Introduction
The reduction in lens transparency caused by
cataracts induces severe visual impairment (1), which can seriously effect patients'
quality of life. Currently, cataract-induced blindness accounts for
~50% of blindness cases worldwide (2). It is well-recognized that damage
resulting from oxidative stress in the ocular lens is a major cause
of cataracts (3). The lens is a
transparent organ that consists of a single layer of epithelial
cells, which are the main target of oxidative stress (4). Oxidative stress caused by exposure to
hydrogen peroxide (H2O2) results in DNA
damage, and impairs the function of cells and tissues (5). H2O2, which is a
non-free radical member of the active oxygen family, generates
hydroxyl radicals that irreversibly damage the lens epithelium,
resulting in cell death and cataract formation (6). Some patients with cataracts have
markedly increased levels of H2O2 within
their lenses (7), indicating the
possible involvement of H2O2 in the genesis
of nuclear cataracts in humans. Therefore, a better understanding
of the mechanisms underlying H2O2-induced
apoptosis of lens cells may provide information regarding the cause
of cataract formation.
ELL-associated factor 2 (Eaf2) is a protein that was
discovered based on its ability to bind with a second protein
(eleven-nineteen lysine-rich leukemia protein; ELL), which is
independently coded by a gene that is upregulated by androgen in
the rat prostate gland and is also named human U19 (8). As a regulator of transcription, Eaf2
interacts with ELL to efficiently stimulate the extension activity
of RNA polymerase II (9). It has
previously been suggested that Eaf2 serves as a tumor suppressor in
prostate cancer (10).
Furthermore, knockdown of Eaf2 has been reported to promote
tumorigenesis in mouse models of adenocarcinoma and hepatocellular
carcinoma (11). In addition to
its role in cancer, Eaf2 serves important roles in embryonic
development via its involvement in non-canonical Wnt signaling
(12,13), which is thought to be essential for
convergence and extension movements, as well as the midline
convergence of organ precursors (14,15).
Liu et al (16)
demonstrated that Eaf2 acts as an upstream modulator of
non-canonical Wnt signaling to mediate convergence and extension
movements. In addition, the Wnt family of secreted signaling
proteins has important roles in organogenesis, tissue homeostasis
and tumor formation (17).
Overexpression of Wnt3a has been reported to promote the
proliferation of human lens epithelial (HLE) cells (18). Furthermore, Eaf2 is required for
normal eye development and the regulation of crystalline lens
development and maturation in Xenopus laevis (12,13).
Recently, a related study identified an important role for Eaf2 in
ultraviolet-induced cataract formation (19). However, the mechanism underlying
the effects of Eaf2 on lenses undergoing oxidative stress remains
unknown.
The present study aimed to investigate the role of
Eaf2 in HLE cells undergoing H2O2-induced
apoptosis, and to determine the underlying molecular mechanism. The
results indicated that in HLE cells, Eaf2 protects against
H2O2-induced cell death by inhibiting caspase
3 enzymatic activity and activating the Wnt3 signaling pathway.
Materials and methods
Cell culture
HLE-B3 cells were purchased from the Cell Bank of
the Chinese Academy of Science (Shanghai, China). HLE-B3 cells were
cultured in minimum essential medium (MEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere containing 5% CO2 at 37°C.
Induction of apoptosis in HLE-B3
cells
H2O2-induced HLE-B3 cells used
in subsequent experiments were gradually deprived of serum via an
overnight culture in MEM containing 2% FBS, prior to being treated
with 50 µM H2O2 for various time periods (4,
8 and 12 h) at 37°C.
Vector construction and cell
transfection
To induce Eaf2 overexpression, the full-length cDNA
(NC_000003.12) for the human Eaf2 gene was obtained from the
total RNA of human HLE-B3 cells isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
cloned into a pcDNA3.0 vector (Invitrogen; Thermo Fisher
Scientific, Inc.) to generate pcDNA-Eaf2, which was subsequently
designated as oeEaf2. The pcDNA3.0 empty plasmid was used as a
negative control, which was designated as oeCon. Vector
transfection was performed using Lipofectamine 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol for 48 h. Cells overexpressing
Eaf2 also underwent Wnt3a knockdown. The Wnt3a
short hairpin (sh)RNA plasmid (5′-AGAAGUAUCCGAGUGGGGCCA-3′) used to
knockdown Wnt3a was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) and was designated as shWnt3a. Subsequently,
cells overexpressing Eaf2 were identified and transfected
with shWnt3a and control shRNA (shCon; 5′-ACGUGACACGUUCGGAGAATT-3′)
using Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol for 48 h. Cells were then cultured in
6-well plates at an inoculation density of 2×104
cells/well. The resulting constructs were confirmed using RT-qPCR
and western blot analysis, and were used for subsequent
experiments.
Lactate dehydrogenase (LDH) release
assay
To detect apoptosis, the release of LDH was assessed
using an LDH assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's protocol. This
method is used to measure the loss of membrane integrity by
quantifying the release of LDH into the medium.
Flow cytometric analysis of
apoptosis
Following exposure to the respective treatments,
HLE-B3 cells were washed with PBS and subjected to a PI/Annexin
V-FITC Apoptosis Detection kit (JingMei Biotech, Beijing, China):
Briefly, 5 µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide (PI) were added to the suspended cells, which were
gently vortexed and incubated for 15 min at room temperature in the
dark. Following incubation, the cells were analyzed by flow
cytometry (Accuri C6; BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometric analysis was performed in triplicate.
RNA isolation and RT-qPCR
analysis
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. cDNA was synthesized from the RNA
using SuperScript II RT (200 U/ml; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. β-actin
served as an endogenous control in the qPCR analysis. The following
primers were used: β-catenin, forward 5′-GCCACAAGATTACAAGAACGG-3′,
reverse 5′-TGGGCACCAATATCAAGTCC-3′; caspase 3, forward
5′-TGGTTCATCCAGTCGCTTTG-3′, reverse 5′-AATTCTGTTGCCACCTTTCG-3′;
Eaf2, forward 5′-AGGTGACCATAACTCTGCCAAAT-3′, reverse,
5′-AGCCGACATTCTCCAGTATCA-3′; Wnt3a, forward
5′-GTCCACGCCATTGCCTCAG-3′, reverse, 5′-GACACCATCCCACCAAACTCG-3′;
B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax), forward
5′-GCAAACTGGTGCTCAAGGC-3′, reverse 5′-GCACTCCCGCCACAAAGA-3′; and
Bcl-2, forward 5′-TGTGGCCTTCTTTGAGTTCG-3′ and reverse
5′-ATCCCAGCCTCCGTTATCC-3′; β-actin, forward:
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′, reverse:
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. The RT-qPCR analyses were performed
in triplicate on a Bio-Rad Connect Real-Time PCR platform (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using a 20 µl reaction
mixture containing 10 µl 2X SYBR Premix Ex Taq, 0.5 µl each primer
(2.5 µM), 5 µl cDNA and 4.0 µl ddH2O. The RT-qPCR
procedure was conducted as follows: Initial denaturation at 95°C
for 1 min, followed by 40 cycles of denaturation at 95°C for 5 sec
and annealing extension at 60°C for 20 sec, followed by 72°C for 20
sec. Human β-actin was used as an internal loading control for
RT-qPCR. The 2−ΔΔCq (20) method was used to determine the
relative mRNA expression levels of human β-catenin, caspase 3,
Eaf2, Wnt3a, Bcl-2 and Bax. These levels were normalized to those
of β-actin.
Western blot analysis
Treated HLE-B3 cells were harvested via
centrifugation (14,000 × g 4 min) at 4°C. Subsequently, the
pelleted cells were lysed in a 2X SDS lysis buffer containing 100
mM Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS and 10% glycine. The lysed
cells were then centrifuged at 15,000 × g for 15 min at 4°C, the
supernatant fractions were collected and protein concentrations
were assessed using the bicinchoninic acid protein assay.
Supernatant aliquots containing ~30 µg protein were separated by
12% SDS-PAGE. The proteins were then transferred onto
polyvinylidene fluoride membranes by electrophoresis at 300 mA for
2 h. The membranes were then blocked with 5% non-fat milk, and were
washed with TBST and probed overnight at 4°C with 1:1,000 dilutions
of primary antibodies, all from Cell Signaling Technology, Inc.,
Danvers, MA, USA: β-catenin (8480), GSK3e (12456), p-GSK3β (5558),
Bcl-2 (15071), Bax (5023), caspase 3 (9662), Eaf2 (14159), Wnt3a
(2721), GAPDH (5174). Subsequently, the blots were incubated with
the corresponding horseradish peroxidase-conjugated secondary
antibodies (Cell Signaling Technology, Inc.) for 45 min at 37°C.
Immunodetection was performed using the Super Enhanced
Chemiluminescence Detection reagent according to the manufacturer's
protocol (Thermo Fisher Scientific, Inc.).
Immunocytochemistry
Then, 24 h post-transfection, immunostaining was
performed as previously described (3). Briefly, the treated HLE-B3 cells were
fixed in 4% paraformaldehyde in PBS for 15 min at room temperature,
washed with PBS, and then permeabilized with 0.1% Triton X-100 in
PBS for 5 min. Subsequently, the cells were blocked for 1 h with
PBS containing 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature and were incubated with
primary antibodies (Eaf2; ab28357; Abcam and Wnt3a; ab28472; Abcam)
for 1 h at room temperature. The cells were then incubated with the
corresponding secondary antibodies (BM3894; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) for 1 h at room temperature. DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for
counterstaining. Images of the immunostained cells were captured
using a fluorescence microscope (VANOX-S; Olympus Corporation,
Tokyo, Japan).
Statistical analysis
All statistical data were analyzed using GraphPad
Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA),
and results are expressed as the mean ± standard deviation (n=3).
One-way analysis of variance was performed followed by post hoc
least significant difference test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Construction of an
H2O2-induced apoptotic HLE cell model
HLE-B3 cells were induced to enter apoptosis by
exposure to H2O2 for various time periods (4,
8 and 12 h). The apoptotic cells were detected by flow cytometry
(Fig. 1A). Determination of the
proportion of Annexin V- and PI-stained gated cells revealed that
the population contained cells in both early stage (Annexin
V+/PI−) and late stage (Annexin
V+/PI+) apoptosis. The statistical analysis
indicated that, when compared with the control cells, cell
populations exposed to 50 µM H2O2 for 4
(P<0.01), 8 (P<0.001) and 12 h (P<0.001) exhibited
increased apoptosis (Fig. 1B).
Similar patterns were also recorded in an LDH release assay
(Fig. 1C). To further investigate
the mechanism underlying H2O2-induced
apoptosis, the expression levels of caspase 3 and Eaf2 were
detected in HLE-B3 cells. The results indicated that the mRNA
(Fig. 1D; P<0.01) and protein
expression levels (Fig. 1E) of
caspase 3 were markedly increased in HLE-B3 cells exposed to
H2O2. Conversely, in HLE-B3 cells exposed to
H2O2, the mRNA (Fig. 1D; P<0.05) and protein expression
levels (Fig. 1E) of Eaf2 were
markedly downregulated, as determined by RT-qPCR and western blot
analysis. Therefore, it may be hypothesized that Eaf2 protects HLE
cells from entering apoptosis. H2O2 treatment
for 12 h increased the number of early and late stage apoptotic
cells by 6- and 12-fold, respectively, making those cells lose the
potential to recover viability during subsequent analysis.
Consequently, HLE-B3 cells that underwent
H2O2 treatment for 8 h were used in
subsequent experiments.
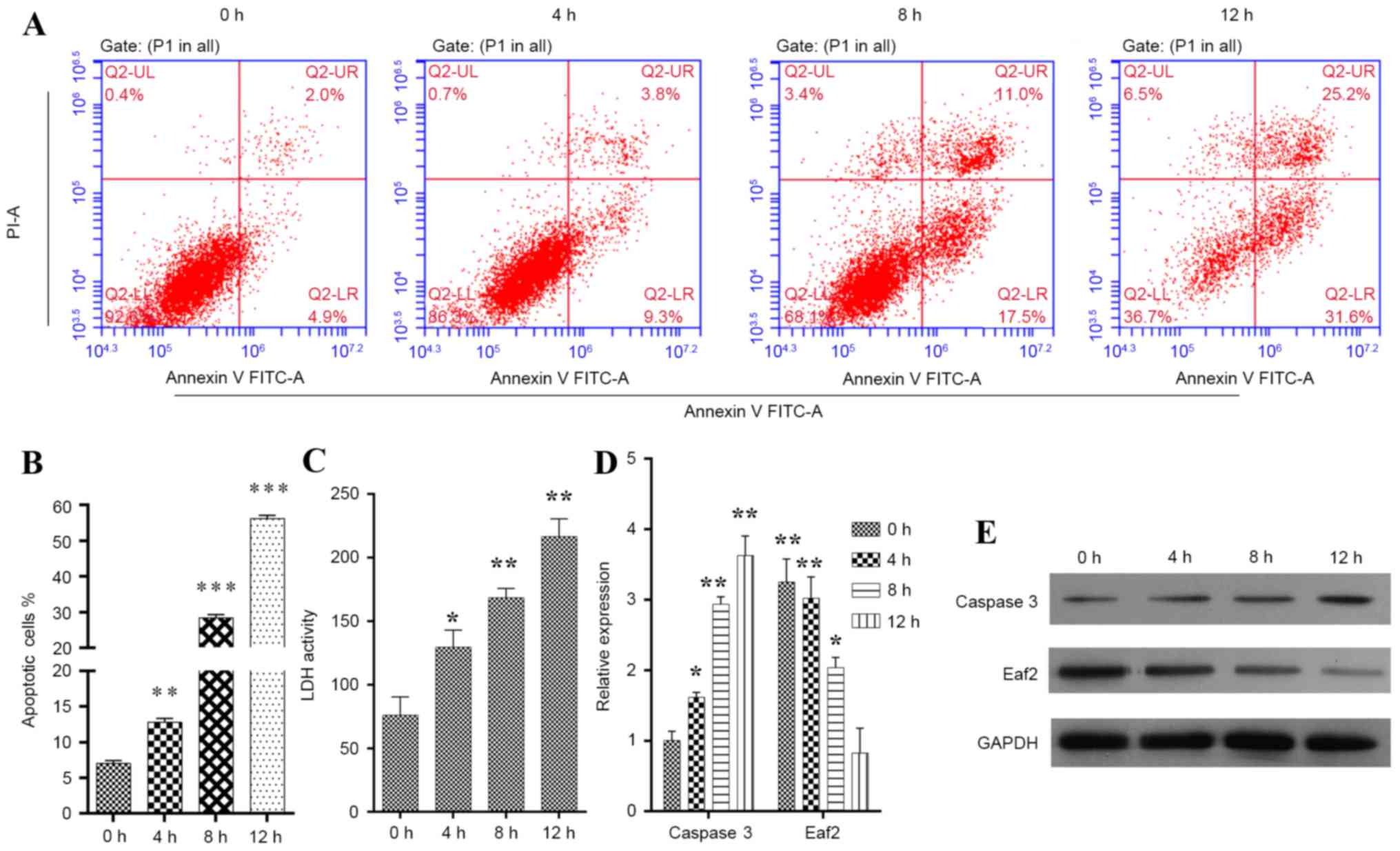 | Figure 1.Construction of the
H2O2-induced apoptotic HLE-B3 cell model. (A)
Representative images showing the time-dependent uptake of Annexin
V/PI by H2O2-induced HLE-B3 cells. (B)
Quantification of overall apoptotic cells in a population of HLE-B3
cells, as determined by flow cytometry. Annexin
V+/PI−, early apoptotic cells; Annexin
V+/PI+, late apoptotic cells. (C)
Quantification of LDH activity in a population of HLE-B3 cells. (D)
mRNA expression levels of caspase 3 and Eaf2 in
H2O2-induced HLE-B3 cells, as determined by
reverse transcription-quantitative polymerase chain reaction
analysis. (E) Protein expression levels of caspase 3 and Eaf2 in
H2O2-induced HLE-B3 cells, as determined by
western blot analysis. *P<0.05, **P<0.01, ***P<0.001 vs.
control. Eaf2, ELL-associated factor 2; FITC, fluorescein
isothiocyanate; H2O2, hydrogen peroxide; HLE,
human lens epithelial; LDH, lactate dehydrogenase; PI, propidium
iodide. |
Overexpression of Eaf2 alleviates
H2O2-induced apoptosis of HLE cells
To further investigate the effects of Eaf2 on
H2O2-induced HLE-B3 cells, cells were induced
to overexpress Eaf2 by transfection with a pcDNA-Eaf2
plasmid, after which various experiments were conducted (Figs. 2 and 3). Apoptosis was analyzed by Annexin V/PI
double staining (Fig. 2A); the
results indicated that a significantly lower percentage of cells
overexpressing Eaf2 had entered apoptosis (Fig. 2B). Overexpression of Eaf2
also decreased LDH activity (Fig.
2C), suggesting that the apoptotic process was suppressed. To
investigate whether Eaf2 improves HLE-B3 cell survival rates by
inhibiting apoptosis, the cellular levels of caspase 3 (a protease
marker for apoptotic cell death) were analyzed by RT-qPCR and
western blotting. The results demonstrated that, when compared with
the control cells, the mRNA (Fig.
2D; P<0.01) and protein expression levels (Fig. 2G) of caspase 3 were markedly
decreased in cells overexpressing Eaf2. Since it has been
reported that a hyperactive Wnt pathway serves a role in early eye
development (21), the present
study examined the expression levels of β-catenin, phosphorylated
(p)-GSK3β and Wnt3a expression in HLE-B3 cells overexpressing
Eaf2. As presented in Fig.
2D-G, cells overexpressing Eaf2 exhibited elevated
expression levels of β-catenin, p-GSK3β, Wnt3a and Bcl-2 while
decreased the expression of Bax, thus suggesting a close
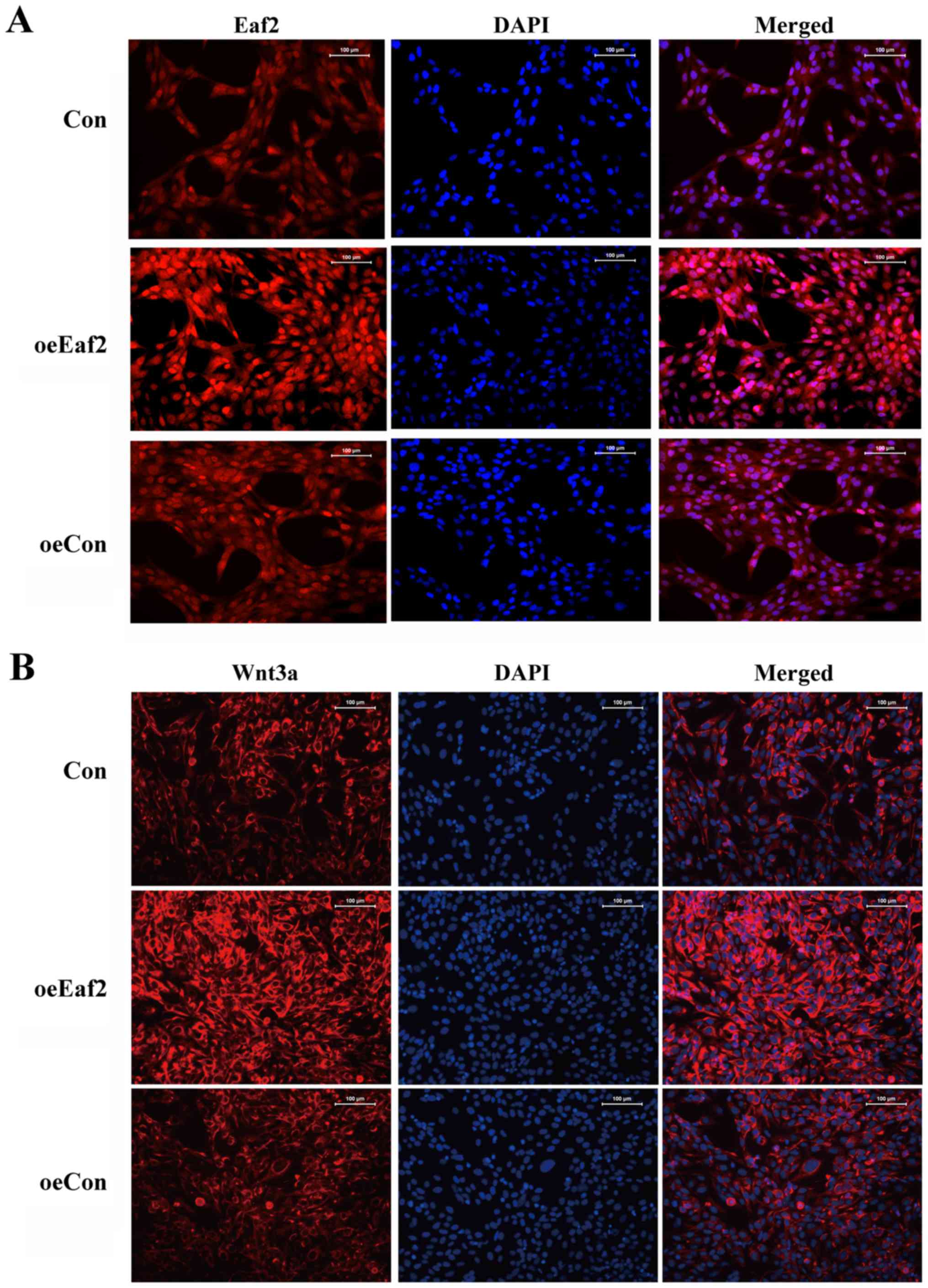
association between Eaf2 and Wnt3a signaling. An immunofluorescence
assay further confirmed the upregulation of Wnt3a in
Eaf2-overexpressing cells compared with the control cells (Fig. 3).
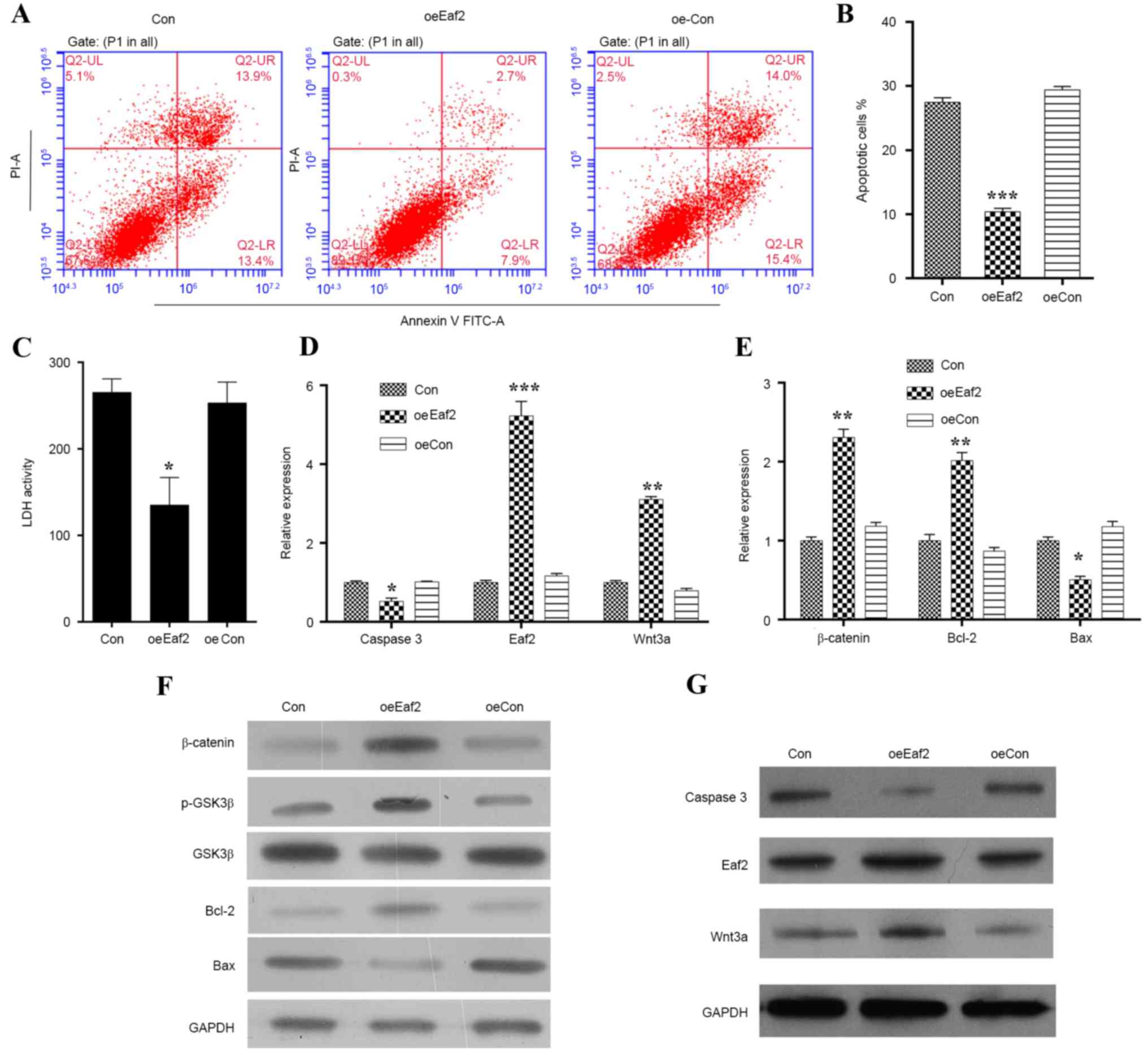 | Figure 2.Effects of Eaf2 overexpression
on H2O2-induced apoptosis of HLE-B3 cells.
(A) Representative images of Annexin V/PI uptake by
H2O2-induced HLE-B3 cells following Eaf2
overexpression, as determined by flow cytometry. (B) Quantification
of apoptotic cells in H2O2-induced HLE-B3
cells overexpressing Eaf2. (C) Quantification of LDH
activity in H2O2-induced HLE-B3 cells
overexpressing Eaf2. Reverse transcription-quantitative
polymerase chain reaction indicating the effects of Eaf2
overexpression on the mRNA expression levels of (D) caspase 3,
Wnt3a and Eaf2, and (E) β-catenin, Bcl-2 and Bax in
H2O2-treated HLE-B3 cells. Western blot
analysis indicating the effects of Eaf2 overexpression on
the protein expression levels of (F) β-catenin, p-GSK3β, GSK3β,
Bcl-2 and Bax, and (G) Wnt3a, Eaf2 and caspase 3 in
H2O2-treated HLE-B3 cells. *P<0.05,
**P<0.01, ***P<0.001 vs. Con or oeCon. Con represents the
control group consisting of normal HLE-B3 cells; oeCon represents
the negative control group consisting of HLE-B3 cells transfected
with empty pcDNA3.0 plasmid; oeEaf2 represents the experimental
group consisting of HLE-B3 cells transfected with pcDNA3.0-Eaf2.
Bax, B-cell lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma
2; GSK3β, glycogen synthase kinase 3β; Eaf2, ELL-associated factor
2; FITC, fluorescein isothiocyanate; H2O2,
hydrogen peroxide; HLE, human lens epithelial; LDH, lactate
dehydrogenase; p, phosphorylated; PI, propidium iodide. |
Eaf2 inhibits
H2O2-induced apoptosis in HLE cells by
regulating Wnt3a
To further investigate whether Eaf2 inhibits
apoptosis of HLE-B3 cells by affecting Wnt3a signaling,
Wnt3a expression was specifically knocked down in cells
overexpressing Eaf2, and the cells were then examined by
Annexin V/PI double staining (Fig.
4A). A statistical analysis indicated that the knockdown of
Wnt3a significantly increased the overall percentage of
Eaf2-overexpressing HLE-B3 cells undergoing apoptosis
(Fig. 4B; P<0.05). In addition,
knockdown of Wnt3a reversed the Eaf2
overexpression-induced suppression of LDH release, thus suggesting
a key role for Wnt3a signaling in the function of Eaf2: That the
role of Eaf2 in HLE-B3 cells depended on the activation of Wnt3a
(Fig. 4C). Furthermore, the
results suggested that Wnt3a knockdown promoted cell
apoptosis by increasing the expression levels of caspase 3 and Bax
while decreasing the expressions of β-catenin, p-GSK3β, Bcl-2 and
Wnt3a (Fig. 4D and G). As
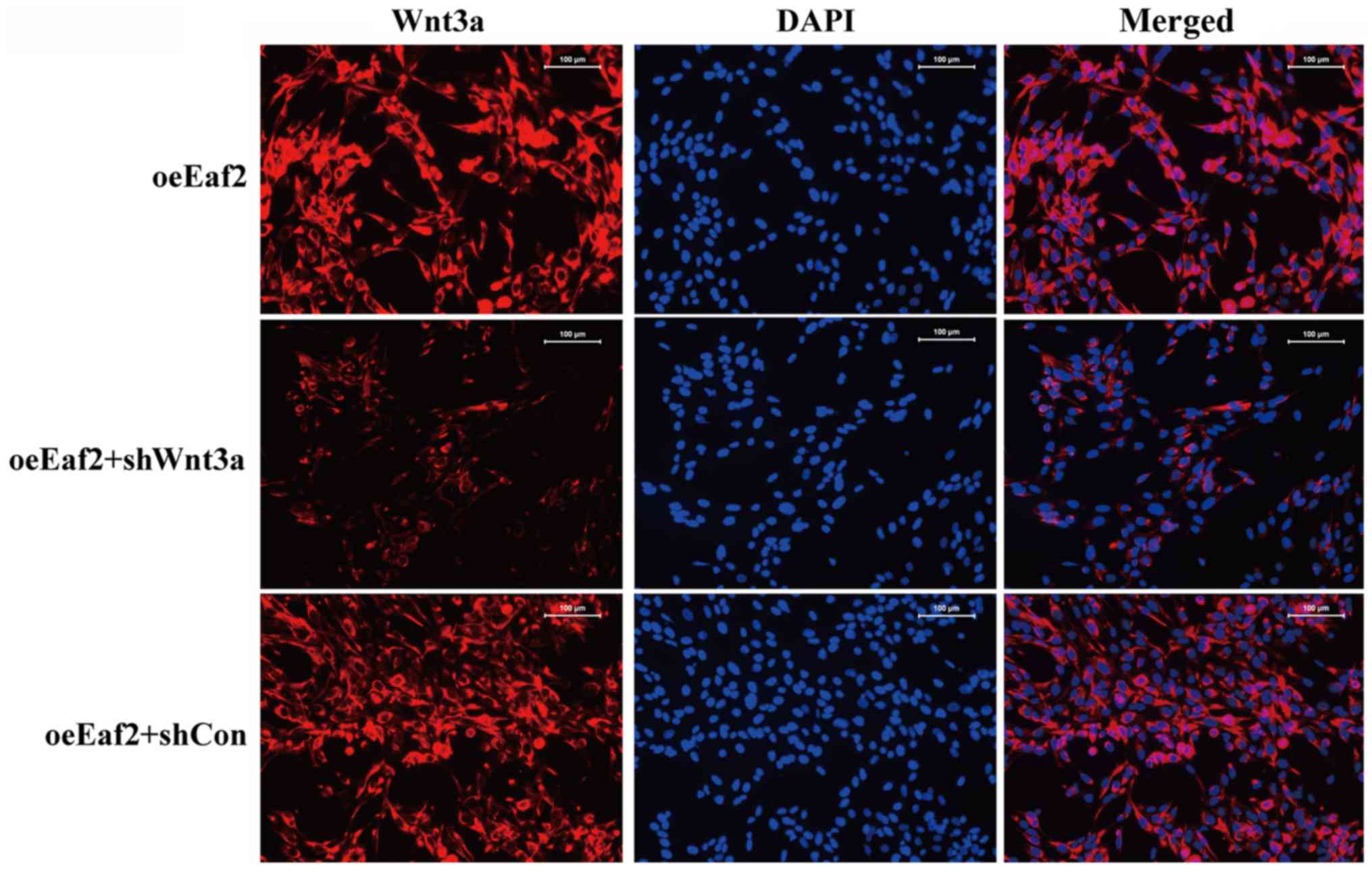
presented in Fig. 5, an
immunofluorescence analysis of Wnt3a expression was conducted in
the various cell groups and the results showed that suppressed
expression and distribution of Wnt3a was achieved in HLE-B3
cells.
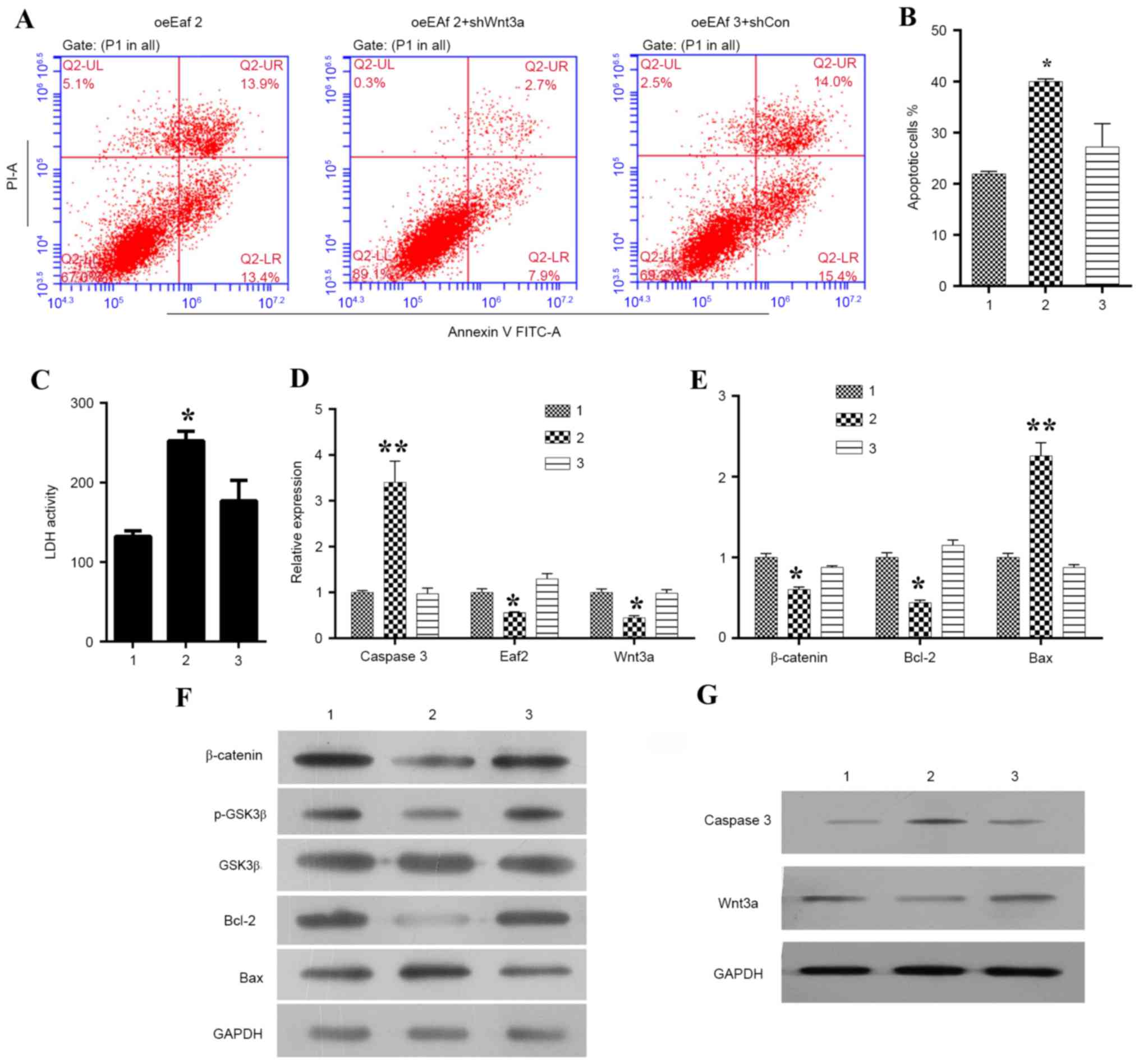 | Figure 4.Effects of Wnt3a silencing on Eaf2
expression in HLE-B3 cells. (A) Representative images of Annexin
V/PI uptake in Eaf2-overexpressing HLE-B3 cells following Wnt3a
knockdown. (B) Quantification of apoptotic cells in
Eaf2-overexpressing HLE-B3 cells following Wnt3a knockdown.
(C) Quantification of LDH activity in Eaf2-overexpressing
HLE-B3 cells following Wnt3a knockdown. Reverse
transcription-quantitative polymerase chain reaction was conducted
to determine the effects of Wnt3a silencing on the mRNA expression
levels of (D) caspase 3, Eaf2 and Wnt3a, and (E) β-catenin, Bcl-2
and Bax in HLE-B3 cells overexpressing Eaf2. Western blot analysis
was conducted to determine the effects of Wnt3a silencing on
the protein expression levels of (F) β-catenin, p-GSK3β, GSK3β,
Bcl-2 and Bax, and (G) caspase 3 and Wnt3a in HLE-B3 cells
overexpressing Eaf2. *P<0.05, **P<0.01 vs. control. Group 1
represents the oeEaf2 group consisting of HLE-B3 cells
overexpressing Eaf2; group 2 represents the oeEaf2 + shWnt3a group
consisting of Eaf2-overexpressing cells transfected with Wnt3a
shRNA; group 3 represents the oeEaf2 + shCon group consisting of
Eaf2-overexpressing cells transfected with control shRNA. Bax,
B-cell lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma 2;
GSK3β, glycogen synthase kinase 3β; Eaf2, ELL-associated factor 2;
FITC, fluorescein isothiocyanate; HLE, human lens epithelial; LDH,
lactate dehydrogenase; p, phosphorylated; PI, propidium iodide;
shRNA, short hairpin RNA. |
Discussion
Oxidative stress is a major cause of cancer and cell
death, and is thought to serve a major role in cataract formation
(22). Previous studies have
demonstrated that H2O2-induced oxidative
stress can stimulate apoptosis of HLE cells, which form a single
layer in the ocular lens (3,5,23).
Furthermore, this type of cellular stress may be considered the
initiating factor for non-congenital cataract formation (24). In the present study, the role of
the tumor suppressor Eaf2 was determined in
H2O2-induced apoptosis of lens cells. The
results of the in vitro study suggested that Eaf2 protects
HLE cells from H2O2-induced apoptosis by
regulating the expression levels of apoptosis-associated proteins.
This conclusion was based on an analysis of data obtained from loss
and gain-of-function experiments. Cellular apoptosis is an
important physiological and pathological process, which results in
the destruction of cell membranes and condensation of chromosomes
(25). Furthermore, the activation
of caspase proteases is regarded as the central mechanism of
apoptosis. The levels of caspase 3 can be used to accurately
reflect the levels of apoptosis in a cell population, particularly
the levels of early stage apoptosis (26). Meanwhile, Bax and Bcl-2 expression
serve as key roles in cell apoptosis. The present study observed
that HLE cells began undergoing apoptosis in a time-dependent
manner following exposure to H2O2.
Furthermore, their entry into apoptosis was accompanied by the
activation of caspase 3 and Bax, and suppression of Bcl-2. In HLE
cells, oxidative stress leads to apoptosis, which is a common
cellular mechanism underlying cataract formation (27).
It has previously been reported that Eaf2 is
spatially regulated in the lenses of embryonic mice (12). Another study demonstrated that Eaf2
is highly enriched in the developing eye, and is essential for
normal eye development (13). In
the present study, gradually decreasing levels of Eaf2 expression
were detected over time in HLE cells undergoing
H2O2-induced apoptosis. A further analysis
indicated that in HLE cells overexpressing Eaf2, the rate of
H2O2-induced apoptosis was decreased, thus
suggesting that it protects HLE cells against oxidative
stress-induced apoptosis.
Overexpression of Eaf2 resulted in a marked
increase in the expression levels of Wnt3a in
H2O2-induced cells. Conversely, knockdown of
Wnt3a decreased Eaf2 expression, and thereby promoted
apoptosis of HLE cells. Wnt3a signaling is reportedly involved in
the regulation of ocular cell proliferation and eye development
(21,28). A previous study demonstrated that
Wnt3a overexpression promotes the proliferation of HLE-B3
cells by increasing the percentage of cells in S phase (18). Furthermore, the loss of Eaf2
function has been reported to result in a loss of eye function, and
the loss of Wnt-4 function can be reversed by Eaf2 (13). Based on these findings, it may be
concluded that Eaf2 suppresses H2O2-induced
apoptosis via its effects on Wnt3a signaling. In addition,
immunocytochemistry studies also revealed that Eaf2 can influence
the expression of Wnt3a, and affect Wnt3a expression levels. This
finding may further explain how Eaf2 regulates the percentage of
lens cells undergoing H2O2-induced
apoptosis.
In conclusion, to the best of our knowledge, these
data are the first to demonstrate that Eaf2 gene
transcription products can protect HLE cells from oxidative damage
caused by exposure to H2O2. In addition, the
results of the present study demonstrated that when overexpressed,
Eaf2 can reduce H2O2-induced cell death by
decreasing the expression levels of caspase 3 and Bax, and
increasing Wnt3a and Bcl-2 expression. These results provide a
theoretical foundation for the development of novel drugs for the
treatment of cataracts. The present study focused on cell apoptosis
and the suppressive effects of Eaf2 on apoptosis; however, further
studies are required to explain how Eaf2 affects apoptosis.
References
|
1
|
Wormstone IM, Collison DJ, Hansom SP and
Duncan G: A focus on the human lens in vitro. Environ Toxicol
Pharmacol. 21:215–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pascolini D and Mariotti SP: Global
estimates of visual impairment: 2010. Br J Ophthalmol. 96:614–618.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Wang D, Li Y and Zhang X:
Protective effects of verapamil against H2O2-induced apoptosis in
human lens epithelial cells. Biomol Ther (Seoul). 22:553–557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ottonello S, Foroni C, Carta A, Petrucco S
and Maraini G: Oxidative stress and age-related cataract.
Ophthalmologica. 214:78–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seomun Y, Kim JT, Kim HS, Park JY and Joo
CK: Induction of p21Cip1-mediated G2/M arrest in H2O2-treated lens
epithelial cells. Mol Vis. 11:764–774. 2005.PubMed/NCBI
|
|
6
|
Spector A: Oxidative stress-induced
cataract: Mechanism of action. FASEB J. 9:1173–1182.
1995.PubMed/NCBI
|
|
7
|
Spector A and Garner WH: Hydrogen peroxide
and human cataract. Exp Eye Res. 33:673–681. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simone F, Luo RT, Polak PE, Kaberlein JJ
and Thirman MJ: ELL-associated factor 2 (EAF2), a functional
homolog of EAF1 with alternative ELL binding properties. Blood.
101:2355–2362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong SE, Banks CA, Shilatifard A, Conaway
JW and Conaway RC: ELL-associated factors 1 and 2 are positive
regulators of RNA polymerase II elongation factor ELL. Proc Natl
Acad Sci USA. 102:pp. 10094–10098. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao W, Zhang Q, Jiang F, Pins M,
Kozlowski JM and Wang Z: Suppression of prostate tumor growth by
U19, a novel testosterone-regulated apoptosis inducer. Cancer Res.
63:4698–4704. 2003.PubMed/NCBI
|
|
11
|
Xiao W, Zhang Q, Habermacher G, Yang X,
Zhang AY, Cai X, Hahn J, Liu J, Pins M, Doglio L, et al: U19/Eaf2
knockout causes lung adenocarcinoma, B-cell lymphoma,
hepatocellular carcinoma and prostatic intraepithelial neoplasia.
Oncogene. 27:1536–1544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Wu X, Zhuang F, Jiang S, Jiang M and
Liu YH: Expression of murine ELL-associated factor 2 (Eaf2) is
developmentally regulated. Dev Dyn. 228:273–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maurus D, Héligon C, Bürger-Schwärzler A,
Brändli AW and Kühl M: Noncanonical Wnt-4 signaling and EAF2 are
required for eye development in Xenopus laevis. EMBO J.
24:1181–1191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heisenberg CP, Tada M, Rauch GJ, Saúde L,
Concha ML, Geisler R, Stemple DL, Smith JC and Wilson SW:
Silberblick/Wnt11 mediates convergent extension movements during
zebrafish gastrulation. Nature. 405:76–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kilian B, Mansukoski H, Barbosa FC, Ulrich
F, Tada M and Heisenberg CP: The role of Ppt/Wnt5 in regulating
cell shape and movement during zebrafish gastrulation. Mech Dev.
120:467–476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu JX, Hu B, Wang Y, Gui JF and Xiao W:
Zebrafish eaf1 and eaf2/u19 mediate effective convergence and
extension movements through the maintenance of wnt11 and wnt5
expression. J Biol Chem. 284:16679–16692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao XL, Song H, Chen Z and Tang X: Wnt3a
promotes epithelial-mesenchymal transition, migration, and
proliferation of lens epithelial cells. Mol Vis. 18:1983–1990.
2012.PubMed/NCBI
|
|
19
|
Jiang Y, Fu R, Zhao J, Wu D, Qiao G, Li R
and Zhang J: Effects of ELL-associated factor 2 on ultraviolet
radiation-induced cataract formation in mice. Mol Med Rep.
12:6605–6611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rasmussen JT, Deardorff MA, Tan C, Rao MS,
Klein PS and Vetter ML: Regulation of eye development by frizzled
signaling in Xenopus. Proc Natl Acad Sci USA. 98:pp. 3861–3866.
2001; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai J, Liu H, Zhou J and Huang K:
Selenoprotein R protects human lens epithelial cells against
d-Galactose-induced apoptosis by regulating oxidative stress and
endoplasmic reticulum stress. Int J Mol Sci. 17:2312016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao YW, Xiang H, Wang J, Korsmeyer S,
Reddan J and Li DW: Human bcl-2 gene attenuates the ability of
rabbit lens epithelial cells against H2O2-induced apoptosis through
down-regulation of the alpha B-crystallin gene. J Biol Chem.
276:43435–43445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takamura Y, Sugimoto Y, Kubo E, Takahashi
Y and Akagi Y: Immunohistochemical study of apoptosis of lens
epithelial cells in human and diabetic rat cataracts. Jpn J
Ophthalmol. 45:559–563. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blankenberg FG and Strauss HW: Recent
advances in the molecular imaging of programmed cell death: Part
I-pathophysiology and radiotracers. J Nucl Med. 53:1659–1662. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li WC, Kuszak JR, Dunn K, Wang RR, Ma W,
Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, et al: Lens
epithelial cell apoptosis appears to be a common cellular basis for
non-congenital cataract development in humans and animals. J Cell
Biol. 130:169–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shang YC, Wang SH, Xiong F, Zhao CP, Peng
FN, Feng SW, Li MS, Li Y and Zhang C: Wnt3a signaling promotes
proliferation, myogenic differentiation, and migration of rat bone
marrow mesenchymal stem cells. Acta Pharmacol Sin. 28:1761–1774.
2007. View Article : Google Scholar : PubMed/NCBI
|