback of cardiotoxicity (2).
Risk of Dox cardiotoxicity increases with both treatment
concentration and duration, and delayed onset of cardiomyopathy can
often occur years after initial treatment (3). The mechanism for Dox-induced
cardiotoxicity is controversial, and several hypotheses have been
proposed. One of the important mechanisms inducing cardiotoxicity
is Dox induction of cardiomyocyte senescence (4,5). Dox
treatment enhanced secretion of senescence-associated cytokines and
augmented the DNA damage-response signaling cascade, thus inducing
cardiomyocyte senescence (5,6).
Therefore, there is an urgent need to find an efficient way to
ameliorate Dox-induced cardiomyocyte senescence in order to prevent
future cardiac complications.
Non-coding RNAs are non-protein-coding transcripts
that function as regulators of RNA molecules. Long non-coding (lnc)
RNAs are non-coding RNAs with at least 200 nucleotides (7) that have been reported to impact a
broad spectrum of biological processes including development,
differentiation, cell division, apoptosis, cellular senescence,
diseases and disorders, thus regulating the complexity of the
organism as a whole (8,9). LncRNA expression patterns are tissue-
and stage-specific, suggesting their considerable importance in
controlling different biological functions, cellular senescence in
particular (10). Among lncRNAs,
long intergenic non-coding (linc) RNA-p21 is closely related to
cellular senescence. Recent research has shown that lincRNA-p21,
which interacts with β-catenin, promoted cellular senescence
(11). Also, lincRNA-p21 has been
identified as a regulator of p53 expression, and reciprocally p53
can regulate the expression of lincRNA-p21, which plays a major
role in pro-senescence networks (12). At the same time, lincRNA-p21 is a
key regulator of age-related heart diseases such as coronary artery
disease (13). However, there has
been no research on the biological function of lncRNAs in
Dox-related cardiotoxicity, so in this study we explored the
involvement of lincRNA-p21 in cardiomyocyte senescence in the
Dox-induced cardiotoxic process.
LncRNAs have been proposed to act in transvia
several mechanisms ranging from repression of gene transcriptional
networks to regulation of mRNA translation and protein stability
(14). The Wnt/β-catenin signaling
pathway is closely associated with ageing-associated impairments in
cardiac regeneration and function (15). A previous study found that the
canonical Wnt signaling pathway was involved in the senescence
process of cardiac stem cells (CSCs) (16). As a post-transcriptional inhibitor
of translation, β-catenin was initially identified as a direct
transcriptional target of lincRNA-p21 (11). Moreover, lincRNA-p21 inhibited
β-catenin signaling, thereby attenuating viability, self-renewal
and glycolysis of colorectal cancer stem cells (17). In addition, in the Dox-induced
dilated cardiomyopathy model, β-catenin signaling was apparently
inhibited by Dox (18). Based on
the role of lincRNA-p21-regulated β-catenin signaling and the
inhibitory effect of Dox on β-catenin signaling, the present study
aimed to determine if modulating lincRNA-p21 could activate
β-catenin signaling and relieve Dox-related cardiotoxicity.
Senescence can be triggered by multiple mechanisms,
including those resulting in the production of reactive oxygen
species (ROS) and oxidative stress (19). Oxidative stress and generation of
ROS are also important mediators of the cellular alterations caused
by Dox exposure (20). Cardiac
senescence induced by Dox correlates with increased generation of
ROS and oxidative stress (21).
LncRNAs have close relationships with oxidative stress, DNA damage
and other types of cellular stress responses (22). With respect to lincRNA-p21, a
recent report observed that UVB-induced apoptosis of keratinocytes
involved increased lincRNA-p21 expression and ROS-associated DNA
damage (23). Furthermore,
endoplasmic reticulum stress induced by lincRNA-p21 has been
suggested to account for its effects on apoptosis induction and
inhibition of hepatocellular carcinoma cell proliferation (24). The current study explored whether
suppressing lincRNA-p21 expression could attenuate oxidative stress
to alleviate cellular senescence induced by Dox and thus exert a
cardioprotective effect.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium and fetal bovine
serum (FBS) were purchased from HyClone (GE Healthcare Life
Sciences, Logan, UT, USA), TRIzol® reagent was from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
the Transcriptor First Strand cDNA Synthesis kit, FastStart
Universal SYBR® Green Master (Rox) and X-tremeGENE HP
DNA transfection reagent were purchased from Roche Diagnostics
(Basel, Switzerland). Rabbit monoclonal antibodies against
β-catenin and β-actin were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA) and horseradish peroxidase-conjugated
anti-rabbit secondary antibodies were from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Small interfering RNAs
(siRNAs) targeting lincRNA-p21 and β-catenin transcripts were
purchased from Thermo Fisher Scientific, Inc. WST-1 Cell
Proliferation and Cytotoxicity Assay kit, Mitochondrial Membrane
Potential Assay kit with JC-1 and Reactive Oxygen Species Assay Kit
were purchased from Beyotime Technology (Jiangsu, China).
Superoxide Dismutase (SOD) Activity Colorimetric Assay and Lipid
Peroxidation (Malondialdehyde; MDA) Assay kits were purchased from
Abcam (Cambridge, UK). N-acetyl cysteine (NAC) was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell culture and cell treatment
HL-1 murine cardiomyocytes were a kind gift from Dr
William C. Claycomb. Cells were maintained in fibronectin-coated
flasks supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml
streptomycin and 2 mM L-glutamine and maintained semi-confluent at
all times. The treatment was carried out by exposing the cell
culture to 5 µM Dox for short periods of time.
WST-1 proliferation assay
HL-1 cells were plated at a density of
1×105 cells/well in a 96-well plate. The cells were
analyzed at 0, 24, 48 and 72 h, using the WST-1 assay. In brief,
the cells were incubated with WST-1 at a concentration of 10 nM for
2 h. The reaction product was quantified by measuring absorbance
using an ELISA reader at 440 and 690 nm. Data were analyzed using
absorbance analysis software.
MTT assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to determine cell viability. Briefly, 300 µl of MTT
reagent was added to each well 2 h prior to harvesting. The
supernatant was then removed and cells were incubated with 400 µl
of dimethylsulfoxide for 10 min. Absorbance at 540 nm was recorded
using the ELISA plate reader. Three repeats were performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of several genes were analyzed
with RT-qPCR. Briefly, total cellular RNA was isolated using
TRIzol® reagent and reversed transcribed using the
transcriptor First Stand cDNA Synthesis Kit according to the
manufacturer's instructions. RT-qPCR was carried out using the Fast
Start Universal SYBR Master and fluorescence quantitative PCR
system. The quantification number of cycles (Cq) was set within the
exponential phase of the PCR. The ΔCq value for each
target gene was calculated by subtracting the Cq value of
GAPDH (internal control) from the target gene. Relative gene
expression levels were calculated by comparing the ΔCq
values between control and experimental conditions for each target
PCR using the following equation: Relative gene
expression=2−(ΔCq sample-ΔCq control). The primer pairs
used to detect the mRNA levels of target genes are listed in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Sequences |
|---|
| LincRNA-p21 | F:
5′-CCTGTCCACTCGCTTTC-3′ |
|
| R:
5′-GGAACTGGAGACGGAATGTC-3′ |
| p53 | F:
5′-GGATGCCCATGCTACAGAGGAGTCT-3′ |
|
| R:
5′-GTCTGAGTCAGGCCCCACTTTCTTG-3′ |
| p16 | F:
5′-TTGGCCCAAGAGCGGGGACA-3′ |
|
| R:
5′-GCGGGCTGAGGCCGGATTTA-3′ |
| telomere
length | F:
5′-TGAAAGTAGAGGATTGCCACTG-3′ |
|
| R:
5′-AGCCAGAACAGGAACGTAGC-3′ |
| β-catenin | F:
5′-TAGTGTGACAAGCTGAGTATGCGA-3′ |
|
| R:
5′-CTGGAGCGTCTGATGAG-3′ |
| GAPDH | F:
5′-GGAGCCAAAAGGGTCATCAT-3′ |
|
| R:
5′-GTGATGGCATGGACTGTGGT-3′ |
|
siRNA-LincRNA-p21 |
UGAAAAGAGCCGUGAGCUA |
|
siRNA-β-catenin |
CTCACTTGCAATAATTACAAA |
| siRNA-NT |
CTCUCCGAACGUGUCACGUTT |
Relative telomere length
measurement
Relative telomere length quantification in HL-1
cells was performed using a qPCR approach based on a previously
established method (25), with
GAPDH as the normalizing gene. The primer pairs used to
detect telomere length are listed in Table I.
Relative telomerase activity
measurement
Telomerase activity of HL-1 cells was examined using
the Telo TAGGG Telomerase PCR ELISA PLUS kit according to the
manufacturer's instructions. Cell lysates were centrifuged for 20
min at 4°C and 3 µl of cell extract were used for each telomeric
repeat PCR amplification reaction and 3 µl of inactivated cell
lysate were used for Telomeric Repeat Amplification Protocol (TRAP)
reactions according to the manufacturer's recommendations. Each
TRAP reaction was performed with amplification of an internal
control from the kit to validate the absence of a PCR inhibitor.
Using the ELISA method, the amplified products were immobilized on
streptavidin-coated microtiter plates via biotin-streptavidin
interaction. Thereafter, the amplifications were detected by
anti-digoxigenin antibodies conjugated to peroxidase. After
addition of the peroxidase substrate
(3,3′,5,5′-tetramethylbenzidine), the amount of TRAP products was
determined by measurement of absorbance at 450 nm using the ELISA
plate reader.
Western blot analysis
To obtain total protein, HL-1 cells were lysed with
ice-cold lysis buffer (Beyotime Biotechnology). Expression of
β-catenin and β-actin were evaluated by western blot. Cellular
extracts were prepared according to the manufacturer's instruction.
Protein samples were quantified and separated with SDS-PAGE.
Western blot assay was performed as described previously (26).
Plasmid transfection
LincRNA-p21 siRNA, β-catenin siRNA and adenoviral
vectors expressing lincRNA-p21 (Ad-lnc-p21) were designed and
synthesized. Scrambled non-targeting siRNA (siRNA-NT) and
adenoviral vectors expressing a control scrambled sequence
(Ad-Ctrl) were used as negative controls. HL-1 cells were
transfected using Lipofectamine® 2000 (Invitrogen) at a
final concentration of 100 nM.
Evolution of ΔΨm
Cells in a 96-well microtiter plate were grown at
37°C for 1 day in complete culture medium to reach 1×105
cells per well. The cells were then washed with PBS and loaded at
37°C for 15 min with 5 µg/ml JC-1. After two wash cycles with PBS,
the time-dependent JC-1 fluorescence was recorded using the ELISA
plate reader. The fluorescent probe was excited at 490 nm and the
emission was alternately read at 530 and 590 nm.
ROS measurement
Levels of intracellular ROS were determined using
2,7-dichlorodihydrofluorescein diacetate (Beyotine Institute of
Biotechnology, Nantong, China), following the manufacturer's
instructions. The fluorescence intensity of the cells was measured
using a fluorescence spectrophotometer, with excitation and
emission wavelengths of 488 and 525 nm, respectively.
SOD activity
SOD activity in HL-1 cells was determined using a
colorimetric assay kit (Abcam) according to the manufacturer's
protocol. Briefly, protein was isolated from HL-1 cells using lysis
buffer, and SOD activity was measured in 10 µg of total protein
extract. Absorbance was measured at 450 nm.
Lipid peroxidation assays
Lipid peroxidation was monitored using an assay kit
(Abcam) to measure the formation of MDA according to the
manufacturer's protocol. Briefly, HL-1 cells (1×106
cells) were homogenized on ice in 300 µl of MDA lysis buffer (with
3 µl of 100× butylated hydroxytoluene), then centrifuged (13,000 ×
g, 10 min) to remove insoluble material. The supernatant (200 µl)
was added to 600 µl of thiobarbituric acid and incubated at 95°C
for 60 min. The samples were cooled to room temperature in an ice
bath for 10 min, and the absorbance at 532 nm was measured
spectrophotometrically.
Statistical analysis
Data were expressed as means ± standard deviation.
Differences among groups were tested with one-way analysis of
variance followed by Tukey's post hoc test, and comparisons between
two groups were evaluated with Student's t-tests using SPSS package
v19.0 (IBM, Armonk, NY, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
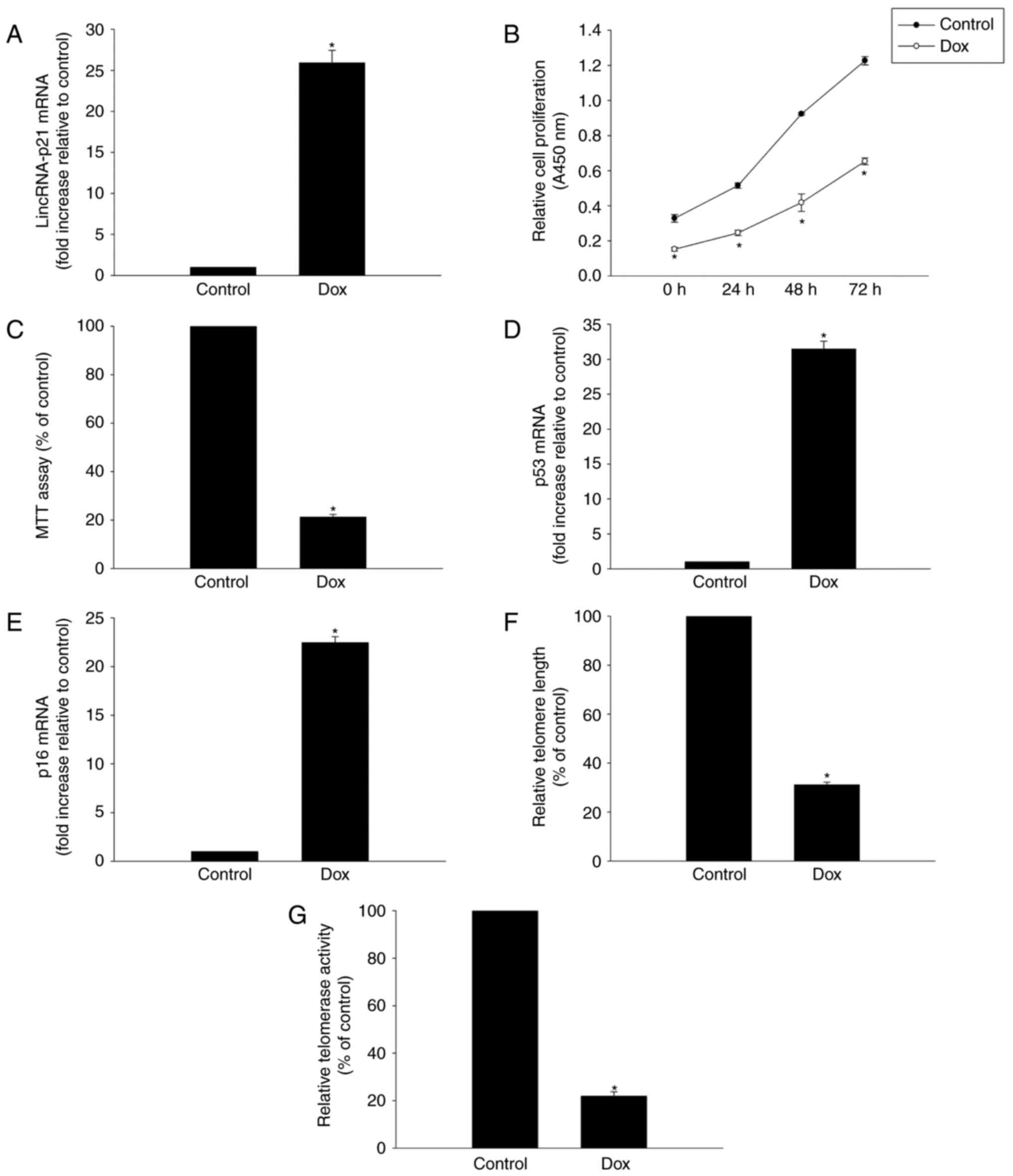
Dox induced cellular senescence and
generation of lincRNA-p21
To determine whether lincRNA-p21 is involved in
Dox-induced cardiac senescence, its expression was evaluated in
HL-1 murine cardiomyocytes exposed to 5 µM Dox for 24 h. RT-qPCR
analysis revealed a significant increase of linRNA-p21 in HL-1
cells following Dox treatment (Fig.
1A).
To further explore the role of Dox in inducing
senescence in HL-1 cells, we tested HL-1 cell viability and
proliferation and demonstrated that both proliferation and the
percentage of viable cells were decreased following Dox treatment
(Fig. 1B and C). Furthermore,
expression of senescence-related genes p53 and p16 was markedly
increased in the Dox treatment group (Fig. 1D, E). Finally, we demonstrated that
treating cells with Dox resulted in decreasing telomere length and
telomerase activity (Fig. 1F and
G).
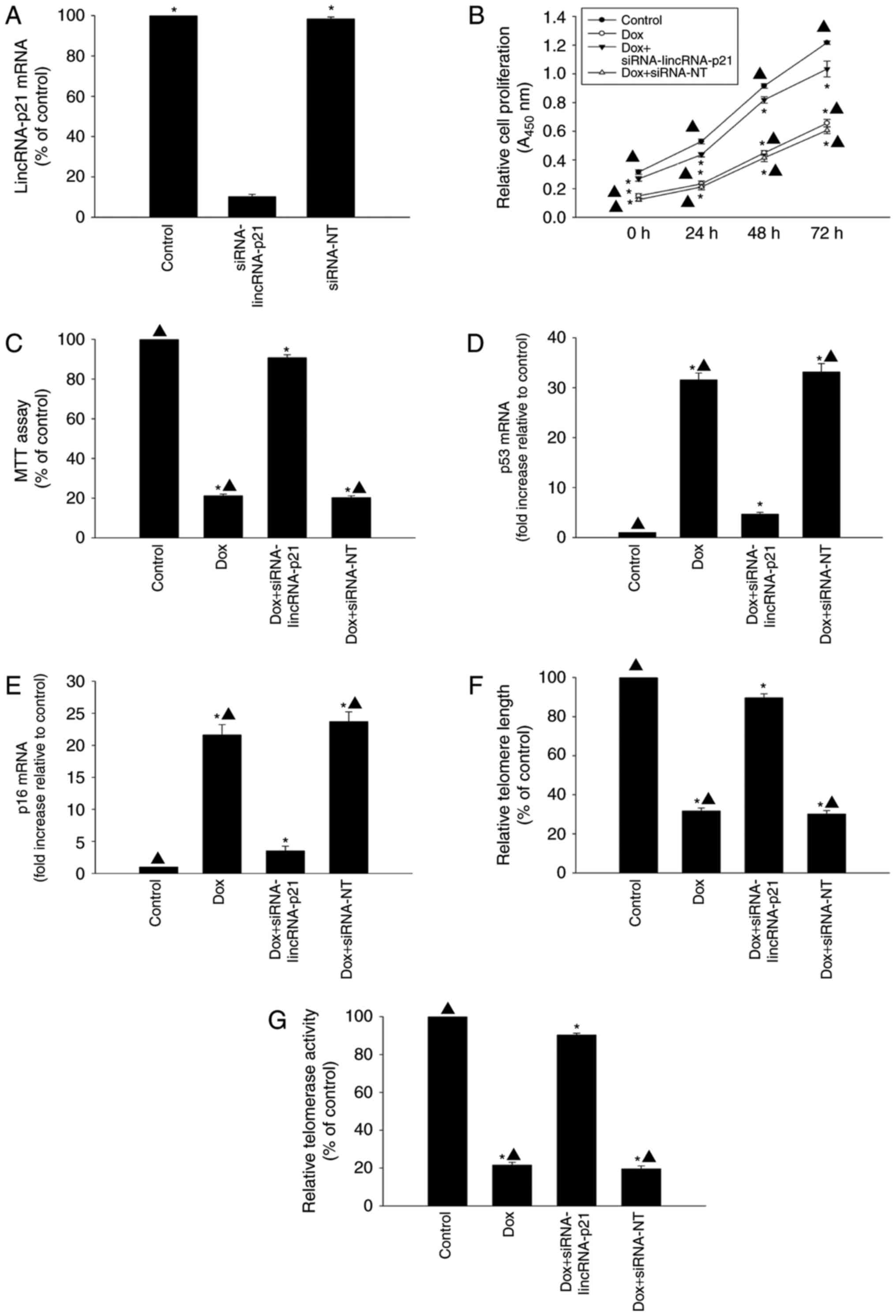
Modulating lincRNA-p21 attenuated
cellular senescence induced by Dox
The role of lincRNA-p21 in cellular senescence
induced by Dox was further investigated by knockdown of endogenous
lincRNA-p21 by siRNA. LincRNA-p21 expression levels were
significantly reduced by transfection with lincRNA-p21 siRNA
compared with siRNA-NT (Fig. 2A).
Knockdown of lincRNA-p21 was associated with significantly
increased proliferation and cellular viability of HL-1 cells
(Fig. 2B and C) compared with the
HL-1 cells treated with Dox only. In addition, inhibition of
lincRNA-p21 in the presence of Dox decreased the expression of
senescence-related genes p53 and p16 (Fig. 2D and E), while telomere length and
telomerase activity increased (Fig. 2F
and G), compared to cells treated with Dox without inhibition
of lincRNA-p21. In contrast, siRNA-NT treatment did not attenuate
cellular senescence induced by Dox.
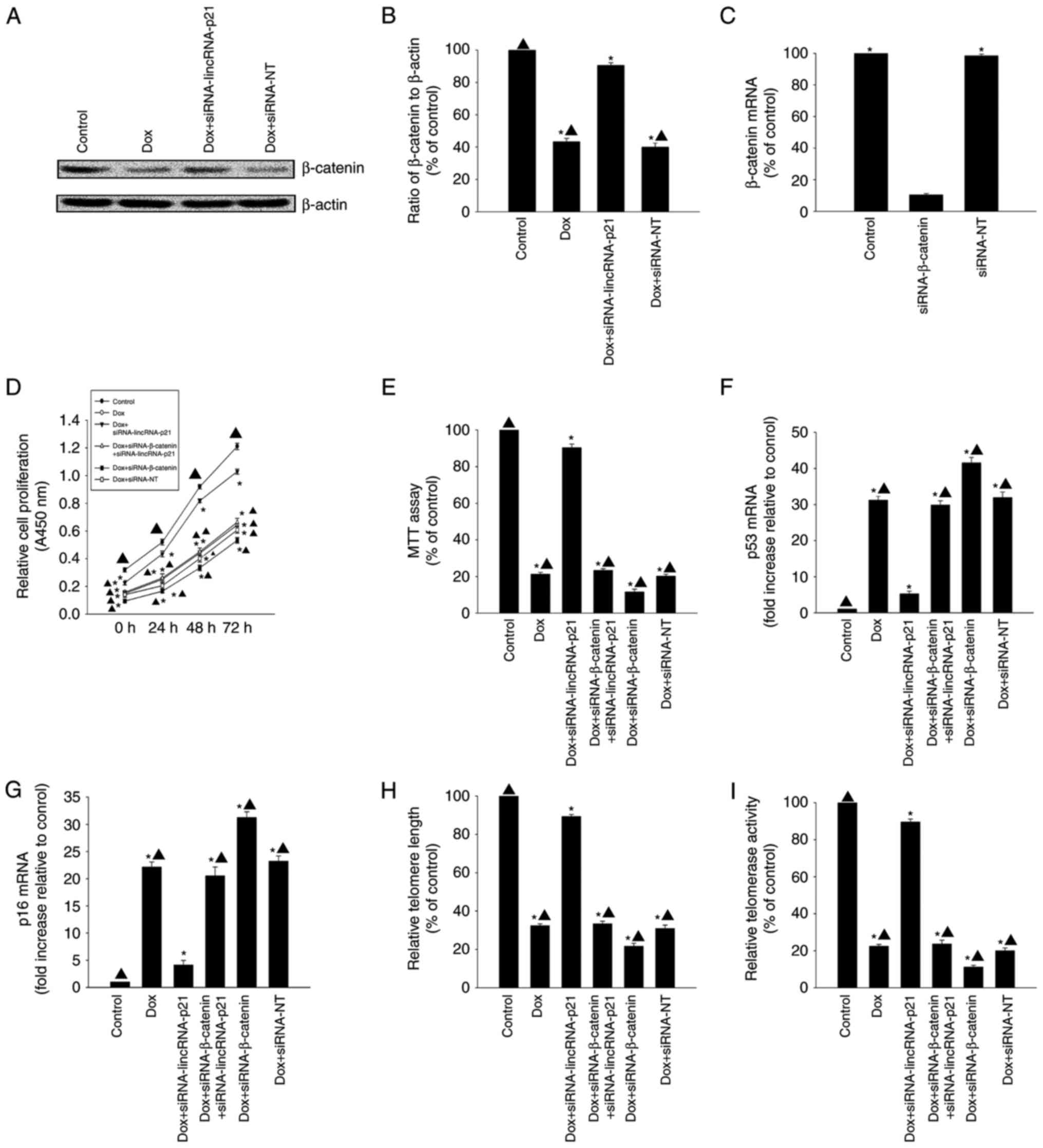
LincRNA-p21-β-catenin signaling was
involved in Dox-related cellular senescence
LincRNA-p21 has previously been demonstrated to
reduce β-catenin protein levels in CSCs (17). In the present study, β-catenin
protein expression levels were decreased in the Dox-treated group
when compared with the control group; however, after silencing
lincRNA-p21, β-catenin protein expression levels increased
(Fig. 3A and B). To further
investigate the mechanism underlying the effect of lincRNA-p21on
Dox-related cellular senescence, we used siRNA to silence
β-catenin. β-catenin mRNA expression levels were significantly
reduced in cells transfected with siRNA-β-catenin compared to
transfection with siRNA-NT control (Fig. 3C). Knockdown of lincRNA-p21
reversed the decrease in proliferation and viability and the
increase in expression of senescence-related genes p53 and p16 in
HL-1 cells induced by Dox (Fig.
3D-G); it also increased telomere length and telomerase
activity (Fig. 3H and I). However,
these effects were abolished by silencing β-catenin.
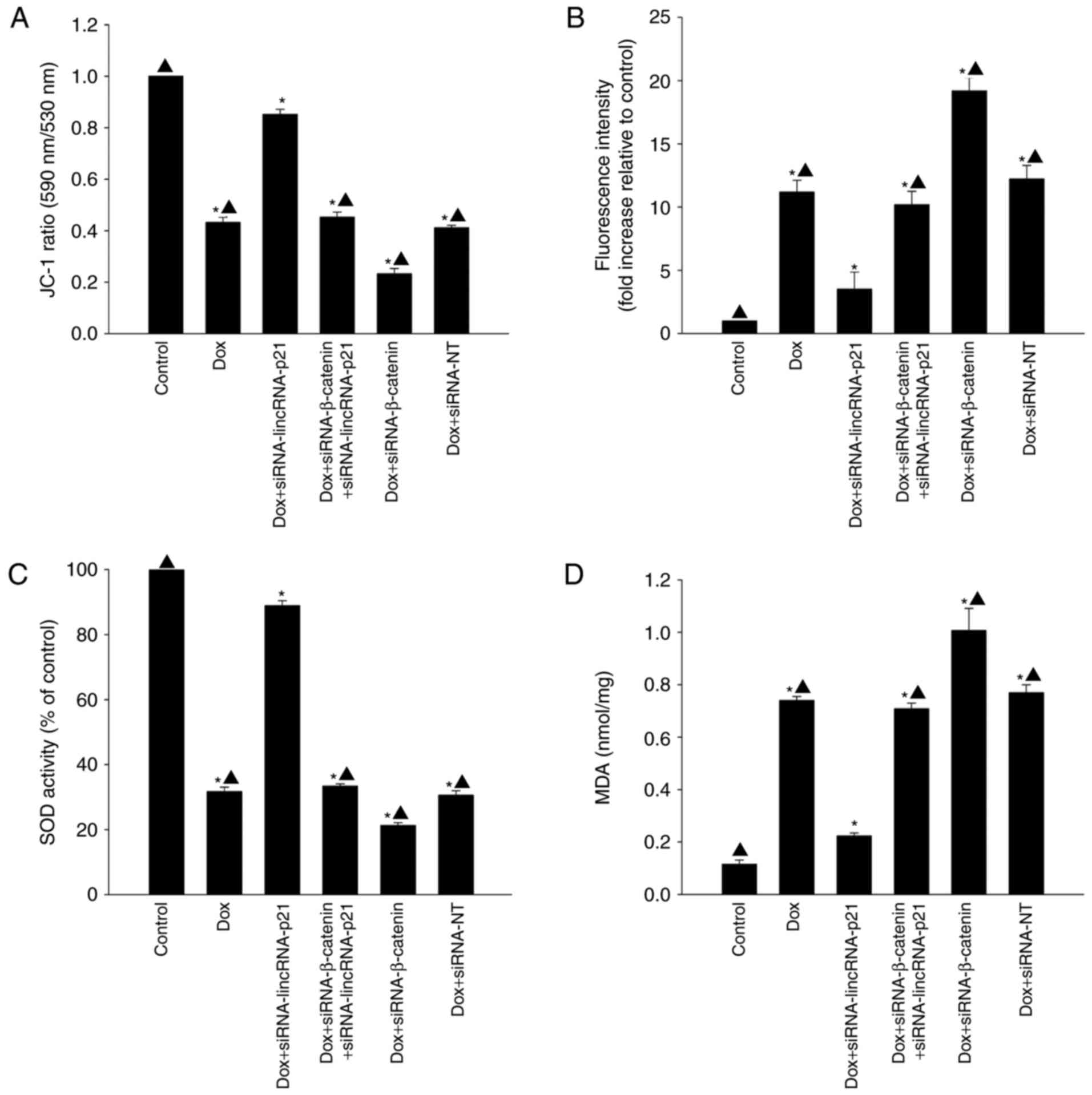
LincRNA-p21 participated in cellular
senescence by inducing oxidative stress
To determine whether lincRNA-p21 induced cellular
senescence by increasing oxidative stress in the presence of Dox,
we examined mitochondrial transmembrane potential, generation of
ROS, activation of SOD and lipid peroxidation by MDA assay. Dox
significantly decreased mitochondrial transmembrane potential
(Fig. 4A) and activation of SOD
(Fig. 4C) while increasing
generation of ROS (Fig. 4B) and
MDA activation (Fig. 4D). However,
after silencing lincRNA-p21, mitochondrial transmembrane potential
and the activation of SOD were increased, but generation of ROS and
MDA activation were decreased. siRNA against β-catenin was a potent
blocker of the inhibitory effect of siRNA-lincRNA-p21 on oxidative
stress, resulting in increased generation of ROS and MDA activation
while decreasing mitochondrial transmembrane potential and
activation of SOD (Fig. 4).
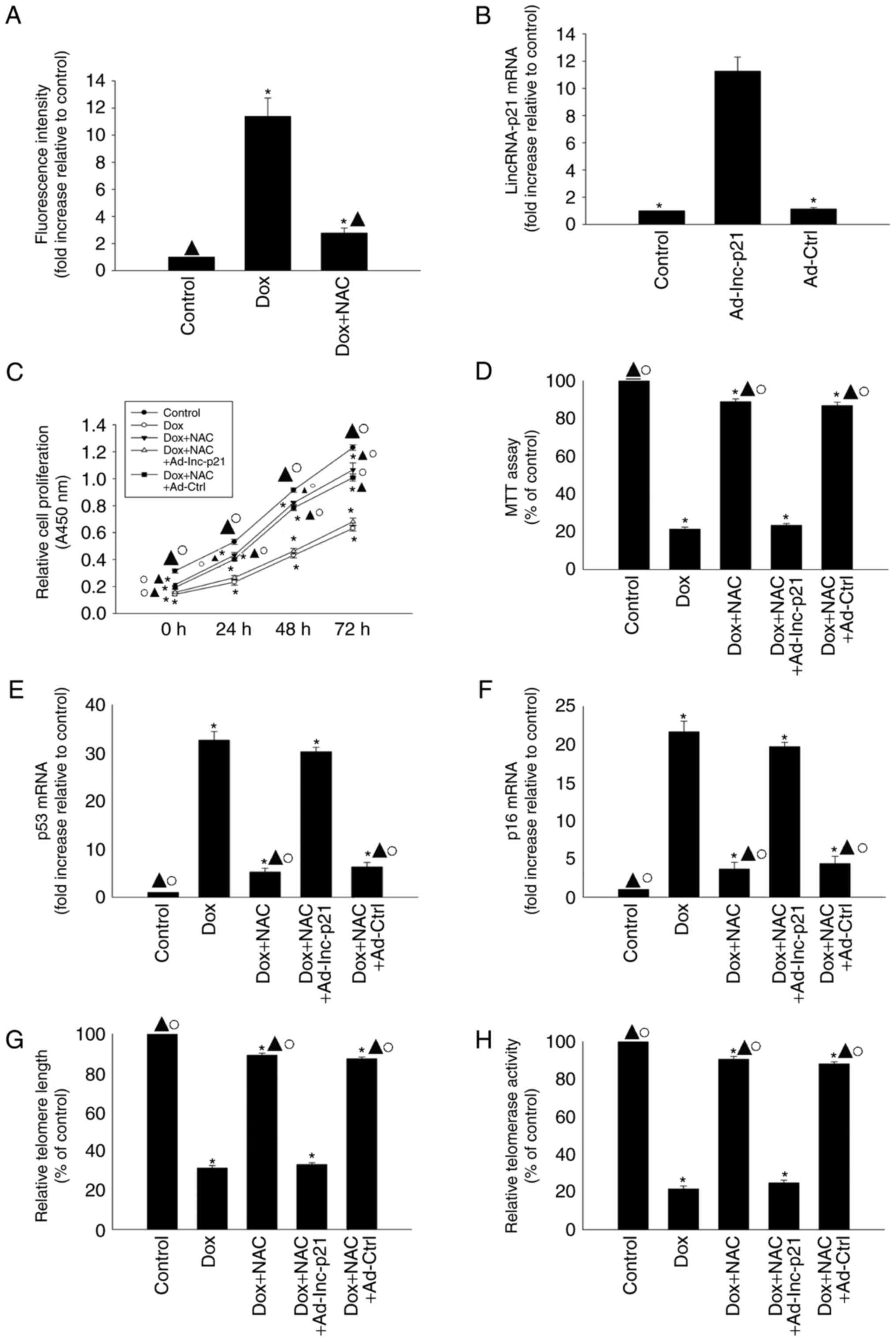
Antioxidant treatment suppressed
cellular senescence induced by Dox
To confirm that the effects of exogenous Dox on
cellular senescence were specifically due to lincRNA-p21-induced
oxidative stress, we investigated the effects of the antioxidant
agent NAC on cellular senescence in Dox-treated HL-1 cells. NAC
treatment apparently decreased the generation of ROS in Dox-treated
HL-1 cells (Fig. 5A). We then
overexpressed lincRNA-p21 using Ad-lnc-p21 transfection (Fig. 5B). We found that NAC reversed the
decrease in proliferation and viability and the increased
expression of senescence-related genes p53 and p16 in HL-1 cells
induced by Dox (Fig. 5C-F), and
also increased telomere length and telomerase activity (Fig. 5G and H); however, these effects
were abolished by lincRNA-p21 overexpression.
Discussion
Dox is among the most widely used chemotherapeutic
agents and has been shown to be effective in a wide range of tumors
(27). However, the clinical
effectiveness of Dox is hampered by the development of
cardiotoxicity that negatively affects patients' outcomes and
severely limits the oncologic therapeutic opportunities (28). Numerous studies have probed the
molecular mechanisms of Dox-related cardiomyopathy. As a result, a
number of molecular elements have been implicated in the
pathogenesis of Dox cardiotoxicity, including DNA and mitochondrial
damage and accumulation of ROS (29,30).
These molecular effects indicated that cardiac cellular senescence
played a substantial role in Dox-induced cardiomyopathy (31). As previous studies showed that
treatment with Dox extensively generated reactive oxidative stress
leading to cardiac senescence, these findings confirmed that a
number of senescence- and stress-associated proteins and genes were
involved in Dox-induced cardiomyopathy (5,32).
In our study, we found that treatment with Dox induced apparent
cellular senescence, showing that increased expression of
senescence related genes p53 and p16 was accompanied by impaired
cellular proliferation and viability. Cellular senescence induced
by Dox is defined as the arrest of cell cycle progression which can
be caused by telomere shortening (5), in agreement with our results finding
that treatment of HL-1cells with Dox induced shortening of telomere
length and decreased telomerase activity.
Epigenetic modifications can also be mediated by
lncRNAs, which play major roles in regulation of gene
transcription, chromatin structure and mRNA stability during cell
development and diseases (33).
They are also involved in the regulation of different cellular
functions such as genome maintenance, post-transcriptional
modifications, structural maintenance of cellular processes and
translational control (34,35).
Several lncRNAs have recently been suggested to be involved in the
regulation of senescence, and recent research has revealed that
lncRNA HOTAIR overexpression reduced adipogenic differentiation of
MSCs, inducing senescence-associated changes (36). In addition, lncRNAs also take part
in the process of cardiac senescence, as related research has
confirmed that the mitochondrial lncRNA ASncmtRNA-2 is induced in
aging and replicative senescence in vascular cells (37). As an important regulator of the
cellular senescence process, lincRNA-p21 exerts its effect in
multiple ways. A previous study suggested that overexpression of
lincRNA-p21 increased p21 expression at both mRNA and protein
levels and impeded cell-cycle progression, and thus was involved in
cellular senescence (14).
LincRNA-p21 has been identified as a regulator of p53 expression,
and reciprocally p53 can regulate the expression of lincRNA-p21,
which plays a major role in pro-senescence networks (12). LincRNA-p21 is necessary for the
recruitment of hnRNPK to the p53 response element and for
increasing the binding efficiency of p53 in the p21 promoter
region, promoting cellular senescence (38). In the present study, we have
characterized the expression profile of lincRNA-p21 in HL-1 cells
treated with Dox and found that its expression was closely related
to HL-1 cellular senescence induced by Dox. Treatment with Dox
induced increased expression of lincRNA-p21 accompanied by
decreased cellular proliferation, viability, telomere length and
telomerase activity, while increasing expression of
senescence-related genes p53 and p16. Furthermore, this senescent
condition was reversed by silencing lincRNA-p21, further confirming
the pro-senescent effect of lincRNA-p21.
Given the impact of cellular senescence in
Dox-associated cardiomyopathy processes, there is much interest in
understanding how to modulate senescence for therapeutic purposes.
The Wnt/β-catenin pathway is closely related to cardiac senescence,
as previous research has revealed a marked decrease in β-catenin in
mouse hearts 8 weeks before the mice developed cardiomyopathy at 21
weeks of age after infection with the coxsakie virus (39). Also, sustained inhibition of the
Wnt/β-catenin pathway was reported to be involved in Dox-induced
cardiomyopathy processes (18). In
our study, as indicated by the results of western blots, expression
of β-catenin was apparently decreased during Dox treatment. As a
target of lincRNA-p21, β-catenin protein has been shown to be
directly downregulated by lincRNA-p21 in various cell types, and
vector-delivered lincRNA-p21 preferentially blocked the activation
of Wnt/β-catenin signaling in CSCs (17). The viability, self-renewal and
tumorigenicity of CSCs in this study were compromised by
lincRNA-p21 overexpression. It has also been reported that
lincRNA-p21 inhibited the Wnt/β-catenin pathway so that inhibition
of lincRNA-p21 caused decreased proliferation of hepatic stellate
cells (40). In agreement with
these previous findings, our research confirmed that treatment with
Dox inhibited Wnt/β-catenin signaling, which was reversed by
silencing lincRNA-p21. In contrast, inactivating the Wnt/β-catenin
pathway using siRNA blocked the anti-senescent effect of silencing
lincRNA-p21, as indicated by decreased cellular proliferation and
viability, reduced telomere length and telomerase activity and
increased expression of p53 and p16.
Oxidative stress has been shown to be a central
mediator of cellular senescence (41). Cellular senescence is accompanied
by ROS generation, increased oxidant enzyme activity and diminished
antioxidant enzyme activity (42).
Dox-induced oxidative stress triggered cardiotoxicity leading to
cardiomyopathy and heart failure (20). Several theories, including
mitochondrial dysfunction, increased ROS production and contractile
failure have been proposed as plausible underlying mechanisms for
Dox-induced cardiomyopathy (43,44).
Regulatory lncRNAs have been identified as key modulators of
senescence, oxidative stress-induced apoptosis and cell cycle
arrest and have great effects during the cellular senescence
process (12,45). LincRNA-p21 was associated with
cellular DNA damage and endoplasmic reticulum stress under
oxidative stress, thus inducing growth regression of HepG2 cells
and apoptosis of hepatocellular carcinoma cells (23). Herein, we found that treatment with
Dox induced oxidative stress, decreased mitochondrial transmembrane
potential and activation of SOD, while increasing generation of ROS
and stimulating MDA activation. These oxidative effects were
relieved by silencing lincRNA-p21. To further confirm the
alleviation of oxidative stress by lincRNA-p21 in Dox-induced
cardiac senescence, we used the antioxidant NAC to alleviate ROS
generation and found that it could ameliorate cellular senescence
induced by Dox. In addition, the ameliorative effects were
abolished by overexpression of lincRNA-p21, confirming that
oxidative stress relieved by lincRNA-p21 played a substantial role
in Dox-induced cardiac senescence.
In conclusion, our study indicated that lincRNA-p21
is involved in cardiac cellular senescence. Enhancing lincRNA-p21
expression may relieve cardiac senescence induced by Dox by
regulating oxidative stress via the Wnt/β-catenin signaling
pathway. We demonstrated that attenuation of cardiac senescence may
have important therapeutic implications for Dox-induced
cardiomyopathy. Targeting lincRNA-p21 expression in cardiomyocytes
may be a useful strategy in treatment of Dox-induced
cardiomyopathy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500261 to M.H.;
and grant no. 81600278 to W.Z.X) and the Medical Science and
Technology Project of Zhejiang Province (grant no. 2018236627 to
M.H.).
References
|
1
|
Binaschi M, Bigioni M, Cipollone A, Rossi
C, Goso C, Maggi CA, Capranico G and Animati F: Anthracyclines:
Selected new developments. Curr Med Chem Anticancer Agents.
1:113–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrans VJ, Clark JR, Zhang J, Yu ZX and
Herman EH: Pathogenesis and prevention of doxorubicin
cardiomyopathy. Tsitologiia. 39:928–937. 1997.PubMed/NCBI
|
|
3
|
Kumar S, Marfatia R, Tannenbaum S, Yang C
and Avelar E: Doxorubicin-induced cardiomyopathy 17 years after
chemotherapy. Tex Heart Inst J. 39:424–427. 2012.PubMed/NCBI
|
|
4
|
Bartlett JJ, Trivedi PC and Pulinilkunnil
T: Autophagic dysregulation in doxorubicin cardiomyopathy. J Mol
Cell Cardiol. 104:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du WW, Yang W, Chen Y, Wu ZK, Foster FS,
Yang Z, Li X and Yang BB: Foxo3 circular RNA promotes cardiac
senescence by modulating multiple factors associated with stress
and senescence responses. Eur Heart J. 38:1402–1412.
2017.PubMed/NCBI
|
|
6
|
Ghosh AK, Rai R, Park KE, Eren M, Miyata
T, Wilsbacher LD and Vaughan DE: A small molecule inhibitor of
PAI-1 protects against doxorubicin-induced cellular senescence.
Oncotarget. 7:72443–72457. 2016.PubMed/NCBI
|
|
7
|
Atianand MK, Cafferey DR and Fitzgerald
KA: Immunobiology of long noncoding RNAs. Annu Rev Immunol.
35:177–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kour S and Rath PC: Age-related expression
of a repeat-rich intergenic long noncoding RNA in the rat brain.
Mol Neurobiol. 54:639–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Wang Z, Chen D, Zhang B, Tian RR,
Wu J, Zhang Y, Xu K, Yang LM, Cheng C, et al: Annotation and
cluster analysis of spatiotemporal- and sex-related lncRNA
expression in rhesus macaque brain. Genome Res. 27:1608–1620. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon JH, Abdelmohsen K, Srikantan S, Yang
X, Martindale JL, De S, Huarte M, Zhan M, Becker KG and Gorospe M:
LincRNA-p21 suppresses target mRNA translation. Mol Cell.
47:648–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdelmohsen K and Gorospe M: Noncoding RNA
control of cellular senescence. Wiley Interdiscip Rev RNA.
6:615–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: LincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis, and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimitrova N, Zamudio JR, Jong RM, Soukup
D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA and Jacks
T: LincRNA-p21 activates p21 in cis to promote Polycomb target gene
expression and to enforce the G1/S checkpoint. Mol Cell.
54:777–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwörer S, Becker F, Feller C, Baig AH,
Köber U, Henze H, Kraus JM, Xin B, Lechel A, Lipka DB, et al:
Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9
developmental signals. Nature. 540:428–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura T, Hosoyama T, Murakami J, Samura
M, Ueno K, Kurazumi H, Suzuki R, Mikamo A and Hamano K: Age-related
increase in Wnt inhibitor causes a senescence-like phenotype in
human cardiac stem cells. Biochem Biophys Res Commun. 487:653–659.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Lei ZJ, Guo Y, Wang T, Qin ZY,
Xiao HL, Fan LL, Chen DF, Bian XW, Liu J and Wang B:
miRNA-regulated delivery of lincRNA-p21 suppresses β-catenin
signaling and tumorigenicity of colorectal cancer stem cells.
Oncotarget. 6:37852–37870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen KH, Chen CH, Wallace CG, Chen YT,
Yang CC, Sung PH, Chiang HJ, Chen YL, Chua S, Yip HK and Cheng JT:
Combined therapy with melatonin and exendin-4 effectively
attenuated the deterioration of renal function in rat cardiorenal
syndrome. Am J Transl Res. 9:214–229. 2017.PubMed/NCBI
|
|
19
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du Q, Zhu B, Zhai Q and Yu B: Sirt3
attenuates doxorubicin-induced cardiac hypertrophy and
mitochondrial dysfunction via suppression of Bnip3. Am J Transl
Res. 9:3360–3373. 2017.PubMed/NCBI
|
|
21
|
Przybylska D, Janiszewska D, Goździk A,
Bielak-Zmijewska A, Sunderland P, Sikora E and Mosieniak G: NOX4
downregulation leads to senescence of human vascular smooth muscle
cells. Oncotarget. 7:66429–66443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng Q, Wang Q, Chen X, Xia K, Tang J,
Zhou X, Cheng Y, Chen Y, Huang L, Xiang H, et al: Analysis of
lncRNAs expression in UVB-induced stress responses of melanocytes.
J Dermatol Sci. 81:53–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hall JR, Messenger ZJ, Tam HW, Phillips
SL, Recio L and Smart RC: Long noncoding RNA lincRNA-p21 is the
major mediator of UVB-induced and p53-dependent apoptosis in
keratinocytes. Cell Death Dis. 6:e17002015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang N, Fu Y, Zhang H, Hui S, Zhu N and
Yang G: LincRNA-p21 activates endoplasmic reticulum stress and
inhibits hepatocellular carcinoma. Oncotarget. 6:281512015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crepin T, Carron C, Roubiou C, Gaugler B,
Gaiffe E, Simula-Faivre D, Ferrand C, Tiberghien P, Chalopin JM,
Moulin B, et al: ATG-induced accelerated immune senescence:
Clinical implications in renal transplant recipients. Am J
Transplant. 15:1028–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia W, Zhang F, Xie C, Jiang M and Hou M:
Macrophage migration inhibitory factor confers resistance to
senescence through CD74-dependent AMPK-FOXO3a signaling in
mesenchymal stem cells. Stem Cell Res Ther. 6:822015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang F, Teves SS, Kemp CJ and Henikoff S:
Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys
Acta. 1845:84–89. 2014.PubMed/NCBI
|
|
28
|
Cardinale D, Colombo A, Bacchiani G,
Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N,
Curigliano G, et al: Early detection of anthracycline
cardiotoxicity and improvement with heart failure therapy.
Circulation. 131:1981–1988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singal PK and Iliskovic N:
Doxorubicin-induced cardiomyopathy. N Engl J Med. 339:900–905.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suliman HB, Carraway MS, Ali AS, Reynolds
CM, Welty-Wolf KE and Piantadosi CA: The CO/HO system reverses
inhibition of mitochondrial biogenesis and prevents murine
doxorubicin cardiomyopathy. J Clin Invest. 117:3730–3741.
2007.PubMed/NCBI
|
|
32
|
Minotti G, Ronchi R, Salvatorelli E, Menna
P and Cairo G: Doxorubicin irreversibly inactivates iron regulatory
proteins 1 and 2 in cardiomyocytes: Evidence for distinct metabolic
pathways and implications for iron-mediated cardiotoxicity of
antitumor therapy. Cancer Res. 61:8422–8428. 2001.PubMed/NCBI
|
|
33
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quinodoz S and Guttman M: Long noncoding
RNAs: An emerging link between gene regulation and nuclear
organization. Trends Cell Biol. 24:651–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andersson R, Refsing Andersen P, Valen E,
Core LJ, Bornholdt J, Boyd M, Heick Jensen T and Sandelin A:
Nuclear stability and transcriptional directionality separate
functionally distinct RNA species. Nat Commun. 5:53362014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kalwa M, Hänzelmann S, Otto S, Kuo CC,
Franzen J, Joussen S, Fernandez-Rebollo E, Rath B, Koch C, Hofmann
A, et al: The lncRNA HOTAIR impacts on mesenchymal stem cells via
triple helix formation. Nucleic Acids Res. 44:10631–10643. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bianchessi V, Badi I, Bertolotti M, Nigro
P, D'Alessandra Y, Capogrossi MC, Zanobini M, Pompilio G, Raucci A
and Lauri A: The mitochondrial lncRNA ASncmtRNA-2 is induced in
aging and replicative senescence in endothelial cells. J Mol Cell
Cardiol. 81:62–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim C, Kang D, Lee EK and Lee JS: Long
Noncoding RNAs and RNA-binding proteins in oxidative stress,
cellular senescence and age-related diseases. Oxid Med Cell Longev.
2017:20623842017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim BK, Xiong D, Dorner A, Youn TJ, Yung
A, Liu TI, Gu Y, Dalton ND, Wright AT, Evans SM, et al:
Coxsackievirus and adenovirus receptor (CAR) mediates
atrioventricular-node function and connexin 45 localization in the
murine heart. J Clin Invest. 118:2758–2770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu F, Guo Y, Chen B, Shi L, Dong P, Zhou M
and Zheng J: LincRNA-p21 Inhibits the Wnt/β-catenin pathway in
activated hepatic stellate cells via sponging MicroRNA-17-5p. Cell
Physiol Biochem. 41:1970–1980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim YY, Jee HJ, Um JH, Kim YM, Bae SS and
Yun J: Cooperation between p21 and Akt is required for
p53-dependent cellular senescence. Aging Cell. 16:1094–1103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rigaud VO, Ferreira LR, Ayub-Ferreira SM,
Ávila MS, Brandão SM, Cruz FD, Santos MH, Cruz CB, Alves MS, Issa
VS, et al: Circulating miR-1 as a potential biomarker of
doxorubicin-induced cardiotoxicity in breast cancer patients.
Oncotarget. 8:6994–7002. 2017.PubMed/NCBI
|
|
44
|
Ichikawa Y, Ghanefar M, Bayeva M, Wu R,
Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ and Ardehali
H: Cardiotoxicity of doxorubicin is mediated through mitochondrial
iron accumulation. J Clin Invest. 124:617–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang D, Lee H, Haspel JA and Jin Y: Long
noncoding RNA FOXD3-AS1 regulates oxidative stress-induced
apoptosis via sponging microRNA-150. FASEB J. 31:4472–4481. 2017.
View Article : Google Scholar : PubMed/NCBI
|