Introduction
As the most common primary malignant bone tumor
occurring in children and adolescents, osteosarcoma is
characterized by early lung metastasis and high recurrence rate
(1). Surgery, combined with
neoadjuvant chemotherapy and other comprehensive treatment
approaches, can significantly improve the disease, but some local
osteosarcomas still recur; at present, there have been no effective
approaches for the treatment of recurrent and metastatic
osteosarcoma (2). Although
neoadjuvant chemotherapy combined with surgery improves the
long-term survival rate of patients with osteosarcoma, short-term
survival rate of patients has not been further improved in recent
years (3).
Autophagy is an ancient biological phenomenon in the
evolution of creatures, and extensively occurs in plants and
low-ranking organisms as an important ways to maintain the
stability of cellular homeostasis; autophagy is also one of the
most important means by which mammals can eliminate tumor cells.
Autophagy is closely related to the incidence, development,
prognosis and treatment of tumor cells (4). When the body grows tumor cells,
autophagy will be activated to remove damaged organelles and
degrade harmful substances in cells such as peroxides, so as to
prevent normal cells from developing into tumor cells (5). Some scholars observed autophagosomes
wrapped parts of the nucleolus, suggesting that autophagy is
involved in the metabolism of genetic materials in cells and in the
elimination of mutant chromosomes (6). Regulating the expression of autophagy
combined with chemotherapy drugs has synergistic or antagonistic
effects in the growth of tumor cells. Compared with chemotherapy
alone, the regulation autophagy changes the apoptosis rate of tumor
cells (7). Inhibiting the
expression of autophagy during radiotherapy or chemotherapy may
cause tumor cells to be unable to eliminate damaged organelles,
thereby accelerating the death of tumor cells, so as to enhance the
efficacy of treatment (8).
Akt (also known as protein kinase B) is a regulator
located at the downstream of the PI3K pathway. Mammalian target of
rapamycin (mTOR), a kind of serine/threonine protein kinase, is a
member of phosphatidylinositol 3-kinase family (9). mTOR exists in multi-protein
complexes, such as mTORC1 and mTORC2. The PI3K-Akt-mTOR signaling
pathway serves important roles in cells, regulating a variety of
cellular behaviors such as growth, survival, proliferation,
apoptosis, angiogenesis and autophagy of cells (10). Many diseases, including cancer,
autoimmune diseases and neuropathy, are caused by disorders of the
PI3K-Akt-mTOR signaling pathway (10). The PI3K-Akt-mTOR signaling pathway
is related to several important mechanisms of cell growth, so a
better understanding of the PI3K-Akt-mTOR signaling pathway
contributes to the development of cancer drugs, and mTOR inhibitors
have been previously developed and applied as novel antitumor
targeting drugs (11).
As one of the two major active constituents of
Magnolia officinalis, honokiol can effectively fight against
bacteria, oxidation, inflammation and tumors, inhibit central
nervous and muscular relaxation, kill pathogenic microorganisms and
lower cholesterol levels, and is generally used in the treatment of
acute enteritis, bacterial or amoebic dysentery and chronic
gastritis (12). A large number of
studies have demonstrated that honokiol inhibits the proliferation
and apoptosis of cancer cells and prevents from angiogenesis in
vivo, indicating that it has good therapeutic effect on
different tumors (13). Honokiol
has a better curative effect if combined with other anticancer
drugs. A study suggested that Honokiol downregulates the
phosphorylation of Akt and upregulates the expression of PTEN to
realize the negative regulation of PI3K/Akt/mTOR pathway, so as to
inhibit breast cancer (14).
Honokiol promoted cycle arrest and apoptosis of breast cancer cells
through the downregulation of the c-Src/EGFR signaling pathway.
Furthermore, the authors investigated whether honokiol induces
autophagy and apoptosis of osteosarcoma, and analyzed the possible
mechanisms underlying these anticancer effects.
Materials and methods
Cell culture
The human OS cell line, MG-63, was purchased from
the Wuhan Cell Bank of Sciences China (Wuhan, China) and maintained
in Dulbecco's modified Eagles medium, which contains 10%
heat-inactivated FBS (both from Gibco; Thermo Fisher Scientific,
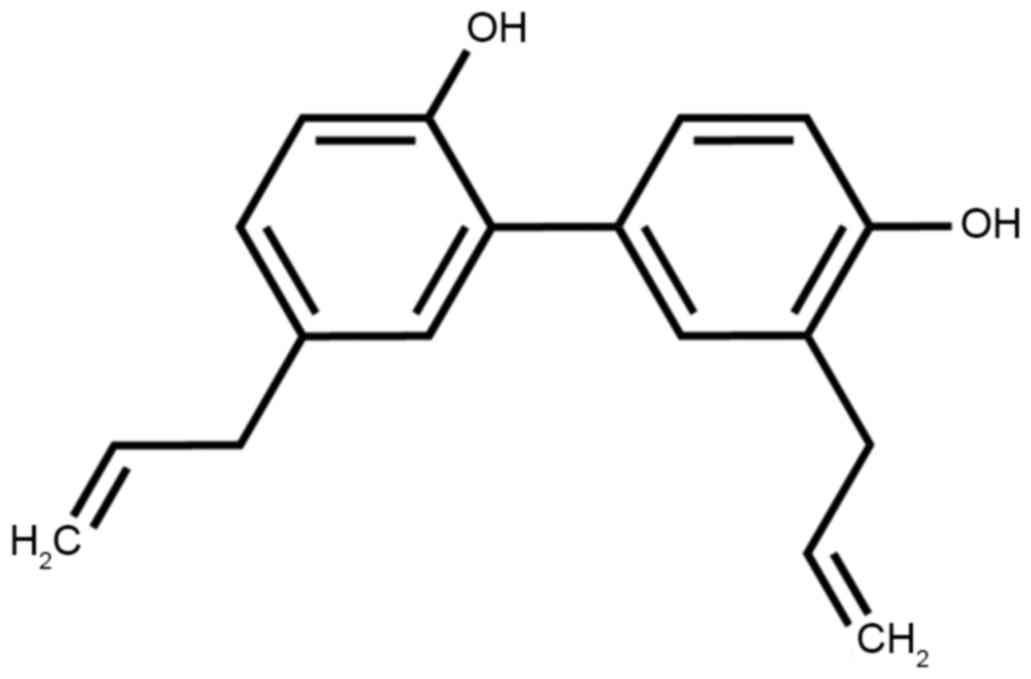
Inc., Waltham, MA, USA) at 37°C in 5% CO2. Honokiol was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and
its constitutional formula is presented in Fig. 1.
MTT assay
MG-63 cells were seeded in 96-well plates at a
density of 1×104 cells/well overnight and then treated
with various concentrations of honokiol (0, 5, 10 and 20 µg/ml) for
24, 48 and 72 h. A total of 20 µl MTT solution (5 g/l; Thermo
Fisher Scientific, Inc.) was added to each well and incubated for 4
h. DMSO (Thermo Fisher Scientific, Inc.) was added to cells and
dissolved for 20 min. The absorbance value was read using an
automatic multiwell spectrophotometer (PowerWave HT; Bio-Tek
Instruments, Inc., Winooski, VT, USA) at 570 nm.
Apoptosis
MG-63 cells were seeded in 6-well plates at a
density of 1×106 cells/well overnight and then treated
with various concentrations of honokiol (0, 5, 10 and 20 µg/ml) for
48 h. MG-63 cells were washed three times with PBS and stained
using the Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) assay (BD Biosciences, Franklin Lakes, NJ, USA)
following the manufacturer's protocol. Apoptotic rate was detected
using flow cytometry (FACSCanto™) and analyzed by CellQuest™
software (version 3.2) (both from BD Biosciences).
Caspase-3 and western blotting
analysis
MG-63 cells were seeded in six-well plates at a
density of 1×106 cells/well overnight and then treated
with various concentrations of Honokiol (0, 5, 10 and 20 µg/ml) for
48 h. MG-63 cells were resuspended in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
at 4°C for 30 min and the lysate was centrifuged at 120,000 × g for
10 min at 4°C. Protein contents were detected using the
Bicinchoninic Acid protein assay kit (Thermo Fisher Scientific,
Inc.). A total of 5–10 µg protein was incubated with Ac-DEVD-pNA
for 1–1.5 h at 37°C. The absorbance value was read using an
automatic multiwell spectrophotometer (PowerWave HT; Bio-Tek
Instruments, Inc.) at 405 nm.
Next, equal amounts of total protein (50 µg) were
separated on 6–12% SDS-PAGE gel and transferred to polyvinylidene
difluoride membranes (Thermo Fisher Scientific, Inc.). The
membranes were blocked with fat-free milk solution (5%, w/v) for 12
h and then incubated with rabbit anti-B-cell lymphoma-2 (Bcl-2,
1:500, cat no. sc-783) and anti-Bcl-2-like protein 4 (Bax, 1:500,
cat no. sc-6236), p53 (cat no. sc-6243), cyclin D1 (1:500, cat no.
sc-717), LC3II (1:500, cat no. sc-292354), PI3K (1:500, cat no.
sc-7174), p-Akt (1:550, cat no. sc-16646-R), p-mTOR (1:500, cat no.
sc-101738) and GAPDH (1:500, cat no. sc-25778) (all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) primary antibodies at 4°C for
12 h. After washing 3 times with TBS and 0.1% Tween-20, membrane
was incubated with horseradish peroxidase-conjugated anti-rabbit
IgG antibody (1:5,000, cat no. sc-2004; Santa Cruz Biotechnology,
Inc.) at 37°C for 1 h and were visualized using Western Blotting
Chemiluminescence Reagent (BD Biosciences). Blots blank was
quantified using BandScan software (version 5.0; Glyko Inc.,
Novato, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-tests were performed for the comparison of results
between different groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Honokiol inhibited cell proliferation
of osteosarcoma cells
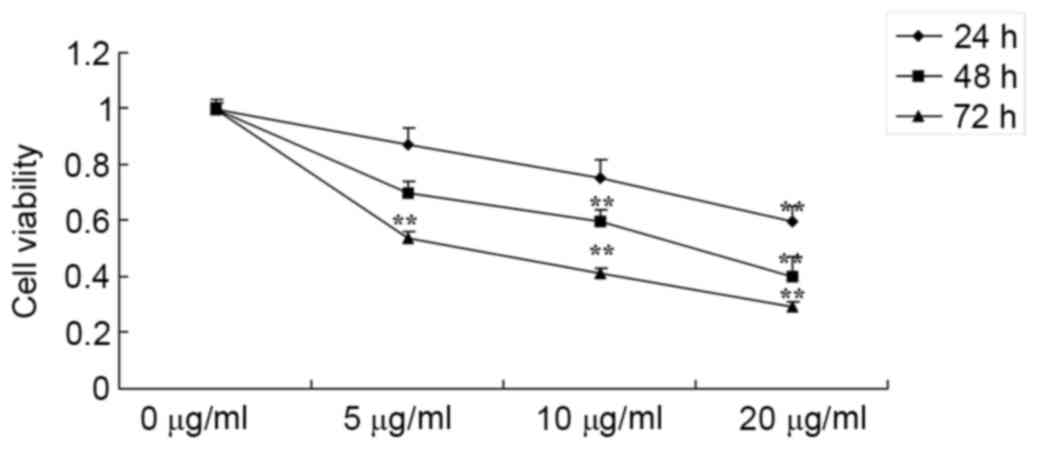
As presented in Fig.
2, an MTT assay demonstrated that various concentrations of
honokiol inhibited cell proliferation of osteosarcoma cells (MG-63)
in a dose- and time-dependent manner. Treatment with osteosarcoma
cell (5, 10 and 20 µg/ml honokiol for 72 h), or 10 and 20 µg/ml
honokiol for 48 h or 20 µg/ml honokiol for 24 h significantly
inhibited cell proliferation of MG-63 cells, compared with the
control group (0 µg/ml group).
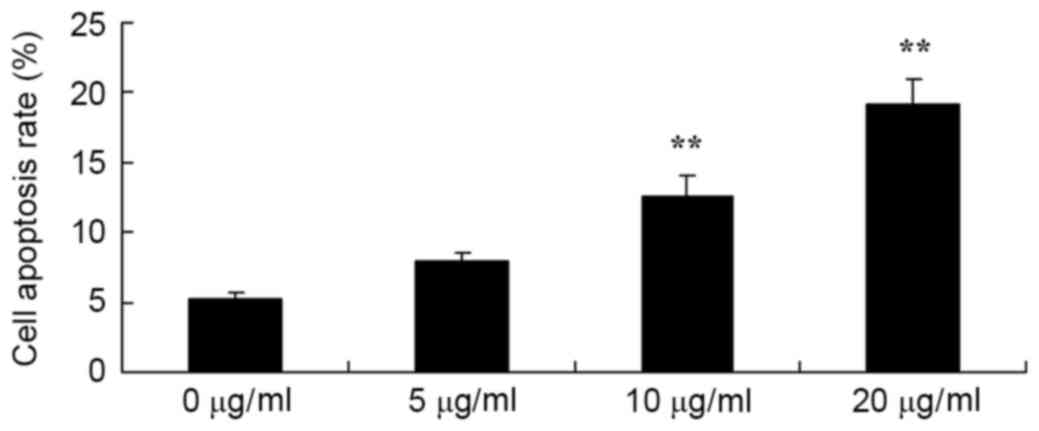
Honokiol induced apoptotic rate of
osteosarcoma cell
Therefore, the authors detected the apoptotic rate
of osteosarcoma cells (MG-63) by honokiol for 48 h. As demonstrated
in Fig. 3, Annexin V-FITC/PI
indicated that 10 and 20 µg/ml honokiol significantly induced
apoptotic rate of MG-63 cells, compared with the control group (0
µg/ml group).
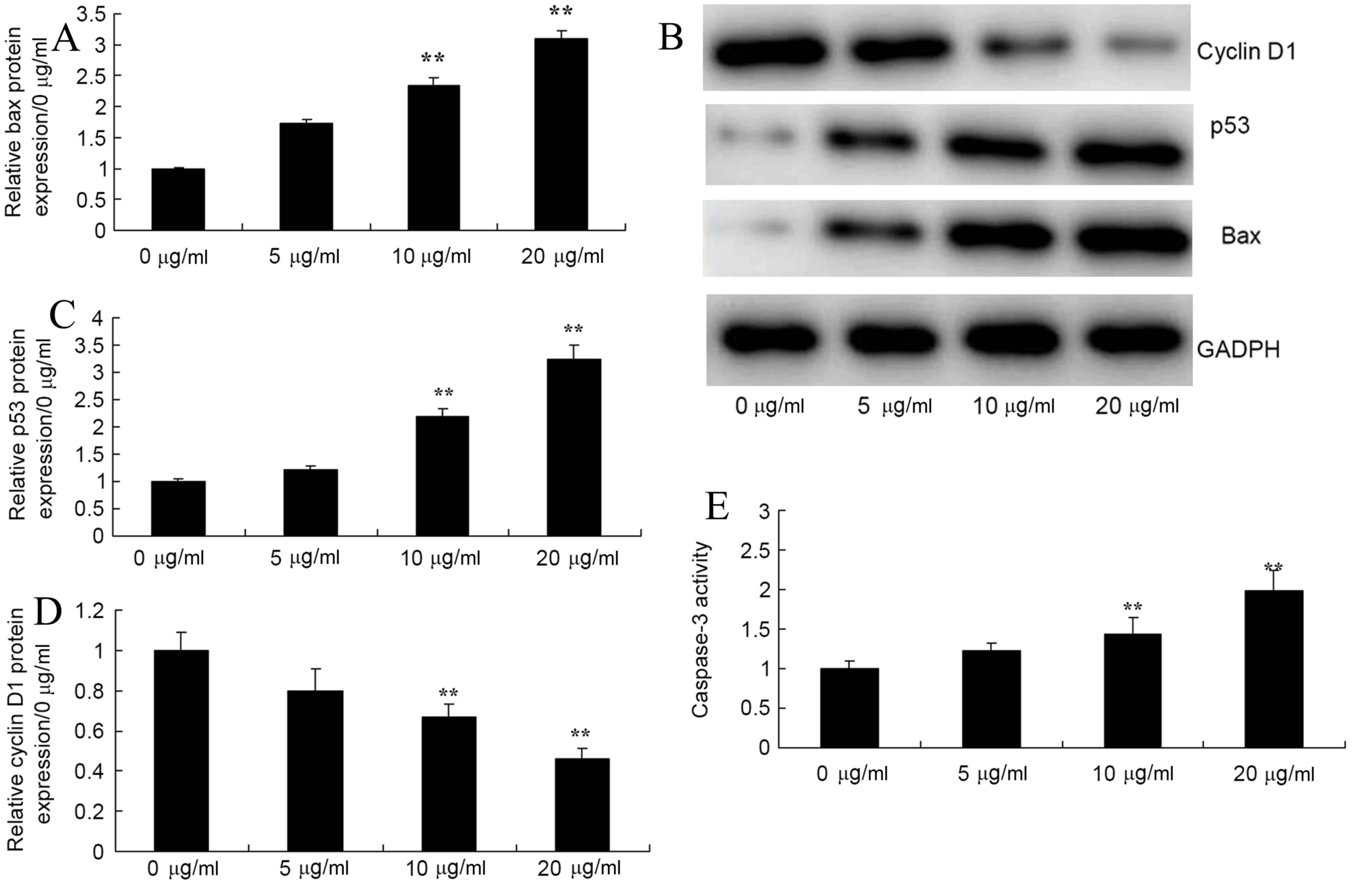
Honokiol induced Bax, p53 and cyclin
D1 protein expression and caspase-3 activity of osteosarcoma
cells
To investigate apoptosis mechanism of Honokiol on
osteosarcoma cell, the authors firstly detected Bax, p53 and cyclin
D1 protein expression and caspase-3 activity in osteosarcoma cell
(MG-63) by honokiol for 48 h. In Fig.
4, 10 and 20 µg/ml honokiol significantly induced bax and p53,
and significantly inhbited cyclin D1 protein expression levels
compared with the control. Honokiol treatment led to increased
caspase-3 activity of MG-63 cells, compared with the control group
(0 µg/ml group).
Honokiol promoted LC3, PI3K, p-Akt and
p-mTOR protein expression of osteosarcoma cells
In order to test the role of autophagy in the
anticancer effects of honokiol on osteosarcoma cells, LC3II protein
expression of MG-63 cell was measured. Western blotting revealed
that 10 and 20 µg/ml honokiol significantly promoted LC3 protein
expression of MG-63 cell, compared with the control group (0 µg/ml
group; Fig. 5A and B). The authors
then examined the autophagy mechanism of anticancer effects of
honokiol on osteosarcoma cells. As presented in Fig. 5A and C-E, PI3K, p-Akt and p-mTOR
protein expression levels of MG-63 cells was significantly
suppressed by 10 and 20 µg/ml honokiol in MG-63 cells, compared
with the control group (0 µg/ml group).
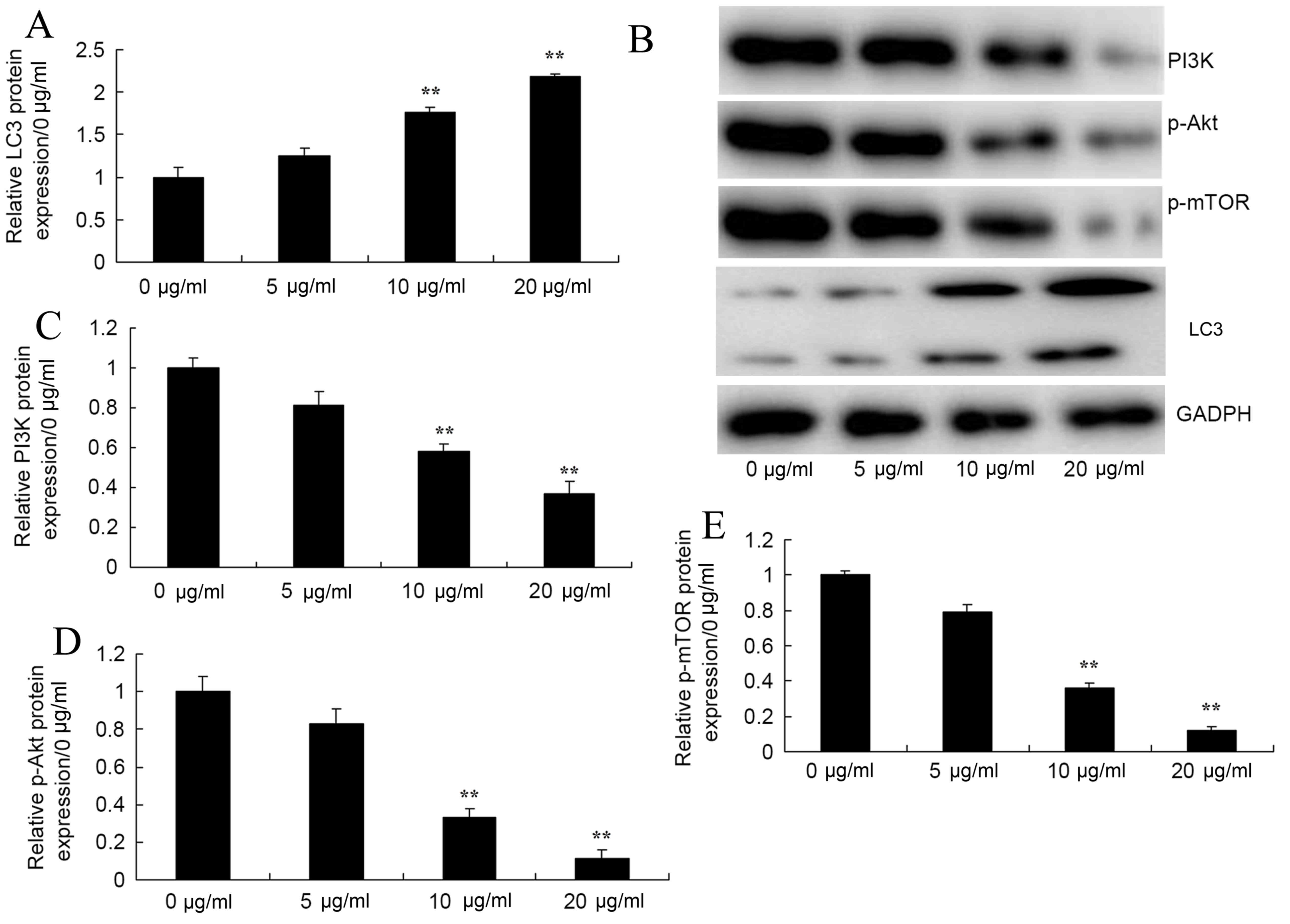 | Figure 5.Honokiol promoted LC3, PI3K, p-Akt and
p-mTOR protein expression of osteosarcoma cells. Honokiol induced
(A and B) LC3, (B and C) PI3K, (B and D) p-Akt and (B and E) p-mTOR
protein expression of osteosarcoma cells by western blotting and
statistical analysis. 0 µg/ml, 0 µg/ml honokiol; 5 µg/ml, 5 µg/ml
honokiol; 10 µg/ml, 10 µg/ml honokiol; 20 µg/ml, 20 µg/ml honokiol.
**P<0.01 vs. 0 µg/ml honokiol group. PI3K, phosphoinositide
3-kinase; p-Akt, phosphorylated protein kinase B; p-mTOR,
phosphorylated mammalian target of rapamycin. |
Discussion
Osteosarcoma is the bone malignancy most commonly
occurring in adolescents (15).
Neoadjuvant chemotherapy significantly improves the survival rate
of patients with osteosarcoma, and reduces the rate of recurrence
and metastasis, but it has a poor chemotherapy effect in some
patients, easy leading to recurrence and metastasis (15,16).
Therefore, revealing the mechanism of the incidence and development
of osteosarcoma from a new perspective to fundamentally cure tumor
has always been the focus and challenge in the study of orthopedics
(16). The present study
demonstrated that honokiol significantly inhibited cell
proliferation and induced apoptotic rate of MG-63 cells.
Cell autophagy removes excessive and harmful
proteins or organelles with long half-life from cells, to protect
the components of cells and provide raw materials for the
reconstruction, regeneration and repair of cells, thus realizing
recycle and reuse of components in cells (17). Cell autophagy is closely associated
with cancers through multiple signaling pathways, such as the
PI3K/Akt/mTOR signaling pathway, the LKB1/AMPK/mTOR signaling
pathway, the p53 signaling pathway and the BCL-2 pathway, as well
as endoplasmic reticulum stress (18). In the present study, honokiol
significantly induced Bax and p53 protein expression, increased
caspase-3 activity and suppressed cyclin D1 of MG-63 cells.
Cell autophagy removes excessive and harmful
proteins or organelles with long half-life from cells to protect
the components of cells (5). Under
normal circumstances, autophagy is conducive to the survival of
cells, so the dysfunction of autophagy causes many diseases, such
as neurodegenerative diseases, bacterial infection, intestinal
inflammation, aging and cancers (19,20).
In normal cells, autophagy pathways are regulated by the signaling
network, which centers on mTOR (21). Nutrients and growth factors can
activate the PI3K/Akt/mTOR signaling pathway, thereby inhibiting
the activity of the autophagy pathway, to promote the growth and
proliferation of cells (22). When
cells are lacking in nutrients and growth factors, or under the
condition of hypoxia and other stresses, PI3K/Akt/m TOR signaling
pathways will be inhibited and thus, the autophagy pathway be
activated, inhibiting cell growth and proliferation (23). The autophagy pathway basically
serves the same role in tumor cells as it does in normal cells,
only the PI3K/Akt/mTOR signaling pathway is damaged and interacts
with other signaling pathways, which makes the autophagy pathway in
tumor cells more complex (24).
The current study demonstrated that honokiol significantly promoted
LC3 protein expression of MG-63 cells. Lv et al (19) revealed that honokiol increased the
expression of p62 and LC3 I in the A549 and H460 cells.
The PI3K-AKT pathway is improperly activated in
various types of cancer. PI3K-AKT can be activated through two
major mechanisms, such as the activation of specific nodes in
pathway and the activation of receptor tyrosine kinase, so
understanding the activation mechanisms of PI3K is vital for the
development of effective therapies and PI3K inhibitors (10,18).
It is often reported that the PI3K/Akt/mTOR signaling pathway works
abnormally in cancer cells, and even being constitutively
activated, which promotes the growth and proliferation of tumor
cells (18). This may be due to
one or more cellular events closely related to the incidence and
development of cancers, such as mutation or deletion of PTEN,
mutation of TSC1/2, tyrosine kinase receptor growth factor and type
I PI3K, overexpression of Akt, inhibition of mTOR, exposure to
carcinogens, which may lead to the activation of PI3K/Akt/mTOR,
thereby inhibiting the autophagy of cells (25,26).
The results demonstrated that honokiol significantly suppressed
PI3K, p-Akt and p-mTOR protein expression of MG-63 cell. Lin et
al (27) reported that
honokiol induces autophagic cell death via regulation of the
p53/PI3K/Akt/mTOR signaling pathway in malignant glioma (27).
In summary, the present study demonstrates, honokiol
significantly inhibited cell proliferation, induced apoptotic rate,
and induced Bax and p53 protein expression, increased caspase-3
activity and suppressed cyclin D1 of MG-63 cells, which may induces
autophagy through the PI3K/Akt/mTOR signaling pathway. In summary,
the current study indicated that honokiol may be a promising
strategy for osteosarcoma therapy or clinical applications.
Acknowledgements
The present study was supported by the Natural
Science Foundation Research Project of Shaanxi Province (grant no.
2014JM4120).
References
|
1
|
Nataraj V, Batra A, Rastogi S, Khan SA,
Sharma MC, Vishnubhatla S and Bakhshi S: Developing a prognostic
model for patients with localized osteosarcoma treated with uniform
chemotherapy protocol without high dose methotrexate: A
single-center experience of 237 patients. J Surg Oncol.
112:662–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tai BC, Machin D, White I and Gebski V;
EOI (The European Osteosarcoma Intergroup), : Competing risks
analysis of patients with osteosarcoma: A comparison of four
different approaches. Stat Med. 20:661–684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
London CA, Gardner HL, Mathie T, Stingle
N, Portela R, Pennell ML, Clifford CA, Rosenberg MP, Vail DM,
Williams LE, et al: Impact of toceranib/piroxicam/cyclophosphamide
maintenance therapy on outcome of dogs with appendicular
osteosarcoma following amputation and carboplatin chemotherapy: A
multi-institutional study. PLoS One. 10:e01248892015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li L, Li Y, Zhao J, Fan S, Wang L and Li
X: CX-5461 induces autophagy and inhibits tumor growth via
mammalian target of rapamycin-related signaling pathways in
osteosarcoma. Onco Targets Ther. 9:5985–5997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Q, Ou YS, Tao Y, Yin H and Tu PH:
Apoptosis and autophagy induced by pyropheophorbide-a methyl
ester-mediated photodynamic therapy in human osteosarcoma MG-63
cells. Apoptosis. 21:749–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma K, Zhang C, Huang MY, Li WY and Hu GQ:
Cinobufagin induces autophagy-mediated cell death in human
osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway.
Oncol Rep. 36:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang B, Yu X and Xia H: The flavonoid
luteolin enhances doxorubicin-induced autophagy in human
osteosarcoma U2OS cells. Int J Clin Exp Med. 8:15190–15197.
2015.PubMed/NCBI
|
|
8
|
Zhou J, Wu S, Chen Y, Zhao J, Zhang K,
Wang J and Chen S: microRNA-143 is associated with the survival of
ALDH1+CD133+ osteosarcoma cells and the chemoresistance of
osteosarcoma. Exp Biol Med (Maywood). 240:867–875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu K, Dai HB and Qiu ZL: mTOR signaling in
osteosarcoma: Oncogenesis and therapeutic aspects (Review). Oncol
Rep. 36:1219–1225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Sun Y, Wu Y and Zhang J:
Cucurbitacin E inhibits osteosarcoma cells proliferation and
invasion through attenuation of PI3K/AKT/mTOR signaling. Biosci
Rep. pii:BSR201601652016.
|
|
12
|
Averett C, Bhardwaj A, Arora S, Srivastava
SK, Khan MA, Ahmad A, Singh S, Carter JE, Khushman M and Singh AP:
Honokiol suppresses pancreatic tumor growth, metastasis and
desmoplasia by interfering with tumor-stromal cross-talk.
Carcinogenesis. 37:1052–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herrmann D, Schreiber A, Ciotkowska A,
Strittmatter F, Waidelich R, Stief CG, Gratzke C and Hennenberg M:
Honokiol, a constituent of Magnolia species, inhibits adrenergic
contraction of human prostate strips and induces stromal cell
death. Prostate Int. 2:140–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crane C, Panner A, Pieper RO, Arbiser J
and Parsa AT: Honokiol-mediated inhibition of PI3K/mTOR pathway: A
potential strategy to overcome immunoresistance in glioma, breast,
and prostate carcinoma without impacting T cell function. J
Immunother. 32:585–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Felgenhauer JL, Nieder ML, Krailo MD,
Bernstein ML, Henry DW, Malkin D, Baruchel S, Chuba PJ, Sailer SL,
Brown K, et al: A pilot study of low-dose anti-angiogenic
chemotherapy in combination with standard multiagent chemotherapy
for patients with newly diagnosed metastatic Ewing sarcoma family
of tumors: A Children's Oncology Group (COG) Phase II study
NCT00061893. Pediatr Blood Cancer. 60:409–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Altaf S, Enders F, Jeavons E, Krailo M,
Barkauskas DA, Meyers P and Arndt C: High-BMI at diagnosis is
associated with inferior survival in patients with osteosarcoma: A
report from the children's oncology group. Pediatr Blood Cancer.
60:2042–2046. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang C, Shogren KL, Goyal R, Bravo D,
Yaszemski MJ and Maran A: RNA-dependent protein kinase is essential
for 2-methoxyestradiol-induced autophagy in osteosarcoma cells.
PLoS One. 8:e594062013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH,
He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, et al: Pro-apoptotic and
pro-autophagic effects of the Aurora kinase A inhibitor alisertib
(MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the
activation of mitochondria-mediated pathway and inhibition of p38
MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther.
9:1555–1584. 2015.PubMed/NCBI
|
|
19
|
Lv X, Liu F, Shang Y and Chen SZ: Honokiol
exhibits enhanced antitumor effects with chloroquine by inducing
cell death and inhibiting autophagy in human non-small cell lung
cancer cells. Oncol Rep. 34:1289–1300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Guo M, Chen JH, Wang Z, Du XF,
Liu PX and Li WH: Osteopontin knockdown inhibits av, b3
integrin-induced cell migration and invasion and promotes apoptosis
of breast cancer cells by inducing autophagy and inactivating the
PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 33:991–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Liu Y, Shi F, Cheng L and She J:
Knockdown of Rap1b enhances apoptosis and autophagy in gastric
cancer cells via the PI3K/Akt/mTOR pathway. Oncol Res. 24:287–293.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai H, Li H, Li W, Gui T, Yang J, Cao D
and Shen K: The PI3K/AKT/mTOR pathway is a potential predictor of
distinct invasive and migratory capacities in human ovarian cancer
cell lines. Oncotarget. 6:25520–25532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roy R, Singh SK, Chauhan LK, Das M,
Tripathi A and Dwivedi PD: Zinc oxide nanoparticles induce
apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition.
Toxicol Lett. 227:29–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar D, Das B, Sen R, Kundu P, Manna A,
Sarkar A, Chowdhury C, Chatterjee M and Das P: Andrographolide
analogue induces apoptosis and autophagy mediated cell death in
U937 cells by inhibition of PI3K/Akt/mTOR pathway. PLoS One.
10:e01396572015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xishan Z: Peer review report 1 on ‘P53
suppresses cell proliferation, metastasis, and angiogenesis of
osteosarcoma through inhibition of the PI3K/AKT/mTOR pathway’. Int
J Surg. 13 Suppl 1:S322015. View Article : Google Scholar
|
|
26
|
Liu Y, Bi T, Dai W, Wang G, Qian L, Shen G
and Gao Q: Lupeol induces apoptosis and cell cycle arrest of human
osteosarcoma cells through PI3K/AKT/mTOR pathway. Technol Cancer
Res Treat. 15:NP16–NP24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh
MH, Lin YW and Chen RM: Honokiol induces autophagic cell death in
malignant glioma through reactive oxygen species-mediated
regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl
Pharmacol. 304:59–69. 2016. View Article : Google Scholar : PubMed/NCBI
|