Introduction
Psoriasis is an immune-mediated autoimmune skin
disorder, which is characterized histologically by inflammatory
cell infiltrate, hyperproliferative keratinocytes in the epidermis
and hyperplasia of the dermal papilla, and affects 1–3% of the
population worldwide (1). Several
methods are used to treat psoriasis, including ultraviolet
treatment, topical or systemic drugs and biological therapy.
However, the limited long-term effectiveness, severe side effects,
high cost and frequency of relapse associated with these
treatments, and satisfactory treatment in only 27% of patients,
novel therapies are required (2).
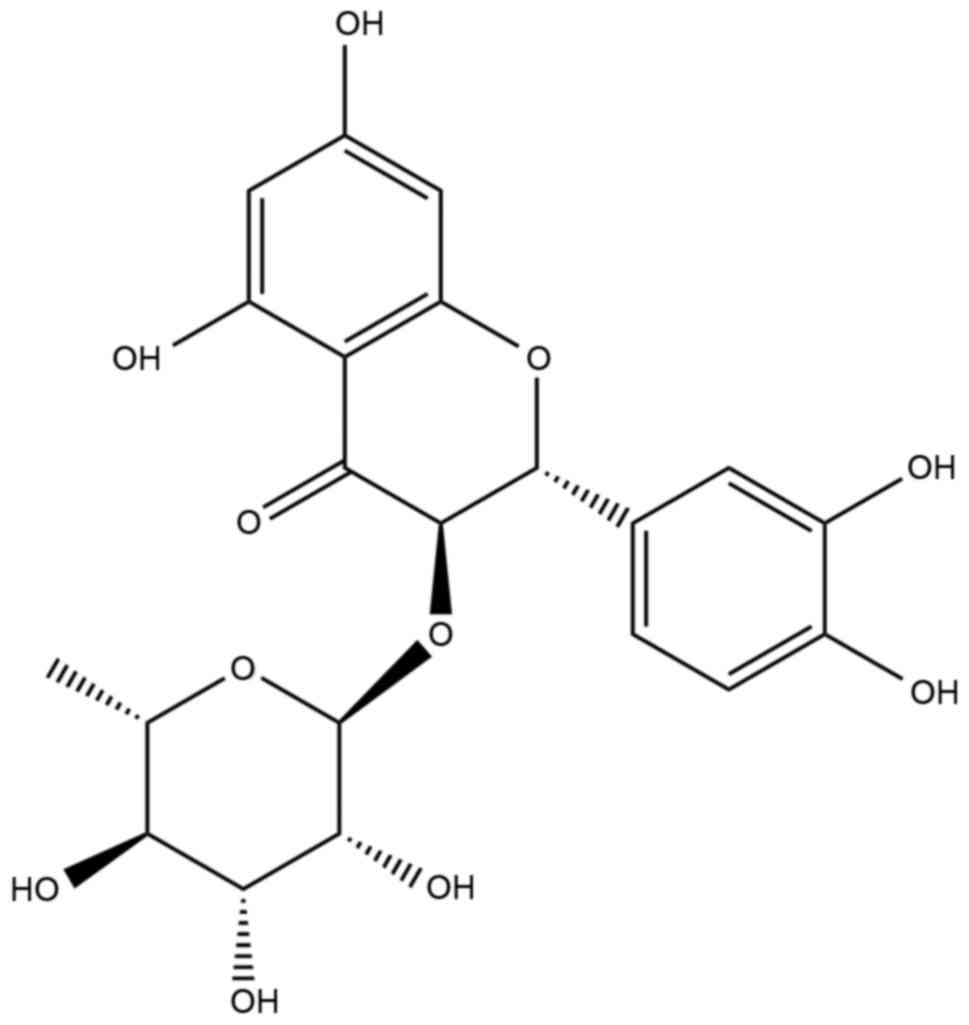
Astilbin,
3,3′,4′,5,7-pentahydroxyflavanone-3-[6-deoxy-(1-mannopyranoside)],
the structure of which is shown in Fig. 1, exists widely in herbs, including
Smilax glabra Roxb (3),
Dimorphandra nollis (4),
Engelhardis roxburghiana (5) and Astilbe chinensis (6). Astilbin has a wide range of
pharmacological effects, including inhibiting the adhesion of T
lymphocytes (7), effects against
experimental diabetic nephropathy (8), inhibiting contact hypersensitivity
(9), inducing immunosuppression
(10), and decreasing functionally
activated T and B cells (11). All
of these properties are associated with the mechanisms controlling
psoriasis.
Therefore, in the present study, the effect of
astilbin emulsion on a propranolol hydrochloride-induced guinea pig
model of psoriasis was investigated, and the underlying mechanism
was examined in an immortalized human keratinocyte (HaCaT) cell
line stimulated by lipopolysaccharide (LPS). The results showed
that astilbin had anti-psoriatic effects, and the mechanism
involved inhibiting p38 mitogen-activated protein kinase (MAPK),
with subsequent inhibition of interleukin (IL)-6 and IL-22.
Materials and methods
Drugs and reagents
Astilbin (purity >98%) was purchased from Purui
Technology Company (Chengdu, China). Propranolol chloride (purity
>98%) was purchased from Maiyuan Chemical Reagent Co. (Wuhan,
China), and the 9.5% astilbin microemulsion and 5% propranolol
emulsion were prepared at the Pharmaceutical Laboratory of
Guangdong Hospital of Chinese Medicine (Guangzhou, China).
RPMI-1640 medium, fetal bovine serum (FBS) and
streptomycin-penicillin were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). TRIzol was purchased from
Takara Biotechnology Co., Ltd. (Dalian, China). Methyl thiazolyl
tetrazolium (MTT) and LPS were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). The reverse transcription kit and
SYBR-Green/Fluorescein qPCR Master mix were purchased from Thermo
Fisher Scientific, Inc. (Carlsbad, CA, USA). The mRNA primers for
human β-actin, IL-6, IL-17 and IL-22 were synthesized by Thermo
Fisher Scientific, Inc. (Shanghai, China). The IL-6, IL-17A and
IL-22 enzyme-linked immunosorbent assay (ELISA) kits and the ELISA
buffer kit were purchased from PeproTech EC, Ltd. (London, UK). The
bicinchoninic acid protein assay kit was purchased from Thermo
Fisher Scientific, Inc. The Clarity™ Western ECL substrate and
Precision Plus Protein Kaleidoscope were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). RIPA buffer (cat. no.
9806S), p38 (cat. no. 8690S), phosphorylated p38 (cat. no. 9211S),
extracellular signal-regulated kinase (ERK)1/2 (cat. no. 4695S),
phosphorylated ERK1/2 (cat. no. 4370S) and GAPDH (cat. no. 2118S)
antibodies, and anti-rabbit IgG, horseradish peroxidase-conjugated
antibody (cat. no. 7074S) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Animals and cell lines. Male albino guinea pigs,
weighing 250–300 g, were purchased from the Medical Laboratory
Animal Center of Guangdong (Guangzhou, China) at 4 weeks of age.
All animal experiments were performed according to the NIH
guidelines for the Care and Use of Laboratory Animals, and were
approved by the Animal and Ethics Review Committee of Guangzhou
University of Chinese Medicine (protocol approval no. 2011001) on
24th December 2010.
The HaCaT cell line was purchased from the China
Centre for Type Culture Collection (Wuhan, China). The cells were
cultured in RPMI-1640 supplemented with 5% FBS, 10 µg/ml of
streptomycin and 10 U/ml of penicillin, and were incubated in a
humidified atmosphere with 5% CO2 and 95% air at
37°C.
In vivo experiments
The animals were housed in a 24°C
humidity-controlled room with 12-h light-dark cycle and allowed
free access to standard diet and tap water. following
acclimatization for 1 week, the guinea pigs were randomly divided
into a control group (n=6) and model group (n=12). The ears of the
animals were shaved in the model group, and a 5% propranolol
emulsion (0.2 ml/ear) was administered topically three times a day
to induce psoriasis-like lesions. After 8 days, the model was
established (12).
The animals in model group were then separated into
a model control (n=6) and experimental (n=6) group. A 9.5%
microemulsion of astilbin was administered topically to animals in
the experimental group at a dose of 0.2 g/ear once per day, whereas
the animals in the model control group were topically administered
with the same quantity of blank emulsion. After 1 week, all guinea
pigs were sacrificed and the ears were cut for the preparation of
paraffin sections with thickness of ~5 µm, which were stained using
hematoxylin and eosin for histopathological evaluations using the
Olympus microscope CX31 (Olympus Corporation, Tokyo, Japan), Baker
scores were assigned (13)
according to the criteria shown in Table I.
 | Table I.Baker score criteria. |
Table I.
Baker score criteria.
| Region | Pathological
change | Score |
|---|
| Corneous layer | Munro abscess | 2.0 |
|
| Hyperkeratosis | 0.5 |
|
| Parakeratosis | 1.0 |
| Epidermis | Lack or attenuation
of granular layer | 1.0 |
|
| Acanthosis | 1.0 |
|
| Elongation of the
rete ridges | 0.5–1.5 |
| Dermis | Lymphocytic
infiltrate | 0.5–2.0 |
|
| Papillary papillae
congestion | 0.5 |
|
| Telangiectasia of
superficial derma | 0.5 |
Cell growth assay
A 5.0-mg quantity of astilbin (>98% in purity)
was precisely weighed and dissolved into 50 µl dimethyl sulfoxide
as a stock solution (222 mM). Prior to experiments, the stock
solution of astilbin was diluted in PBS and then prepared to the
experimental doses.
The growth inhibition induced by astilbin in HaCaT
cells was assessed using an MTT assay. The cells were seeded in a
96-well plate at a density of 2,000 cells/well and cultured for 24
h to allow the cells to adhere to the plates. Subsequently, the
cells were exposed to the presence or absence of different
concentrations of astilbin, (6.66, 13.88, 27.75, 55.50 and 222.00
µM) in RPMI-1640 medium. The plates were placed at 37°C for 24 h,
following which 20 µl MTT (5 mg/ml) was added to each well of
cells, and the reaction was allowed to proceed at 37°C for 4 h in
the dark. The supernatant was then collected and the cells were
washed with PBS. The formazan salts produced were dissolved with
dimethyl sulfoxide, and the absorbance was measured at 570 nm using
a Vector 5 multifunctional immune analyzer (PerkinElmer, Inc.,
Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of the mRNA expression
levels of IL-6, IL-17 and IL-22
Following pretreatment with astilbin at different
concentrations (2.22 and 11.10 µM) for ٢٤ h and subsequent
treatment with LPS (5 µg/ml) for 2 h, total RNA from the HaCaT
cells in 6-well plates was isolated using TRIzol reagent. cDNA was
generated from 1 µg total RNA and reverse transcriptase using the
First Strand cDNA synthesis kit on a 7500 Fast Real-Time PCR system
(ABI; Thermo Fisher Scientific, Inc.). The specific primer pairs
are shown in Table II.
 | Table II.Primer sets used for reverse
transcription-quantitative polymerase chain reactions. |
Table II.
Primer sets used for reverse
transcription-quantitative polymerase chain reactions.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-actin |
CGGGAAATCGTGCGTGACAT |
CAGGAAGCAAGGCTGGAAGA |
| IL-6 |
CTGAGGGCTCTTCGGCAAAT |
GCCCAGTGGACAGGTTTCTG |
| IL-17A |
GCCTTCAAGACTGAACACCG |
TGACATGCCATTCCTCAGGG |
| IL-22 |
AACTAACCCCCTTTCCCTGC |
AACGCAGGGGTTCATTTGGA |
The reaction products were detected by measuring the
binding of SYBR Green I to DNA using SYBR Green PCR Master mix
(Thermo Fisher Scientific, Inc.). Optimization of the amplification
reaction was assured through dissociation curve analysis. The basic
protocol for the RT-qPCR procedure was an initial incubation step
at 95°C for 7 min, followed by 45 cycles of 95°C for 10 sec and
60°C for 30 sec. All experiments were run in triplicate, and the
relative expression values were normalized to the expression value
of β-actin, followed by analysis using the 2−ΔΔCq method
(14).
ELISA for IL-6, IL-17A and IL-22
Following pretreatment with astilbin at different
concentrations (2.22 and 11.10 µM) for ٢٤ h and subsequent
treatment with LPS (5 µg/ml) for 2 h, the incubation media of the
HaCaT cells was collected and centrifuged at 3,000 × g for 3 min at
room temperature to remove floating cells. The clear supernatant
was collected to perform ELISA analyses, according to the
manufacturer's protocol, to assess the expression levels of IL-6,
IL-17A and IL-22. The 96-well plates loaded with the samples were
read at 450 nm on a Vector 5 multifunctional immune analyzer. The
levels of human IL-6, IL-17 and IL-22 in each well calculated as
pg/ml.
Western blot analysis of protein
levels of phosphorylated p38 MAPK and phosphorylated ERK1/2
The HaCaT cells were washed twice with ice-cold PBS
and lysed in ice-cold RIPA lysis buffer containing 1% proteinase
inhibitors and 1% phosphatase inhibitors. The lysates were
centrifuged at 20,800 × g for 15 min at 4°C to obtain the total
protein. The protein concentrations were determined using the
bicinchoninic acid protein assay kit, and cell lysates containing
30 µg total proteins were separated on a 12% SDS-PAGE and
transferred electrophoretically onto polyvinylidene fluoride
membranes. Following incubation in blocking solution (5% non-fat
milk solution in Tris-buffered saline with Tween-20) for 1 h at
room temperature, the membranes were incubated with 1:1,000
dilutions of primary antibodies against p38 MAPK, ERK1/2,
phosphorylated ERK1/2 or GAPDH overnight at 4°C. GAPDH was used as
control. The membranes were then washed three times using Tris-HCl
with 20% Tween-80 and incubated with a 1:2,000 dilution of
secondary antibody for 1 h at 4°C. Finally, the membranes were
washed three more times. Protein expression on the membranes was
visualized using an enhanced chemiluminescence detection system.
Densitometric analysis was performed using Quantity One software
(version 4.6.2; Bio-Rad Laboratories, Inc.) to scan the signals.
The signal quantities shown were standardized to the background and
normalized for loading. The controls were set as 1.0, and the
fold-changes were plotted in relation to the control value. The
results represent the average values of three independent
experiments.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data analysis was performed using the SPSS statistical
software package (version 13.0; SPSS, Inc., Chicago, IL, USA).
Differences between the experimental groups and control group were
estimated using one-way analysis of variance (homogeneity of
variances was P>0.05) for the in vivo experiment, and
analyzed using an independent-sample t-test for the in vitro
experiment. P<0.05 was considered to indicate a statistically
significant difference.
Results
Astilbin relieve psoriasis-like
lesions induced by propranolol chloride
Topical therapy is one of the most convenient
methods for psoriasis. Therefore, astilbin was prepared into a
microemulsion in the present study as a topical carrier for
dermatological treatment, and its effect on guinea pigs was
evaluated. The results showed that, following stimulation with
propranolol chloride, the ears of the guinea pigs became red and
swollen, accompanied by silvery scales and lesions, which remained
until the end of experiment. However, the lesions on the animals
treated with astilbin were milder, compared with those in the
disease control group.
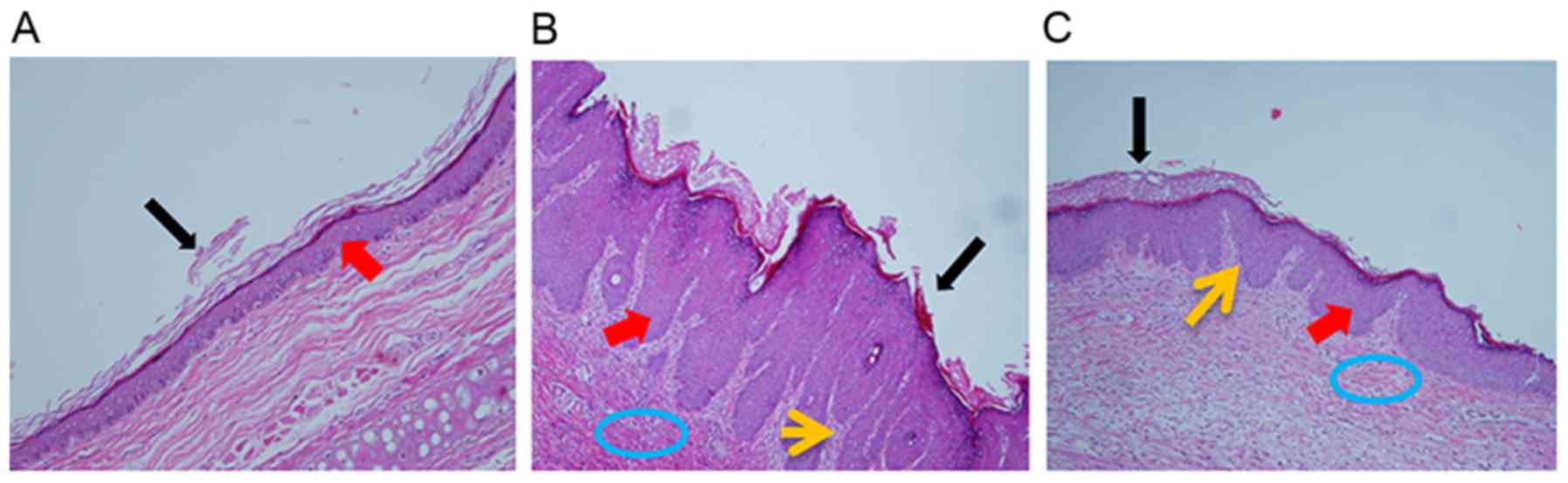
From the microscopic structure of the skin tissues,
as shown as Fig. 2, the tissues in
the disease control group exhibited parakeratosis and
hyperkeratinization in the epidermis, thickened spinous layers and
papillary papillae congestion in the derma; these histopathological
characteristics are similar to those of psoriatic lesions in
humans. By contrast, the astilbin microemulsion reduced the lesions
in the cuticular layer, the granular layer appeared, and the
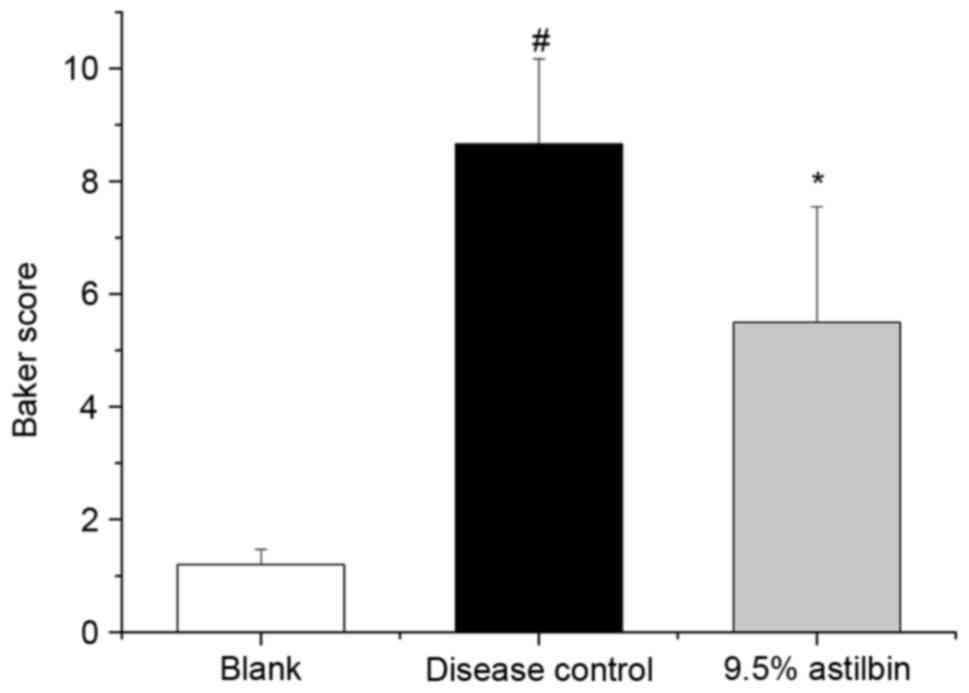
spinous layers and derma decreased. Based on the Baker scores, the
score of the disease control group was significantly increased,
compared with that of the blank group. Topical administration of
the astilbin microemulsion resulted in a significant decrease in
the Baker score, as shown in Fig.
3, with an inhibition ratio of 36.5% (P<0.05). This
suggested that the astilbin microemulsion ameliorated the negative
effects in the psoriasis-like model in guinea pigs.
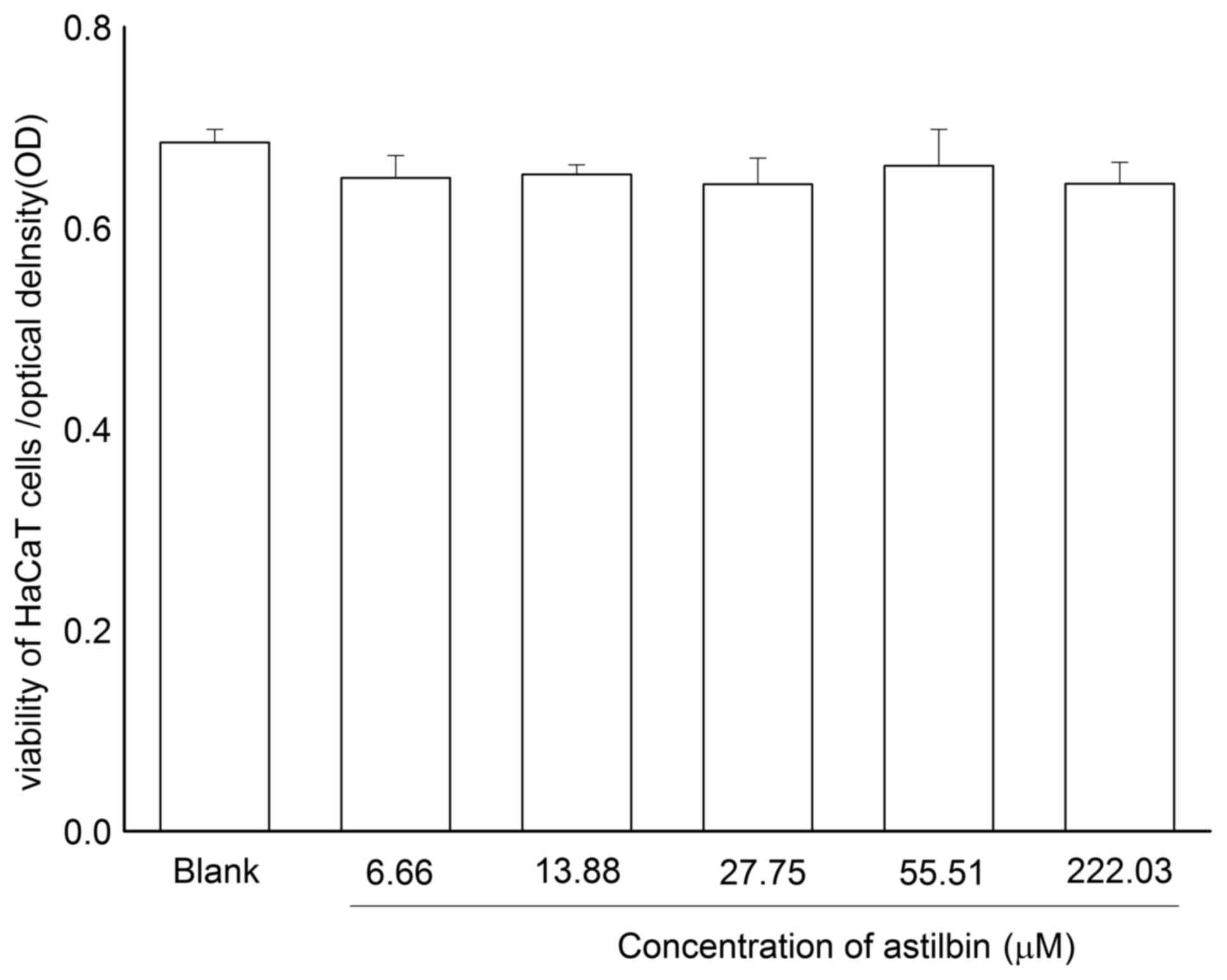
Astilbin has no effect on the
viability of HaCaT cells
To investigate the effect of astilbin on HaCaT cell
proliferation, an MTT method was used to detect the viability of
HaCaT cells co-incubated with astilbin (6.66, 13.88, 27.75, 55.50
and 222.00 µM). As shown in Fig.
4, no significant changes in the viability of the HaCaT cells
were observed following co-incubation with astilbin at different
concentrations for a 24-h incubation period. This result suggested
that astilbin was non-toxic towards the HaCaT cells at the
experimental dose range and may have no effect on the normal
epidermis.
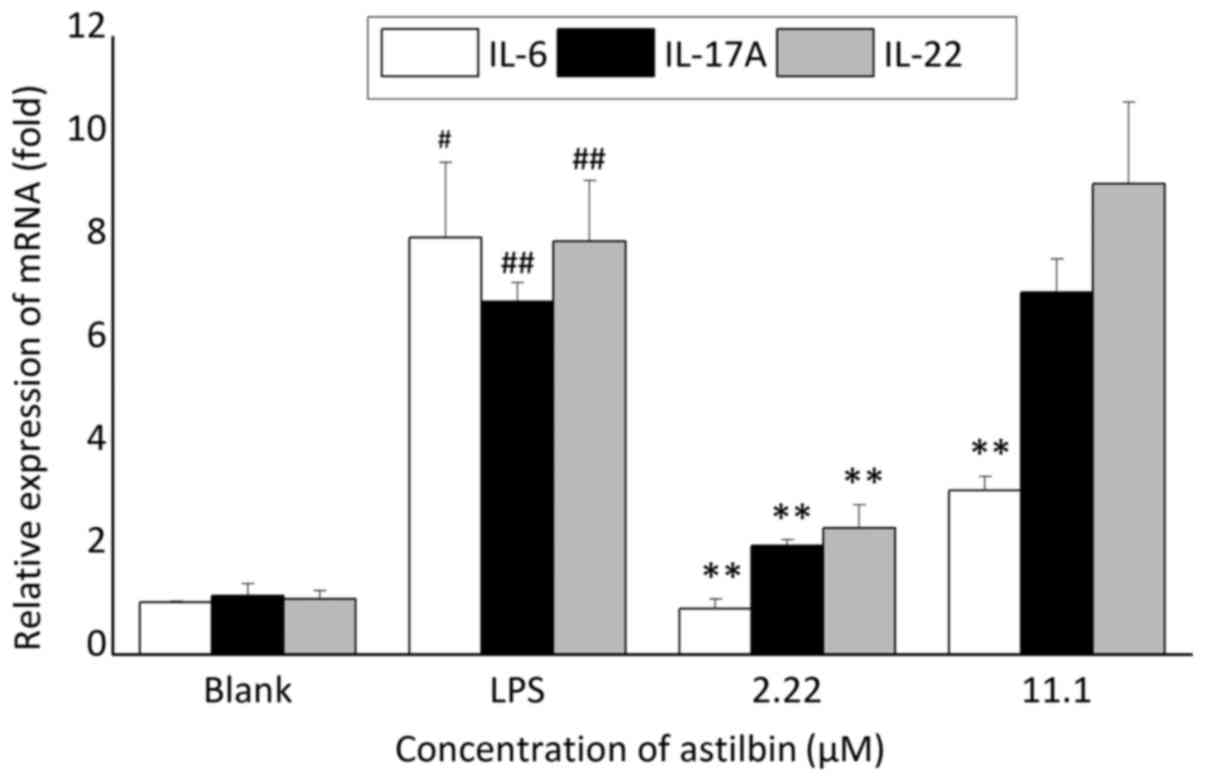
Astilbin inhibits the mRNA expression
levels of IL-6, IL-17A and IL-22 in inflammation
In order to identify the cytokines targeted by
astilbin in the inflamed skin microenvironment, the inflammatory
model of HaCaT cells co-cultured with LPS for 2 h was established,
and the mRNA expression levels of inflammatory cytokines associated
with psoriasis, including IL-6, IL-4, IL-10 and IL-23 (some data
not shown) were determined. The results showed that LPS increased
the mRNA expression levels of IL-6, IL-17A and IL-22 in the HaCaT
cells, whereas astilbin inhibited the mRNA expression levels of
IL-6, IL-17A and IL-22 (Fig. 5).
At a concentration of 2.22 µM, the inhibition ratio of astilbin was
89, 69.1 and 69.3% for IL-6, IL-17A and IL-22, respectively
(P<0.01); however, at a concentration of 11.00 µM, astilbin only
significantly decreased the mRNA expression of IL-6 (60.6%;
P<0.01), with no significant effects on IL-17A or
IL-22.
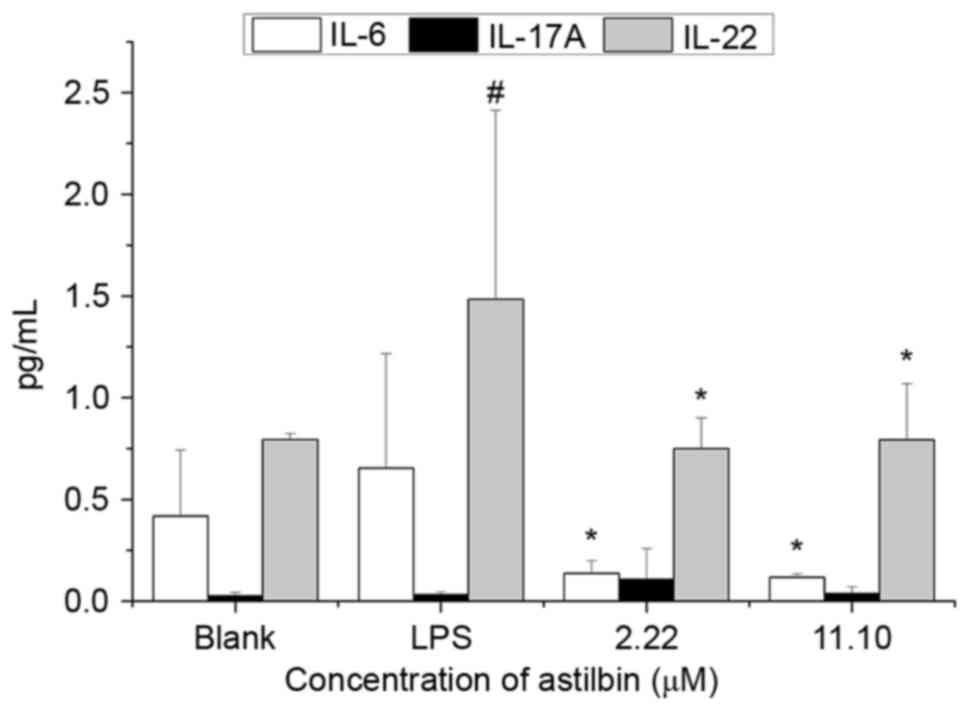
Astilbin inhibits the protein
expression levels of IL-6 and IL-22 stimulated by LPS in HaCaT
cells
Whether changes in gene expression affect protein
function depends on the subsequent protein translation. Therefore,
the effects of astilbin on the protein expression levels of IL-6,
IL-17A and IL-22 were detected in the same model using ELISA. As
shown in Fig. 6, LPS increased the
protein expression of IL-6 and IL-22 by 36.0 and 46.5%,
respectively. Compared with the LPS-stimulated group, 2.22 µM
astilbin markedly decreased the expression levels of IL-6 and IL-22
by 79.2 and 49.5%, respectively (P<0.05). The inhibition ratio
of 11.10 µM astilbin on IL-٦ was ٨٢.٢٪. These levels were similar
to the results of the RT-qPCR analysis. However, unlike the results
of the RT-qPCR analysis, 11.10 µM astilbin also significantly
decreased the expression of IL-22 by 46.5%. Neither LPS nor
astilbin had a significant effect on the expression of IL-17A.
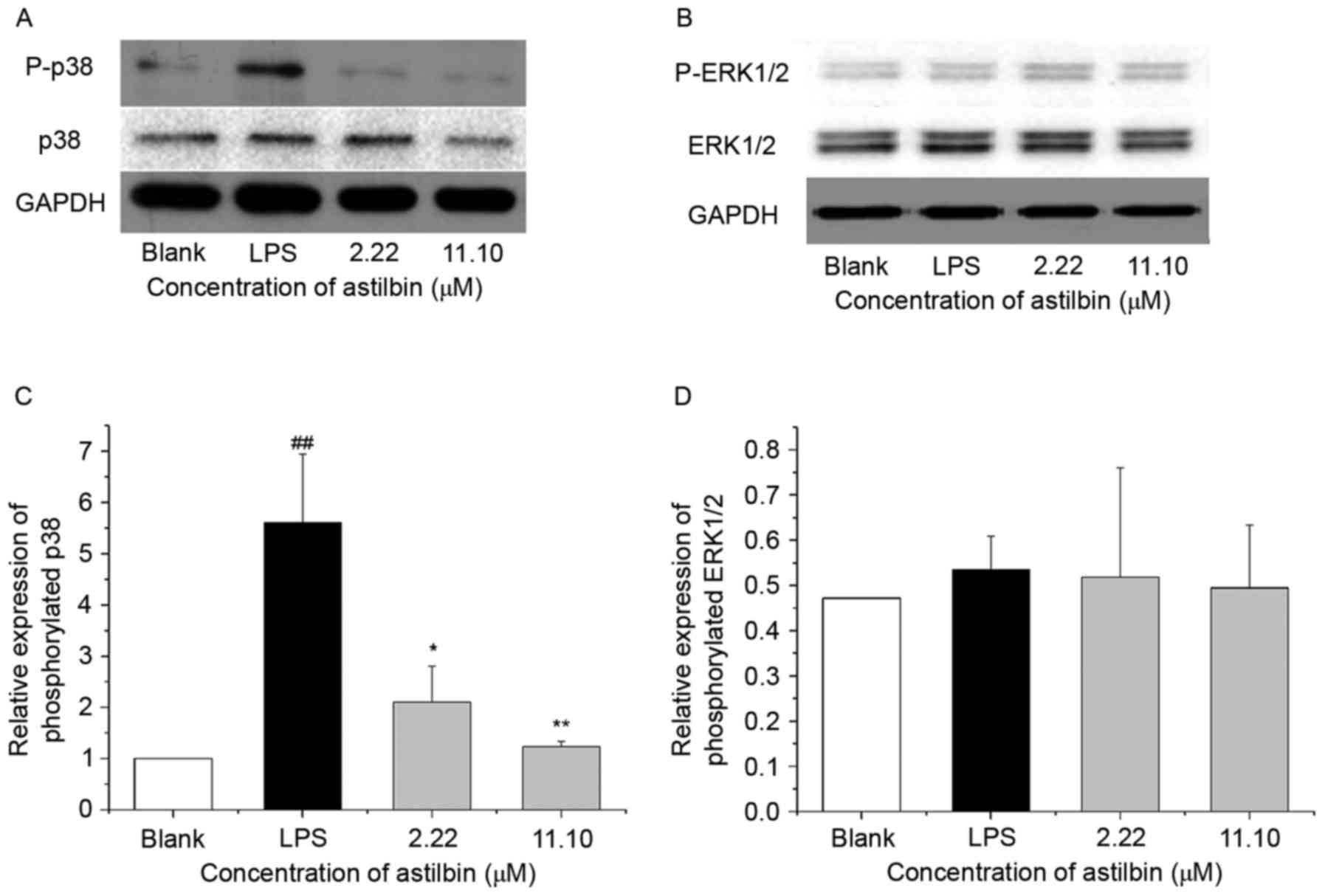
Astilbin inhibits the expression of
phosphorylated p38 in HaCaT cells
MAPK signaling is an important signaling pathway in
psoriasis by regulating inflammation and proliferation. To examine
whether astilbin inhibits IL-6 and IL-22 through MAPK signaling,
the present study performed western blot analysis to analyze the
expression of p38 MAPK and ERK1/2. As shown in Fig. 7, treatment with 2.22 and 11.10 µM
astilbin reduced the phosphorylation activity of p38 MAPK by 62.4
and 78.0%, respectively (P<0.01) but had no significant effect
on the phosphorylation of ERK1/2. These data support the hypothesis
that astilbin targets p38 MAPK signaling to regulate downstream
proteins, including inflammatory factors IL-6 and IL-22.
In order to confirm whether astilbin had a similar
effect on the expression of IL-6 and IL-22 with p38 MAPK
inhibition, the present study used SB203580, a p38 MAPK inhibitor,
for co-culture with the HaCaT cells prior to LPS treatment. As
shown in Fig. 8, the p38 MAPK
inhibitor suppressed the overexpression of IL-6 and IL-22 in the
LPS-induced HaCaT cells, with inhibition ratios of 83.4 and 43.7%,
respectively (P<0.05), exhibiting the same effect as astilbin,
which suggested that astilbin had a similar role as the p38 MAPK
inhibitor in the inflammatory HaCaT cell model.
Discussion
Psoriasis is one of the most challenging diseases
worldwide, which lacks complete treatment efficacy. For the
majority of patients with mild-to-moderate psoriasis, topical
treatment is the first option. Currently, there is an emphasis on
the development of more effective drugs with fewer side effects for
the treatment of psoriasis.
Astilbin is an active compound of the Chinese herb
Smilax glabra Roxb. Accumulated evidence has shown that
astilbin exerts varied activities, which may be associated with
psoriasis (15). However, whether
astilbin can be developed into a topical agent for psoriasis
remains to be fully elucidated. In order to investigate this, the
present study successfully prepared astilbin into a microemulsion
and examined its effect on a psoriasis-like model in guinea pigs.
The results showed that the topical administration of astilbin
significantly ameliorated the psoriasis-like lesions stimulated by
propranolol.
Based on the in vivo results of astilbin, the
present study aimed to define the mechanism of astilbin in direct
contact with inflamed or hyperplastic epidermal cells. Therefore,
inflammatory HaCaT cells induced by LPS were used in the present
study to examine how astilbin affected the epidermis cells.
Astilbin was expected to suppress the proliferation of HaCaT cells,
however, the results showed that astilbin had no effect on the
proliferation of HaCaT cells, suggesting that anti-proliferative
activity is not involved in the mechanism of astilbin.
Previous studies have indicated that astilbin has
immunosuppressive activity in certain inflammatory diseases,
including contact dermatitis, liver injury and colitis (16,17),
and that certain anti-inflammatory drugs, including
glucocorticoids, have anti-psoriatic effects. This suggested that
the anti-psoriatic effect of astilbin may be through
anti-inflammatory effects. Cytokines are indicators of
inflammation, and IL-6, IL-17A, IL-22, IL-23, IL-4 and IL-10
(18–20) are all upregulated in psoriatic
lesions, a number of which have been used as a drug target and
associated with disease severity. Therefore, the effect of astilbin
on these cytokines was investigated in the present study. The
results showed that astilbin decreased the expression of IL-6 and
IL-22 at the mRNA and protein levels, and inhibited the expression
of IL-17A at the mRNA level only. Taken together, these results
suggested that astilbin targeted IL-6 and IL-22 to improve the
microenvironment in the epidermis, rather than suppressing the
normal proliferation of keratinocytes.
IL-6, IL-17 and IL-22 were all elevated in psoriatic
skin. IL-6 is a multifunctional cytokine, which can be produced by
keratinocytes in response to certain stimuli, including LPS
(21). In psoriasis, IL-6 can
directly promote epidermal hyperplasia and affect the function of
dermal inflammatory cells (22).
According to previous reports, astilbin decreased the serum
cytokine level of IL-6 in lupus-prone mice and reduced the mRNA
expression of IL-6 in LPS-stimulated J774A mice (23–25).
The results of these studies are in accordance with the present
study and suggested that decreasing the expression of IL-6 is an
anti-inflammatory function of astilbin, associated with the
inhibition of hyperplasia and inflammatory infiltration in the
psoriatic epidermis.
IL-22 was another cytokine targeted by astilbin in
the present study. It has been reported that IL-22 induces features
of psoriatic lesions, including inhibiting the differentiation and
cornification of keratinocytes, disturbing the barrier function of
the epidermis, facilitating immune cell infiltration and
restructuring of the epidermis (26). Astilbin may have suppressed IL-22
to improve lesions in the psoriasis-like model. The association
between astilbin and IL-22 in HaCaT cells has not been demonstrated
previously. The downregulation of IL-22 may be a novel mechanism
underlying the effect of astilbin on inflammatory diseases.
IL-17A is a pro-inflammatory cytokine, which has
effects on neutrophil recruitment, host defense and
immuno-inflammatory diseases, and is secreted predominantly by Th17
cells, mast cells, neutrophils and dendritic cells (27). Previous reports have indicated that
astilbin decreases the serum cytokine level of IL-17A in
lupus-prone mice (23). However,
the present study showed that astilbin only marginally decreased
the mRNA expression of IL-17A and had no effect on the protein
expression of IL-17A. The reason for astilbin having different
effects on mRNA and protein levels of IL-17A is unclear, however,
it may be associated with the effect of LPS on the expression of
IL-17A. Although there are reports of IL-17A responding to
LPS-induced lung inflammation or other microbial infections
(28,29), these reports are based on in
vivo experiments. In isolated epithelial cells, LPS or
microbial infection may activate the gene activity of IL-17A and
promote its transcription, however, the translation to protein
depends on multiple factors, including the stability of mRNA, the
abundance of specific codons and the longevity of proteins
(30–32). Therefore, further investigations
are required.
The expression of inflammatory cytokines are always
regulated by a signaling molecule and, in order to identify the
signaling pathway through which astilbin functions in the
inflammatory model, p38 MAPK and EKR1/2 MAPK were selected for
investigation. p38 MAPK and ERK1/2MAPK belong to MAPKs, and have
been found to contribute to the pathogenesis of psoriasis. p38 MAPK
inhibitors have been developed into potential biologicals for
several inflammatory diseases, including arthritis and psoriasis
(33,34). Zou et al (35) reported that astilbin suppressed the
expression of p38 MAPK in an acute heart allograft rejection model,
which was involved in inhibiting activated T cells. Consistent with
this, the present study found that astilbin inhibited the
phosphorylation of p38 MAPK in the HaCaT cells but had no effect on
the phosphorylation of ERK1/2. This suggested that astilbin may
suppress cytokines via p38 MAPK and ameliorate psoriasis. In order
to investigate this, the present study used SB203580, a p38 MAPK
inhibitor, for co-culture with the HaCaT cells prior to LPS
exposure. The results showed that SB203580 inhibited the expression
levels of IL-6 and IL-22 in the same manner as astilbin, which
suggested that astilbin was involved as a p38 MAPK inhibitor in the
inflamed HaCaT cell model.
In conclusion, the present study identified the
anti-psoriatic activity of astilbin through the use of a psoriasis
model in guinea pigs. The molecular mechanism involved the
suppression of inflammatory cytokines IL-6 and IL-22 via the
inhibition of p38 MAPK signaling. These results indicate that
astilbin, as a natural micromolecule, offers potential for
development as an effective drug to treat psoriasis by targeting
p38 MAPK/IL-6/IL-22.
Acknowledgements
This study was supported by the Teamwork Project of
the Natural Science Foundation of Guangdong Province of China
(grant no. S2013030011515), the Project of the Traditional Chinese
Medicine Hospital of Guangdong Province (grant no. YK2013B1N11),
and the Project of the State Administration of Traditional Chinese
Medicine Clinical Base Fund (grant no. JDZX2015206).
References
|
1
|
Abe R, Yamagishi S, Fujita Y, Hoshina D,
Sasaki M, Nakamura K, Matsui T, Shimizu T, Bucala R and Shimizu H:
Topical application of anti-angiogenic peptides based on pigment
epithelium-derived factor can improve psoriasis. J Dermatol Sci.
57:1–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richards HL, Fortune DG, O'Sullivan TM,
Main CJ and Griffiths CE: Patients with psoriasis and their
compliance with medication. J Am Acad Dermatol. 41:581–583. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen T, Li J, Cao J, Xu Q, Komatsu K and
Namba T: A new flavanone isolated from Rhizoma Smilacis glabrae and
the structural requirements of its derivatives for preventing
immunological hepatocyte damage. Planta Med. 65:56–59. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Xu Q and Chen T: Quantitative
determination of astilbin in rabbit plasma by liquid
chromatography. J Chromatogr B Analyt Technol Biomed Life.
805:357–360. 2004. View Article : Google Scholar
|
|
5
|
Huang H, Cheng Z, Shi H, Xin W, Wang TT
and Yu LL: Isolation and chacterization of two flavonoids,
engeletin and astilbin from the leaves of Engelhardia roxburghiana
and their potential anti-inflammatory properties. J Agric Food
Chem. 59:4562–4569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bezerra GP, Góis RW, de Brito TS, de Lima
FJ, Bandeira MA, Romero NR, Magalhães PJ and Santiago GM:
Phytochemical study guided by the myorelaxant activity of the crude
extract, fractions and constituent from stem bark of Hymeana
courbaril L. J Ethnopharmacol. 149:62–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi HW, Lu XM, Fang F, Wang J and Xu Q:
Astilbin inhibits the adhesion of T lymphocytes via decreasing
TNF-alpha and its associated MMP-9 activity and CD44 expression.
Int Immunopharmacol. 18:1467–1474. 2008. View Article : Google Scholar
|
|
8
|
Li GS, Jiang WL, Yue XD, Qu GW, Tian JW,
Wu J and Fu FH: Effect of astilbin on experimental diabetic
nephropathy in vivo and in vitro. Planta Med. 75:1470–1475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fei M, Wu X and Xu Q: Astilbin inhibits
contact hypersensitivity through negative cytokine regulation
distinct from cyclosporine A. J Allergy Clin Immunol.
116:1350–1356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou S, Shen X, Tang Y, Fu Z, Zheng Q and
Wang Q: Astilbin suppresses acute heart allograft rejection by
inhibiting maturation and function of dendritic cells in mice.
Transplant Proc. 42:pp. 3798–3802. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo L, Liu W, Lu T, Guo W, Gao J, Luo Q,
Wu X, Sun Y, Wu X, Shen Y and Xu Q: Decrease of functional
activated T and B cells and treatment of glomerulonephitis in
Lupus-prone mice using a natural flavonid astilbin. PLoS One.
10:e01240022015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gaylarde PM, Brock AP and Sarkany I:
Psoriasiform changes in guinea-pig skin from propranolol. Clin Exp
Dermatol. 3:157–160. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baker BS, Brent L, Valdimarsson H, Powles
AV, al-Imara L, Walker M and Fry L: Is epidermal cell proliferation
in psoriatic skin-grafts on nude-mice driven by T-cell derived
cytokines. Br J Dermatol. 12:105–110. 1992. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diao H, Kang Z, Han F and Jiang W:
Astilbin protects diabetic rat heart against ischemia-reperfusion
injury via blockade of HMGB1-dependent NF-κB signaling pathway.
Food Chem Toxicol. 63:104–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fei MJ, Wu XF and Xu Q: Astilbin inhibits
contact hypersensitivity through negative cytokine regulation
distinct from cyclosporin A. J Allergy Clin Immunol. 116:1350–1356.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding Y, Liang Y, Deng B, Qiao A, Wu K,
Xiao W and Gong W: Induction of TGF-β and IL-10 production in
dendritic cells using astilbin to inhibit dextran sulfate
sodium-induced colitis. Biochem Biophys Res Commun. 446:529–534.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Puig L: Cardiovascular risk and psoriasis:
The role of biologic therapy. Actas Dermosifiliogr. 103:853–862.
2012. View Article : Google Scholar
|
|
19
|
Menter A, Gottlieb A, Feldman SR, Van
Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA,
Korman NJ, et al: Guidelines of care for the management of
psoriasis and psoriatic arthritis-section 1. Overview of psoriasis
and guidelines of care for the treatment of psoriasis with
biologics. J Am Acad Dermatol. 58:826–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pietrzak AT, Zalewska A, Chodorowska G,
Krasowska D, Michalak-Stoma A, Nockowski P, Osemlak P, Paszkowski T
and Roliński JM: Cytokines and anticytokines in psoriasis. Clin
Chim Acta. 394:7–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin J, Sundararaj KP, Samuvel DJ, Zhang X,
Li Y, Lu Z, Lopes-Virella MF and Huang Y: Different signaling
mechanisms regulating IL-6 expression by LPS between gingival
fibroblasts and mononuclear cells: Seeking the common target. Clin
Immunol. 143:188–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grossman RM, Krueger J, Yourish D,
Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB and
Gottlieb AB: Interleukin 6 is expressed in high levels in psoriatic
skin and stimulates proliferation of cultured human keratinocytes.
Proc Natl Acad Sci USA. 86:pp. 6367–6371. 1989; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo L, Liu W, Lu T, Guo W, Gao J, Luo Q,
Wu X, Sun Y, Wu X, Shen Y and Xu Q: Decrease of functional
activated T and B cells and treatment of glomerulonephitis in
lupus-prone mice using a natural flavonoid astilbin. PLoS One.
10:e01240022015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang H, Cheng Z, Shi H, Xin W, Wang TT
and Yu L: Isolation and characterization of two flavonoids,
engeletin and astilbin, from the leaves of engelhardia roxburghiana
and their potential anti-inflammatory properties. J Agric Food
Chem. 59:4562–4569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song SH, Shen XY, Liu F, Tang Y, Wang ZM
and Fu ZR: Protective effects of astilbin on renal
ischemia-reperfusion injury in rats. Zhong Xi Yi Jie He Xue Bao.
7:753–757. 2009.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao JQ: Targeting Interleukin-22 in
psoriasis. Inflammation. 37:94–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grygorczuk S, Świerzbińska R, Moniuszko A,
Kondrusik M, Zajkowska J, Czupryna P, Dunaj J and Pancewicz S:
Synthesis of Th17 cytokines in the culture of peripheral blood
mononuclear cells stimulated with Borrelia burgdorferi sensu lato.
Ann Agric Environ Med. 23:242–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SR, Kim HJ, Kim DI, Lee KB, Park HJ,
Jeong JS, Cho SH and Lee YC: Blockade of interplay between IL-17A
and endoplasmic reticulum stress attenuates LPS-induced lung
injury. Theranostics. 5:1343–1362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waggoner SA and Liebhaber SA: Regulation
of alpha-globin mRNA stability. Exp Biol Med. 228:387–395. 2003.
View Article : Google Scholar
|
|
31
|
Pesole G, Gissi C, Grillo G, Licciulli F,
Liuni S and Saccone C: Analysis of oligonucleotide AUG start codon
context in eukariotic mRNAs. Gene. 261:85–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bachmair A, Finley D and Varshavsky A: In
vivo half-life of a protein is a function of its amino-terminal
residue. Science. 234:179–186. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mavropoulos A, Rigopoulou EI, Liaskos C,
Bogdanos DP and Sakkas LI: The role of p38 MAPK in the
aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev
Immunol. 2013:5697512013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johansen C, Kragballe K, Westergaard M,
Henningsen J, Kristiansen K and Iversen L: The mitogen-activated
protein kinases p38 and ERK1/2 are increased in lesional psoriatic
skin. Br J Dermatol. 152:37–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou S, Shen X, Tang Y, Fu Z, Zheng Q and
Wang Q: Astilbin suppresses acute heart allograft rejection by
inhibiting maturation and function of dendritic cells in mice.
Transplant Proc. 42:pp. 3798–3802. 2010; View Article : Google Scholar : PubMed/NCBI
|