Introduction
Damaged tendons heal slowly and rarely regain the
structural integrity and mechanical strength of normal, undamaged
tendons (1). Tissue engineering
may be used to repair tendons (2).
Recent research has focused on the use of active mesenchymal cells
and growth factors as alternative strategies for tendon repair
(3).
Mesenchymal stem cells (MSCs) are adult stem cells
that have multilineage differentiation potential (4,5).
MSCs are easy to obtain, have low immunogenicity and are associated
with fewer ethical issues than embryonic stem cells (6). MSCs are primarily found in the bone
marrow; however, stem/progenitor cells with MSC-like features have
been reported in various other tissues and organs (7). It has previously been reported that
cells isolated from mouse cortical bone exhibit the morphological
and immunological phenotype of MSCs, and possess osteogenic,
adipogenic and chondrogenic differentiation potentials (8). Therefore, MSCs from murine compact
bone may be considered a suitable candidate for tendon
bioengineering.
Growth factors are frequently required for the
regulation of cell metabolism, cell proliferation, differentiation
and extracellular matrix production (9,10).
Growth differentiation factor (GDF)-5 is a member of the GDF
family, which serves an important role in limb development and
maturation (11,12). It is a member of the tumor growth
factor (TGF)-β superfamily, which is involved in cell growth and
differentiation in embryonic and adult tissues (11). Transfer of the GDF-5 gene into
chick embryos enhanced the number of cells in S phase by 53.6%
(11), and GDF-5 induced the
proliferation of rat adipose-derived MSCs (3). It has previously been reported that
GDF-5 gene therapy increases the tensile strength of rat Achilles
tendon without inducing bone or cartilage formation within the
healed tendon (13). GDF-5 is
involved in osteogenic and cartilaginous differentiation (12); however, the role of GDF-5 in
musculotendinous differentiation has rarely been reported.
Tenomodulin is primarily expressed in tendons,
ligaments and eyes (14), and
serves an important role in tendon tissue growth (15). Tenomodulin and chondromodulin I act
together as regulators of cell proliferation and differentiation
(14). An association between
tendon formation and tenomodulin expression has previously been
reported (16,17). Deletion of the tenomodulin gene
reduced the mitotic activity of tendon cells, and affected the
formation and size of collagen fibers (14). In addition, previous studies have
demonstrated that tenomodulin is involved in regulation of the
tendon maturation process (18,19).
Therefore, these results suggest that tenomodulin may have a key
role in tendon tissue growth.
p38 mitogen-activated protein kinase (MAPK) is a
classical signaling pathway in mammals. p38 signal transduction is
involved in cell growth, development, differentiation, apoptosis,
and a series of physiological and pathological processes. In
addition, deletion of the gene encoding myostatin (alternatively
known as GDF-8) affected the expression of tenomodulin via p38
(20). p38 activation is involved
in the expression of numerous factors involved in tendon remodeling
(21), including tenomodulin
(20); however, p38 may be a
non-obligate modulator of tenomodulin (22).
Previous literature has suggested the existence of
certain associations between the p38 MAPK signaling pathway and
tenomodulin expression; therefore, the present study aimed to
investigate the effects of GDF-5 on the regulation of tenomodulin
expression via the p38 MAPK signaling pathway. These results may
improve understanding of the underlying molecular mechanisms
involved in tendon repair.
Materials and methods
Animals
C57BL/6 female mice (age, 2–3 weeks; weight, 15–20
g) were purchased from the Laboratory Animal Center of The First
Affiliated Hospital of Harbin Medical University (Harbin, China).
The mice were maintained at 22±2°C and 40% humidity in a specific
pathogen-free room under a 12 h light/dark cycle. The mice were
given free access to standard mouse chow and tap water. All
procedures and animal experiments were approved by the Animal Care
and Use Committee of Harbin Medical University (Harbin, China).
Isolation and culture of MSCs from
murine compact bone
MSCs were isolated from compact bones, as previously
described (8). Four mice were
anaesthetized with 3% sodium pentobarbital (50 mg/kg
intraperitoneally), sacrificed by cervical dislocation and placed
in a beaker with 100 ml 75% (v/v) ethanol for 3 min. Mice were
placed in a 100-mm sterile glass dish and a circular incision was
made from the lower part of the abdomen (ventral side), from the
shoulder to the middle back. The skin was pulled and the femurs and
humeri were cut below the femoral heads and at the axillae. The
bones were cleaned of muscles and tendons, and were stored in a
sterile glass dish with 5 ml α-minimal essential medium (α-MEM;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing
0.1% (v/v) penicillin/streptomycin (Hyclone; GE Healthcare Life
Sciences) and 2% (v/v) fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences). The marrow cavity was exposed by
severing the epiphyses and was flushed thoroughly with α-MEM until
the bone turned pale. The humeri, tibiae and femurs were cut into
small sections (~1 mm3) and transferred into a
25-cm2 flask with 3 ml α-MEM containing 10% (v/v) FBS
and 0.15% (w/v) collagenase II (Sigma-C-6885; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). The sections were digested for 1–2 h in
a shaking incubator at 37°C and 200 rpm. The digestion was
terminated when the sections became loosely attached to each other.
The sections were washed ≥3 times and were subsequently transferred
into fresh 25-cm2 plastic flasks in α-MEM supplemented
with 10% (v/v) FBS and 0.1% (v/v) penicillin/streptomycin. The
flask was placed at 37°C in a 5% CO2 incubator. The
cultured medium was replaced on day 4. When cells reached 70–80%
confluence, cells were dissociated with trypsin-EDTA and passaged
at a ratio of 1:3.
Flow cytometric analysis
To identify the surface markers of MSCs from murine
compact bone, cells from passages 3–10 were harvested by trypsin
digestion and incubated with the following antibodies at room
temperature for 30 min: 2.0 µg antibody/1×106 cells/ml
fluorescein isothiocyanate (FITC) anti-mouse cluster of
differentiation (CD)44 (cat. no. 553133), peridinin chlorophyll
protein complex (PerCP) anti-mouse CD45 (cat. no. 561047),
phycoerythrin (PE) anti-mouse CD73 (cat. no. 550741) and
allophycocyanin (APC) anti-mouse CD90.2 (cat. no. 553007). In
addition, cells were incubated with the following isotype control
antibodies: 2.0 µg antibody/1×106 cells/ml FITC rat
immunoglobulin (Ig)G2b (cat. no. 556923), PerCP rat IgG2b (cat. no.
552991), PE rat IgG2a (cat. no. 555844) and APC rat IgG2a (cat. no.
551139). All antibodies were purchased from BD Biosciences
(Franklin Lakes, NJ, USA). MSCs from murine compact bone were
identified as exhibiting low expression levels of the hematopoietic
stemness marker CD45, and high expression levels of the mesenchymal
stemness markers CD44, CD73 and CD90 (23). Flow cytometry was conducted using a
FACSCalibur and data were analyzed using Cell Quest™ Pro
5.2.1, (both from BD Biosciences).
Adipogenic differentiation
MSCs from the third passage were seeded in a 24-well
plate at a density of 2×104 cells/well, and cultured at
37°C and 5% CO2. When cells reached ~100% confluence,
the medium was carefully aspirated from each well and adipogenic
induction medium was added, which contained 10−6 M
dexamethasone, 0.5 µM isobutylmethylxanthine and 10 ng/ml insulin
(Cyagen Biosciences, Santa Clara, CA, USA). α-MEM supplemented with
10% FBS served as the negative control. The medium was replaced
every 2–3 days. On day 14, the MSCs that committed to the
adipogenic lineage were filled with lipid-rich vacuoles and were
identified by staining with Oil red O. The cells were observed
under an IX53 light microscope (Olympus Corporation, Tokyo,
Japan).
Osteogenic differentiation
MSCs at the third passage were seeded in a 24-well
plate at a density of 3×103 cells/well and cultured at
37°C and 5% CO2. When cells were ~60% confluent, the
medium was carefully aspirated and osteogenic induction medium
containing 10−7 M dexamethasone, 10 mM β-glycerol
phosphate and 50 µM ascorbate-2-phosphate (Cyagen Biosciences) was
added. α-MEM supplemented with 10% FBS served as the negative
control. The medium was replaced every 2–3 days. After 3 weeks of
differentiation, the cells were fixed with 4% formalin, stained
with Alizarin red and observed under an IX53 light microscope
(Olympus Corporation).
Chondrogenic differentiation
MSCs at the third passage were seeded in 15-ml
polypropylene culture tubes at a density of 1×106
cells/tube, centrifuged at 150 × g for 5 min at room temperature,
and subsequently cultured at 37°C and 5% CO2. The cells
were pelleted every 2–3 days and incubated with complete
chondrogenic medium containing 10−7 M dexamethasone, 1%
insulin-transferrin-sodium selenite, 50 µM ascorbate-2-phosphate, 1
mM sodium pyruvate, 50 µg/ml proline and 20 ng/ml TGF-β3 (Cyagen
Biosciences). α-MEM supplemented with 10% FBS served as the
negative control. The medium was replaced every 2–3 days.
Chondrogenic pellets were harvested after 21 days. Pellets were
fixed in 4% formalin and embedded in paraffin for Alcian blue
staining, which was used to detect synthesis of proteoglycans by
chondrocytes, and observed under a IX53 light microscope (Olympus
Corporation).
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was performed to evaluate the effects of GDF-5 on
the viability of MSCs. Cells at passage 3 were seeded in 96-well
plates at a density of 1×103 cells/well with 100 µl
medium, which was replaced every 2 days. To study the concentration
kinetics of GDF-5, MSCs were maintained in culture medium
containing GDF-5 protein (PeproTech, Inc., Rocky Hill, NJ, USA) at
0, 1, 10, 100 and 1,000 ng/ml at 37°C for 4 days. In this
experiment, 100 ng/ml GDF-5 exhibited the most significant
difference compared with other concentrations tested; therefore,
100 ng/ml GDF-5 was selected for subsequent experiments. Cells were
cultured with or without 100 ng/ml GDF-5 and were analyzed after 3,
6, 9 and 12 days. CCK-8 was added and the plates were incubated for
1–2 h. The absorbance was measured at a wavelength of 490 nm using
a Synergy H4 microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA) and cell viability was calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
MSCs at the third passage were seeded in a 6-well
plate at a density of 5×104 cells/well and cultured at
37°C and 5% CO2. To study the concentration kinetics of
GDF-5, cells were maintained in culture medium supplemented with
GDF-5 at 0, 1, 10, 100 and 1,000 ng/ml at 37°C for 4 days. On day
4, cells were harvested for RNA extraction. Total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RNA purity was determined via absorbance at 260
and 280 nm (A260/280). cDNA synthesis was performed using the
Accupower RocketScript RT PreMix (Bioneer Corporation, Daejeon,
Korea) according to the manufacturer's protocol. Using the
SYBR®-Green Real-Time PCR master mix kit (Tiangen
Biotech Co., Ltd., Beijing, China), qPCR was performed with 25 µl
SYBR®-Green master mix and the following primers:
Tenomodulin, forward 5′-CACCAGACAAGCAAGCGAG-3′, reverse
5′-GCAGTAGGGGTATGGGTAGTAG-3′; and GAPDH, forward
5′-AAACCCATCACCATCTTCCA-3′ and reverse 5′-GTGGTTCACACCCATCACAA-3′,
on a 7500 Real-Time PCR system (Applied Biosystems, Thermo Fisher
Scientific, Inc.). Cycling conditions were as follows: Initial
denaturation at 95°C for 2 min, followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 30 sec. The quantification cycle (Cq) value
was defined as the number of cycles in which the fluorescence
signal exceeded the detection threshold value. The
2−ΔΔCq quantification method (24) was used to quantify PCR results and
the values were normalized to GAPDH.
Western blotting
MSCs at the third passage were seeded in a 6-well
plate at a density of 5×104 cells/well and cultured at
37°C and 5% CO2. To determine the effects of GDF-5 on
the activation of p38, cells were synchronized and stimulated with
100 ng/ml GDF-5 for 0, 5, 10, 30 and 60 min, and 6 and 24 h, at
37°C; subsequently, the expression levels of relevant proteins were
detected by western blotting.
Alternatively, MSCs at the third passage were seeded
in a 6-well plate at a density of 5×104 cells/well and
cultured at 37°C and 5% CO2. Cells were synchronized and
pre-stimulated with 10 µM SB203580 (Tocris Bioscience, Bristol, UK;
an inhibitor of p38 MAPK) at 37°C for 1 h. The control group was
treated with an equivalent volume of 0.1% dimethyl sulfoxide. The
cells were then treated with 100 ng/ml GDF-5 for 30 min, which
resulted in optimal activation of GDF-5. The expression levels of
the relevant proteins were detected by western blotting 30 min
after GDF-5 induction.
Cell lysates were prepared using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing 0.1 M phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Following extraction, the protein
concentrations of lysates were determined by a bicinchoninic acid
assay (Beyotime Institute of Biotechnology). Equal quantities of
protein (80 µg) were separated by 10% SDS-PAGE prior to transfer
onto nitrocellulose membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). Membranes were blocked with 5% non-fat dry milk
at 4°C overnight. Membranes were subsequently incubated with
primary antibodies against tenomodulin (1:1,000; ab203676; Abcam,
Cambridge, UK), p38, phosphorylated (p)-p38 (1:1,000; B0798 and
A7179; Assay Biotechnology Company, Inc., Sunnyvale, CA, USA) and
β-actin (1:1,000; SC47778; OriGene Technologies, Inc., Beijing,
China) at 4°C overnight. Following washing with PBS, membranes were
incubated with a horseradish peroxidase-conjugated IgG secondary
antibody (1:5,000; ZDR-5118; ZSGB-Bio Co., Ltd.) for 2 h. Proteins
were detected using an Enhanced Chemiluminescence reagent (Pierce;
Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
of three independent experiments. Statistical significance was
evaluated by a Student's t-test or one-way analysis of variance
with Student-Newman-Keuls post hoc test. All statistical analyses
were conducted using SPSS version 12.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of MSCs from murine
compact bone
Cells were observed growing from the bone specimens
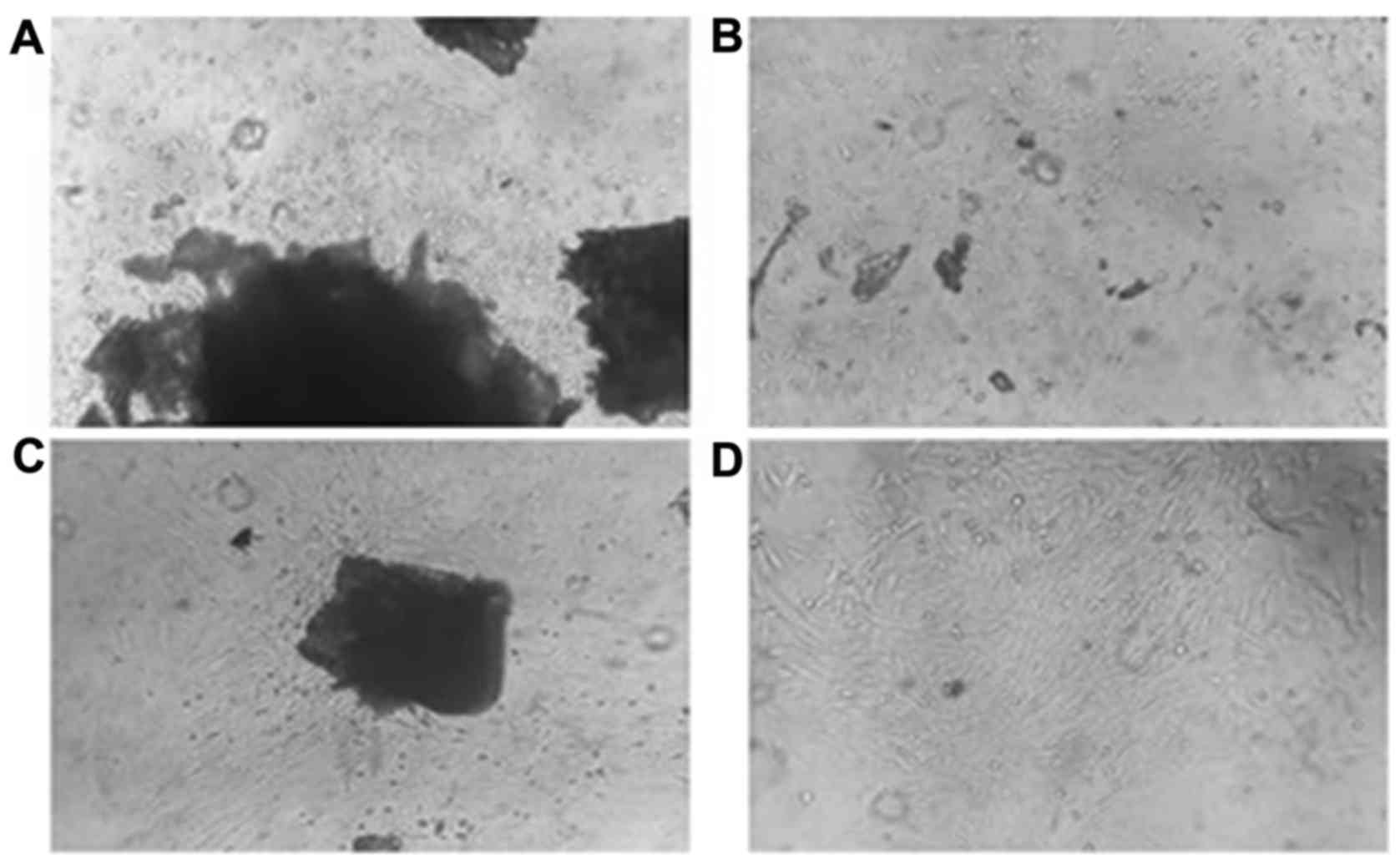
after 48 h cultivation (Fig. 1A).
The bone specimens were removed from the flask after the third
passage; Fig. 1B demonstrates the
distribution of the cells observed in the culture flask without
bone specimens. After 96 h culture, cells were observed surrounding
the bone pieces, adhering to the plate and exhibited a radial
growth typical of MSCs (Fig. 1C).
After the third generation, cell morphology was stable and
proliferation was rapid (Fig.
1D).
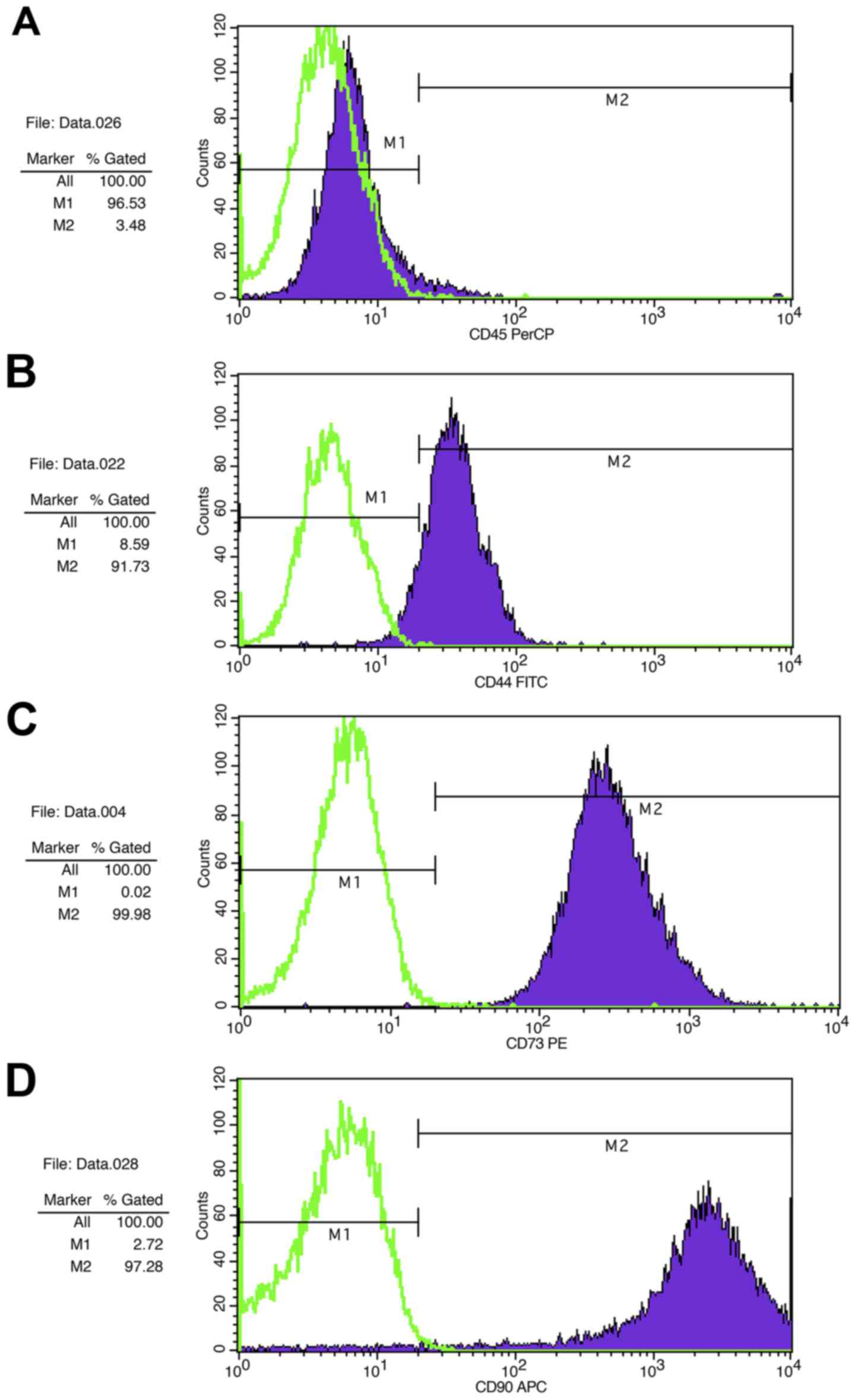
Flow cytometric analysis revealed that MSCs from
murine compact bone had a classical MSC phenotype, as they
exhibited low expression of the hematopoietic stem marker CD45
(Fig. 2A), and high expression of
the mesenchymal stem markers CD44 (Fig. 2B), CD73 (Fig. 2C) and CD90 (Fig. 2D).
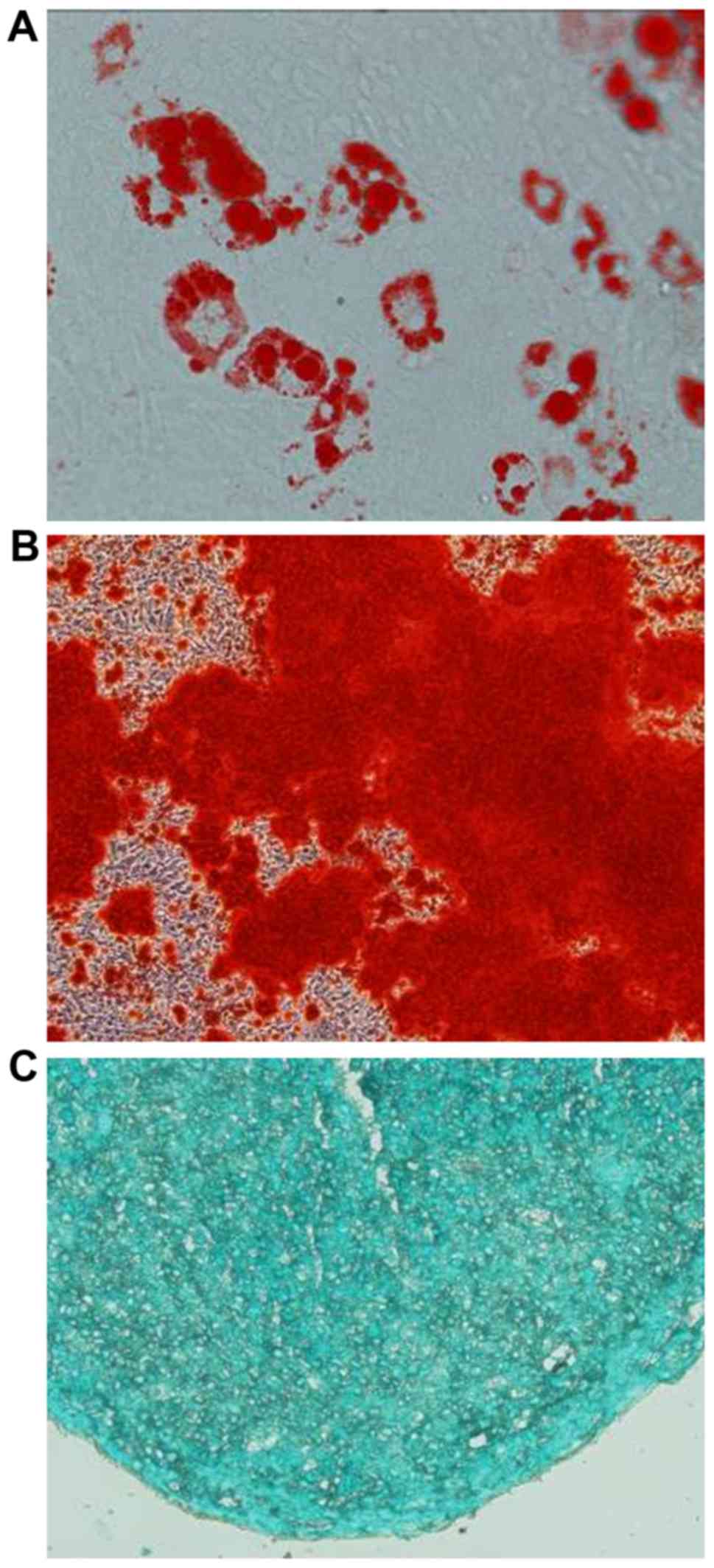
As shown in Fig. 3,
MSCs isolated from murine compact bone were pluripotent and
exhibited adipogenic, osteoblastic and chondrocyte differentiation
potentials.
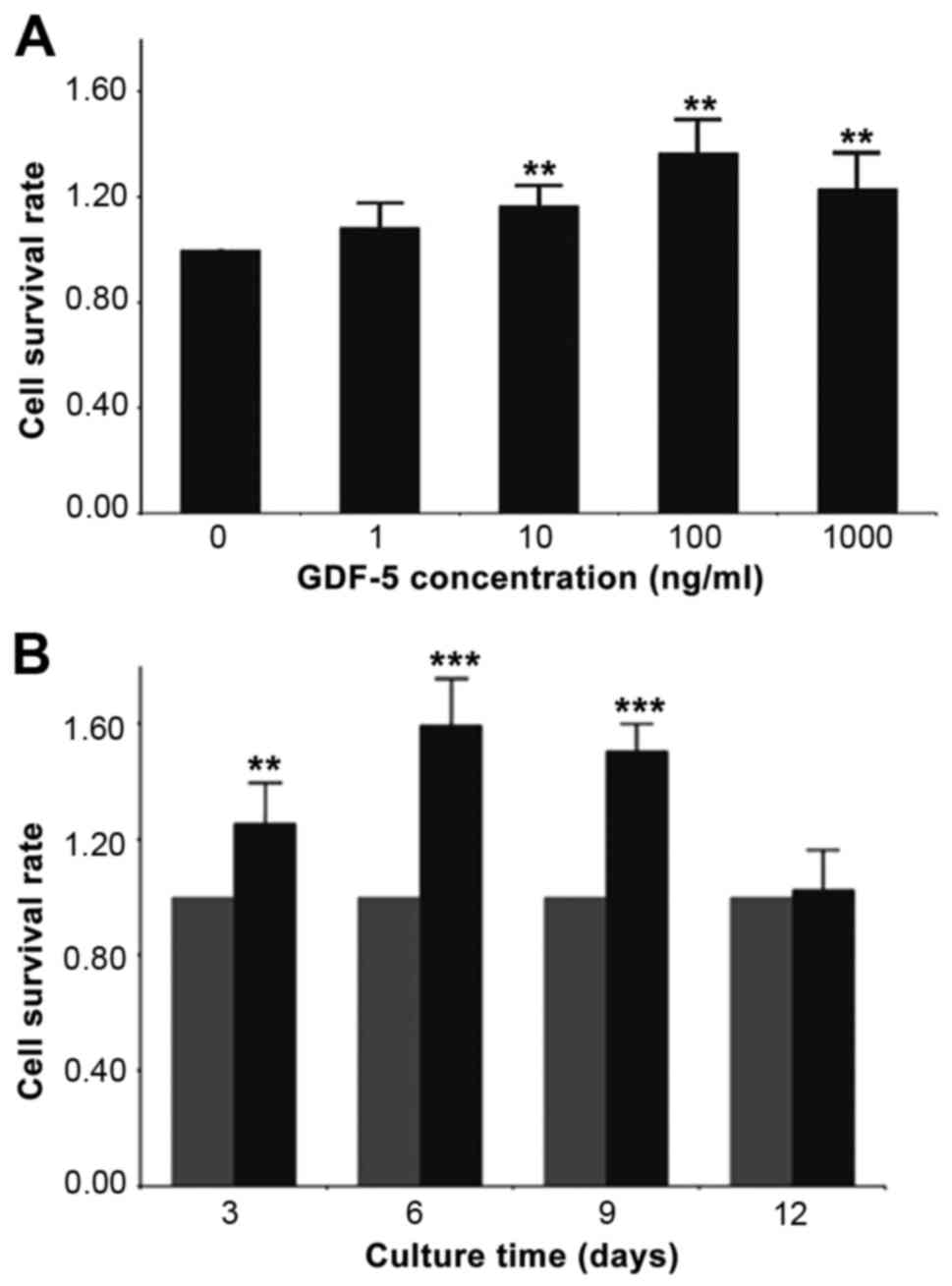
Effects of concentration and duration
of GDF-5 treatment on MSC viability
Compared with the control group (0 ng/ml GDF-5),
treatment with 10–1,000 ng/ml GDF-5 enhanced cell viability (10
ng/ml, +16.7%; 100 ng/ml, +36.9%; 1,000 ng/ml, +23.2%; P<0.01;
Fig. 4A); the greatest difference
was achieved using 100 ng/ml GDF-5. When determining the effects of
various GDP-5 treatment durations, results revealed that GDF-5
enhanced cell viability from day 3 to 9 compared with the control
group (P<0.01; Fig. 4B), and
reached a peak on day 6 (day 3, +25.6%; day 6, +56.6%; day 9,
+50.5%). There was no difference compared with the control on day
12 (P>0.05).
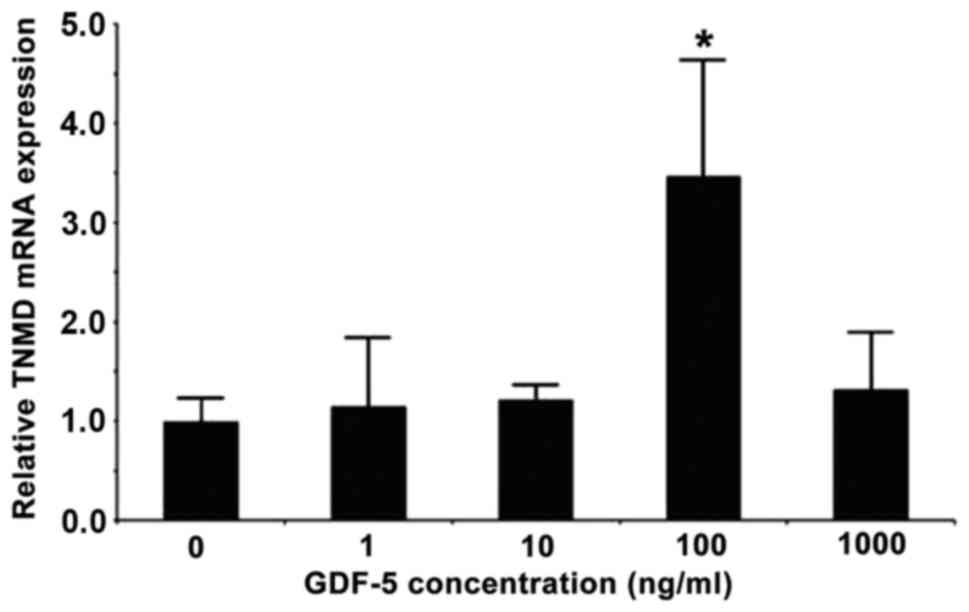
Effects of GDF-5 on tenomodulin mRNA
expression in MSCs from compact bone
Compared with the control group (0 ng/ml GDF-5), the
mRNA expression levels of tenomodulin were increased following
treatment with 100 ng/ml GDF-5 (+249%; P<0.05; Fig. 5). However, there was no difference
in expression after treatment with 1, 10 or 1,000 ng/ml GDF-5
(P>0.05 vs. 0 ng/ml GDF-5; Fig.
5).
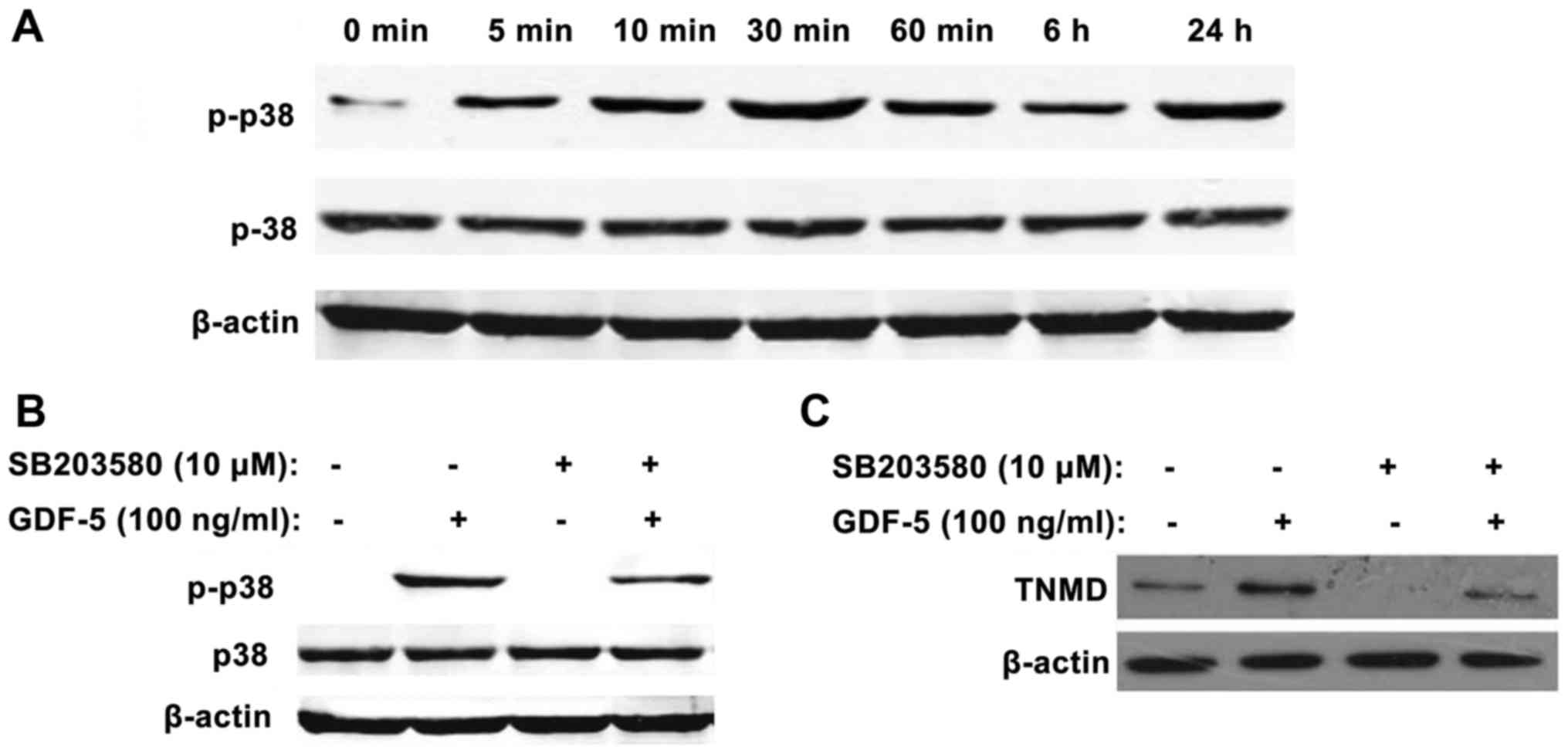
GDF-5 treatment increases the protein
expression of tenomodulin via phosphorylation of p38 in MSCs from
murine compact bone
Treatment of MSCs with GDF-5 increased the protein
expression levels of p-p38; this increase was initially observed
after 5 min, reached a peak at 30 min and declined from 60 min to
24 h (Fig. 6A), compared with the
control group (0 ng/ml GDF-5). These results suggested that p38 may
be activated by GDF-5, thus increasing the expression levels of
p-p38.
MSCs were cultured for 60 min with SB203580 prior to
culture with GDF-5 for 30 min. In the control group, the expression
levels of p-p38 were markedly enhanced by GDF-5. Compared with the
control group, the expression of p38 was not affected by SB203580
treatment; however, the expression of p-p38 was markedly inhibited
in GDF-5-treated cells by SB203580 (Fig. 6B).
Compared with the control group, the protein
expression levels of tenomodulin were enhanced following 4 days of
incubation with GDF-5. However, when comparing the GDF-5 + SB203580
group with the GDF-5 treatment group, the expression of tenomodulin
was suppressed by 1 h pretreatment with 10 µM SB203580 (Fig. 6C).
Discussion
GDF-5 serves a role in tissue development and
tenomodulin serves an important role in the development of tendons;
however, the effects of GDF-5 on MSC viability and its use in
tendon bioengineering remain to be elucidated. The present study
aimed to investigate the effects of GDF-5 on viability and
tenomodulin expression of MSCs from murine compact bone. GDF-5
treatment promoted MSC viability and increased the protein
expression levels of tenomodulin in MSCs from murine compact bone.
Therefore, the use of GDF-5 may be of value for tendon
engineering.
Previous studies (25,26)
have investigated bone marrow-derived MSC extraction, screening,
purification, culture and applications. However, the culture of
mouse bone marrow-derived MSCs has certain disadvantages. Notably,
mouse MSC isolation often yields a low number of stem cells
(27): ~1 per million (28). In addition, when the whole bone
marrow adherence method is utilized, mouse bone marrow
hematopoietic stem cells are difficult to remove due to their
strong adherence ability, which markedly affects results.
Furthermore, the methods of separation are complex and expensive
(27,28). The present study suggested that
MSCs may be successfully isolated from murine compact bone. To
achieve optimal results, bone chips should be ~1 mm3, as
large chips may impair cell migration, whereas small chips may
result in cells escaping during digestion. However, the
optimization of isolation and purification of MSCs from murine
compact bone remains to be achieved.
Stem cells are required for tendon tissue
engineering, and these cells have to be in adequate numbers
(29). During preparation, stem
cells may suffer from apoptosis due to alterations in the physical
and chemical environment. Therefore, induction of stem cells may
strengthen their proliferative activity, in order to improve the
success rate of tendon tissue engineering (29). Park et al (3) reported that GDF-5 may induce the
proliferation of rat adipose-derived MSCs. An MTT assay revealed
that 10 ng/ml GDF-5 enhanced the proliferation of cells, and 100
ng/ml GDF-5 achieved greater effects (3). Although 1,000 ng/ml GDF-5
additionally improved cell viability, it was limited (3). These findings were concordant with
the results of the present study, which used the CCK-8 method to
analyze cell viability. Results revealed that cell viability was
not increased following treatment with 1 ng/ml GDF-5; however,
viability was increased with 10 ng/ml GDF-5 and peaked using 100
ng/ml GDF-5. Conversely, viability was lower following treatment
with 1,000 ng/ml GDF-5. These results suggested that there was a
dose-dependent association between GDF-5 and MSC viability.
In the time-effect experiments, cell viability was
greatest on day 6 and declined by day 9. The viability of
GDF-5-treated cells was similar to control cells by day 12. This
may be due to contact inhibition (cells growing to confluence) and
the availability of nutrients. In the present study, a 2D culture
method was utilized, thus limiting the culture space. However, the
results demonstrated a time-dependent association between GDF-5
treatment and MSC viability.
Treatment with 100 ng/ml GDF-5 may enhance
tenomodulin expression levels in adipose tissue-derived MSCs
(3) and in MSCs cultured on
scaffolds (30); these previous
findings supported the results of the present study. Therefore, it
may be suggested that in the process of constructing
tissue-engineered tendons, the presence of GDF-5 in seeded cells
may induce tendon cell differentiation.
GDF-5 is known to activate p38-MAPK (31,32)
and extracellular signal-regulated kinase 1/2 (33) signaling pathways, which are
involved in cell growth, proliferation and death. In addition, a
mouse model demonstrated that knockdown of GDF-5 resulted in
reduced p38 phosphorylation (34).
p38 activation affects the expression of numerous factors involved
in tendon remodeling (21),
including tenomodulin (20).
However, p38 may be a non-obligate modulator of tenomodulin
(22). The present study revealed
that GDF-5 treatment increased tenomodulin expression; however,
pretreatment with the p38-specific inhibitor, SB203580, resulted in
reduced tenomodulin expression. These results suggested that p38
may be involved in GDF-5-mediated MSC viability. The present study
did not investigate the underlying molecular mechanisms of this
process. Additional studies are required to determine this
association and whether it may be used for tendon
bioengineering.
In conclusion, GDF-5 treatment promoted MSC
viability, and increased the protein expression levels of
tenomodulin via phosphorylation of p38 in MSCs from murine compact
bones. These results may aid the future development of tendon
bioengineering.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 30500508)
and the Natural Science Foundation of Heilongjiang Province of
China (grant no. D201112).
References
|
1
|
Sharma P and Maffulli N: Biology of tendon
injury: Healing, modeling, and remodeling. J Musculoskelet Neuronal
Interact. 6:181–190. 2006.PubMed/NCBI
|
|
2
|
DeFranco MJ, Derwin K and Iannotti JP: New
therapies in tendon reconstruction. J Am Acad Orthop Surg.
12:298–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park A, Hogan MV, Kesturu GS, James R,
Balian G and Chhabra AB: Adipose-derived mesenchymal stem cells
treated with growth differentiation factor-5 express
tendon-specific markers. Tissue Eng Part A. 16:2941–2951. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou S, Yates KE, Eid K and Glowacki J:
Demineralized bone promotes chondrocyte or osteoblast
differentiation of human marrow stromal cells cultured in collagen
sponges. Cell Tissue Bank. 6:33–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howard D, Buttery LD, Shakesheff KM and
Roberts SJ: Tissue engineering: Strategies, stem cells and
scaffolds. J Anat. 213:66–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
da Silva Meirelles L, Chagastelles PC and
Nardi NB: Mesenchymal stem cells reside in virtually all post-natal
organs and tissues. J Cell Sci. 119:2204–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H, Guo ZK, Jiang XX, Li H, Wang XY,
Yao HY, Zhang Y and Mao N: A protocol for isolation and culture of
mesenchymal stem cells from mouse compact bone. Nat Protoc.
5:550–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oshin AO, Caporali E, Byron CR, Stewart AA
and Stewart MC: Phenotypic maintenance of articular chondrocytes in
vitro requires BMP activity. Vet Comp Orthop Traumatol. 20:185–191.
2007.PubMed/NCBI
|
|
10
|
Aspenberg P: Stimulation of tendon repair:
Mechanical loading, GDFs and platelets. A mini-review. Int Orthop.
31:783–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buxton P, Edwards C, Archer CW and
Francis-West P: Growth/differentiation factor-5 (GDF-5) and
skeletal development. J Bone Joint Surg Am. 83-A Suppl 1:S23–S30.
2001.
|
|
12
|
Daans M, Luyten FP and Lories RJ: GDF5
deficiency in mice is associated with instability-driven joint
damage, gait and subchondral bone changes. Ann Rheum Dis.
70:208–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolt P, Clerk AN, Luu HH, Kang Q, Kummer
JL, Deng ZL, Olson K, Primus F, Montag AG, He TC, et al: BMP-14
gene therapy increases tendon tensile strength in a rat model of
Achilles tendon injury. J Bone Joint Surg Am. 89:1315–1320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Docheva D, Hunziker EB, Fässler R and
Brandau O: Tenomodulin is necessary for tenocyte proliferation and
tendon maturation. Mol Cell Biol. 25:699–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aslan H, Kimelman-Bleich N, Pelled G and
Gazit D: Molecular targets for tendon neoformation. J Clin Invest.
118:439–444. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukunami C, Takimoto A, Miura S,
Nishizaki Y and Hiraki Y: Chondromodulin-I and tenomodulin are
differentially expressed in the avascular mesenchyme during mouse
and chick development. Cell Tissue Res. 332:111–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shukunami C, Takimoto A, Oro M and Hiraki
Y: Scleraxis positively regulates the expression of tenomodulin, a
differentiation marker of tenocytes. Dev Biol. 298:234–247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pisani DF, Pierson PM, Massoudi A, Leclerc
L, Chopard A, Marini JF and Dechesne CA: Myodulin is a novel
potential angiogenic factor in skeletal muscle. Exp Cell Res.
292:40–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukunami C and Hiraki Y: Chondromodulin-I
and tenomodulin: The negative control of angiogenesis in connective
tissue. Curr Pharm Des. 13:2101–2112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mendias CL, Bakhurin KI and Faulkner JA:
Tendons of myostatin-deficient mice are small, brittle, and
hypocellular. Proc Natl Acad Sci USA. 105:pp. 388–393. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Popov C, Burggraf M, Kreja L, Ignatius A,
Schieker M and Docheva D: Mechanical stimulation of human tendon
stem/progenitor cells results in upregulation of matrix proteins,
integrins and MMPs and activation of p38 and ERK1/2 kinases. BMC
Mol Biol. 16:62015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartz AJ, Sarver DC, Sugg KB,
Dzierzawski JT, Gumucio JP and Mendias CL: p38 MAPK signaling in
postnatal tendon growth and remodeling. PLoS One. 10:e01200442015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pereira RF, Halford KW, O' Hara MD, Leeper
DB, Sokolov BP, Pollard MD, Bagasra O and Prockop DJ: Cultured
adherent cells from marrow can serve as long-lasting precursor
cells for bone, cartilage, and lung in irradiated mice. Proc Natl
Acad Sci USA. 92:pp. 4857–4861. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prockop DJ: Marrow stromal cells as stem
cells for non hematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varma MJ, Breuls RG, Schouten TE, Jurgens
WJ, Bontkes HJ, Schuurhuis GJ, van Ham SM and van Milligen FJ:
Phenotypical and functional characterization of freshly isolated
adipose tissue-derived stem cells. Stem Cells Dev. 16:91–104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phinney DG, Kopen G, Isaacson RL and
Prockop DJ: Plastic adherent stromal cells from the bone marrow of
commonly used strains of inbred mice: Variations in yield, growth,
and differentiation. J Cell Biochem. 72:570–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin Z, Chen X, Chen JL and Ouyang HW: Stem
cells for tendon tissue engineering and regeneration. Expert Opin
Biol Ther. 10:689–700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farng E, Urdaneta AR, Barba D, Esmende S
and McAllister DR: The effects of GDF-5 and uniaxial strain on
mesenchymal stem cells in 3-D culture. Clin Orthop Relat Res.
466:1930–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura K, Shirai T, Morishita S, Uchida
S, Saeki-Miura K and Makishima F: p38 mitogen-activated protein
kinase functionally contributes to chondrogenesis induced by
growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res.
250:351–363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coleman CM and Tuan RS: Functional role of
growth/differentiation factor 5 in chondrogenesis of limb
mesenchymal cells. Mech Dev. 120:823–836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe H, de Caestecker MP and Yamada Y:
Transcriptional cross-talk between Smad, ERK1/2 and p38
mitogen-activated protein kinase pathways regulates transforming
growth factor-beta-induced aggrecan gene expression in chondrogenic
ATDC5 cells. J Biol Chem. 276:14466–14473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zaidi SH, Huang Q, Momen A, Riazi A and
Husain M: Growth differentiation factor 5 regulates cardiac repair
after myocardial infarction. J Am Coll Cardiol. 55:135–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|