Introduction
Parkinson's disease (PD) is a common chronic
neurodegenerative disorder, which affects >0.1% of individuals
>40 years of age (1,2). There is a loss of dopaminergic
nigrostriatal neurons in the substantia nigra (SN) pars compacta in
PD (3,4), and clinical characteristics for the
majority of patients with PD include motor disorder, slowness of
movement, tremor at rest, rigidity and disturbances in balance.
However, it is difficult to diagnose PD in its early stages due to
the high rate of misdiagnosis with other movement disorders, which
can share certain features with PD, including essential tremor,
multiple system atrophy (MSA) and progressive supranuclear palsy
(5). Therefore, it is necessary
and urgent to screen diagnostic or therapeutical biomarkers, which
can be utilized in the diagnosis or treatment of PD.
In previous years, numerous significant factors have
been correlated with the development of PD, which has improved
current understanding of PD. For example, the level of S100 calcium
binding protein B (S100B) is higher in the post-mortem SN of
patients with PD, compared with control tissue, and the ablation of
S100B results in neuroprotection, reduces microgliosis and
decreases the expression of tumor necrosis factor-α and the
receptor for advanced glycation end products (6). The reduced expression level of
lysosomes-associated membrane protein 2A in dopaminergic cell lines
leads to reduced chaperone-mediated autophagy and decreases the
metabolism of wild-type α-synuclein (SNCA), which is a cause
of PD (7). In addition, a set of
molecules, which offer potential in identifying individuals at risk
for PD, and assist in the diagnosis and management of PD have been
identified, including SNCA (8) and DJ-1 (9). Furthermore, based on microarray
analysis, Papapetropoulos et al identified 11 candidate
genes with deregulated expression in brain regions from patients
with PD, and the significant role of mitochondria ribosomal protein
S6 (MRPS6) in the oxidative phosphorylation system was
reported (10). In addition, a
previous study identified a set of differentially expressed genes
(DEGs) in PD, and the candidate compound, alvespimycin, was
selected as a lead compound for PD, based on the gene expression
data from the study by Papapetropoulos et al (10,11).
In organisms, each protein exerts its functions via interactions
with other proteins and regulation of a set of regulators. However,
interactions and regulators of DEGs in PD, which assist in
obtaining a more comprehensive understanding of the pathogenesis of
PD, were not fully investigated in the studies of Papapetropoulos
et al and Gao et al (10,11);
there may be additional potential key genes associated with PD,
which remain undetected.
The present study aimed to examine additional
potential key genes associated with PD, in addition to examining
their interactions and regulation. The microarray data of
Papapetropoulos et al (10)
were used to identify DEGs between patients with PD and those
without PD. The potential crucial genes associated with PD were
then examined. Subsequently, transcription factors (TFs) and
protein-protein interactions (PPIs) of the DEGs were analyzed. A
second microarray dataset (12) of
PD, which included additional post-mortem brain samples, was
selected to validate the expression of key genes in PD. The results
may provide novel information for investigation of the molecular
mechanisms underlying the pathogenesis of PD.
Materials and methods
Gene expression data of PD
The GSE7621 gene expression data (10) were obtained from the Gene
Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) based on
[HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array
platform (GPL570; Affymetrix, Inc., Santa Clara, CA, USA). A total
of 25 post-mortem SN samples, including 16 samples from patients
with idiopathic PD and nine samples from aged controls without PD,
were included in the GSE7621 expression data. For the patients with
PD, the ratio of men to women was 13:3, and the mean age was 74
years, ranging between 60 and 88. For the controls, the ratio of
men to women was 4:5, and the mean age was 78.2 years, ranging
between 46 and 90. The age, post-mortem interval (PMI), and brain
pH of the two subject groups were matched as closely as possible.
Analysis of the quality control parameters showed no significant
differences in age, gender, brain pH, PMI or RNA QC values between
the aged control and PD groups (10).
Another gene expression dataset, GSE8397 (12), was used for validation, which was
also downloaded from the GEO, which was based on the platforms of
Affymetrix Human Genome U133A Array (GPL96) and Affymetrix Human
Genome U133B Array (GPL97; Affymetrix, Inc.). A total of 47
individual tissue samples analyzed using one A and one B GeneChip
per sample [15 medial SN (MSN) samples and nine lateral SN (LSN)
samples obtained from patients with idiopathic PD, eight MSN
samples and seven LSN samples obtained from controls without PD,
five superior frontal gyrus samples from patients with PD, and
three superior frontal gyrus samples from controls] were included
in this dataset. The LSN and MSN samples were obtained from the
same cohort of patients with idiopathic PD or controls. For the
patients with PD, the ratio of men to women was 9:6, and the mean
age was 80 years, ranging between 68 and 89; for the controls, the
ratio of men to women was 6:2, and the mean age was 70.6 years,
ranging between 46 and 81. The subjects in the PD and control
groups were well matched in age, PMI and brain pH (12). Among the 47 brain tissue samples,
only the data of 16 LSN samples and 23 MSN samples from the
patients with PD and controls produced by the GPL96 platform were
used in the present study.
Data preprocessing and identification
of DEGs
The CEL files and probe annotation files were
downloaded from the database. The raw data were preprocessed via
background correction, quantile normalization, and calculating
expression using the robust multi-array average (RMA) algorithm
(13). The probes were then mapped
into corresponding gene names. The probe, which corresponded to a
plurality of genes was removed. If one gene symbol was matched by
multiple probe IDs, the mean expression value was selected as the
expression level of this gene. The Samr package in R software
(version 3.1; http://cran.r-project.org/web/packages/samr/index.html)
(14) was used to select the DEGs
between the PD and control samples. The P-value for each gene was
calculated using an unpaired t-test, and adjusted into a false
discovery rate (FDR) using the Benjamini-Hochberg (BH) method
(15). Only the genes with
FDR<0.05 and |log2fold change (FC)|>1, as commonly
used criteria, were selected as DEGs.
Prediction of crucial PD-associated
genes
The Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway database (http://www.kegg.jp/kegg/pathway.html) is a collection
of manually drawn pathway maps representing current knowledge on
the molecular interaction and reaction networks for metabolism,
cellular processes and human diseases (16). Firstly, the online Database for
Annotation, Visualization and Integrated Discovery (DAVID;
https://david.ncifcrf.gov/), which
integrates a set of functional annotation tools to understand the
biological meaning of numerous genes (17), was used to perform KEGG pathway
enrichment analysis for the identified DEGs. The P-value calculated
by Fisher's exact test of P<0.05, and a gene count >2 were
set as the cut-off criteria.
Secondly, in order to obtain the PD-associated
genes, the DEGs were submitted to the Genetic Association Database
(http://geneticassoiationdb.nih.gov/),
which is a database of genetic association data from complex
diseases and disorders. PD-associated genes with P<0.05 were
considered significant.
Finally, to identify the crucial PD-associated
genes, sequence alignment of the protein sequences of the genes,
which were involved in the nervous system-associated KEGG pathways
and PD-associated genes was performed using the BLASTP program
(version 2.2.31; https://blast.ncbi.nlm.nih.gov/Blast.cgi?;
E<10−10). The smaller the E value was, the more
reliable the alignment result. If the protein sequence of the gene
involved in the nervous system-associated KEGG pathways was matched
to multiple protein sequences of PD-associated genes, and had the
lowest E-value, this gene was considered to be a crucial
PD-associated gene.
Gene ontology (GO) enrichment analysis
of crucial PD-associated genes
The GO database collects biological data in the
format of gene-to-annotation, and is a suitable and commonly used
method for high-throughput bioinformatics scanning for enrichment
analysis (18). GO enrichment
analysis of the predicted crucial PD-associated genes was performed
using DAVID. Only the GO terms with the common criterion of
FDR<0.01 were considered significant.
Prediction of TFs for the crucial
PD-associated genes
TF binding sites (TFBSs) are short DNA motifs
in genes, which bind with TFs. TFBSs often occur in close proximity
to each other forming cis-regulatory modules, which suggest
the existence of a combinatorial code for transcriptional
regulation (19). Based on the
known TFBS information in the University of California Santa Cruz
(UCSC) database (http://genome.ucsc.edu/) in DAVID (20), the TFs in the UCSC database
targeting the crucial PD-associated genes were identified.
FDR<0.01 was set as the cut-off criterion.
PPI network analysis
PPI analysis of the screened DEGs was performed
using the online Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING; version 10.0; http://string-db.org/) software, which integrates
known and predicted protein interaction data (21) (combined score>0.4). The PPI
networks containing the predicted crucial PD-associated genes were
then extracted and visualized using Cytoscape software (version
3.2.0; http://www.cytoscape.org/), which is an
open source software project for integrating biomolecular
interaction networks (22). In a
network, a node represents a protein (gene), and lines represent
interactions of the proteins. The ‘degree’ of each node is equal to
the number of nodes interacting with this node. The higher the
degree is, the closer the connections with other nodes are,
indicating a higher importance of the node in the network.
Validation of the expression level of
crucial PD-associated genes
The DEGs were identified in the PD and control
samples from another dataset, GSE8397 (Moran et al. 2006)
using the same method as that used for GSE7621. The identified DEGs
were then compared with those in the GSE7621 dataset.
Results
Anlayses of DEGs and crucial
PD-associated genes
Following normalization of the raw data in the
GSE7621 dataset, a total of 670 DEGs were identified in the SN of
PD, including 398 upregulated genes and 272 downregulated genes
(data not shown). Based on the criteria of P<0.05 and gene count
>2, these DEGs were associated with the pathways of axon
guidance (P=0.003347; gene count, 12; http://www.genome.jp/dbget-bin/show_pathway?
hsa04360+1630+4893+8440+3265
+219699+80031+7852+56920+6092+9423+4775+6585) and endocytosis
(P=0.017984; gene count=13; http://www.genome.jp/dbget-bin/show_pathway?
hsa04144+5979+3265+
79643+80230+3303+3791+3305+7852+2870+57154+10938+ 8218+22905),
which were associated with the nervous system. The DEGs, including
deleted in colorectal cancer (DCC), NCL adaptor protein 2
(NCK2) and C-X-C chemokine receptor type 4 (CXCR4),
were significantly involved in the axon guidance pathway, and a set
of DEGs, including CXCR4 and clathrin heavy chain like 1
(CLTCL1), were associated with the endocytosis pathway
(Table I).
 | Table I.Kyoto Encyclopedia of Genes and
Genomes pathways associated with the nervous system for
differentially expressed genes. |
Table I.
Kyoto Encyclopedia of Genes and
Genomes pathways associated with the nervous system for
differentially expressed genes.
| Term | P-value | Genes |
|---|
| hsa04360 Axon
guidance | 0.003347 | DCC, NRAS, NCK2,
HRAS, UNC5B, SEMA6D, CXCR4, SEMA3G, ROBO2, NTN1, NFATC3,
SLIT1 |
| hsa04144
Endocytosis | 0.017984 | RET, HRAS,
CHMP6, RUFY1, HSPA1A, KDR, HSPA1L, CXCR4, GRK6, SMURF1, EHD1,
CLTCL1, EPN2 |
A total of 10 DEGs, which may be correlated with PD
were identified from the Genetic Association Database, including
heat shock 70 kDa protein 1-like (HSPA1L), Cytochrome P450
family 27 subfamily A1 (CYP27A1), dopamine receptor D2
(DRD2), solute carrier family 6 member 3 (SLC6A3),
PARK2 coregulated (PACRG), tyrosine hydroxylase (TH),
nuclear receptor subfamily 4 A2 (NR4A2), solute carrier
family 18 member A2 (SLC18A2), lutathione S-transferase ζ1
(GSTZ1) and heat shock protein family A member 1A
(HSPA1A). Based on the DEGs enriched in the pathways of axon
guidance and endocytosis, in addition to DEGs correlated with PD,
which were identified using the Genetic Association Database, a
total of 10 genes were predicted to be closely associated with PD
via sequence alignment, including CXCR4, DCC, EH
domain-containing 1 (EHD1), epsin-2 (EPN2), G
protein-coupled receptor kinase 6 (GRK6), NCK2,
roundabout guidance receptor 2 (ROBO2), RUN and FYVE domain
containing 1 (RUFY1), semaphorin 6D (SEMA6D) and
SLIT1. These were defined as crucial PD-associated
genes.
GO enrichment analysis
To further reveal the biological functions of the
predicted crucial PD-associated genes, GO enrichment analysis was
performed. The majority of GO terms of the predicted crucial
PD-associated genes, including NCK2, CXCR4,
SL1T1, ROBO2 and DCC, were involved in neuron
development and differentiation, and cell morphogenesis.
EHD1, RUFY1, and EPN2 were associated with GO
terms associated with endocytosis (Table II). No significant GO terms were
enriched by the other two crucial PD-associated genes
(SEMA6D and GRK6).
 | Table II.Gene ontology enrichment analysis of
the crucial Parkinson's disease-associated genes. |
Table II.
Gene ontology enrichment analysis of
the crucial Parkinson's disease-associated genes.
| Gene | Gene Ontology
terms |
|---|
| SLIT1 | Cell morphogenesis,
neuron development, neuron projection development, cell part
morphogenesis, cell projection morphogenesis, cell morphogenesis
involved in differentiation, neuron projection morphogenesis, cell
morphogenesis involved in neuron differentiation, axonogenesis,
cell motion, cell projection organization, axon guidance, neuron
differentiation, cellular component morphogenesis |
| RUFY1 | Regulation of
endocytosis, membrane organization, membrane invagination,
endocytosis |
| ROBO2 | Cell morphogenesis,
neuron development, neuron projection development, cell part
morphogenesis, cell projection morphogenesis, cell morphogenesis
involved in differentiation, neuron projection morphogenesis, cell
morphogenesis involved in neuron differentiation, axonogenesis,
cell motion, cell projection organization, axon guidance, neuron
projection, neuron differentiation, cellular component
morphogenesis |
| NCK2 | Cell motion, cell
projection organization, cell motility, localization of cell, cell
migration |
| EPN2 | Endocytic vesicle,
regulation of endocytosis, membrane organization, membrane
invagination, endocytosis |
| EHD1 | Endocytic vesicle,
membrane organization, membrane invagination, endocytosis |
| DCC | Cellular component
morphogenesis, cell morphogenesis, neuron development, neuron
projection development, cell part morphogenesis, cell projection
morphogenesis, cell morphogenesis involved in differentiation,
neuron projection morphogenesis, cell morphogenesis involved in
neuron differentiation, axonogenesis, cell motion, cell projection
organization, axon guidance, neuron migration, neuron projection,
cell motility, localization of cell, cell migration, neuron
differentiation |
| CXCR4 | Cellular component
morphogenesis, cell morphogenesis, neuron development, neuron
projection development, cell part morphogenesis, cell projection
morphogenesis, cell morphogenesis involved in differentiation,
neuron projection morphogenesis, cell morphogenesis involved in
neuron differentiation, axonogenesis, cell motion, cell projection
organization, axon guidance, neuron migration, neuron projection,
cell motility, localization of cell, cell migration, neuron
differentiation |
Analyses of TFs and PPI interaction
networks
To further investigate the regulatory factors and
interactions of the crucial PD-associated genes, TFs targeting the
genes were predicted. A total of 55 TFs were found to regulate the
10 crucial PD-associated genes. DCC, CXCR4,
NCK2, SLIT1, ROBO2 and GRK6 were all
regulated by a set of TFs, including GATA, E2F and E4
promoter-binding protein 4 (E4BP4) (Table III).
 | Table III.List of the 55 transcription factors
targeting the crucial Parkinson's disease-associated genes. |
Table III.
List of the 55 transcription factors
targeting the crucial Parkinson's disease-associated genes.
| Gene | Transcription
factors |
|---|
| DCC | IK2, SP1, HNF3B,
MYOGNF1, TATA, TCF11, GFI1, NKX25, FAC1, PAX2, MEIS1B, HOXA9, OLF1,
ARP1, HEN1, CEBPB, GATA1, HOXA3, HAND1E47, S8, CDP, RSRFC4, SOX5,
NFY, CDP, CR3HD, SREBP1, MZF1, NRSF, MYC, MAX, AHRARNT, PAX5, MSX1,
TAX, CREB, MYB, COUP, GATA2, IRF1, AP4, GATA, MRF2, PAX3, AP2REP,
AREB6, SRY, MEF2, GRE, MIF1, USF, BRACH, PAX6, COMP1, AML1,
LMO2COM, E2F, YY1, E4BP4 |
| EPN2 | IK2, SP1, HNF3B,
TCF11, GFI1, NKX25, PAX2, ARP1, HEN1, GATA1, HAND1E47, S8, RSRFC4,
CDP, CR3HD, MZF1, NRSF, MYC, MAX, PAX5, MYB, AP4, AP2REP, SRY,
MEF2, GRE, MIF1, USF, BRACH, PAX6, AML1, LMO2COM, E4BP4 |
| CXCR4 | IK2, MYOGNF1,
TATA, TCF11, GFI1, NKX25, FAC1, PAX2, MEIS1B, HOXA9, HAND1E47, S8,
CDP, SOX5, NRSF, MYC, MAX, AHRARNT, MSX1, MYB, GATA2, IRF1, GATA,
PAX3, AP2REP, AREB6, SRY, MEF2, USF, BRACH, PAX6, COMP1, AML1,
LMO2COM, E2F, E4BP4 |
| NCK2 | IK2, HNF3B,
MYOGNF1, TATA, TCF11, NKX25, PAX2, MEIS1B, HOXA9, CEBPB, GATA1,
HOXA3, S8, CDP, RSRFC4, SOX5, NFY, CDP, CR3HD, MZF1, NRSF, MYC,
MAX, PAX5, MSX1, IRF1, AP4, GATA, MRF2, AP2REP, AREB6, MEF2, GRE,
USF, BRACH, COMP1, AML1, E2F, E4BP4 |
| SLIT1 | IK2, HNF3B,
MYOGNF1, TATA, TCF11, GFI1, NKX25, FAC1, PAX2, MEIS1B, HOXA9, OLF1,
ARP1, HEN1, CEBPB, GATA1, HAND1E47, S8, CDP, RSRFC4, SOX5, CDP,
CR3HD, SREBP1, NRSF, MYC, MAX, AHRARNT, PAX5, MSX1, TAX, CREB, MYB,
COUP, GATA2, AP4, GATA, MRF2, PAX3, AP2REP, AREB6, SRY, MEF2, GRE,
MIF1, USF, BRACH, PAX6, COMP1, AML1, LMO2COM, E2F, YY1,
E4BP4 |
| SEMA6D | IK2, HNF3B,
TATA, TCF11, GFI1, NKX25, FAC1, PAX2, MEIS1B, HOXA9, ARP1, HEN1,
CEBPB, GATA1, HOXA3, HAND1E47, S8, CDP, NFY, CDP, CR3HD, SREBP1,
MZF1, MYC, MAX, PAX5, MSX1, TAX, CREB, MYB, COUP, GATA2, IRF1, AP4,
GATA, MRF2, AREB6, SRY, MEF2, GRE, MIF1, USF, BRACH, PAX6, COMP1,
AML1, LMO2COM, E2F, YY1, E4BP4 |
| RUFY1 | SP1, HNF3B,
TATA, NKX25, PAX2, MEIS1B, HOXA9, OLF1, ARP1, CEBPB, GATA1, HOXA3,
HAND1E47, S8, CDP, RSRFC4, NFY, CDP, CR3HD, SREBP1, MZF1, NRSF,
AHRARNT, PAX5, MSX1, TAX, CREB, IRF1, AP4, GATA, MRF2, AP2REP,
AREB6, SRY, MEF2, GRE, USF, BRACH, PAX6, AML1, LMO2COM, YY1,
E4BP4 |
| EHD1 | SP1, MYOGNF1,
TATA, TCF11, GFI1, PAX2, OLF1, ARP1, HEN1, GATA1, HOXA3, SOX5, NFY,
SREBP1, MZF1, NRSF, MYC, MAX, AHRARNT, PAX5, TAX, CREB, MYB, COUP,
IRF1, AP4, PAX3, AP2REP, AREB6, MEF2, MIF1, USF, BRACH, PAX6,
COMP1, AML1, LMO2COM, E2F, YY1, E4BP4 |
| ROBO2 | HNF3B, MYOGNF1,
TATA, TCF11, GFI1, NKX25, FAC1, PAX2, MEIS1B, HOXA9, OLF1, HEN1,
CEBPB, GATA1, HOXA3, HAND1E47, S8, CDP, RSRFC4, SOX5, NFY, CDP,
CR3HD, SREBP1, MZF1, NRSF, MYC, MAX, AHRARNT, MSX1, TAX, CREB, MYB,
COUP, GATA2, IRF1, AP4, GATA, MRF2, PAX3, AP2REP, AREB6, SRY, MEF2,
GRE, MIF1, USF, BRACH, PAX6, COMP1, AML1, LMO2COM, E2F, YY1,
E4BP4 |
| GRK6 | MYOGNF1, TATA,
TCF11, GFI1, FAC1, PAX2, MEIS1B, HOXA9, OLF1, ARP1, HEN1, CEBPB,
GATA1, HOXA3, HAND1E47, S8, NFY, SREBP1, MZF1, NRSF, MYC, MAX,
AHRARNT, PAX5, MSX1, TAX, CREB, COUP, AP4, PAX3, AREB6, MEF2, GRE,
MIF1, USF, BRACH, PAX6, COMP1, AML1, LMO2COM, E2F, YY1,
E4BP4 |
Furthermore, the PPIs of the crucial PD-associated
genes were searched, and a PPI network was constructed. In the PPI
networks, the downregulated DCC and upregulated CXCR4
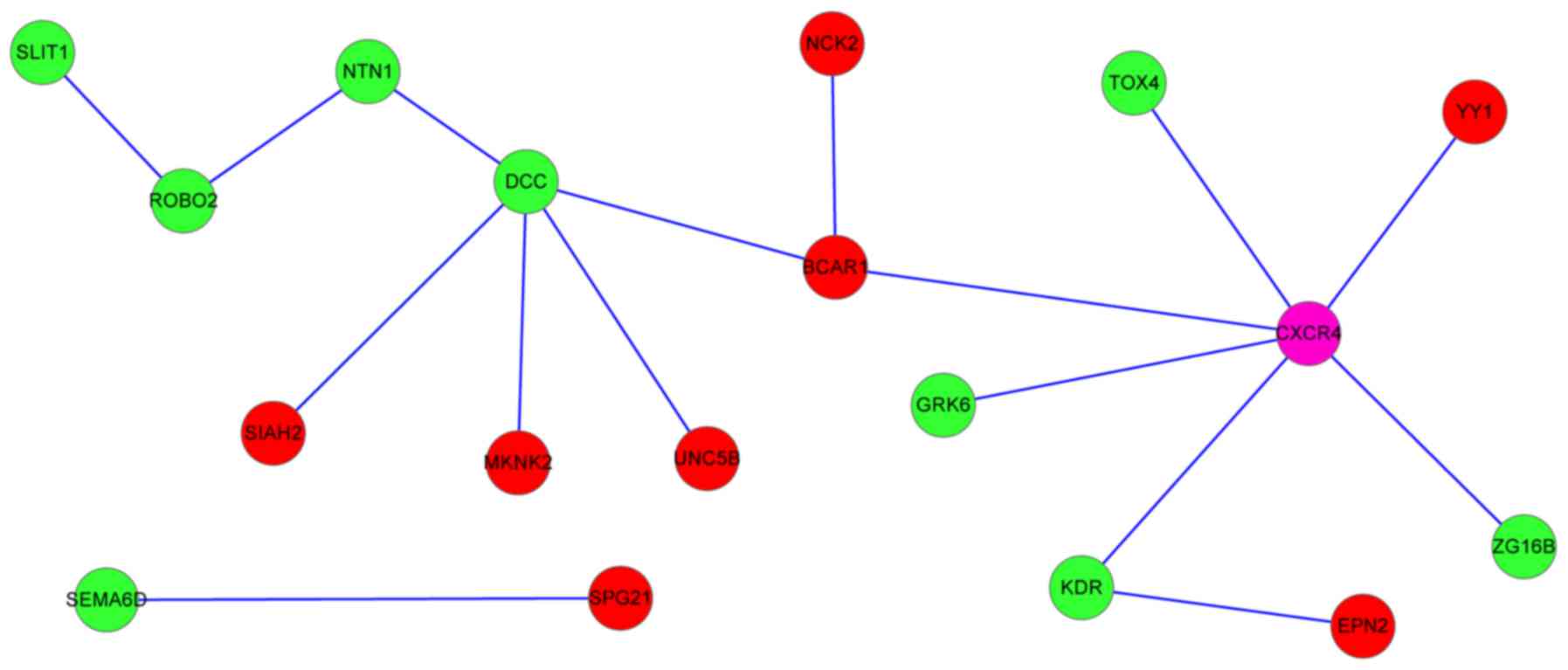
were the hub nodes, interacting with multiple genes (Fig. 1). DCC, CXCR4 and
NCK2 interacted with breast cancer anti-estrogen resistance
1 (BCAR1), and netrin 1 (NTN1) interacted with
ROBO2, which interacted with SLIT1.
Validation of the expression level of
crucial PD-associated genes
To confirm the expression level of the potential
crucial PD-associated genes, DEGs in another microarray dataset,
GSE8397 (12), were identified,
and the mean probe expression levels of each common DEG in the two
datasets were compared manually. In the other microarray dataset of
PD (12), two crucial
PD-associated genes (CXCR4 and NCK2) were also
upregulated in the PD samples, compared with the controls, which
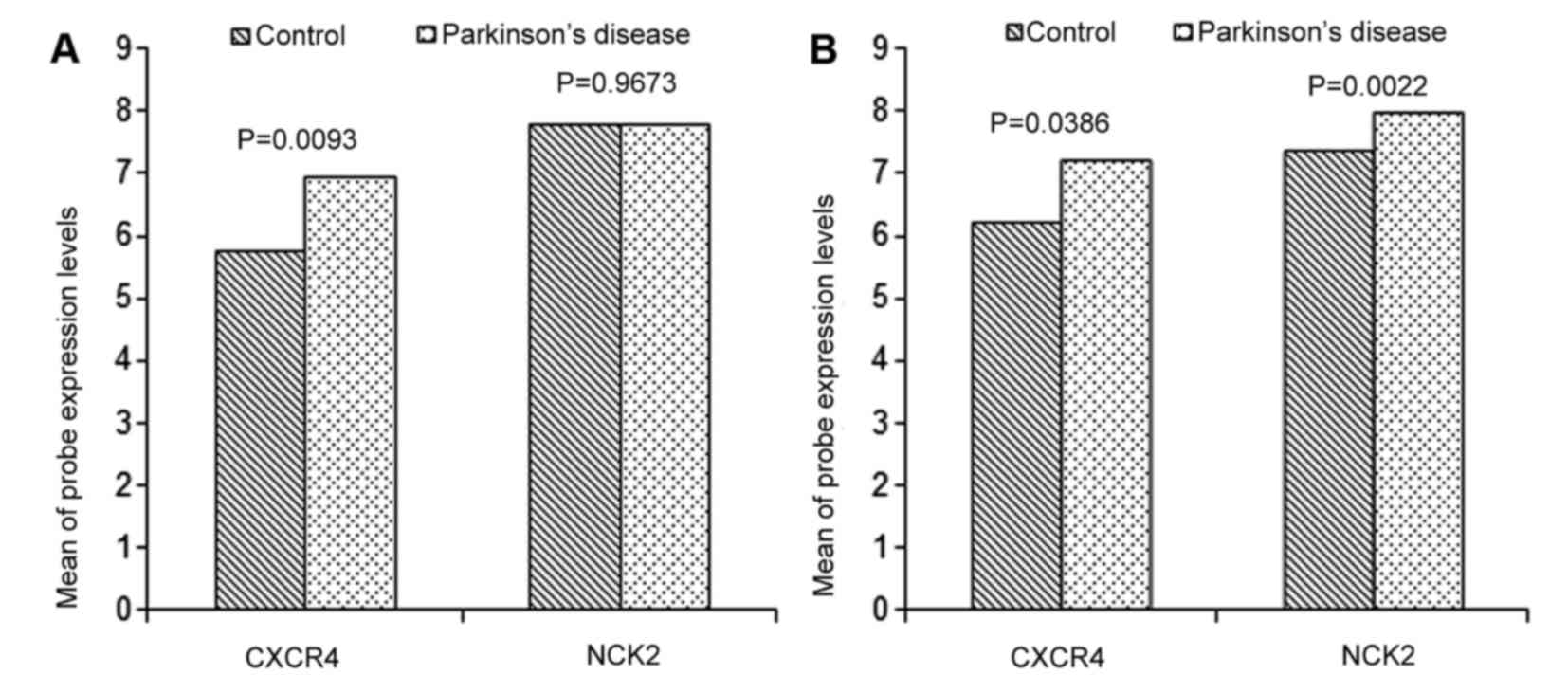
was consistent with that in the first dataset (10). CXCR4 was significantly
upregulated in the LSN and MSN samples (Fig. 2A and B), and NCK2 was
upregulated in the MSN samples (Fig.
2B).
Discussion
In the present study, based on the analysis of the
GSE7621 dataset, 398 upregulated genes and 272 downregulated genes
were identified in the SN from the patients with PD, compared with
the controls. A total of 10 DEGs (CXCR4, DCC,
EHD1, EPN2, GRK6, NCK2, ROBO2,
RUFY1, SEMA6D and SLIT1) were predicted to be
closely associated with PD. Among these 10 genes, downregulated
DCC and upregulated CXCR4 were the hub proteins,
which interacted with multiple genes in the PPI network. These two
proteins were distinctly enriched in the axon guidance pathway, and
GO terms associated with neuron development and
differentiation.
In the PPI network, DCC interacted with
NTN1. DCC encodes an NTN1 receptor, a member of the
immunoglobulin superfamily, which mediates the axon guidance of
neuronal growth cones towards sources of NTN1 ligand (23). NTN1 binds to the DCC
receptor and signals through downstream proteins, which regulate
cytoskeletal reorganization, causing growth cone extension and
neurite growth (24). Another
study reported that NTN1 and DCC are able to attract
axons to the nervous system midline, and mutations in DCC
lead to midline guidance defects in humans (25). There is also evidence that
DCC-mediated NTN1 signaling affects the
formation/maintenance of mesocorticolimbic DA topography (26). Polymorphisms in NTN1 and
DCC are associated with the loss of multiple functions and
increased the risk of PD (27,28).
These results suggested that the decreased expression of DCC
and NTN1 may lead to a loss of dopaminergic nigrostriatal
neurons in the SN, resulting in the onset of PD. In the PPI
network, NTN1 interacted with ROBO2, which interacted
with SLIT1. The ROBO2 protein belongs to the Robo
family, which is important for axon guidance across the midline
during central nervous system development (29). ROBO2 has also previously
been found to be deregulated in PD (30). Loss of Slit1 expression results in
an abnormal course of the nigrostriatal pathway through the
diencephalon (31). Although the
genes of the DCC-NTN1-ROBO2-SLIT1 interaction pathway have
been previously reported to be associated with the nervous system,
their interactions in the present study are a novel finding.
CXCR4 encodes a CXC chemokine receptor
specific for stromal cell-derived factor-1 (32). In the SN of patients with PD,
higher expression of CXCR4 and its natural ligand,
CXCL12, have been detected, compared with control subjects
(28,33), which is consistent with the results
of the present study. The activation of CXCR4 has been shown
to reduce dopamine levels in the striatum, and induce cell death
and tissue loss in the SN and striatum (34,35).
Furthermore, CXCL12 and CXCR4 are neurotoxic to
dopamine neuronal populations through the release of neurotoxins
from microglia (36). Therefore,
CXCR4 may be pivotal in the etiology of PD by reducing
dopamine levels in the striatum and/or releasing neurotoxins from
microglia to induce the death of dopamine neurons. In the PPI
network, CXCR4 interacted with other gene proteins,
including TOX high mobility group box family member 4
(TOX4), YY1, GRK6, kinase insert domain
receptor (KDR) and zymogen granule protein 16B
(ZG16B). Among these genes, YY1 has been reported to
regulate the expression of SNCA, which is an established
susceptibility gene for PD (37).
In the present study, YY1 was predicted as a TF targeting
DCC, ROBO2, SLIT1 and GRK6. Therefore,
YY1 may also be involved in PD via regulating the expression
of these genes. In an animal model of PD, the expression of
GRK6 was found to be altered in the caudate/putamen and
ventral striatum (38), and the
altered expression of GRK6 can result in the dysregulation
of DA receptors, thus contributing to the core motor deficits
observed in PD (39). Other genes,
including TOX4, KDR and ZG16B, and their
interactions with CXCR4, have not been correlated with PD in
previous studies; thus, these are novel potential genes, which may
be associated with PD.
In the present study, NCK2 was upregulated in
the SN of PD based on the analysis of the two datasets. NCK2
encodes a member of the NCK family of adaptor proteins, which are
involved in the regulation of receptor protein tyrosine kinases
(40). It has been reported that
NCK2 is expressed in neurons and glia (41). There is evidence that NCK2
interacts with particularly interesting cysteine histidine-rich
protein and Dock180 protein, which are involved in
neuropathological processes (42,43).
In the PPI network, NCK2, CXCR4 and DCC all
interacted with BCAR1. BCAR1 is involved in various
cellular events, including migration, survival and transformation
(44). A previous study has
reported the differential expression of BCAR1 in PD
(45). The cerebellum may have
certain roles in the pathophysiology of PD (46). p130Cas/BCAR1 was found to be
involved in cellular mechanisms regulating different stages of
cerebellar development (47),
suggesting that the abnormal expression of BCAR1 may be
associated with PD. However, there is no evidence to confirm the
association of NCK2 with the progression of PD.
Collectively, these findings indicated that the upregulation of
NCK2 may be responsible for the progression of PD, via
interaction with BCAR1 or regulation by TFs.
In addition, according to the TF analysis for
crucial PD-associated genes in the present study, a series of TFs
were predicted to target the 10 crucial PD-associated genes.
DCC, CXCR4, NCK2, SLIT1, ROBO2
and GRK6 were all regulated by a set of TFs, including
GATA, E2F and E4BP4. A previous study reported that
GATA-1 and GATA-2 regulate the expression of
SNCA dopaminergic cells (48). The pRb/E2F cell-cycle pathway
mediates cell death in PD, and E2F-1-deficient mice are
significantly more resistant to dopaminergic cell death, compared
with their wild-type littermates (49). E4BP4, also known as nuclear
factor, interleukin 3 regulated is a transcriptional regulator of
the human interleukin-3 promoter (50). A previous study reported that
E4BP4 is reportedly associated with neuron disease (51), and is upregulated in the SN tissue
from patients with PD (52).
Collectively, the DCC, CXCR4 and NCK2 genes
are involved in the pathogenesis of PD, not only via the axon
guidance pathway, but also likely via the regulation of multiple
TFs, including GATA, E2F and E4BP4.
Of note, although the present study reanalyzed the
public data deposited by others, the analytical procedure of the
present study was different from that of previous studies. The
criteria of the FDR<0.05 and |log2 FC|>1 were more
strict, compared with those (P<0.05; |FC|≥1.3) used in the
previous study by Papapetropoulos et al (10), indicating that the DEGs identified
in the present study show a higher level of differential
expression. In the present study, the P-value of each gene was
adjusted using the BH method to reduce false positives, however,
the P-value was not adjusted in the previous study. Furthermore, in
the present study, the protein interactions of DEGs and the TFs
regulating DEGs were analyzed, which assisted in providing a more
comprehensive understanding of the pathogenesis of PD, whereas
these were not investigated in the previous study. Despite the
significance, there were certain limitations in the present study.
For example, only two datasets were analyzed, whereas performing a
meta-analysis using multiple datasets, including multiple types of
data, may produce additional significant results. This is an
important aim of further investigations.
In conclusion, the present study identified a set
of potential crucial PD-associated genes, including CXCR4,
DCC and NCK2, which were associated with neuron
development and differentiation. The DCC-NTN1-ROBO2-SLIT1
interaction pathway and several genes, including TOX4,
KDR and ZG16B, which interacted with CXCR4,
were novel findings. Genes, including DCC, CXCR4,
NCK2, SLIT1 and ROBO2 were also regulated by
multiple TFs, including GATA, E2F and E4BP4.
Additionally, CXCR4 and NCK2 were detected to be
upregulated in the SN of patients with PD following the analysis of
two datasets. These genes, interactions of proteins and TFs may be
key in the progression of PD. These findings contribute to an
improved understanding of the pathogenesis of PD. However, further
experiments are required to confirm the results of the present
study and further elucidate the pathogenesis of PD.
References
|
1
|
Dawson TM and Dawson VL: Molecular
pathways of neurodegeneration in Parkinson's disease. Science.
302:819–822. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armentero MT, Pinna A, Ferré S, Lanciego
JL, Müller CE and Franco R: Past, present and future of A(2A)
adenosine receptor antagonists in the therapy of Parkinson's
disease. Pharmacol Ther. 132:280–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michell AW, Lewis SJ, Foltynie T and
Barker RA: Biomarkers and Parkinson's disease. Brain.
127:1693–1705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berendse HW, Booij J, Francot CM, Bergmans
PL, Hijman R, Stoof JC and Wolters EC: Subclinical dopaminergic
dysfunction in asymptomatic Parkinson's disease patients' relatives
with a decreased sense of smell. Ann Neurol. 50:34–41. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galvin JE, Lee VM and Trojanowski JQ:
Synucleinopathies: Clinical and pathological implications. Arch
Neurol. 58:186–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sathe K, Maetzler W, Lang JD, Mounsey RB,
Fleckenstein C, Martin HL, Schulte C, Mustafa S, Synofzik M,
Vukovic Z, et al: S100B is increased in Parkinson's disease and
ablation protects against MPTP-induced toxicity through the RAGE
and TNF-α pathway. Brain. 135:3336–3347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez-Erviti L, Rodriguez-Oroz MC,
Cooper JM, Caballero C, Ferrer I, Obeso JA and Schapira AH:
Chaperone-mediated autophagy markers in Parkinson disease brains.
Arch Neurol. 67:1464–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malek N, Swallow D, Grosset KA, Anichtchik
O, Spillantini M and Grosset DG: Alpha-synuclein in peripheral
tissues and body fluids as a biomarker for Parkinson's disease-a
systematic review. Acta Neurol Scand. 130:59–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devic I, Hwang H, Edgar JS, Izutsu K,
Presland R, Pan C, Goodlett DR, Wang Y, Armaly J, Tumas V, et al:
Salivary α-synuclein and DJ-1: Potential biomarkers for Parkinson's
disease. Brain. 134:e1782011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papapetropoulos S, Ffrench-Mullen J,
McCorquodale D, Qin Y, Pablo J and Mash DC: Multiregional gene
expression profiling identifies MRPS6 as a possible candidate gene
for Parkinson's disease. Gene Expr. 13:205–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao L, Zhao G, Fang JS, Yuan TY, Liu AL
and Du GH: Discovery of the neuroprotective effects of alvespimycin
by computational prioritization of potential anti-Parkinson agents.
FEBS J. 281:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moran LB, Duke DC, Deprez M, Dexter DT,
Pearce RK and Graeber MB: Whole genome expression profiling of the
medial and lateral substantia nigra in Parkinson's disease.
Neurogenetics. 7:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tibshirani R, Chu G, Narasimhan B and Li
J: SAM: Significance Analysis of Microarrays. Version 2.0. The
Comprehensive R Archive Network. 2011.
|
|
15
|
Glueck DH, Mandel J, Karimpour-Fard A,
Hunter L and Muller KE: Exact calculations of average power for the
Benjamini-Hochberg procedure. Int J Biostat. 4:Article
112008.PubMed/NCBI
|
|
16
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40:D109–D114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine M and Tjian R: Transcription
regulation and animal diversity. Nature. 424:147–151. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin TM, Plautz SA and Pannier AK:
Network analysis of endogenous gene expression profiles after
polyethyleneimine-mediated DNA delivery. J Gene Med. 15:142–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serrato-Combe A: Lindebmayer
Systems-experimenting with software string rewriting as an assist
to the study and generation of architectural form. Proceedings of
the 9th Iberoamerican Congress of Digital Graphics. SIGRADI; Lima.
pp. 161–166. 2005;
|
|
22
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keino-Masu K, Masu M, Hinck L, Leonardo
ED, Chan SS, Culotti JG and Tessier-Lavigne M: Deleted in
colorectal cancer (DCC) encodes a netrin receptor. Cell.
87:175–185. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu B, Goldman JS, Rymar VV, Forget C, Lo
PS, Bull SJ, Vereker E, Barker PA, Trudeau LE, Sadikot AF and
Kennedy TE: Critical roles for the netrin receptor deleted in
colorectal cancer in dopaminergic neuronal precursor migration,
axon guidance, and axon arborization. Neuroscience. 169:932–949.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Engle EC: Human genetic disorders of axon
guidance. Cold Spring Harb Perspect Biol. 2:a0017842010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manitt C, Mimee A, Eng C, Pokinko M, Stroh
T, Cooper HM, Kolb B and Flores C: The netrin receptor DCC is
required in the pubertal organization of mesocortical dopamine
circuitry. J Neurosci. 31:8381–8394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin L, Lesnick TG, Maraganore DM and
Isacson O: Axon guidance and synaptic maintenance: Preclinical
markers for neurodegenerative disease and therapeutics. Trends
Neurosci. 32:142–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lesnick TG, Papapetropoulos S, Mash DC,
Ffrench-Mullen J, Shehadeh L, de Andrade M, Henley JR, Rocca WA,
Ahlskog JE and Maraganore DM: A genomic pathway approach to a
complex disease: Axon guidance and Parkinson disease. PLoS Genet.
3:e982007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sundaresan V, Mambetisaeva E, Andrews W,
Annan A, Knöll B, Tear G and Bannister L: Dynamic expression
patterns of Robo (Robo1 and Robo2) in the developing murine central
nervous system. J Comp Neurol. 468:467–481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bossers K, Meerhoff G, Balesar R, van
Dongen JW, Kruse CG, Swaab DF and Verhaagen J: Analysis of gene
expression in Parkinson's disease: Possible involvement of
neurotrophic support and axon guidance in dopaminergic cell death.
Brain Pathol. 19:91–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bagri A, Marı́n O, Plump AS, Mak J,
Pleasure SJ, Rubenstein JL and Tessier-Lavigne M: Slit proteins
prevent midline crossing and determine the dorsoventral position of
major axonal pathways in the mammalian forebrain. Neuron.
33:233–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wegner SA, Ehrenberg PK, Chang G, Dayhoff
DE, Sleeker AL and Michael NL: Genomic organization and functional
characterization of the chemokine receptor CXCR4, a major entry
co-receptor for human immunodeficiency virus type 1. J Biol Chem.
273:4754–4760. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimoji M, Pagan F, Healton EB and
Mocchetti I: CXCR4 and CXCL12 expression is increased in the
nigro-striatal system of Parkinson's disease. Neurotox Res.
16:318–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bachis A, Aden SA, Nosheny RL, Andrews PM
and Mocchetti I: Axonal transport of human immunodeficiency virus
type 1 envelope protein glycoprotein 120 is found in association
with neuronal apoptosis. J Neurosci. 26:6771–6780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nosheny RL, Bachis A, Aden SA, De Bernardi
MA and Mocchetti I: Intrastriatal administration of human
immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line
derived neurotrophic factor levels and causes apoptosis in the
substantia nigra. J Neurobiol. 66:1311–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bezzi P, Domercq M, Brambilla L, Galli R,
Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J
and Volterra A: CXCR4-activated astrocyte glutamate release via
TNFalpha: Amplification by microglia triggers neurotoxicity. Nat
Neurosci. 4:702–710. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizuta I, Takafuji K, Ando Y, Satake W,
Kanagawa M, Kobayashi K, Nagamori S, Shinohara T, Ito C, Yamamoto
M, et al: YY1 binds to α-synuclein 3′-flanking region SNP and
stimulates antisense noncoding RNA expression. J Hum Genet.
58:711–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bezard E, Gross CE, Qin L, Gurevich VV,
Benovic JL and Gurevich EV: L-DOPA reverses the MPTP-induced
elevation of the arrestin2 and GRK6 expression and enhanced ERK
activation in monkey brain. Neurobiol Dis. 18:323–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Managò F, Espinoza S, Salahpour A,
Sotnikova TD, Caron MG, Premont RT and Gainetdinov RR: The role of
GRK6 in animal models of Parkinson's disease and L-DOPA treatment.
Sci Rep. 2:3012012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tu Y, Li F and Wu C: Nck-2, a novel Src
homology2/3-containing adaptor protein that interacts with the
LIM-only protein PINCH and components of growth factor receptor
kinase-signaling pathways. Mol Biol Cell. 9:3367–3382. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ritchie MD: Using prior knowledge and
genome-wide association to identify pathways involved in multiple
sclerosis. Genome Med. 1:652009. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rearden A, Hurford R, Luu N, Kieu E,
Sandoval M, Perez-Liz G, Del Valle L, Powell H and Langford TD:
Novel expression of PINCH in the central nervous system and its
potential as a biomarker for human immunodeficiency
virus-associated neurodegeneration. J Neurosci Res. 86:2535–2542.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi L: Dock protein family in brain
development and neurological disease. Commun Integr Biol.
6:e268392013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brinkman A, van der Flier S, Kok EM and
Dorssers LC: BCAR1, a human homologue of the adapter protein
p130Cas, and antiestrogen resistance in breast cancer cells. J Natl
Cancer Inst. 92:112–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hourani M, Mendes A, Berretta R and
Moscato P: Genetic biomarkers for brain hemisphere differentiation
in Parkinson's disease. AIP Conference Proceedings. 952:pp.
207–216. 2007; View Article : Google Scholar
|
|
46
|
Wu T and Hallett M: The cerebellum in
Parkinson's disease. Brain. 136:696–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Furuichi T, Shiraishi-Yamaguchi Y, Sato A,
Sadakata T, Huang J, Shinoda Y, Hayashi K, Mishima Y, Tomomura M,
Nishibe H and Yoshikawa F: Systematizing and cloning of genes
involved in the cerebellar cortex circuit development. Neurochem
Res. 36:1241–1252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Scherzer CR, Grass JA, Liao Z, Pepivani I,
Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B, et
al: GATA transcription factors directly regulate the Parkinson's
disease-linked gene alpha-synuclein. Proc Natl Acad Sci USA.
105:pp. 10907–10912. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Höglinger GU, Breunig JJ, Depboylu C,
Rouaux C, Michel PP, Alvarez-Fischer D, Boutillier AL, Degregori J,
Oertel WH, Rakic P, et al: The pRb/E2F cell-cycle pathway mediates
cell death in Parkinson's disease. Proc Natl Acad Sci USA. 104:pp.
3585–3590. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang W, Zhang J, Kornuc M, Kwan K, Frank
R and Nimer SD: Molecular cloning and characterization of NF-IL3A,
a transcriptional activator of the human interleukin-3 promoter.
Mol Cell Biol. 15:6055–6063. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hulme DJ, Blair IP, Dawkins JL and
Nicholson GA: Exclusion of NFIL3 as the gene causing hereditary
sensory neuropathy type I by mutation analysis. Hum Genet.
106:594–596. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu WC: Parkinson disease is a TH17
dominant autoimmune disorder against accumulated alpha-synuclein.
Nature Preced. 61762011.
|