Introduction
Proliferative vitreoretinopathy (PVR) is one of the
most important causes of blindness following surgery for retinal
detachment and in diabetic retinopathy (1). The formation of scar-like tissue
causes subsequent traction retinal detachment resulting in serious
vision complications (2). Retinal
pigment epithelial (RPE) cells have a central role in the early
stages of PVR induction and are modulated by a wide range of growth
factors, including transforming growth factor-β (TGF-β) and
epidermal growth factor (EGF) (3).
EGF and TGF-β signaling has been shown to be one of the most potent
inducers of epithelial-mesenchymal transition (EMT) in various
types of cancer and is associated with both invasion and nodal
metastasis (4,5). In addition, stimulation with EGF and
TGF-β triggers a variety of PVR-associated phenotypical changes and
proliferation of RPE cells (6,7).
Cigarette smoke (CS) is the single most important
environmental risk factor for age-related macular degeneration,
which is the major cause of legal blindness in developed countries
(8,9). Exposure to CS results in oxidative
stress and damage or modification to RPE cells (10). Oxidative stress can alter RPE cells
to express growth factors, including EGF, proteins of the
EGF-related growth factor family, and vascular endothelial growth
factor (VEGF) (11–13). Stimulation of lung cancer cells
with CS or hydrogen peroxide (H2O2) induces
aberrant phosphorylation of EGF receptor (EGFR) and activates
downstream signaling pathways (14). EGFR is expressed in various
different types of cancer including lung cancer and has a critical
role in aberrant cell proliferation and development of cancer
(15,16). The combination of EGF and TGF-β1
induces dramatic fibroblast-like morphologies and characteristics
of EMT in intestinal epithelium (17). CS-induced TGF-β1 release induces
downstream signaling via the Sma- and Mad-related family 2/3
(Smad2/3) cascade to promote EMT processes in human bronchial
epithelial cells (18,19). In addition, stimulation of human
lung cancer cells with TGF-β1 induces migration and invasion
through Smad2/3 and focal adhesion kinase (FAK)/Src proto-oncogene,
non-receptor tyrosine kinase (Src) signaling (20). However, it remains unclear whether
CS-induced abnormal EGFR activation has a critical role in the
migration of RPE cells and how TGF-β1, which is released by
CS-induced EGFR activation, transduces the signal needed to trigger
the EMT process in RPE cells.
Erlotinib, an EGFR tyrosine kinase inhibitor (TKI),
inhibits EGFR signaling, thereby impeding tumor proliferation
(21). EGFR TKI treatment also
inhibits TGF-β-induced cell motility (22). In addition, treatment with
erlotinib inhibits pathological neovascularization of the retina
and prevents posterior capsule opacification after cataract surgery
(23,24). Stimulation with various growth
factors including EGF, hepatocyte growth factor and keratinocyte
growth factor induces 12-lipoxygenase (12-LOX), leading to a marked
increase in 12(S)-hydroxyeicosatetraenoic acid (HETE). On the other
hand, baicalein, an inhibitor of 12-LOX, blocks EGF-dependent cell
growth and the production of 12(S)-HETE in rabbit corneal
epithelial cells (25,26). 12-LOX and its product HETE also has
an important role in retinal neovascularization by upregulating
production of VEGF (27). These
findings suggest that erlotinib and baicalein may have a beneficial
effect on preventing and treating abnormal EGFR-associated retinal
diseases by downregulating TGF-β1-mediated signaling in RPE cells.
Furthermore, the cellular mechanisms by which cigarette smoke
causes EMT in PVR through the regulation VEGF or TGF-β1 production
remain unclear.
In this study, the signaling pathways downstream of
CS extract (CSE)-induced abnormal EGFR activation that are involved
in promoting EMT were investigated using ARPE-19 human RPE cells.
Additionally, whether the EGF-like role of CSE induces EMT by
modulating VEGF or TGF-β production was examined. Finally, the
ability of erlotinib or baicalein to inhibit EMT processes was
investigated in CSE-stimulated RPE cells.
Materials and methods
Cell culture and reagents
Primary human RPE (HRPEpi) cells were purchased from
ScienCell Research Laboratories, Inc. (San Diego, CA, USA).
ARPE-19, a human RPE cell line, was purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
maintained in Dulbecco's modified Eagle's medium (DMEM)/F12
supplemented with 10% fetal bovine serum (FBS) (both from (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA) and antibiotics under
a humidified atmosphere with 5% CO2. Baicalein
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in
sterile dimethyl sulfoxide. PP1 (Src inhibitor) and Bay-61-3606
[spleen associated tyrosine kinase (Syk) inhibitor] were purchased
from Calbiochem (Merck KGaA, Darmstadt, Germany). Erlotinib (EGFR
TKI) was obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Recombinant EGF (rEGF) and recombinant VEGF (rVEGF) were
purchased from Tocris Biosciences (Bristol, UK). LY2109761 (dual
TGF-β receptor I and II kinase inhibitor) was purchased from
Selleck Chemicals (Houston, TX, USA).
Preparation of cigarette smoke
extracts
Research-grade cigarettes (3R4F) were obtained from
the Kentucky Tobacco Research Council (University of Kentucky,
Lexington, KY, USA). Each cigarette contained 0.726 mg nicotine,
9.4 mg tar, and 10.9 mg total particulate matter. CSE consisted of
an extract of mainstream CS. Briefly, smoke was bubbled through 25
ml DMEM/F12 medium without FBS for 2 min and the resulting solution
was used as a stock (100%) and diluted further. The pH was adjusted
to 7.2, the obtained CSE was filtered through a 0.22-µm pore filter
(EMD Millipore, Billerica, MA, USA) for sterilization. CSE was
standardized by measuring the absorbance at a wavelength of 320 nm
and was used within 30 min after preparation (19). ARPE-19 cells were seeded into a
6-well plate at a density of 2×105 cells/2 ml in
DMEM/F12 containing 10% FBS. Following overnight incubation at
37°C, the cells were either treated with rEGF (100 ng/ml) or
exposed to 5% CSE for 4 h at 37°C, and then incubated with complete
medium for an additional 24 h at 37°C. To analyze the effect of
VEGF, ARPE-19 cells were treated with either 50 ng/ml rVEGF or 5%
CSE alone, or co-treated (50 ng/ml rVEGF + 5% CSE) for 4 h at 37°C,
subsequently washed out, and then incubated with complete medium
for an additional 24 h at 37°C. To analyze the effect of EGFR and
TGF-β blocking, ARPE19 cells were exposed to 5% CSE or 100 ng/ml
rEGF for 4 h at 37°C, subsequently washed out, and then incubated
with complete medium or 50 nM erlotinib (EGFK tyrosin kinase
inhibitor) or 100 nM LY2109761 (TGF-β receptor I and II kinase
inhibitor) for an additional 24 h at 37°C. To investigate the
activation of the Syk/Src pathway, ARPE19 cells were exposed to 5%
CSE or 100 ng/ml rEGF for 4 h at 37°C, subsequently washed out, and
then incubated with complete medium, 200 nM PP1 (Src inhibitor) or
200 nM Bay 61–3606 (Syk inhibitor) for an additional 24 h at 37°C.
To investigate the regulatory effect of baicalein on EMT, ARPE19
cells were exposed to 5% CSE or 100 ng/ml rEGF for 4 h at 37°C,
subsequently washed out, and then incubated with complete medium or
20 µM baicalein for an additional 24 h at 37°C.
Small interfering RNA (siRNA)
transfection
Experimentally verified human FAK-siRNA duplex
(5′-GGUUCAAGCUGGAUUAUUTT-3′) and negative control-siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from Bioneer
Corporation (Daejeon, Korea). Cells were seeded at a concentration
of 1×105/well in a T75 flask and cultured overnight.
Cells in each T75 flask were then transfected with 200 nM siRNAs
using Lipofectamine® RNAiMAX reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. Cells were used for further
experiments 48 h after transfection.
Immunoblotting
Cells were harvested and lysed in NP-40 buffer
(Elpis Biotech, Inc., Daejeon, Korea) supplemented with a protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). To address
phosphorylation events, an additional set of phosphatase inhibitors
(Cocktail II; Sigma-Aldrich; Merck KGaA) was added to the NP-40
buffer. Protein concentration was determined using a bicinchoninic
acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). The same
volume of 2X Laemmli sample buffer (Elpis Biotech, Inc.) was added
to each lysate, and each protein sample (10 µg/sample) was
immediately boiled for 5 min at 100°C. Insoluble material was
centrifuged at 14,000 × g at 25°C. Total cell lysates
(5×106 cells/sample) were subjected to SDS-PAGE (10
µg/well) on a gel containing 15% (w/v) acrylamide under reducing
conditions. Separated proteins were transferred to nitrocellulose
membranes (EMD Millipore). The membranes were blocked with 5% skim
milk and western blot analysis was performed. Chemiluminescence was
detected using an enhanced chemiluminescence kit (Advansta, Inc.,
Menlo Park, CA, USA) and an Amersham Imager 600 (GE Healthcare Life
Sciences, Little Chalfont, UK). Primary antibodies against the
following proteins were incubated with membranes overnight at 4°C:
E-cadherin (cat. no. 3195; 1:1,000), vimentin (cat. no. 5741;
1:1,000), tight junction protein 1 (ZO-1; cat. no. 8193; 1:1,000),
EGFR (cat. no. 2232; 1:1,000), phospho-Smad2/3
(Ser465/467/Ser423/425; cat. no. 8828;
1:1,000), Smad2/3 (cat. no. 5678; 1:1,000), phospho-FAK
(Tyr397; cat. no. 3283; 1:1,000), phospho-FAK
(Tyr925; cat. no. 3284; 1:1,000), FAK (cat. no. 3285;
1:1,000), phospho-Syk (Tyr323; cat. no. 2715; 1:1,000),
phospho-Syk (Tyr525/526; cat. no. 2710; 1:1,000), Syk
(cat. no. 13,198; 1:1,000), phospho-Src (Tyr416; cat.
no. 6943; 1:1,000), Src (cat. no. 2123; 1:1,000), extracellular
signal-regulated kinase (ERK; cat. no. 9102; 1:1,000), phospho-ERK
(cat. no. 9101; 1:1,000), phospho-p38 (cat. no. 9211; 1:1,000), p38
(cat. no. 9212; 1:1,000), c-Jun N-terminal kinase (JNK; cat. no.
9258; 1:1,000) phospho-JNK (cat. no. 4671; 1:1,000), and β-actin
(cat. no. 4967; 1:1,000; Cell Signaling Technology, Inc.); and VEGF
receptor 1 (VEGF-R1; cat. no. sc-271789; 1:500), VEGF-R2 (cat. no.
sc-393163; 1:500), 12-LOX (cat. no. sc-365194; 1:500) and 15-LOX
(cat. no. sc-133085; 1:500; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA); and α-smooth muscle actin (α-SMA; cat. no. bs-10196R;
1:500; BIOSS, Beijing, China) Following this, the membranes were
incubated with the following secondary antibodies for 1 h at room
temperature: Goat anti-mouse-horseradish peroxidase (HRP; cat. no.
K0211589; 1:3,000) or goat anti-rabbit-HRP (cat. no. K0211708;
1:3,000) (both from Koma Biotech, Seoul, Korea).
Enzyme-linked immunosorbent assay
(ELISA)
At 24 h, conditioned media was collected and the
concentration of active TGF-β1 (cat. no. DY240) and VEGF (cat. no.
DY293B) secreted by ARPE-19 or CSE-treated ARPE-19 cells was
measured using the Single Cytokine ELISA Assay kit (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's instructions. HETE concentrations in the culture
supernatants were quantified using a single cytokine ELISA kit
(Abcam, Cambridge, UK). Data are expressed as the mean value for
biological replicates ± standard deviation (SD).
Wound healing assay
Wound healing assays were performed to measure the
migration ability of ARPE-19 cells. CSE-treated or untreated
ARPE-19 cells were plated in 6-well plates. As the cell layers
reached confluence, a uniform wound consisting of a straight line
was created in each well using a 200-µl micropipette tip and the
wounded layers were washed with PBS to remove all cell debris. The
cells were cultured in 5% CO2 at 37°C, and images were
captured at 0 and 24 h after scratching using an inverted phase
contrast microscope at ×100 magnification.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analysis was conducted using one-way analysis of variance (ANOVA)
using SigmaPlot software (version 10.0; Systat Software, Inc., San
Jose, CA, USA). Bonferroni post hoc analysis was performed
following the ANOVA for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
EGFR activation by CSE stimulates EMT
processes in RPE cells by activating the TGF-β1-related
pathway
It was initially analyzed whether CSE promotes the
induction of EGFR expression and mesenchymal characteristics of RPE
cells, and also whether exposure to CSE has an effect on the
production of TGF-β1 and VEGF. Treatment with EGF or CSE increased
the expression of EGFR, mesenchymal markers (vimentin and α-SMA),
but reduced the levels of E-cadherin and ZO-1 compared with the
control (Fig. 1A). Although
CSE-exposed ARPE-19 cells acquired a fibroblast-like mesenchymal
appearance (Fig. 1B), treatment
with erlotinib restored epithelial characteristics and suppressed
the cellular migration of CSE-stimulated ARPE-19 cells (Fig. 1C). Stimulation of RPE cells with a
combination of EGF or CSE and erlotinib, an EGFR tyrosine kinase
inhibitor (TKI), efficiently blocked the upregulation of
mesenchymal markers and migration of CSE- or EGF-induced ARPE-19
cells (Fig. 1A and C). In
addition, secretion of TGF-β1 and VEGF in CSE- or EGF-treated
ARPE-19 cells was efficiently blocked by treatment with erlotinib
(Fig. 1D). Stimulation with EGF or
CSE had no effect on the activation of mitogen-activated protein
kinases (MAPKs), including ERK, JNK and p38 MAPK (Fig. 2A). Additionally, ARPE-19 cells
treated with EGF or CSE had increased expression of VEGFR and
Smad2/3 phosphorylation, an intracellular signaling adaptor of
TGF-β1 (Fig. 2A). Inhibition of
the TGF-β1 pathway in EGF- or CSE-exposed ARPE cells using
LY2109761, a small molecule inhibitor of the TGF-β receptor type
I/type II kinases, disrupted Smad2/3 phosphorylation, blocked the
suppression of E-cadherin and ZO-1 and decreased the expression of
mesenchymal markers (Fig. 2A).
Furthermore, considering that EGF or CSE treatment of ARPE-19 cells
increased the production of VEGF (Fig.
1D), the present study also investigated whether co-treatment
with CSE and VEGF enhanced the migration of ARPE-19 cells. Combined
treatment of ARPE-19 cells with VEGF and CSE led to further
enhancement of cell migration activity compared with either
recombinant VEGF or CSE alone (Fig.
2B). These results suggested that CSE has an EGF-like effect on
TGF-β1 and VEGF production through abnormal EGFR activation, and
that TGF-β1-mediated signaling triggers EMT processes in cigarette
smoke-exposed RPE cells.
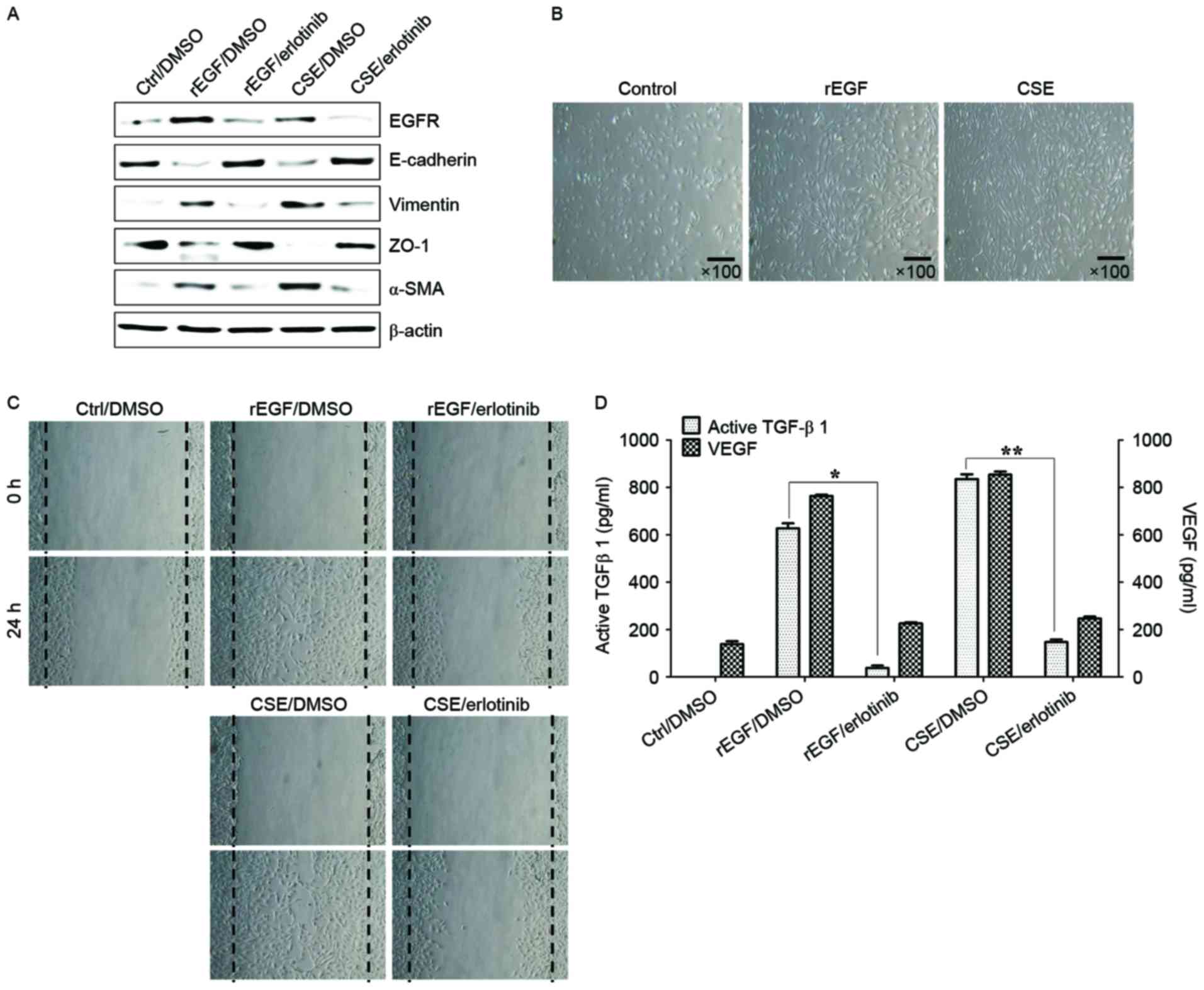 | Figure 1.CSE-mediated EGFR activation induces
EMT of RPE cells through secretion of VEGF and TGF-β1. ARPE-19
cells were exposed to 5% CSE or 100 ng/ml rEGF for 4 h,
subsequently washed out, and then incubated with complete medium or
50 nM erlotinib for an additional 24 h. (A) Total cell lysates were
immunoblotted with the indicated antibodies. β-actin served as an
internal control. (B) CSE induces mesenchymal morphology in ARPE-19
cells. Morphology was observed with an inverted phase-contrast
microscope. The scale bar represents 100 µm, and photographs were
taken at ×100 magnification using a digital camera. (C) Cell
motility was increased by CSE as measured by a wound-healing assay.
Cells were wounded (0 h) and maintained for 24 h in complete
medium. Dotted lines indicate the edges of the wounds. (D)
Concentrations of active TGF-β1 and VEGF in the culture
supernatants were quantified by ELISA assay. Data are presented as
the mean ± standard deviation of three independent experiments.
Results are representative of three independent experiments.
*P<0.05 and **P<0.01. Ctrl, control; DMSO, dimethyl
sulfoxide; rEGF, recombinant epidermal growth factor; CSE,
cigarette smoke extract; EGFR, epidermal growth factor receptor;
ZO-1, tight junction protein 1; α-SMA, α-smooth muscle actin;
TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial
growth factor. |
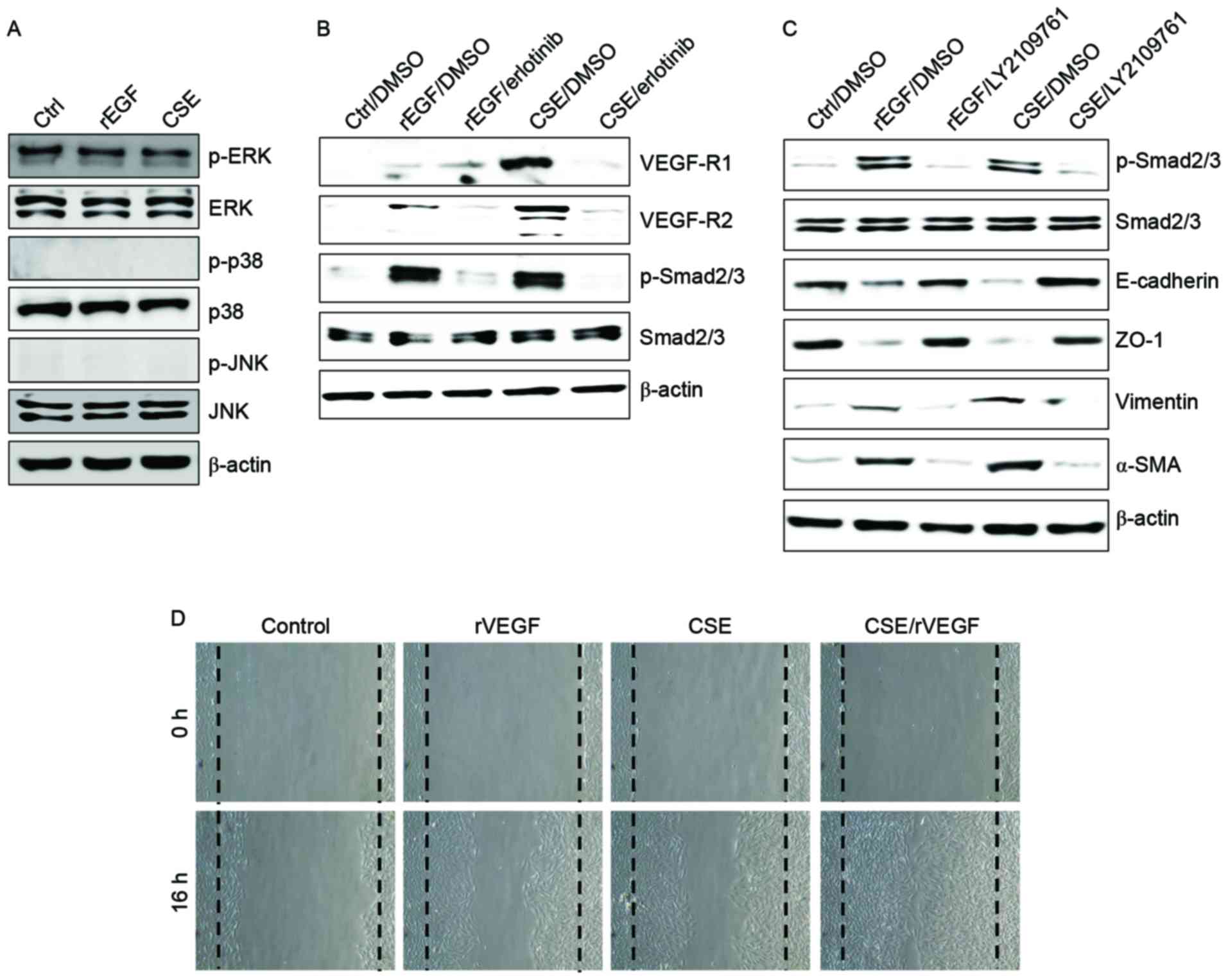 | Figure 2.CSE induces upregulation of VEGFR and
activation of the transforming growth factor-β1-associated pathway.
(A-C) ARPE-19 cells were exposed to 5% CSE or 100 ng/ml rEGF for 4
h, subsequently washed out, and then incubated with complete medium
or 50 nM erlotinib or 100 nM LY2109761 for an additional 24 h.
Total cell lysates were immunoblotted with the indicated
antibodies. β-actin served as an internal control. (D) ARPE19 cells
were treated with either 50 ng/ml rVEGF or 5% CSE alone, or
co-treated (50 ng/ml rVEGF + 5% CSE) for 4 h, subsequently washed
out, and then incubated with complete medium for an additional 24
h. Dotted lines indicate the edges of the wounds. Results are
representative of three independent experiments. Ctrl, control;
rEGF, recombinant epidermal growth factor; CSE, cigarette smoke
extract; p, phospho; ERK, extracellular regulated kinase; JNK,
c-Jun N-terminal kinase; DMSO, dimethyl sulfoxide; VEGF-R, vascular
endothelial growth factor receptor; Smad, Sma- and Mad-related
family; ZO-1, tight junction protein 1; α-SMA, α-smooth muscle
actin; rVEGF, recombinant vascular endothelial growth factor. |
CSE-mediated TGF-β1 activates the
phosphorylation of FAK, Syk and Src to induce EMT processes in RPE
cells
TGF-β induces EMT processes in various normal and
cancer cells, and is also responsible for FAK-mediated scar
formation in chronic fibrotic disease (28,29).
The activation of the FAK/Src pathway in ARPE-19 cells was
previously reported to be associated with collagen gel contraction
in an in vitro model of PVR (30). It was subsequently investigated
whether CSE-induced TGF-β1 activates the FAK signaling pathway in
ARPE-19 cells. Stimulation with CSE induced FAK phosphorylation and
stimulated the phosphorylation of Src and Syk kinase; however,
pretreatment with erlotinib or LY2109761 efficiently blocked
CSE-induced phosphorylation of FAK, Syk, and Src (Fig. 3A). In addition, treatment with
LY2109761 significantly inhibited migratory activity, and TGF-β1
and VEGF production in CSE-stimulated ARPE cells (Fig. 3B and C). These results suggested
that EGF-like CSE stimulation increases the production of TGF-β1
and activation of FAK-associated downstream signaling in ARPE-19
cells.
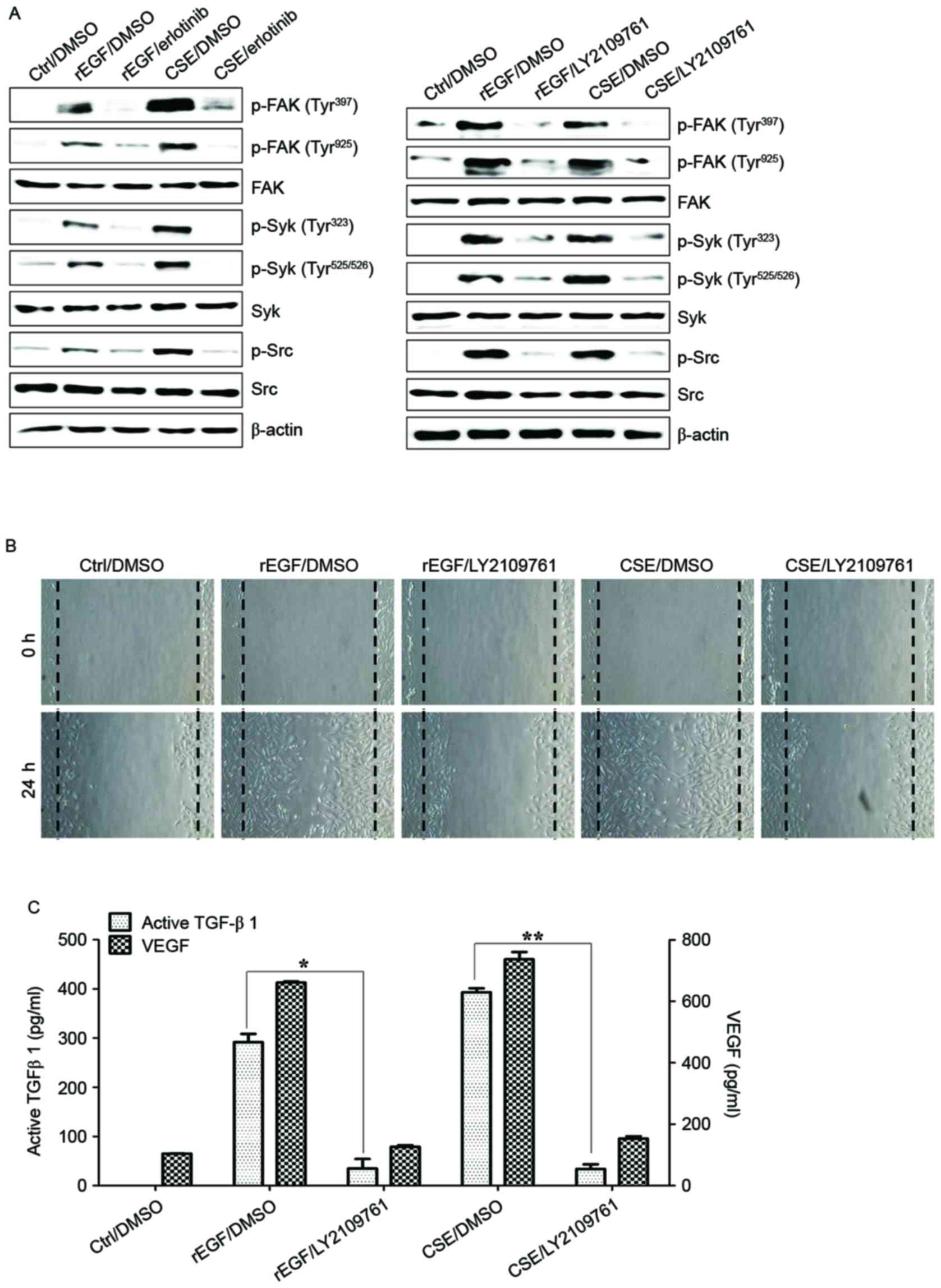 | Figure 3.CSE-mediated TGF-β1 activates the
phosphorylation of Syk, Src and FAK for EMT in retinal pigment
epithelial cells. ARPE19 cells were exposed to 5% CSE or 100 ng/ml
rEGF for 4 h, subsequently washed out, and then incubated with
complete medium or 50 nM erlotinib or 100 nM LY2109761 for an
additional 24 h. (A) Total cell lysates of each condition were
harvested and immunoblotted with the indicated antibodies. β-actin
served as an internal control. (B) CSE- or rEGF-induced cell
migration was decreased by LY2109761 treatment as measured by a
wound healing assay. Cells were wounded (0 h) and maintained for 24
h in complete medium. Dotted lines indicate the edges of the
wounds. (C) Concentrations of active TGF-β1 and VEGF in the culture
supernatants were quantified by ELISA. Data are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05 and **P<0.01. Ctrl, control; rEGF, recombinant
epidermal growth factor; CSE, cigarette smoke extract; p, phospho;
FAK, focal adhesion kinase; Syk, spleen associated tyrosine kinase;
Src, Src proto-oncogene non-receptor tyrosine kinase; TGF-β1,
transforming growth factor-β1; VEGF, vascular endothelial growth
factor. |
FAK-mediated Syk/Src activation
promotes the production of TGF-β1 and VEGF in CSE-stimulated RPE
cells
It was also investigated whether CSE-mediated FAK
activation has an effect on TGF-β1 production and cellular
motility. The association between FAK and Syk or Src signaling in
ARPE-19 cells stimulated with CSE was determined. Knockdown of FAK
in ARPE-19 cells inhibited phosphorylation of Syk and Src kinase
following CSE stimulation (Fig.
4A). Downregulation of FAK with siRNA significantly suppressed
the phosphorylation of Smad2/3 and expression of mesenchymal
markers (vimentin and α-SMA), and also restored the expression of
epithelial markers (E-cadherin and ZO-1) in CSE-stimulated APRE-19
cells (Fig. 4A). Gene silencing of
FAK by RNA interference resulted in significant reduction of
CSE-activated VEGF and TGF-β1 release, and blocked cell migration
activity (Fig. 4B and C).
Pharmacological inhibitors of Src and Syk were also used to further
analyze the role that these kinases have in TGF-β1-mediated FAK
signaling in ARPE-19 cells exposed to CSE. Treatment with PP1 (Src
inhibitor) and Bay 61–3606 (Syk inhibitor) effectively inhibited
the activation of Src and Syk in CSE-treated ARPE cells, but did
not block the phosphorylation of FAK (Fig. 5A). Levels of mesenchymal markers
and expression of phosphorylated Smad2/3 in CSE-activated ARPE
cells were reduced by the inhibitors (Fig. 5A). In addition, VEGF and TGF-β1
secretion was attenuated by addition of the inhibitors, as was the
wound healing capacity of CSE-stimulated ARPE cells (Fig. 5B and C). Taken together, these
results suggested that FAK-mediated Syk/Src signaling can activate
EMT processes in CSE-treated ARPE-19 cells.
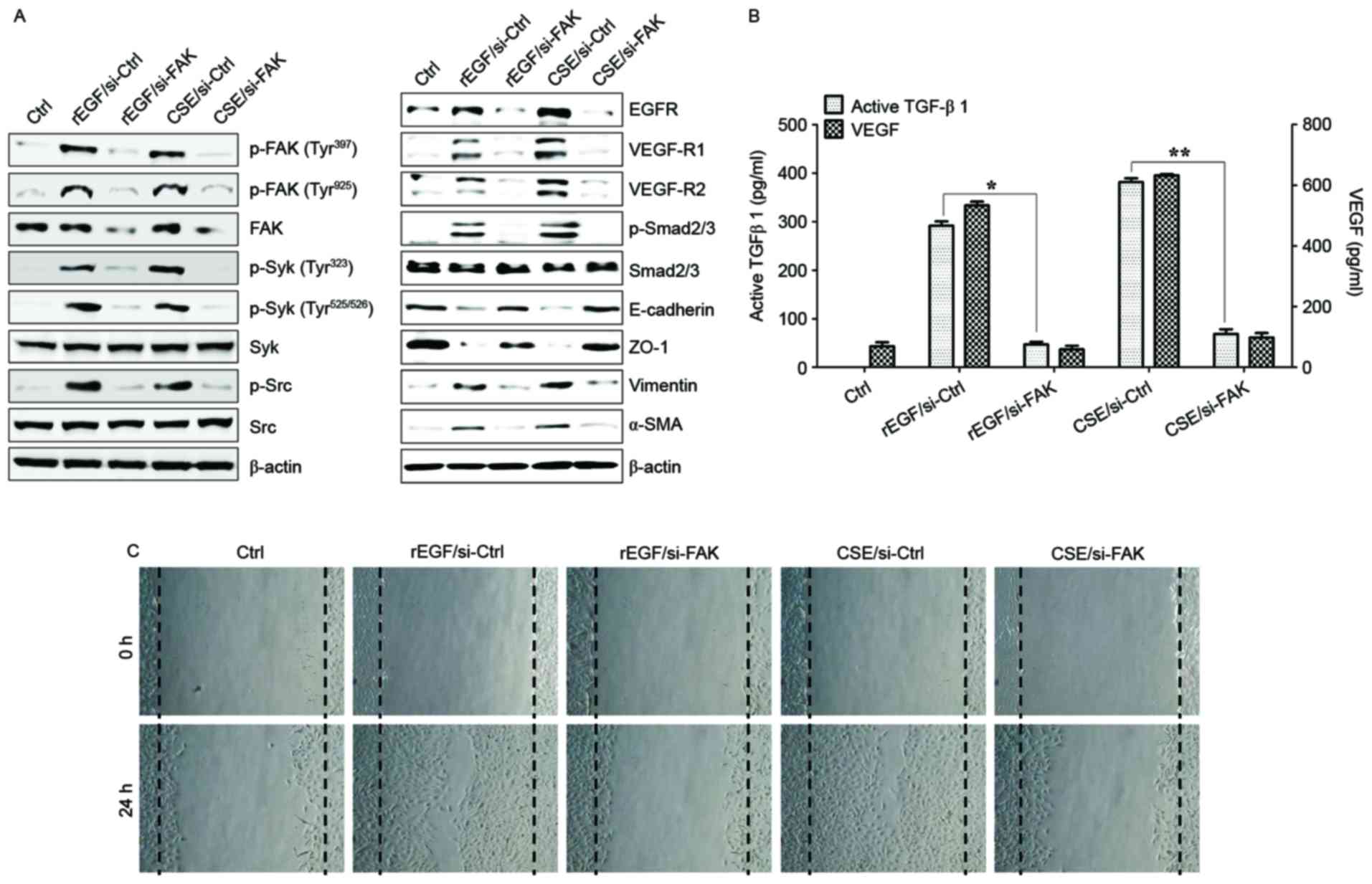 | Figure 4.FAK is an upstream signal of
CSE-induced Syk/Src activation and secretion of VEGF and TGF-β1.
ARPE19 cells were exposed to 5% CSE or 100 ng/ml rEGF for 4 h,
subsequently washed out, and then incubated with complete medium
for an additional 4 h. Cell were transfected with either
si-FAK-siRNA (200 nM) or si-Ctrl for 36 h prior to experiments. (A)
Following transfection, protein levels of cells were detected by
western blotting with the indicated antibodies. β-actin was used as
a loading control. (B) Concentrations of active TGF-β1 and VEGF in
the culture supernatants were quantified by ELISA assay. Data are
presented as the mean ± standard deviation of three independent
experiments. (C) CSE- or rEGF-induced cell migration inhibited by
FAK knockdown as measured by a wound healing assay. Cells were
wounded (0 h) and maintained for 24 h in complete medium. Dotted
lines indicate the edges of the wounds. Results are representative
of three independent experiments. *P<0.05 and **P<0.01. Ctrl,
control; rEGF, recombinant epidermal growth factor; si, small
interfering RNA; FAK, focal adhesion kinase; CSE, cigarette smoke
extract; p, phospho; Syk, spleen associated tyrosine kinase; Src,
Src proto-oncogene non-receptor tyrosine kinase; EGFR, epidermal
growth factor receptor; VEGF-R, vascular endothelial growth factor
receptor; Smad, Sma- and Mad-related family; ZO-1, tight junction
protein 1; α-SMA, α-smooth muscle actin; TGF-β1, transforming
growth factor-β1; VEGF, vascular endothelial growth factor. |
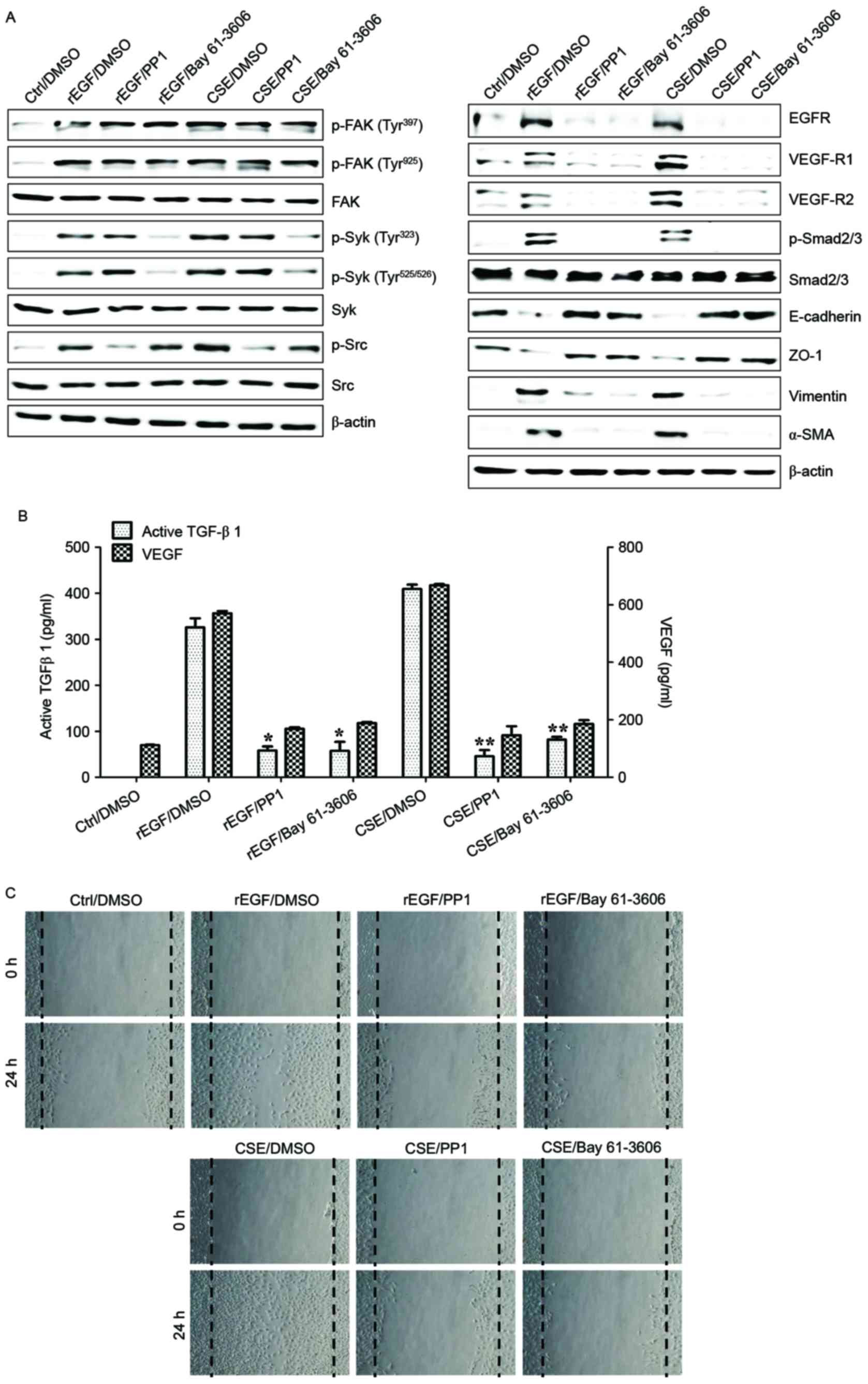 | Figure 5.Syk/Src activation regulates the
secretion of VEGF and TGF-β1 in CSE-stimulated ARPE19 cells. ARPE19
cells were exposed to 5% CSE or 100 ng/ml rEGF for 4 h,
subsequently washed out, and then incubated with complete medium,
200 nM PP1 (Src inhibitor) or 200 nM Bay 61–3606 (Syk inhibitor)
for an additional 24 h. (A) Protein levels were detected by western
blotting with the indicated antibodies. β-actin was used as a
loading control. (B) Concentration of active TGF-β1 and VEGF in the
culture supernatants were quantified by ELISA assay. Data are
presented as the mean of three independent experiments, and error
bars represent the standard deviation of the means. (C) PP1 or Bay
61–3606 treatment blocked CSE- or rEGF-induced cell migration as
measured by wound healing assay. Cells were wounded (0 h) and
maintained for 24 h in complete medium. Dotted lines indicate the
edges of the wounds. Results are representative of three
independent experiments. *P<0.05 and **P<0.01. Ctrl, control;
DMSO, dimethyl sulfoxide; rEGF, recombinant epidermal growth
factor; CSE, cigarette smoke extract; p, phospho; FAK, focal
adhesion kinase; Syk, spleen associated tyrosine kinase; Src, Src
proto-oncogene non-receptor tyrosine kinase; EGFR, epidermal growth
factor receptor; VEGF-R, vascular endothelial growth factor
receptor; Smad, Sma- and Mad-related family; ZO-1, tight junction
protein 1; α-SMA, α-smooth muscle actin; TGF-β1, transforming
growth factor-β1; VEGF, vascular endothelial growth factor. |
Baicalein treatment modulates
CSE-mediated signaling pathways involved in EMT processes in ARPE
cells
Baicalein, an inhibitor of 12-LOX, prevents the
EGF-dependent signaling involved in cell growth in rabbit corneal
epithelial cells (26). In the
current study, it was also examined whether baicalein may alter the
regulation of CSE-dependent TGF-β1 production, cell migration and
associated signaling transduction in CSE-exposed ARPE-19 cells.
Compared with ARPE-19 cells treated with EGF or CSE, treatment with
baicalein reduced the expression of EGFR, VEGF-R1 and -R2,
activation of Smad2/3 phosphorylation, and upregulation of
mesenchymal markers in CSE-exposed ARPE-19 cells (Fig. 6A). Furthermore, baicalein
efficiently blocked the phosphorylation of FAK, Src, and Syk in
CSE-stimulated ARPE-19 cells (Fig.
6A). The activation of 12/15-LOX in CSE- or EGF-stimulated
ARPE-19 cells was also markedly attenuated by baicalein treatment
(Fig. 6A). FAK-knockdown or
targeted inhibition of Syk/Src kinase attenuated the expression of
12/15-LOX in CSE-exposed ARPE-19 cells (Fig. 6B and C). In addition, pre-exposure
to baicalein significantly suppressed the production of TGF-β1,
VEGF and 12(S)-HETE in CSE-treated ARPE-19 cells (Fig. 6D and E). Finally, the effect of
baicalein on migration activity and underlying signaling pathway in
HRPEpi cells following treatment with EGF or CSE was determined.
Levels of EGFR, VEGFR and phosphorylated Smad2/3 in CSE-treated
HRPEpi cells were suppressed by treatment with baicalein or
erlotinib (Fig. 7A). Treatment
with baicalein of CSE-exposed HRPEpi resulted in restoration of
epithelial markers (E-cadherin and ZO-1) and reduction of
mesenchymal markers (vimentin and α-SMA; Fig. 7A). Furthermore, levels of
phosphorylated FAK, Syk, and Src were reduced by baicalein
treatment compared with erlotinib treatment (Fig. 7A). Cell migratory activity and
secretion of EMT-associated cytokines, including TGF-β1 and VEGF,
were blocked following treatment with baicalein or erlotinib in
CSE-stimulated HRPEpi cells (Fig. 7B
and C). Treatment of CSE-activated HRPEpi cells with baicalein
or erlotinib markedly downregulated the expression of 12/15-LOX and
the production of 12(S)-HETE compared with non-treated
CSE-stimulated HRPEpi (Fig. 7D).
Together, these results suggested that baicalein could suppress the
activation of 12/15-LOX in RPE cells affected by cigarette smoking
via the inhibition of FAK-related signaling pathway in retinal
pathology.
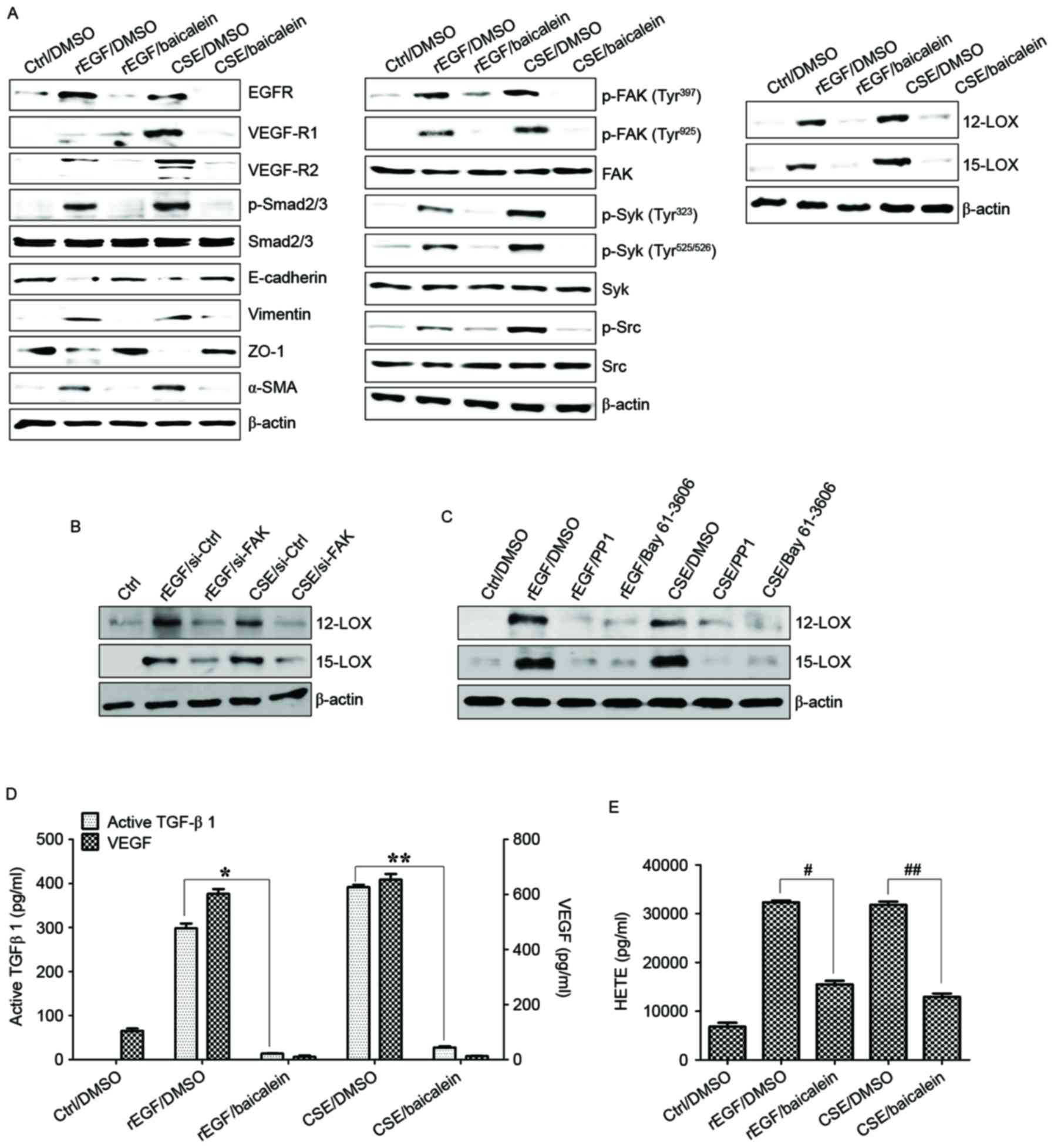 | Figure 6.Baicalein treatment modulates the
CSE-mediated signaling pathway for EMT processes in ARPE19 cells.
(A) Cells were exposed to 5% CSE or 100 ng/ml rEGF for 4 h,
subsequently washed out, and then incubated with complete medium or
20 µM baicalein for an additional 24 h. (B) Cells were exposed to
5% CSE or 100 ng/ml rEGF for 4 h, subsequently washed out, and then
incubated with complete medium for an additional 4 h. Cell were
transfected with either FAK-siRNA (200 nM) or control-siRNA for 36
h prior to experiments. (C) Cells were exposed to 5% CSE or 100
ng/ml rEGF for 4 h, subsequently washed out, and then incubated
with complete medium, 200 nM PP1 (Src inhibitor), or 200 nM Bay
61–3606, (Syk inhibitor) for an additional 24 h. Total cell lysates
of each condition were harvested and immunoblotted with the
indicated antibodies. β-actin served as an internal control. (D)
Concentrations of active TGF-β1 and VEGF in the culture
supernatants were quantified by ELISA assay. Data are presented as
the mean of three independent experiments, and error bars represent
the standard deviation. (E) Concentrations of HETE in the culture
supernatants were quantified by ELISA assay. Data are presented as
the mean of three independent experiments, and error bars represent
the standard deviation. *P<0.05 and **P<0.01;
#P<0.005 and ##P<0.001. Results are
representative of three independent experiments. Ctrl, control;
DMSO, dimethyl sulfoxide; rEGF, recombinant epidermal growth
factor; CSE, cigarette smoke extract; EGFR, epidermal growth factor
receptor; VEGF-R, vascular endothelial growth factor receptor;
Smad, Sma- and Mad-related family; ZO-1, tight junction protein 1;
α-SMA, α-smooth muscle actin; p, phospho; FAK, focal adhesion
kinase; Syk, spleen associated tyrosine kinase; Src, Src
proto-oncogene non-receptor tyrosine kinase; LOX, lipoxygenase;
TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial
growth factor; HETE, hydroxyeicosatetraenoic acid. |
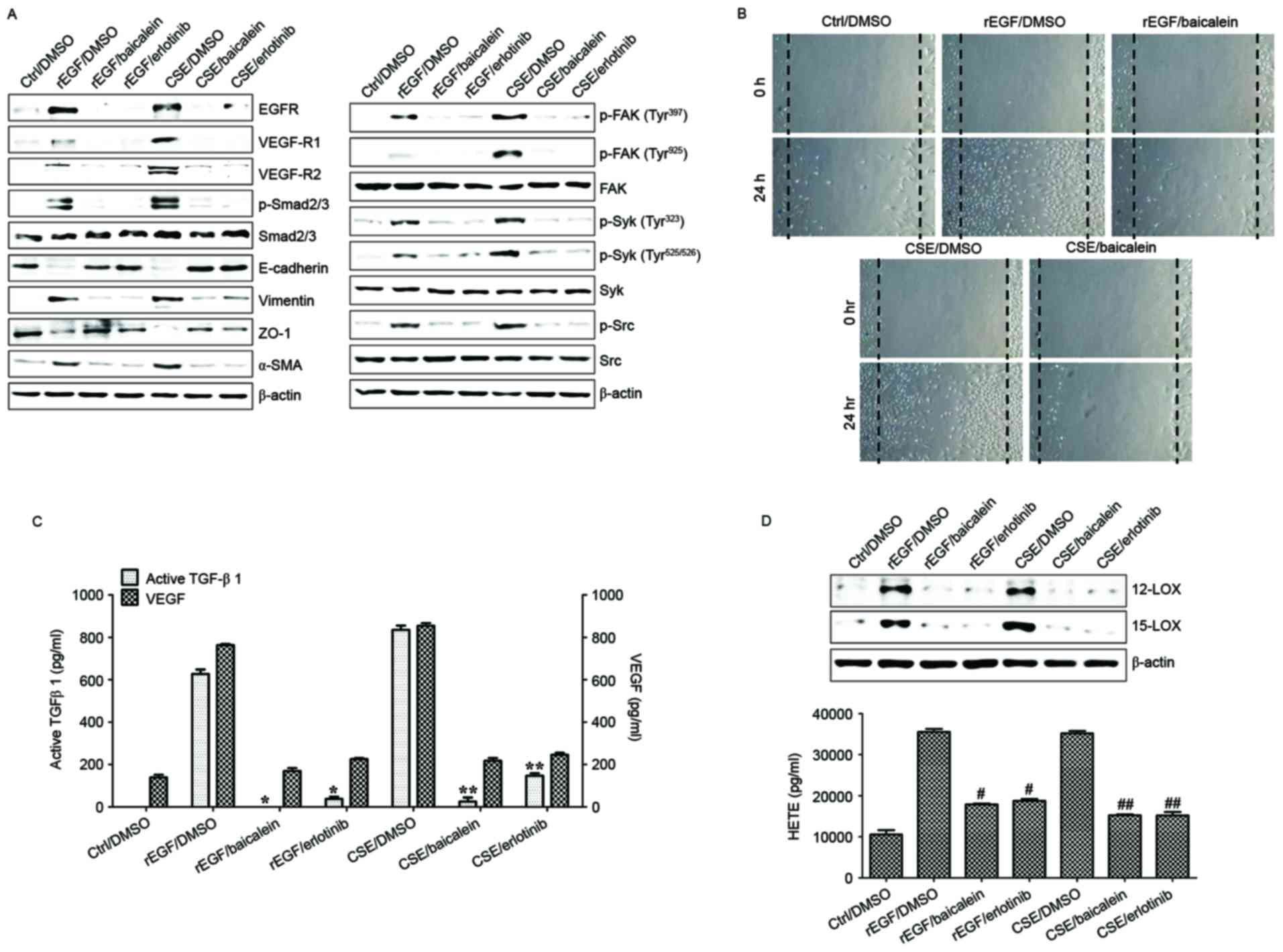 | Figure 7.Baicalein treatment modulates the
CSE-mediated signaling pathway for EMT processes in HRPEpi. HRPEpi
were exposed to 5% CSE or 100 ng/ml rEGF for 4 h, subsequently
washed out, and then incubated with complete medium, 20 µM
baicalein, or 50 nM erlotinib for an additional 24 h. (A) Total
cell lysates of each condition were harvested and immunoblotted
with the indicated antibodies. β-actin served as an internal
control. (B) Baicalein treatment blocked CSE- or rEGF-induced cell
migration as measured by wound healing assay. Cells were wounded (0
h) and maintained for 24 h in complete medium. Dotted lines
indicate the edges of the wounds. Wound closure (measured after 24
h) was faster in cells treated with CSE or rEGF than in those
treated with baicalein. (C) Concentrations of active TGF-β1 and
VEGF in the culture supernatants were quantified by ELISA assay.
Data are presented as the mean of three independent experiments,
and error bars represent the standard deviation. (D) Upregulation
of 12/15-LOX and production of HETE by CSE- or rEGF treatment was
decreased by baicalein or erlotinib treatment. Total proteins were
extracted from cell lysates and western blotting was performed for
12/15-LOX protein. Concentrations of HETE in the culture
supernatants of ARPE19 cells were quantified by ELISA assay. Data
are presented as the mean of three independent experiments, and
error bars represent the standard deviation. Results are
representative of three independent experiments. *P<0.05 and
**P<0.01; #P<0.005 vs. rEGF/DMSO and
##P<0.001 vs. CSE/DMSO. HRPEpi, human primary retinal
pigment epithelial cells; Ctrl, control; DMSO, dimethyl sulfoxide;
rEGF, recombinant epidermal growth factor; CSE, cigarette smoke
extract; EGFR, epidermal growth factor receptor; VEGF-R, vascular
endothelial growth factor receptor; p, phospho; Smad, Sma- and
Mad-related family; ZO-1, tight junction protein 1; α-SMA, α-smooth
muscle actin; FAK, focal adhesion kinase; Syk, spleen associated
tyrosine kinase; Src, Src proto-oncogene non-receptor tyrosine
kinase; TGF-β1, transforming growth factor-β1; VEGF, vascular
endothelial growth factor; LOX, lipoxygenase; HETE,
hydroxyeicosatetraenoic acid. |
Discussion
The presence of TGF-β1 and EGF in the subretinal
fluid of patients with retinal detachment has been confirmed
previously (2,3). Exposure of human airway epithelial
cells to either CS or H2O2 results in an
increase in EGFR activation and activation of metalloproteinases
(31). APRE-19 cells stimulated
with TGF-β1 and EGF have enhanced expression of α-SMA and vimentin,
and upregulated metalloproteinases activity (32), indicating a role for these factors
in the pathogenesis of PVR and EMT processes in RPE cells. Based on
these results, blocking abnormal EGFR expression, and consequently
its pathways, may inhibit cellular migration and invasion of RPE
cells. Furthermore, the effects and underlying molecular mechanisms
of smoking on EMT processes in PVR remain unclear. In the current
study, ARPE cells exposed to CSE exhibited high levels of EGFR
expression and activated downstream FAK-mediated Syk/Src signaling.
CSE-mediated FAK activation also had an important effect on TGF-β1
and VEGF release in APRE-19 cells. Specifically, treatment with
erlotinib or baicalein efficiently blocked CSE-dependent TGF-β1
release and downstream signal transduction, resulting in
suppression of EMT processes (Fig.
8). These results suggest that suppression of EGFR-dependent
FAK activation may be a key mechanism for preventing deterioration
of PVR in chronic smokers.
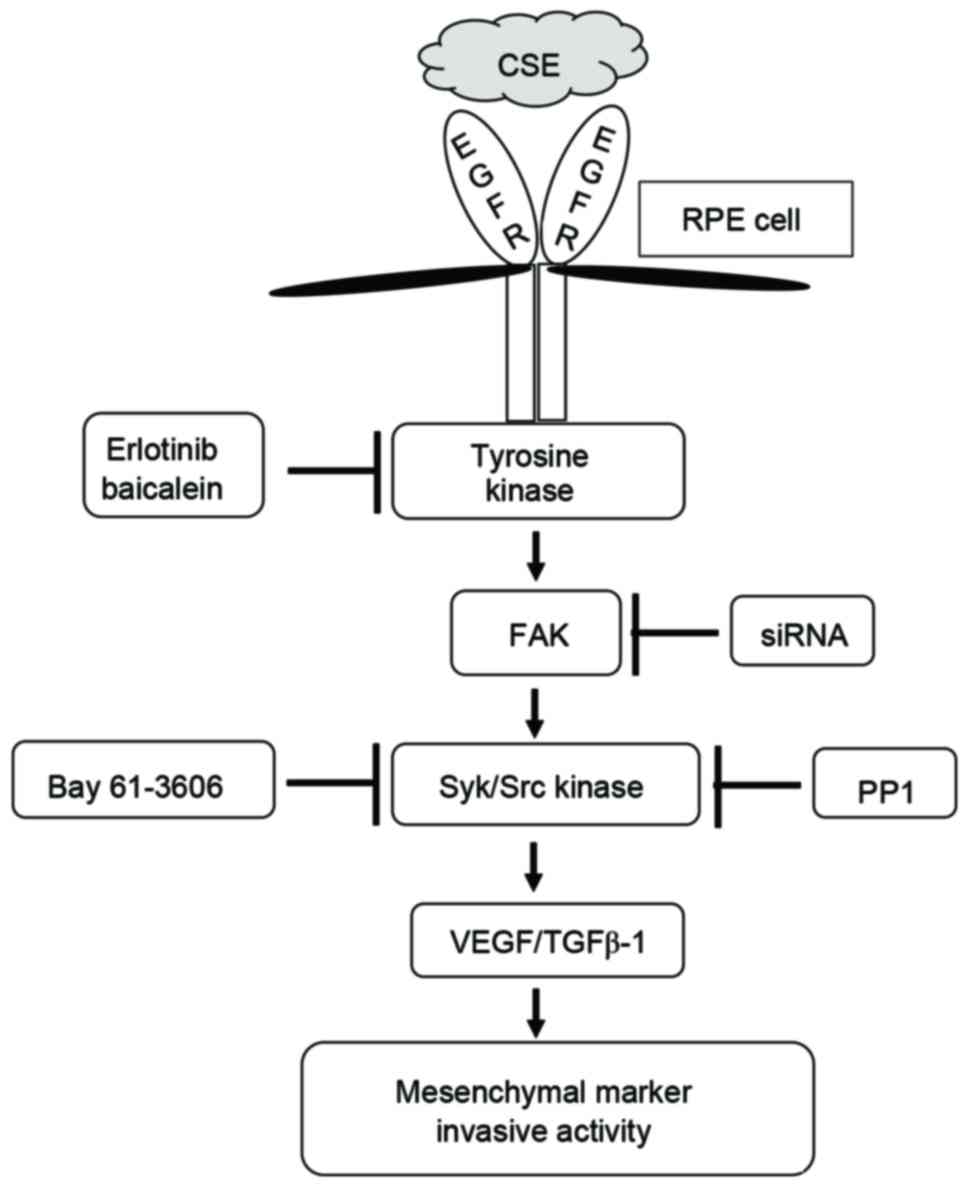 | Figure 8.Schematic diagram of intracellular
signaling mechanisms that induce epithelial mesenchymal transition
during CSE-induced aberrant EGFR activation in human RPE cells. CSE
induces the expression of EGFR and increases the activity of FAK,
Syk, and Src in RPE cells. These signaling molecules promote the
production of TGF-β1 and VEGF and induce expression of EMT markers.
CSE, cigarette smoke extract; EGFR, epidermal growth factor
receptor; RPE cell, retinal pigment epithelial cell; FAK, focal
adhesion kinase; siRNA, small interfering RNA; Syk, spleen
associated tyrosine kinase; Src, Src proto-oncogene non-receptor
tyrosine kinase; VEGF, vascular endothelial growth factor; TGF-β1,
transforming growth factor-β. |
EGF synergistically stimulates TGF-β-mediated EMT
processes (33), and FAK also acts
as a critical mediator of EMT and metastasis stimulated by TGF-β in
hepatocytes (34). Additionally,
CSE-activated FAK signaling decreases cell motility and induces
apoptosis in human bronchial cells (35,36),
and CSE disrupts focal adhesion complexes and decreases FAK
activity in cultured lung endothelial cells (37). These findings suggest that the role
of FAK, particularly when induced by TGF-β1, is
cell-type-dependent. In addition, it remains unclear whether FAK
facilitates EGF-mediated TGF-β1-dependent signaling for enhancing
EMT processes in retina-associated pathological conditions. In
CSE-exposed RPE cells, the TGF-β1-mediated Smad2/3 signaling
pathway was associated with FAK-induced migration activity.
Activated FAK in CSE-stimulated APRE cells also promoted the
production of TGF-β1 and VEGF. These results suggest that FAK
activation is able to regulate the EMT processes of RPE cells in
CSE-associated retinal diseases, and that FAK signaling may be a
novel therapeutic target for regulating pathological conditions of
the retina.
Src signaling promotes the transition of cells with
an adhesive phenotype to a more motile phenotype in cancer
(38). Unlike EGF-activated EGFR,
CS-stimulated EGFR triggers aberrant Src phosphorylation in lung
cancer cells (39). However,
EGF-treated or CSE-exposed RPE cells enhanced the phosphorylation
of Src in the present study. This result suggests that CSE
stimulation of RPE cells may promote an EGFR-associated signaling
pathway induced by EGF. The activated Src-induced FAK pathway
facilitates TGF-β1-mediated EMT by diverse mechanisms required for
migration and invasion in different cell types (38). TGF-β-induced phosphorylated FAK is
also involved in EMT (40,41); however, direct evidence of the
connection and role of Src and FAK in CSE-stimulated RPE cells has
yet to been reported. Based on existing data, the link between Src
phosphorylation and FAK activation in CSE-exposed ARPE19 cells was
investigated in the current study. CSE stimulation induced the
phosphorylation of FAK, Syk and Src. Although inhibitors of Syk or
Src failed to block the induction of phosphorylated FAK, FAK
knockdown in APRE-19 cells attenuated the activation of Syk/Src
kinases following stimulation with CSE. Syk and Src kinase
inhibitors efficiently suppressed the production of TGF-β1 and VEGF
in CSE-treated APRE-19 cells, and the expression of mesenchymal
markers and cell migration activity were reduced. These results
suggest that FAK-dependent Syk/Src activation is a critical step
for activating EMT processes in RPE cells following exposure to
CSE.
High levels of EGFR expression and paracrine EGF
signaling are strongly associated with poor prognosis of patients
with cancer, and with tumor cell invasion and dissemination
(42). RPE cells also express
various members of the EGF-related growth factor family.
Heparin-binding-EGF-like growth factor produced by RPE cells
stimulates proliferation, migration, and secretion of VEGF under
conditions of oxidative stress (11). In addition, tissue inhibitor of
matrix metalloproteinases-3 and erlotinib have emerged as
attractive candidates for preventing and treating oxidative
stress-induced proliferative retinopathies (23). Although erlotinib is an efficient
and specific TKI for blocking EGFR activation and signaling,
long-term exposure to CS causes alterations in EGFR conformation
leading to smoking-associated TKI resistance (39). Based on these results, the
development of novel therapeutic drugs is required to control
cigarette smoke-associated retinal diseases. Baicalein, a 12-LOX
inhibitor, may protect retinal cells from ischemia-induced
apoptosis by downregulating hypoxia-inducible factor-1α, VEGF and
matrix metalloproteinase-9 (43).
In addition, treatment of rabbit corneal epithelial cells with
baicalein efficiently blocks EGF-mediated cell growth (26). In the present study, treatment with
baicalein also reduced the expression of TGF-β1 and suppressed the
activation of FAK-associated signaling pathways in CSE-treated
HRPEpi cells. These results suggest for the first time, to the best
of our knowledge, that baicalein may be useful for treating PVR and
associated retinal diseases. Furthermore, the use of human primary
RPE cells supports the close association between CS and retinal
diseases, rather than individual components of CSE.
In conclusion, the results of the present study
suggest that treatment with erlotinib may prevent progression of
PVR by suppressing the aberrant EGFR activation induced by CS, and
also that baicalein is beneficial for inhibiting the development of
smoke-associated retinal disease.
Acknowledgements
This study was supported by the Basic Science
Research Program of Ministry of Education (grant no.
NRF-2015R1D1A1A01056672) and Ministry of Science, ICT and Future
Planning (grant no. NRF-2015R1C1A2A01053732) through the National
Research Foundation of Republic of Korea. The sponsor had no role
in the design or conduct of this research.
References
|
1
|
Ryan SJ: Traction retinal detachment. XLIX
Edward Jackson Memorial Lecture. Am J Ophthalmol. 115:1–20. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charteris DG, Sethi CS, Lewis GP and
Fisher SK: Proliferative vitreoretinopathy-developments in
adjunctive treatment and retinal pathology. Eye (Lond). 16:369–374.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Charteris DG: Proliferative
vitreoretinopathy: Pathobiology, surgical management, and
adjunctive treatment. Br J Ophthalmol. 79:953–960. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ha GH, Kim JL and Breuer EK: TACC3 is
essential for EGF-mediated EMT in cervical cancer. PLoS One.
8:e703532013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Chen CZ, Gong WR, Li JP and Xing
YQ: Integrin-alpha5 mediates epidermal growth factor-induced
retinal pigment epithelial cell proliferation and migration.
Pathobiology. 77:88–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wang H, Wang F, Gu Q and Xu X: Snail
involves in the transforming growth factor β1-mediated
epithelial-mesenchymal transition of retinal pigment epithelial
cells. PLoS One. 6:e233222011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakravarthy U, Wong TY, Fletcher A,
Piault E, Evans C, Zlateva G, Buggage R, Pleil A and Mitchell P:
Clinical risk factors for age-related macular degeneration: A
systematic review and meta-analysis. BMC Ophthalmol. 10:312010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein R, Knudtson MD, Cruickshanks KJ and
Klein BE: Further observations on the association between smoking
and the long-term incidence and progression of age-related macular
degeneration: The Beaver Dam Eye Study. Arch Ophthalmol.
126:115–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunchithapautham K, Atkinson C and Rohrer
B: Smoke exposure causes endoplasmic reticulum stress and lipid
accumulation in retinal pigment epithelium through oxidative stress
and complement activation. J Biol Chem. 289:14534–14546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hollborn M, Iandiev I, Seifert M,
Schnurrbusch UE, Wolf S, Wiedemann P, Bringmann A and Kohen L:
Expression of HB-EGF by retinal pigment epithelial cells in
vitreoretinal proliferative disease. Curr Eye Res. 31:863–874.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins GT, Wang JH, Dockery P, Cleary PE
and Redmond HP: Induction of angiogenic cytokine expression in
cultured RPE by ingestion of oxidized photoreceptor outer segments.
Invest Ophthalmol Vis Sci. 44:1775–1782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong A, Xie B, Shen J, Yoshida T, Yokoi K,
Hackett SF and Campochiaro PA: Oxidative stress promotes ocular
neovascularization. J Cell Physiol. 219:544–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan EM, Lanir R, Danielson AR and
Goldkorn T: Epidermal growth factor receptor exposed to cigarette
smoke is aberrantly activated and undergoes perinuclear
trafficking. FASEB J. 22:910–917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uttamsingh S, Bao X, Nguyen KT, Bhanot M,
Gong J, Chan JL, Liu F, Chu TT and Wang LH: Synergistic effect
between EGF and TGF-beta1 in inducing oncogenic properties of
intestinal epithelial cells. Oncogene. 27:2626–2634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Araya J, Cambier S, Markovics JA, Wolters
P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard
D, et al: Squamous metaplasia amplifies pathologic
epithelial-mesenchymal interactions in COPD patients. J Clin
Invest. 117:3551–3562. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen HJ, Sun YH, Zhang SJ, Jiang JX, Dong
XW, Jia YL, Shen J, Guan Y, Zhang LH, Li FF, et al: Cigarette
smoke-induced alveolar epithelial-mesenchymal transition is
mediated by Rac1 activation. Biochim Biophys Acta. 1840:1838–1849.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim J and Hwan Kim S: CK2 inhibitor
CX-4945 blocks TGF-β1-induced epithelial-to-mesenchymal transition
in A549 human lung adenocarcinoma cells. PLoS One. 8:e743422013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muraoka-Cook RS, Dumont N and Arteaga CL:
Dual role of transforming growth factor beta in mammary
tumorigenesis and metastatic progression. Clin Cancer Res.
11:937s–943s. 2005.PubMed/NCBI
|
|
23
|
Hewing NJ, Weskamp G, Vermaat J, Farage E,
Glomski K, Swendeman S, Chan RV, Chiang MF, Khokha R, Anand-Apte B
and Blobel CP: Intravitreal injection of TIMP3 or the EGFR
inhibitor erlotinib offers protection from oxygen-induced
retinopathy in mice. Invest Ophthalmol Vis Sci. 54:864–870. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wertheimer C, Liegl R, Kernt M, Mayer W,
Docheva D, Kampik A and Eibl-Lindner KH: EGF receptor inhibitor
erlotinib as a potential pharmacological prophylaxis for posterior
capsule opacification. Graefes Arch Clin Exp Ophthalmol.
251:1529–1540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seth RK, Haque MS and Zelenka PS:
Regulation of c-fos induction in lens epithelial cells by
12(S)HETE-dependent activation of PKC. Invest Ophthalmol Vis Sci.
42:3239–3246. 2001.PubMed/NCBI
|
|
26
|
Ottino P, Taheri F and Bazan HE: Growth
factor-induced proliferation in corneal epithelial cells is
mediated by 12(S)-HETE. Exp Eye Res. 76:613–622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Shabrawey M, Mussell R, Kahook K,
Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY,
Gurel Z, et al: Increased expression and activity of
12-lipoxygenase in oxygen-induced ischemic retinopathy and
proliferative diabetic retinopathy: Implications in retinal
neovascularization. Diabetes. 60:614–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thannickal VJ, Lee DY, White ES, Cui Z,
Larios JM, Chacon R, Horowitz JC, Day RM and Thomas PE:
Myofibroblast differentiation by transforming growth factor-beta1
is dependent on cell adhesion and integrin signaling via focal
adhesion kinase. J Biol Chem. 278:12384–12389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S, Xu SW, Kennedy L, Pala D, Chen Y,
Eastwood M, Carter DE, Black CM, Abraham DJ and Leask A: FAK is
required for TGFbeta-induced JNK phosphorylation in fibroblasts:
Implications for acquisition of a matrix-remodeling phenotype. Mol
Biol Cell. 18:2169–2178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morales SA, Mareninov S, Wadehra M, Zhang
L, Goodglick L, Braun J and Gordon LK: FAK activation and the role
of epithelial membrane protein 2 (EMP2) in collagen gel
contraction. Invest Ophthalmol Vis Sci. 50:462–469. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Adiseshaiah P and Reddy SP:
Matrix metalloproteinase/epidermal growth factor
receptor/mitogen-activated protein kinase signaling regulate fra-1
induction by cigarette smoke in lung epithelial cells. Am J Respir
Cell Mol Biol. 32:72–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee H, O'Meara SJ, O'Brien C and Kane R:
The role of gremlin, a BMP antagonist, and
epithelial-to-mesenchymal transition in proliferative
vitreoretinopathy. Invest Ophthalmol Vis Sci. 48:4291–4299. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buck E, Eyzaguirre A, Barr S, Thompson S,
Sennello R, Young D, Iwata KK, Gibson NW, Cagnoni P and Haley JD:
Loss of homotypic cell adhesion by epithelial-mesenchymal
transition or mutation limits sensitivity to epidermal growth
factor receptor inhibition. Mol Cancer Ther. 6:532–541. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cicchini C, Laudadio I, Citarella F,
Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carter CA: Multiplexed high content
screening reveals that cigarette smoke condensate-altered cell
signaling pathways are accentuated through FAK inhibition in human
bronchial cells. Int J Toxicol. 31:257–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carter CA and Hamm JT: Multiplexed
quantitative high content screening reveals that cigarette smoke
condensate induces changes in cell structure and function through
alterations in cell signaling pathways in human bronchial cells.
Toxicology. 261:89–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Q, Sakhatskyy P, Grinnell K, Newton J,
Ortiz M, Wang Y, Sanchez-Esteban J, Harrington EO and Rounds S:
Cigarette smoke causes lung vascular barrier dysfunction via
oxidative stress-mediated inhibition of RhoA and focal adhesion
kinase. Am J Physiol Lung Cell Mol Physiol. 301:L847–L857. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Avizienyte E and Frame MC: Src and FAK
signalling controls adhesion fate and the epithelial-to-mesenchymal
transition. Curr Opin Cell Biol. 17:542–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Filosto S, Becker CR and Goldkorn T:
Cigarette smoke induces aberrant EGF receptor activation that
mediates lung cancer development and resistance to tyrosine kinase
inhibitors. Mol Cancer Ther. 11:795–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka Y, Kobayashi H, Suzuki M, Kanayama
N and Terao T: Transforming growth factor-beta1-dependent urokinase
up-regulation and promotion of invasion are involved in
Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol
Chem. 279:8567–8576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakamura K, Yano H, Schaefer E and Sabe H:
Different modes and qualities of tyrosine phosphorylation of Fak
and Pyk2 during epithelial-mesenchymal transdifferentiation and
cell migration: Analysis of specific phosphorylation events using
site-directed antibodies. Oncogene. 20:2626–2635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ueno NT and Zhang D: Targeting EGFR in
triple negative breast cancer. J Cancer. 2:324–328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chao HM, Chuang MJ, Liu JH, Liu XQ, Ho LK,
Pan WH, Zhang XM, Liu CM, Tsai SK, Kong CW, et al: Baicalein
protects against retinal ischemia by antioxidation, antiapoptosis,
downregulation of HIF-1α, VEGF and MMP-9 and upregulation of HO-1.
J Ocul Pharmacol Ther. 29:539–549. 2013. View Article : Google Scholar : PubMed/NCBI
|






















