Introduction
Gold nanoparticles (GNPs) have good optical
properties, good biocompatibility and are harmless. Therefore, GNPs
have been applied to biochemical and medical analysis, biosensors,
genomics, immunology, wound healing, and tumor diagnosis and
treatment (1). The nano-gold
complex consists of gold nanoparticles with a diameter of 1–100 nm
(2) surface-modified by covalent
or non-covalent bonding to specific molecules or drugs, which are
used in the diagnosis and treatment of multiple cancers. GNPs
exhibit enormous potential, from basic research to clinical
applications. Its use as a treatment for cancer has been approved
by the FDA and has entered the early stages of clinical trials
(3). The surface of GNPs readily
binds to chemotherapy drugs and functional ligands (4). This combination of capabilities
extends the functionality and applications of GNPs, and they have
also been used as molecular probes, and for optical imaging. Song
et al (5) used gold
nanorods as lymph tracer in a mouse model and successfully obtained
sentinel lymph node photoacoustic imaging in vivo.
Polymer microbubbles are an ultrasound contrast
agent that demonstrates good uniformity, high stability, a long
retention time in vivo, good anti-stress performance and
weak sound attenuation characteristics. Currently the most widely
used is poly (lactic-co-glycolic) acid (PLGA). Néstor et al
(6) evaluated the echogenic power
and stability of air-filled PLGA nanocapsules with a mean diameter
of 370±96 nm in vitro. Kohl et al (7) prepared and evaluated multifunctional
PLGA nanoparticles (NPs) for use in photoacoustic imaging. Xu et
al (8) developed PLGA
nanobubbles (mean diameter 268 nm) for cancer targeting and
imaging. Ke et al (9)
prepared the nanocomposite through combined GNPs with a polymer
microcapsule shell to operate as a new multi-functional agent for
both contrast enhanced ultrasonic imaging and photohyperthermia.
Good ultrasonic imaging results were achieved as a result.
Therefore, it is possible to hypothesize that the
combination of gold and polymer nanobubbles significantly increases
the echo signal of the PLGA nanobubble contrast agent, improving
the ultrasound contrast performance. The aim of the present study
was to prepare a gold-poly(lactic-co-glycolic) acid (Au-PLGA)
complex and investigate the feasibility of using this complex to
enhance ultrasound imaging in vitro.
Materials and methods
Materials
PLGA (50:50; 40k MW) was obtained from Shandong
Jinan Daigang Biomaterial Co., Ltd. (Shandong, China).
Thiol-terminated methoxy polyethylene glycol
(H2N-PEG1000-SH; 1k MW) was obtained from Shanghai Yare
Biotech, Co., Ltd. (Shanghai, China). Methylene chloride,
isopropanol, mannitol, phosphotungstic acid, hydrogen
tetra-chloroaurate (III) hydrate
(HAuCl4•4H2O) and sodium citrate were
obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China). Polyvinyl alcohol (PVA; 88% mole hydrolyzed), camphor (D+),
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and
N-Hydroxysuccinimide (NHS) were obtained from Aladdin Chemistry
Co., Ltd. (Shanghai, China). Deionized (DI) water, used in all
experiments, was purified using a Milli-Q Plus 185 water
purification system (EMD Millipore, Billerica, MA, USA) with
resistivity >18 MΩ cm. All other chemicals and reagents were of
analytical grade.
Preparation of PLGA NPs
PLGA NPs were prepared according to a previously
reported method (10). To make the
first emulsion, PLGA (125 mg) and camphor (12.5 mg) were dissolved
in methylene chloride (5 ml), followed by adding 1 ml PVA (3% w/v).
Then, the mixture was emulsified using an ultrasonic disrupter
04717 (Cole-Parmer Instrument Co., Ltd., Vernon Hills, IL, USA) at
130 W in an ice bath and sonicated for 180 sec at 4 sec on and 2
sec off. To make the second emulsion, the solution was added to 20
ml PVA (3% w/v) and sonicated as above. Following this, the
emulsion was added to 100 ml isopropyl alcohol (5% v/v) and stirred
for 1 h. The samples were subsequently obtained by centrifugation
at 17,800 × g for 10 min at 25°C, washed three times with DI
water, and lyophilized using a freeze dryer (TFD5505; Ilshin Lab
Co., Ltd., Yangju, South Korea). In a vacuum, the PLGA NPs was
filled with perfluoropropane gas (Shanghai Renjieling Optics
Instruments Co., Ltd., Shanghai, China).
Preparation of GNPs
GNPs with an average diameter of ~20 nm were
prepared according to the previously reported method (11,12).
HAuCl4•4H2O aqueous solution (0.5 ml; 1% w/v)
was added into 48 ml DI water, and the solution was stirred and
heated in an oil bath so that the temperature was raised and
stabilized at 137°C. Under vigorous stirring, 1.5 ml sodium citrate
solution (1% w/v) was added, and stirring continued for 20 min
until the solution became a clear red. It was subsequently removed
from the oil bath and cooled to room temperature with constant
stirring.
The GNP solution was prepared by filtration through
a 0.22 µm nitrocellulose membrane, so as to remove electrolytic
ions and aggregated colloidal gold particles. Glassware used for
the experiment was subjected to several rinses with DI water and
acidification.
Preparation of Au-PLGA complex
H2N-PEG1000-SH (30 mg) was dissolved in
10 ml filtered GNP solution and continuously stirred at room
temperature for 4 h. PLGA NPs (30 mg) was fully dissolved in DI
water and kept standing for 20 min to discard large particles,
which precipitated at the bottom. The suspension was centrifuged at
17,800 × g for 10 min at 25°C and washed with DI water three
times to obtain purified PLGA NPs. Subsequently, the PLGA NPs were
mixed with EDC (3 mg) and NHS (2 mg) in 10 ml DI water. The
resulting solution was stirred at room temperature for 2 h, then
centrifuged at 17,800 × g for 10 min at 25°C and washed with
DI water to expose the carboxyl groups (-COOH) of PLGA NPs. Then,
the PLGA NPs were completely suspended in GNP solution and stirred
at room temperature for another 2 h to obtain the Au-PLGA
complex.
Characterization of PLGA NPs, GNPs and
the Au-PLGA complexes
The S-4800 field emission scanning electron
microscope (Hitachi High Technologies Corporation, Tokyo, Japan)
and JEOL JEM-2100 low to high-resolution transmission electron
microscope (TEM; Hitachi High Technologies Corporation, Tokyo,
Japan) were used to observe the morphology and structure of the
nanoparticles. The PLGA NPs were stained with freshly prepared
phosphotungstic acid solution (1%, w/v) prior to TEM observation. A
drop of the PLGA NPs suspension was placed onto copper grids for 3
min and then the copper grids was immersed in phosphotungstic acid
solution for 1 min. The grids were air-dried and observed by TEM.
The size distributions and ζ potentials of the nanoparticles were
evaluated five times for each sample using dynamic light scattering
(Zetasizer Nano ZS model ZEN3690; Malvern Instruments, Ltd.,
Malvern, UK). The Varian 4000 UV-Vis spectrophotometer was used to
measure the ultraviolet (UV)/Vis absorption spectra of the
nanoparticles. To investigate the preserved stability of the
Au-PLGA complex, sample suspensions were sufficiently
ultrasonically dispersed using the mixing function of an ultrasonic
cleaning machine set to 500 W and kept standing for 0, 1, 7 and 14
days at 4°C. They were subsequently measured five times for each
sample to obtain the average diameters and ζ potentials.
In vitro ultrasound imaging of the
Au-PLGA complex
Au-PLGA complexes and PLGA NPs were dispersed in the
degassed deionized water, and different imaging parameters (imaging
times 0, 30, 60, 120 and 180 sec) and conditions (concentration of
sample 3, 2, 1, 0.5 and 0.25 mg/ml) were selected according the
purpose of the different experiments in order to observe the
corresponding imaging ability. The imaging evaluation of the
Au-PLGA complexes and PLGA NPs were performed using an ultrasonic
diagnostic instrument (MyLab Twice; Esaote SpA, Florence, Italy)
and conventional B-mode sonograms (mechanical index=0.06). The
center frequencies of the transducers were 13 and 22 MHz, according
to need.
Statistical analysis
The results were presented as the mean ± standard
deviation.
Results
Preparation and characterization of
nanoparticles
The morphology and structure of PLGA NPs, GNPs and
the Au-PLGA complexes were characterized by transmission electron
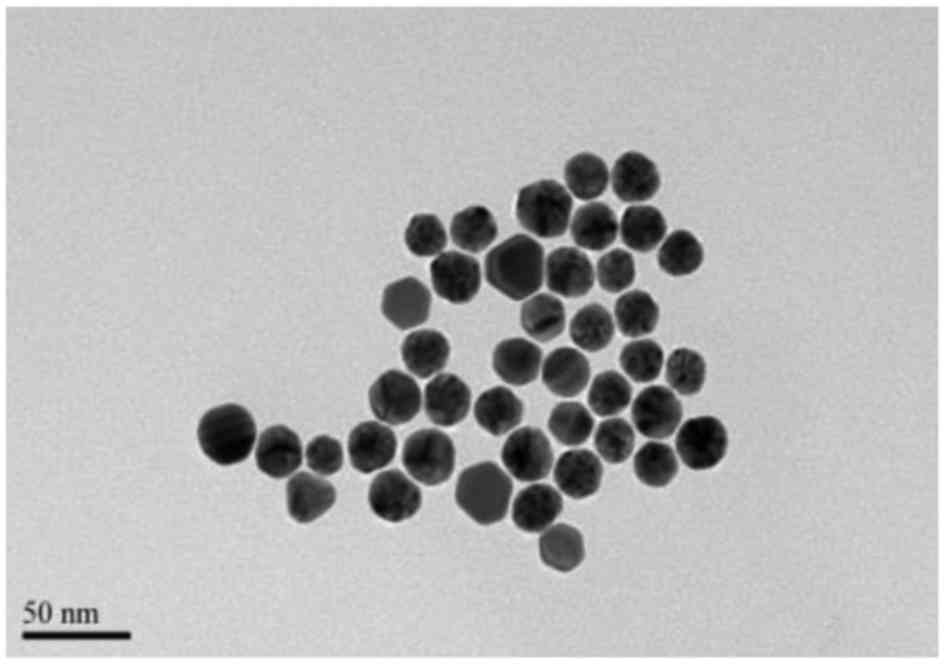
microscopy (TEM). The GNPs (Fig.
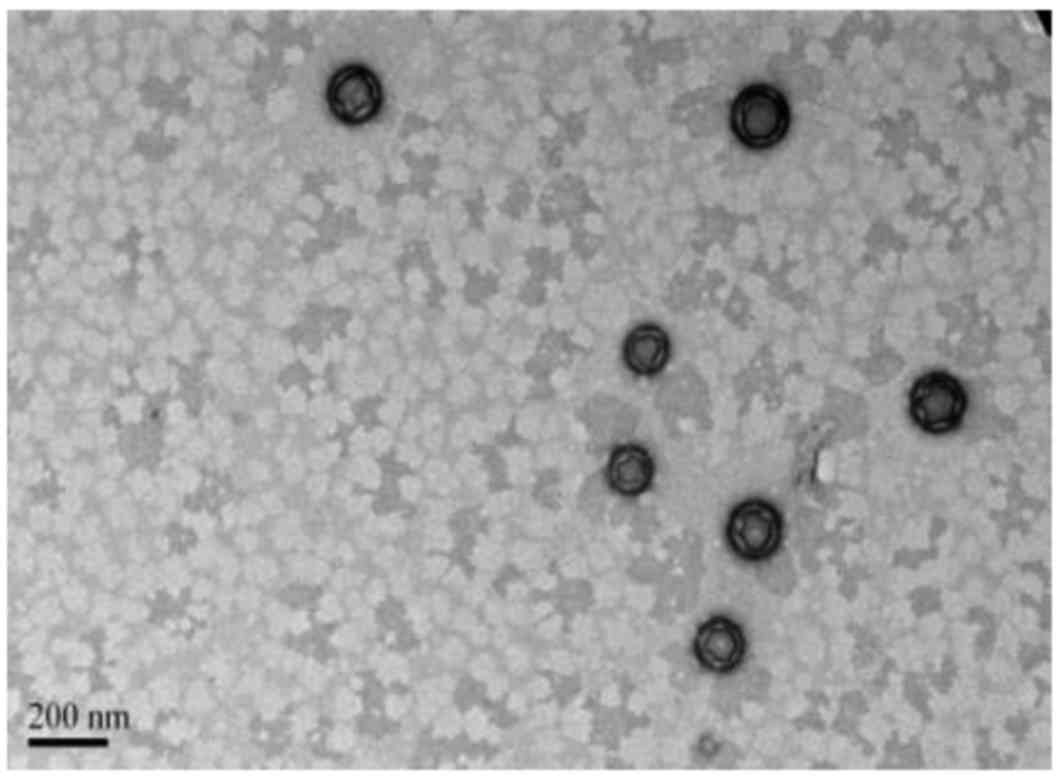
1) and PLGA NPs (Fig. 2)
demonstrated a spherical shape. GNPs had a more uniform size and
PLGA NPs demonstrated good dispersion. TEM revealed the PLGA NPs
had a thin shell that was not smooth but wrinkled, due to the TEM
electron beam across the thin, cavity-rich particles (Fig. 2). DLS revealed that the GNPs and
PLGA NPs had diameters of 21.42±1.56 and 144.5±30.28 nm,
respectively (Table I). The
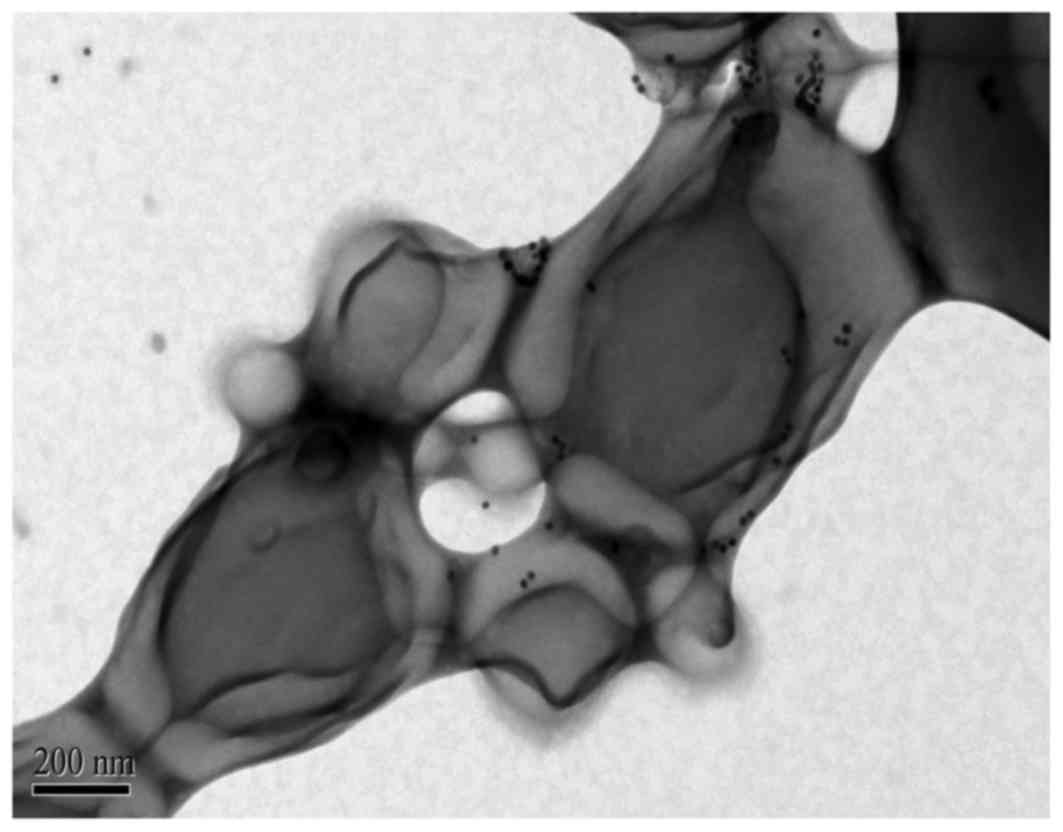
Au-PLGA complexes also had spherical shape following the
combination of GNPs with PLGA NPs (Figs. 3 and 4) and the average diameter of the complex
increased to ~359.4±67.94 nm (Table
I). As time passed from 0 to 14 days, the diameters and ζ
potentials of the Au-PLGA complexes changed from 359.4±67.94 to
398.19±42.34 nm and −4.80±4.52 to −4.96±3.02 mv, respectively,
indicating that it is stable (Table
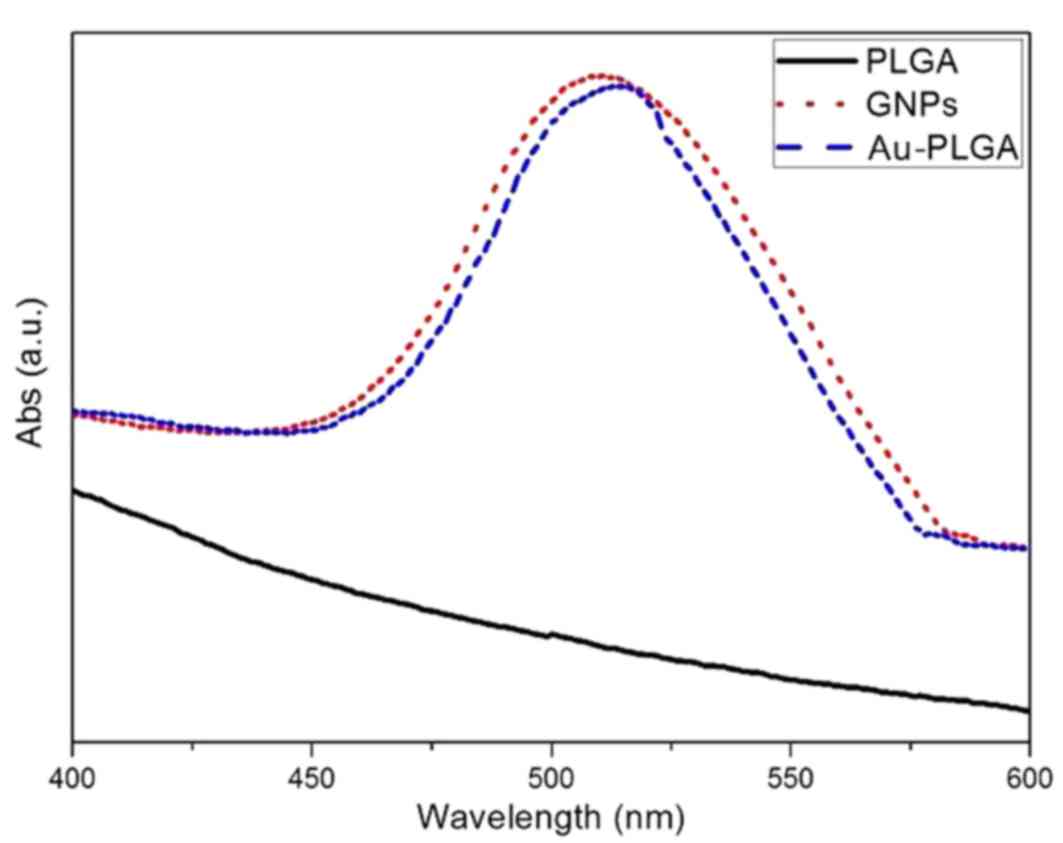
II). UV-visible extinction spectra revealed that GNPs have a
maximum absorption peak at 508 nm, while the Au-PLGA complex had a
maximum absorption peak at 515 nm (Fig. 5). The maximum peak absorption curve
demonstrated redshift, which determined the initial PLGA NPs and
GNPs combined with each other successfully.
 | Table I.Physical characteristics of
nanoparticles (χ ± S). |
Table I.
Physical characteristics of
nanoparticles (χ ± S).
| Nanoparticles | Diameter (nm) | ζ-potential (mv) |
|---|
| GNPs | 21.42±1.56 | −28.8±3.88 |
| PLGA | 144.5±30.28 | −25.20±4.01 |
| Au-PLGA | 359.4±67.94 | −4.80±4.52 |
 | Table II.Physical characteristics of the
Au-PLGA complex (χ ± S). |
Table II.
Physical characteristics of the
Au-PLGA complex (χ ± S).
| Au-PLGA | 0 days | 1 day | 7 days | 14 days |
|---|
| Diameter (nm) | 359.4±67.94 | 365.19±32.47 | 377.16±23.54 | 398.19±42.34 |
| ζ (mv) | −4.80±4.52 | −4.57±2.32 | −4.6±2.7 | −4.96±3.02 |
In vitro imaging
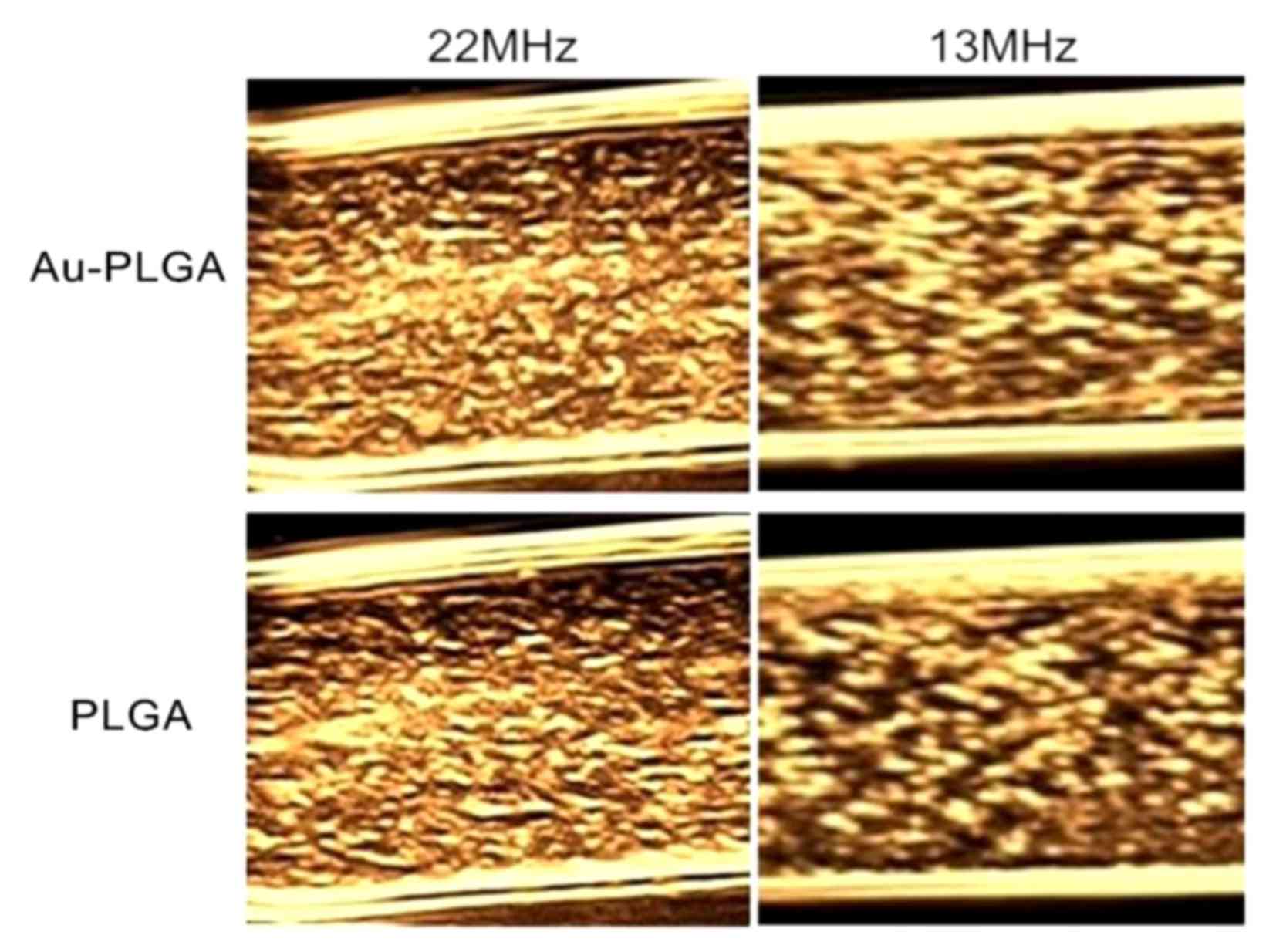
The effect of different frequencies of imaging was
studied under a certain concentration. The same concentrations (3
mg/ml) of the Au-PLGA complex and PLGA NPs ultrasound contrast
agents at 22 MHz and 13 MHz produced good images, but at 22 MHz
demonstrated improved echoing (Fig.
6). At the same frequency under 22 MHz or 13 MHz condition, the
echo signal of the Au-PLGA complexes was stronger than PLGA
NPs.
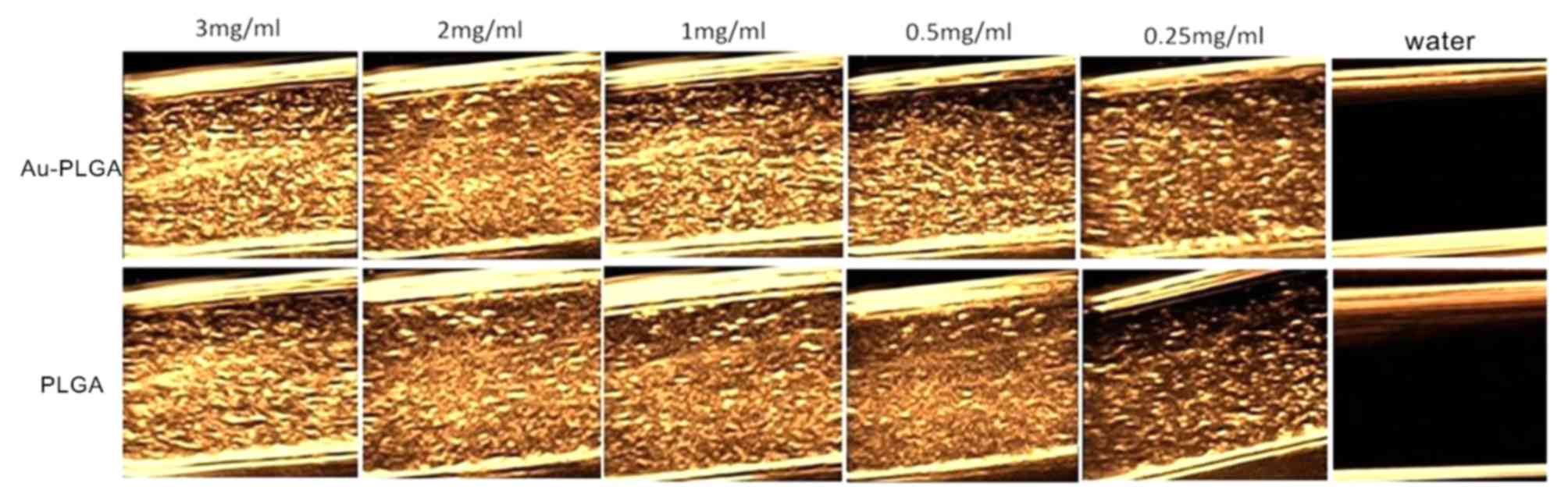
The ultrasound signals gradually decreased with
decreasing concentrations of Au-PLGA complexes and PLGA NPs at 22
MHz (Fig. 7). The strongest echo
was demonstrated at the maximum 3 mg/ml for Au-PLGA complexes and
PLGA NPs. At the same concentration of Au-PLGA complexes and PLGA
NPs, the signal intensity of the former was improved compared with
the latter.
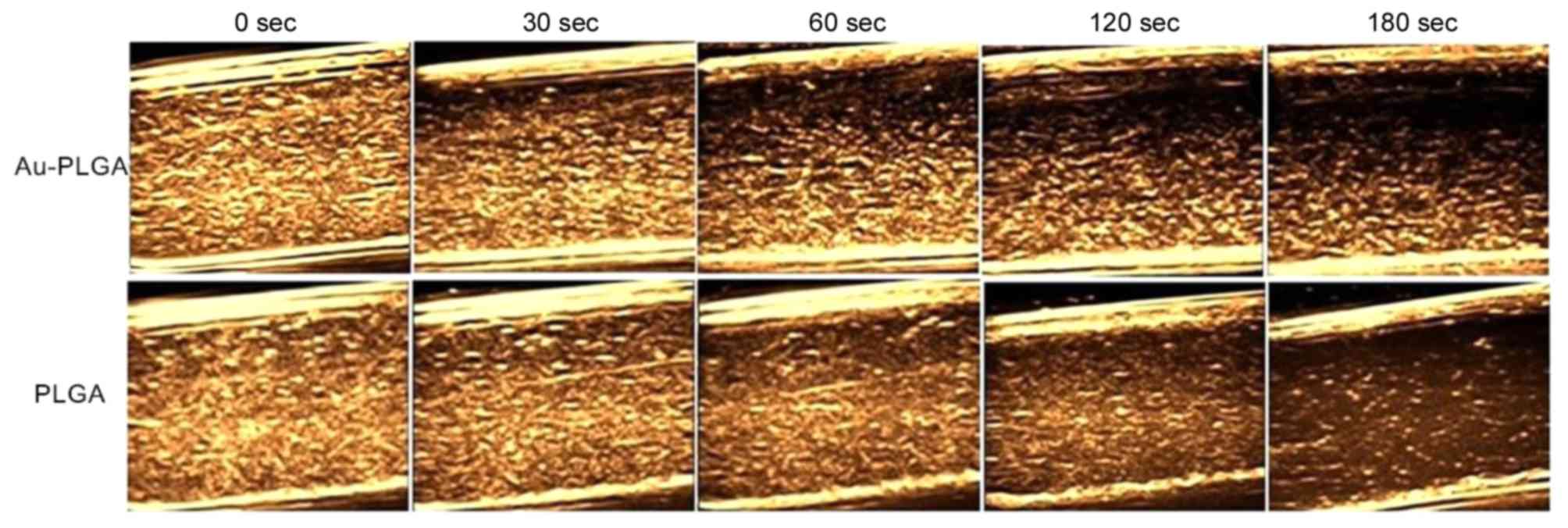
Furthermore, the effect of imaging time on the use
of Au-PLGA complexes and PLGA NPs as ultrasound contrast agents was
investigated in vitro under the conditions of 22 MHz and the
concentration of 2 mg/ml. The imaging ability of the Au-PLGA
complexes and PLGA NPs visibly weakened as time increased (Fig. 8). The echo signal intensity and
strength of the Au-PLGA complexes were superior to the PLGA NPs
across the entire imaging process, but a deposition of the Au-PLGA
complex occurs at 30 sec while PLGA NPs demonstrated a more uniform
ultrasonic signal without deposition across the entire imaging
process. The signals had been distributed and dissipated
evenly.
Discussion
Ultrasound contrast agents alter the fundamental
function of the acoustic wave (absorption, reflection and
refraction), so the sites where the echo signal is enhanced have
significant differences in background. Therefore, ultrasound
contrast agents are used for the diagnosis and identification of
heart, liver, breast and other organ diseases. Previous studies
have demonstrated that the material characteristics of polymer
contrast agents are superior to proteins, lipids, carbohydrates and
surfactants in multiple ways (13,14).
Thus, polymer ultrasound contrast agents have become an important
synthetic material.
Preliminary results revealed that PLGA NP ultrasound
imaging contrast agents, prepared with the method of an adjusted
double emulsion solvent evaporation, had positive effects in in
vitro and in vivo ultrasound imaging experiments
(15). Therefore, the same method
was adopted in the present study: A modified double-emulsion
solvent evaporation method to prepare PLGA NPs with a nanobubble
structure. In the formation process of polymer nanobubbles, the
evaporation of the solvent and PLGA film exhalation result in the
formation of polymer nanospheres that contain water. Results of
previous research have demonstrated that surfactant adsorbs more
readily on the gas/liquid interface when freeze drying, forming a
more stable microbubble structure (16). Following freeze drying, nanospheres
containing moisture and camphor were sublimed and the shape
remained unchanged. Thus, an internal hollow nanocapsule was
obtained. As gas molecules are able to enter the microcapsule by
molecular diffusion or osmosis, an internal, hollow microbubble of
gas is eventually obtained (17).
The double emulsion solvent evaporation method is simple, requires
mild reaction conditions, and it is possible to adjust the
preparation of the micro bubble particle size, uniform and
controllable particle size, shell properties and internal structure
through material selection and preparation technology (18,19).
Thus, suitable polymer nanobubbles were obtained.
TEM images of PLGA following phosphotungstic acid
dyeing revealed that it had a visible shell membrane structure, but
the nanosphere surface was wrinkled. The reason for this was that
PLGA NPs contain voids and the shell is thinner, so when the strong
electron beam of TEM hit the particles the shell membrane retracted
and formed the wrinkles. Thin shell structure permits the use of
smaller amounts of ultrasonic energy that make the nanobubbles
burst. This feature may be advantageous to investigate target
enhancement effects in future experiments concerning targeted
contrast agents in in vivo tumor models. There was a certain
degree of adhesion between the Au-PLGA complexes (Fig. 3). This was again due to the TEM
electron beam, which fused the borders of the polymer nanobubbles.
During the experiment, it was demonstrated that when using
TEM-focused photograph samples, electron microscopy produces a
high-energy electron beam. This will break the shell membrane
structure of polymer materials and the nanobubble will deform and
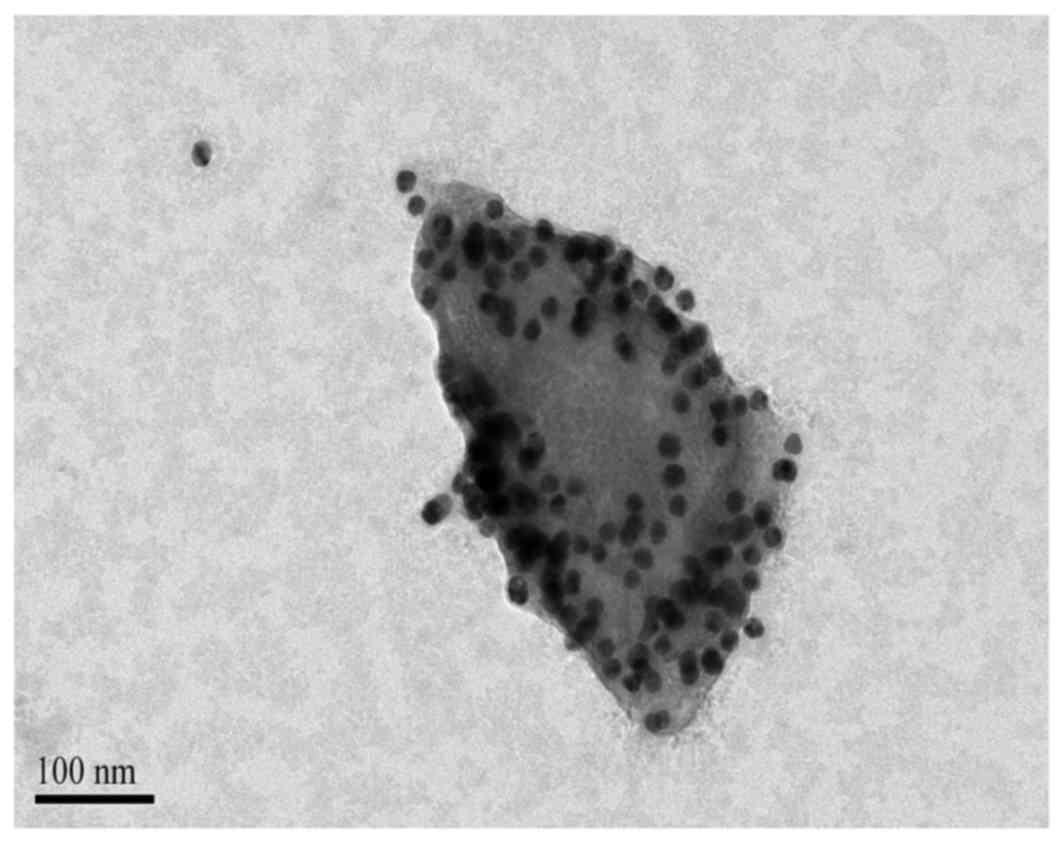
fracture quickly, which is not conducive to observations. Fig. 4 presents a TEM photo of a single
nanobubble complex. It is possible to observe that the complex
nanosphere structure has been destroyed, and this may have been
caused by a bombardment of high-energy electron beams on the
nanobubble. However, more GNPs adhered to the surface of the PLGA
NPs. They appeared as scattered black spots in Fig. 4, ~20 nm in diameter with uniform
size distribution, uniform distribution in the shell and no visible
aggregation.
The oxidation of sodium citrate reduction method is
the earliest method of preparation of water-soluble GNPs, and is
also the most widely used. In the present study, the sodium citrate
reduction method prepared 20 nm GNPs that demonstrated good
physical and chemical properties. The UV visible absorption
spectrum of GNPs revealed a maximum absorption peak of 508 nm. When
the UV spectrum of GNPs prior to and following combination with
PLGA NPs, it is possible to see that the maximum peak of absorption
curve demonstrated redshift. According to a previous report
(20), when GNPs and the
surrounding medium or matrix material interact, it may cause
absorption redshift. This determined that GNPs successfully
combined with PLGA NPs due to the right shift of the maximum
absorption peak. The successful combination was also demonstrated
by TEM. Thiol compounds easily and firmly bind to the surface of
the GNPs. Under the action of the decoupling activator NHS/EDC, the
carboxylic acid group (-COOH) on the surface of the PLGA
nanobubbles was activated, permitting it to combine with amino
groups (-NH2) or form other chemical bonds. In the
present study, the double-ended compound H2N-PEG1000-SH
was used as an intermediary to combine the thiol group with the
surface of the GNPs and the amino group with the activated
carboxylic acid group to form the Au-PLGA complex. This complex has
good stability and the diameter of the Au-PLGA complex
theoretically permits them to enter through the tumor endothelial
gap (400–600 nm) (21).
Previous studies have confirmed that nanoscale
ultrasound contrast agents in blood circulation possess a low echo
reflection, but an accumulation of a large amount of nanoscale
contrast agents significantly enhances the echo of the signal, more
effectively detecting lesions (22,23).
In theory, the Au-PLGA complex, which includes PLGA nanobubbles
containing gas and gold metal atoms, enhances the echo reflected
signal more than nanobubble alone. In vitro ultrasound
imaging results revealed that under conditions of high-frequency
ultrasound, the ultrasound imaging ability of Au-PLGA complexes was
improved compared with PLGA NPs. Under the same concentration, it
has a higher echo signal strength. Compared with PLGA NPs, it
deposits more readily. The reason may be the load of GNPs on the
surface of PLGA NPs, which increases the quality of a single
complex and results in the shortening of the suspension stability
time.
In conclusion, Au-PLGA complexes were successfully
constructed and the results of in vitro studies demonstrated
that the Au-PLGA complexes exhibited effective imaging
capabilities. Thus, Au-PLGA complexes hold potential as ultrasound
contrast agents. However, further experimental verification in
vivo is still required.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shanghai (grant no. 15ZR1425600) and the
National Natural Science Foundation of China (grant no.
81571678).
References
|
1
|
Khlebtsov N, Bogatyrev V, Dykman L,
Khlebtsov B, Staroverov S, Shirokov A, Matora L, Khanadeev V,
Pylaev T, Tsyganova N and Terentyuk G: Analytical and theranostic
applications of gold nanoparticles and multifunctional
nanocomposites. Theranostics. 3:167–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain S, Hirst DG and O'sullivan JM: Gold
nanoparticles as novel agents for cancer therapy. Br J Radiol.
85:101–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim BY, Rutka JT and Chan WC:
Nanomedicine. N Engl J Med. 363:2434–2443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh YC, Creran B and Rotello VM: Gold
nanoparticles: Preparation, properties, and applications in
bionanotechnology. Nanoscale. 4:1871–1880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song KH, Kim C, Maslov K and Wang LV:
Noninvasive in vivo spectroscopic nanorod-contrast photoacoustic
mapping of sentinel lymph nodes. Eur J Radiol. 70:227–231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Néstor MM, Kei NP, Guadalupe NA, Elisa ME,
Adriana GQ and David QG: Preparation and in vitro evaluation of
poly(D, L-lactide-co-glycolide) air-filled nanocapsules as a
contrast agent for ultrasound imaging. Ultrasonics. 51:839–845.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohl Y, Kaiser C, Bost W, Stracke F,
Fournelle M, Wischke C, Thielecke H, Lendlein A, Kratz K and Lemor
R: Preparation and biological evaluation of multifunctional
PLGA-nanoparticles designed for photoacoustic imaging.
Nanomedicine. 7:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu JS, Huang J, Qin R, Hinkle GH, Povoski
SP, Martin EW and Xu RX: Synthesizing and binding dual-mode
poly(lactic-co-glycolic acid) (PLGA) nanobubbles for cancer
targeting and imaging. Biomaterials. 31:1716–1722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke H, Wang J, Dai Z, Jin Y, Qu E, Xing Z,
Guo C, Yue X and Liu J: Gold-nanoshelled microcapsules: A
theranostic agent for ultrasound contrast imaging and photothermal
therapy. Angew Chem Int Ed Engl. 50:3017–3021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu RX, Huang J, Xu JS, Sun D, Hinkle GH,
Martin EW and Povoski SP: Fabrication of indocyanine green
encapsulated biodegradable microbubbles for structural and
functional imaging of cancer. J Biomed Opts. 14:0340202009.
View Article : Google Scholar
|
|
11
|
Frens G: Controlled nucleation for the
regulation of the particle size in monodisperse gold suspensions.
Nature. 241:20–22. 1973.
|
|
12
|
Horisberger M and Rosset J: Colloidal
gold, a useful marker for transmission and scanning electron
microscopy. J Histochem Cytochem. 25:295–305. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cózar-Bernal MJ, Holgado MA, Arias JL,
Muñoz-Rubio I, Martín-Banderas L, Alvarez-Fuentes J and
Fernández-Arévalo M: Insulin-loaded PLGA microparticles: Flow
focusing versus double emulsion/solvent evaporation. J
Microencapsul. 28:430–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shive MS and Anderson JM: Biodegradation
and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv
Rev. 28:5–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CW, Yang SP, Hu H, Du J and Li FH:
Synthesis, characterization and in vitro and in vivo investigation
of C3F8-filled poly(lactic-co-glycolic acid)
nanoparticles as an ultrasound contrast agent. Mol Med Rep.
11:1885–1890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho SH, Kim JY and Kim JD: Dynamic surface
tension of stable air-filled microbubbles prepared by freeze-drying
a solution of lipid/surfactant mixture. Colloids and Surfaces A
Physicochemical and Engineering Aspects. 284–285, 1-457. 2006.
|
|
17
|
Ma XY, Pan GM, Lu Z, Hu JS, Bei JZ, Jia JH
and Wang SG: Preliminary study of oral polylactide microcapsulated
insulin in vitro and in vivo. Diabetes Obes Metab. 2:243–250. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumari A, Yadav SK and Yadav SC:
Biodegradable polymeric nanoparticles based drug delivery systems.
Colloids Surf B Biointerfaces. 75:1–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piñón-Segundo E, Nava-Arzaluz MG and
Lechuga-Ballesteros D: Pharmaceutical polymeric nanoparticles
prepared by the double emulsion-solvent evaporation technique.
Recent Pat Drug Deliv Formul. 6:224–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XY, Yang ZL and Zhou HG: Studying the
absorption spectrum properties of the gold nanosphere-The effect of
the size on the absorption spectra of the anoparticles. J Zhangzhou
Teachers Coll (Nat Sci). 17:31–34. 2004.
|
|
21
|
Yuan F, Dellian M, Fukumura D, Leunig M,
Berk DA, Torchilin VP and Jain RK: Vascular permeability in a human
tumor xenograft: Molecular size dependence and cutoff size. Cancer
Res. 55:3752–3756. 1995.PubMed/NCBI
|
|
22
|
Koo OM, Rubinstein I and Onyuksel H: Role
of nanotechnology in targeted drug delivery and imaging: A concise
review. Nanomedicine. 1:193–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panchapakesan B and Wickstrom E:
Nanotechnology for sensing, imaging, and treating cancer. Surg
Oncol Clin N Am. 16:293–305. 2007. View Article : Google Scholar : PubMed/NCBI
|