Introduction
Cholestasis in infants is a relatively common infant
disease, with an incidence of 1/2,500 among newborns worldwide
(1,2). Delayed treatment of cholestasis may
lead to accumulation of bile salts and other toxic components of
bile in hepatocytes and throughout the body, resulting in
cholestatic hepatic fibrosis and even liver cirrhosis; as such, it
is a notable cause of infant mortality and disability (3). For a variety of reasons, including
infection, poisoning, autoimmune diseases, and genetic defects,
metabolic abnormalities induce hepatocyte dysfunction, or bile duct
obstruction and reduced bile flow, which then induce cholestasis in
infants. Early diagnosis of cholestasis, and timely and effective
treatment measures are required to cease disease progression in
infants (4,5). Due to the nature of infant growth and
development, treatment for cholestasis in infants has been
difficult for pediatricians (6).
Therefore, it is necessary to develop a reliable pharmacological
agent with good efficacy, few side effects and that is suitable for
children.
In recent years, with advances in medical
technology, a number of underlying mechanisms of cholestasis have
been identified (2,6,7).
Diagnosis and treatment of cholestasis in infants has made
substantial progress; however, the overall diagnosis and treatment
for the disease in China lag behind those of other countries
(8). A number of previous studies
have demonstrated that ursodeoxycholic acid (UDCA) is effective for
the treatment of cholestasis (9–11),
and it has also been used as a treatment for many types of
intrahepatic cholestasis (12).
UDCA has been found to possibly replace the toxic hydrophobic bile
salts in enterohepatic circulation, inhibit the absorption of other
bile salts in the ileum, improve the mobility of the basolateral
membrane of the hepatic sinusoid and hepatic-bile capillary
membrane (13), protect the
membranes of hepatocytes, enhance the excretory function of
hepatocytes by improving the gallbladder, and protecting
hepatocytes to inhibit the pathological process of liver fibrosis
and cirrhosis (14).
α-naphthylisothiocyanate (ANIT) has been extensively used for
establishing liver fibrosis models. The present study established a
reversible acute ANIT-induced cholestatic hepatic fibrosis model in
infant rats and used UDCA to treat the disease to simulate UDCA
treatment for cholestatic hepatic fibrosis in infants using the
in vivo model. Through detecting the biochemical markers for
liver function, four serum markers for hepatic fibrosis [hyaluronic
acid (HA), type-III procollagen (PCIII), laminin protein (LN) and
type-IV collagen (cIV)], hepatic fibrosis markers in liver disease,
and pathomorphological changes in different groups of the rat
model, the therapeutic effect of UDCA on cholestatic hepatic
fibrosis in infant rats was evaluated, with the aim of identifying
an effective treatment for early intervention of the disease in
infants.
Materials and methods
Experimental animals and reagents
A total of 49 3-week old healthy Sprague Dawley (SD)
rats (clean grade; weight, 50–70 g; 24 male, 25 female) were
purchased from the Experimental Animal Center of Hebei Province
(Hebei Medical University, Shijiazhuang, Hebei, China; certificate
nos. 1307128, 1308020 and 1308039). Rats were housed in the
Experimental Animal Center at the Third Hospital of Hebei Medial
University (Shijiazhuang, Hebei, China) under standard conditions
(temperature, 25±1°C, humidity ~70%, 12-h light/dark cycle), with
ad libitum access to standard diet and water. Rats were
randomly divided into three groups: ANIT model group (n=20), UDCA
treatment group (n=19) and control group (n=10). ANIT was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). UDCA was
purchased from Losan Pharma GmbH (Neuenburg, Germany) and prepared
as a 1.6% UDCA solution using 0.9% sodium chloride. All procedures
involving animals were reviewed and approved by the Institutional
Animal Care and Use Committee of the Third Hospital of Hebei
Medical University (certificate no. A2013-002-1).
Animal modeling
Different ANIT doses (50, 75 and 100 mg) and
different time points were selected to build a cholestatic hepatic
fibrosis model, and it was identified that 75 mg/kg/kg 1% ANIT for
2 days (once daily) was best to build a good cholestatic hepatic
fibrosis model. A dose of 75 mg/kg ANIT has recently been
demonstrated to induce cholestatic hepatic fibrosis (15). Rats in the present study in the
ANIT model group were administrated 75 mg/kg 1% ANIT via gavage on
the fourth day of experiment; rats in the UDCA treatment group were
administered 100 mg/kg UCDA solution daily from the second day of
the experiment and on the fourth day of experiment administration
of 75 mg/kg 1% ANIT was performed via gavage until sacrifice of all
the rats on the sixth day of the experiment. Rats in the control
group received normal diet, as above, prior to decapitation. Rats
in all groups were fasted for 12 h prior to and following gavage,
with ad libitum access to water to ensure adequate and even
drug absorption. At the time of sacrifice, the weight range of rats
was 50–60 g. All rats were sacrificed via decapitation following
anesthesia via intraperitoneal injection of 0.1 ml/200 g ketamine
hydrochloride/xylazine (Sigma-Aldrich; Merck KGaA).
Experimental methods
According to the results of the preliminary
experiments, rats were administered the appropriate treatment
following 24 h acclimating. Rats were sacrificed at 2 days
following ANIT gavage and 4–5 ml blood was collected, frozen at
−20°C and used for the detection of serum biomarkers of hepatic
fibrosis (HA; PCIII; LN and cIV) and liver functions [alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
γ-glutamyl transpeptidase (γ-GT), total bilirubin (TBIL), direct
bilirubin (DBIL), indirect bilirubin (IBIL), cholinesterase (CHE)
and total bile acids (TBA)]. Following blood collection, the
peritoneal cavities of rats were opened to dissect 1.0×0.8×0.3 cm
liver tissue samples from the right hepatic lobe, followed by
fixing the tissues in 10% neutral formalin at temperature 25°C for
4–6 h at least, conventional dehydration, paraffin embedding and
sectioning at 4–6 µm).
Liver function
A total of 43 serum samples fitted in the
experimental requirements from the 49 samples (6 rats failed to
provide a sufficient blood sample and were excluded): 8 samples
from the control group, 18 samples from the ANIT model group and 17
samples from the UCDA treatment group. Liver function indices (ALT,
AST, TBIL, DBIL, IBIL, γ-GT, CHE, and TBA) were measured using
assay reagents (Aspartate Aminotransferase kit no. 12–0763, Alanine
Aminotransferase kit no. 12-0739, Total Bilirubin kit no. 12063017,
Direct Bilirubin kit no. 12061821, γ-glutamyl transpeptidase kit
no. 12-0777, Cholinesterase kit no. 12-0635 and Total Bile Acids
kit no. 12-0652) purchased from Johnson & Johnson Medical
(Shanghai), Ltd. (Shanghai, China). IBIL was calculated as
TBIL-DBIL. An Olympus AU5400 automatic biochemical analyzer
(Olympus Corporation, Tokyo, Japan) was used for detection of
function indices. Rat blood samples (2–3 ml) were placed in
pro-coagulation tubes, which were then centrifuged at 1,006 × g at
4°C for 3 min to isolate serum for ALT, AST, γ-GT, CHE, and TBA
biochemical assays based on the velocity method, and TBIL, DBIL,
and IBIL for biochemical assays based on colorimetry (8,16).
Some rats failed to provide a sufficient blood sample, so the case
for liver function was less.
Serum biomarkers of hepatic
fibrosis
Chose 43 serum samples fitted in the experimental
requirements from the 49 samples, 8 samples from the control group,
18 samples from the ANIT model group and 17 samples from the UCDA
treatment group. The serum biomarkers (HA, PCIII, LN, and cIV) of
hepatic fibrosis were detected via radioimmunoassay (RIA). Rat
blood samples (2–3 ml) were placed in pro-coagulation tubes, and
centrifuged at 1,006 × g at 4°C for 3 min to isolate serum. To
detect the serum biomarkers of hepatic fibrosis, radioimmunoassay
kits (Hyaluronic acid kit, 012-1186; Type-III procollagen kit,
012–1335; Laminin protein kit, 012–1219; and Type-IV collagen kit,
12–1452; Shanghai Haiyan Biotechnology Institute, Shanghai, China)
and an XH-6020 γ-RIA counter (Xi'an Nuclear Instrument Factory,
Xi'an, China) were used according to the manufacturers' protocols
(8,16).
Hematoxylin and eosin (H&E)
staining
Liver tissues isolated from rats were fixed in 10%
neutral formalin (Zhongshan Beijing Biotechnology Co., Ltd.,
Beijing, China) for at least 4–6 h at 25°C and transferred to 70%
ethanol. Individual hepatic tissues biopsy material was placed in
processing cassettes, dehydrated through a serial alcohol gradient,
and embedded in wax blocks. Sections were cut at 4–6 µm and dewaxed
in xylene, rehydrated through decreasing concentrations of ethanol,
and washed in PBS. The sections were then stained with hematoxylin
for 4–5 min and with eosin for 1–2 min at 25°C (Zhongshan Beijing
Biotechnology Co., Ltd.). Stained liver sections were observed and
photographed under light microscopy (magnification, ×200; BX53-p;
Olympus Corporation) (16).
Picric acid-sirius red staining
Liver tissues isolated from rats were fixed in 10%
neutral formalin (Zhongshan Beijing Biotechnology Co., Ltd.,
Beijing, China) for at least 4–6 h at 25°C and transferred to 70%
ethanol. Individual hepatic tissues biopsy material was placed in
processing cassettes, dehydrated through a serial alcohol gradient,
and embedded in wax blocks. Sections were cut at 4–6 µm and dewaxed
in xylene, rehydrated through decreasing concentrations of ethanol,
and washed in PBS. The sections, mounted on glass slides, were
stained with picric acid-Sirius red (0.1% Sirius red in saturated
aqueous picric acid; Zhongshan Beijing Biotechnology Co., Ltd.) for
5 min at 25°C to detect hepatic fibrosis. Stained sections were
examined with polarizing microscopy at magnifications of ×4, ×10,
×100 and ×400(16).
Immunohistochemical staining
A total of 46 serum samples were selected from the
49 samples, 10 sample from the control group, 13 samples from the
ANIT group and 13 samples from the UCDA group, respectively. For
the immunohistochemical staining of cIV and LN, primary antibodies
were purchased from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China; Laminin A03522 and cIV BA3585-2), and the Goat
Anti-Rabbit secondary antibody was purchased from OriGene
Technologies, Inc. (Beijing, China). Dilution of all antibodies in
immunohistochemical staining was 1:150. PBS was used to replace
primary antibodies as a negative control. Following incubation with
goat serum for 10 min at room temperature, samples were incubated
with primary antibodies (rabbit-anti-rat) for 30 min at 37°C,
washed with PBS 3 times for 3 min each wash and then incubated with
the secondary antibody at 37°C for 20 min. The samples were washed
with PBS 3 times for 3 min each wash and stained with DAB for 5 min
at room temperature. The slides were washed, dehydrated as above,
and observed using an Olympus BX51 optical microscope (Olympus
Corporation). Positive expression of cIV and LN presented as light
yellow or brown granules or linear deposition was localized in the
cytoplasm, membrane or interstitial regions. Images were acquired
of the tissue sections from 5 randomly selected visual fields,
using magnification, ×10 for cIV staining and magnification, ×20
for LN staining. Image Pro-Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA) was used to quantitatively analyze the
integrated optical density in each of the 5 randomly selected
visual fields per tissue section. The following formula was used to
obtain the integrated optical density (IOD) of the
positively-stained region following the removal of the background
value: IOD of the positively stained region/area of positively
stained region-IOD of the negatively stained region/area of
negatively stained region. The mean of the results of each tissue
section from the 5 randomly selected visual fields was then
calculated.
Pathological grading of hepatic
fibrosis
A total of 47 serum samples were selected from the
49 samples, 8 samples from the control group, 20 samples from the
ANIT model group and 19 samples from the UCDA treatment group.
Conventional H&E-stained sections (as above) from different
samples were used to observe liver cell degeneration, the degree of
collagen fiber proliferation and differences in morphology.
Pathological grading of hepatic fibrosis was performed in
accordance with Knodell scores (17), staging criteria, and quantitative
and semi-quantitative scoring methods as follows: 0 points, normal
hepatocyte morphology, no lesion, and no marked collagen fiber
proliferation in hepatic lobules; 1 point, <25% degeneration of
total hepatocytes, presence of collagen fiber proliferation, small
amount of fiber extension in the central vein and portal area, and
tendency for fiber extension towards hepatic lobules, but no septa
formation; 2 points, 25–50% degeneration of total hepatocytes,
notable collagen fiber formation, thickened connective tissues in
the central vein and portal area and fiber extension towards
peripheral regions, formation of incomplete septa, but
substantially retained lobular structure; 3 points, 50–75%
degeneration of total hepatocytes, large amount of collagen fiber
proliferation, some complete septa or incomplete and thickened
septa to form pseudolobuli; 4 points, >75% degeneration of total
hepatocytes, complete and thick septa and a large amount of
pseudolobuli (16).
Statistical analysis
SPSS 21.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis in the present study. Data are
presented as the mean ± standard deviation. Normally distributed
data with homogeneity of variance were compared using
one-way-analysis of variance followed by Dunnett post hoc test for
comparisons with multiple groups. Non-normally distributed date or
data with heterogeneity of variance were analyzed using
Mann-Whitney U test. Analysis of pathological grading indices was
performed using Spearman's correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Impact of UDCA on the liver function
of infant rats with cholestatic liver fibrosis
As demonstrated in Table I, all liver function indices except
for CHE were significantly increased in the ANIT model group
compared with the control group (P<0.01), which indicated that
the cholestatic liver fibrosis model was successfully established
in the infant rats. In the UDCA treatment group, γ-GT and TBA were
significantly lower than in the ANIT model group (P<0.05),
whereas no significant differences were observed in ALT, AST, TBIL,
DBIL, IBIL and CHE between the UDCA treatment and ANIT model
groups.
 | Table I.Effects of UDCA on liver function in
infant cholestatic rats. |
Table I.
Effects of UDCA on liver function in
infant cholestatic rats.
| Parameter | Control (n=8) | ANIT model
(n=18) | UDCA treatment
(n=17) |
|---|
| ALT (U/l) | 47.1±15.8 |
782.6±251.2a | 618.1±195.7 |
| AST (U/l) | 386.4±138.8 |
2265±524.8a | 1990.7±850.6 |
| TBIL (µmol/l) | 3.0±0.9 |
142.3±25.3a | 138.9±14.4 |
| DBIL (µmol/l) | 2.5±1.1 |
129.1±23.0a | 127.1±11.1 |
| IBIL (µmol/l) | 0.5±0.3 |
12.8±5.3a | 11.9±4.7 |
| γ-GT (U/l) | 0.4±3.4 |
15.7±6.1a |
11.1±3.3b |
| CHE (kU/l) | 0.26±0.04 | 0.25±0.05 | 0.25±0.04 |
| TBA (µmol/l) | 194.3±130.9 |
657.5±95.1a |
608.2±63.0b |
Impact of UDCA on serum biomarkers of
hepatic fibrosis in rats with cholestatic hepatic fibrosis
As demonstrated in Table II, serum LN and cIV were
significantly higher in the ANIT model group than in the control
group (P<0.05); whereas no significant differences were observed
in serum HA and PCIII. Serum LN and cIV were significantly lower in
the UDCA treatment group than in the ANIT model group (P<0.01);
whereas no significant differences in serum HA and PCIII were
observed.
 | Table II.Effects of UDCA on potential
biomarkers of liver fibrosis in infant cholestatic rats. |
Table II.
Effects of UDCA on potential
biomarkers of liver fibrosis in infant cholestatic rats.
| Group | Rats (n) | HA (ng/ml) | PCIII (ng/ml) | LN (ng/ml) | cIV (ng/ml) |
|---|
| Control | 8 | 1489.8±215.3 | 40.4±1.6 | 39.7±5.4 | 24.9±4.1 |
| ANIT model | 18 | 1772.7±316.9 | 41.2±11.0 |
48.5±8.8a |
30.8±7.4a |
| UDCA treatment | 17 | 1608.5±409.9 | 41.6±4.6 |
41.7±5.6b |
24.0±7.9b |
Impact of UDCA on LN and cIV
expression in the liver tissues of the infant rats with cholestatic
hepatic fibrosis
As demonstrated in Table III, the LN and cIV expression in
the liver tissues of the ANIT model group were significantly higher
than in the control group (P<0.01); whereas the LN and cIV
expression in the liver tissues of the UDCA treatment group were
significantly lower than in the ANIT model groups (P<0.01).
 | Table III.Effects of UDCA on
immunohistochemistry of liver tissue in neonatal cholestatic
rats. |
Table III.
Effects of UDCA on
immunohistochemistry of liver tissue in neonatal cholestatic
rats.
| Group | Rats (n) | LN (IOD) | cIV (IOD) |
|---|
| Control | 10 | 0.041±0.013 | 0.017±0.003 |
| ANIT model | 13 |
0.058±0.008a |
0.022±0.003a |
| UDCA treatment | 13 |
0.038±0.008b |
0.018±0.003b |
Impact of UDCA on the pathological
grading of cholestatic hepatic fibrosis in infant rats
Table IV
demonstrated that the degree of hepatic fibrosis in the liver
tissues of the ANIT model group was significantly higher than in
the control group (P<0.01), which suggested that the cholestatic
hepatic fibrosis model was successfully established. The degree of
hepatic fibrosis in the liver tissues of the UDCA treatment group
was significantly lower than in the ANIT model group
(P<0.01).
 | Table IV.Effects of UDCA on pathological
grading of liver fibrosis in infant cholestatic rats. |
Table IV.
Effects of UDCA on pathological
grading of liver fibrosis in infant cholestatic rats.
| Group | Rats (n) | Pathological
score |
|---|
| Control | 8 | 0.250±0.463 |
| ANIT model | 20 |
2.100±0.641a |
| UDCA treatment | 19 |
1.000±0.577b |
H&E and picric acid-Sirius red
staining
H&E and picric acid-Sirius red staining are
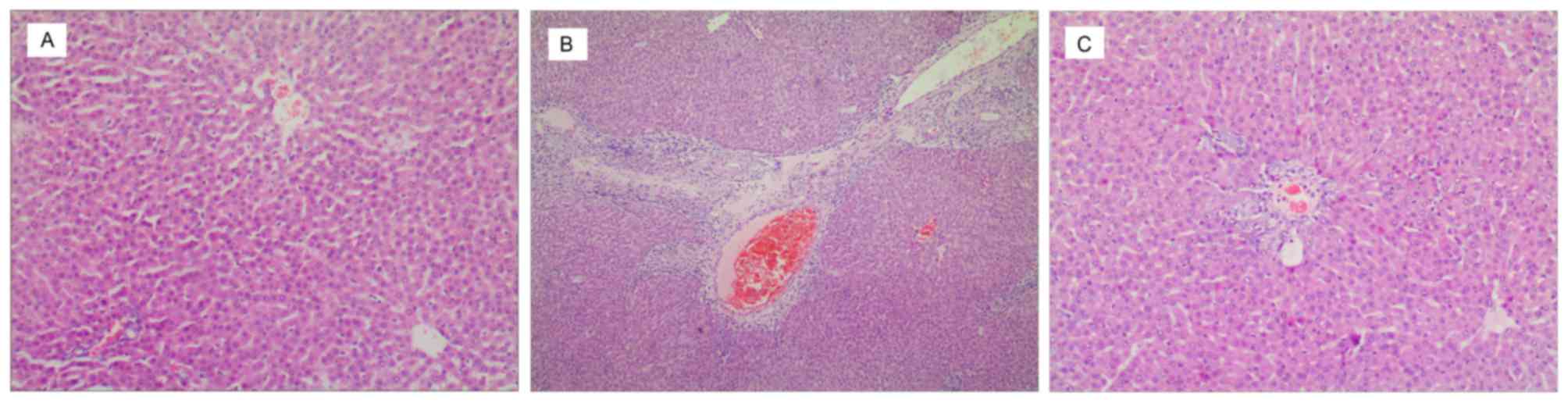
presented in Figs. 1 and 2, respectively. In the control group,
H&E staining showed a complete and clear hepatic lobule, with
hepatocytes arranged neatly, which extended to peripheral regions.
No abnormal proliferation of connective tissue was found in the
central vein and portal area (Fig.
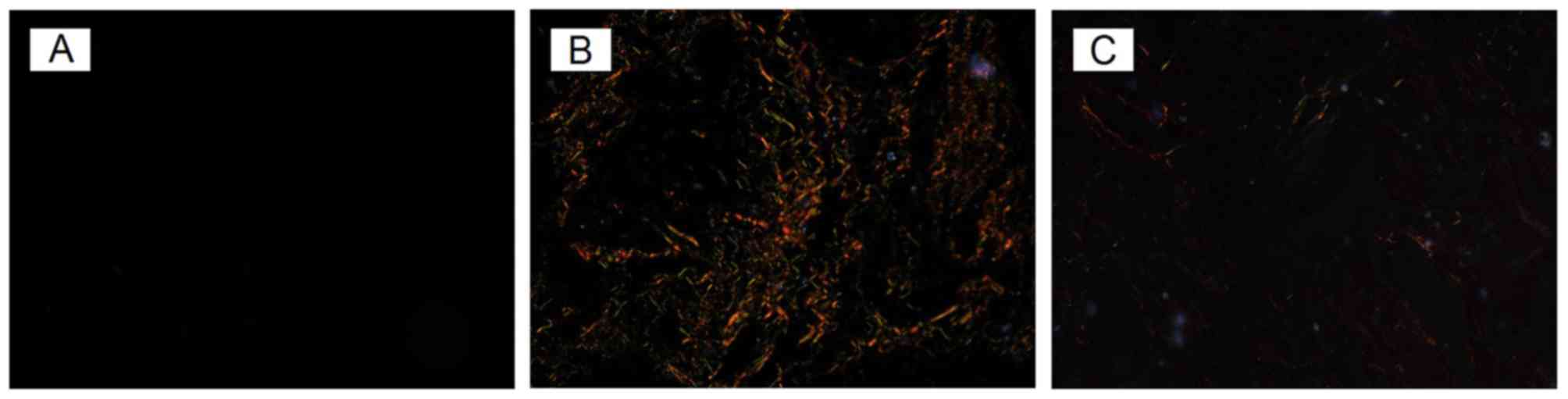
1A). With picric acid-Sirius red staining, tissues in the
control group exhibited almost no fiber staining (Fig. 2A).
H&E staining in the ANIT model group (Fig. 1B) indicated that the normal hepatic
lobular structure was destroyed; 25–75% of liver tissues exhibited
fatty degeneration, necrosis, and ballooning degeneration, with
visible cholestasis in hepatocytes and small bile ducts. Notable
proliferation of fibrous tissues was detected in the necrotic
portal area and lobules that were accompanied by a number of
neutrophil and eosinophil infiltrations. The proliferated fibrous
tissues divided the hepatic lobules into pseudolobuli. With picric
acid-Sirius red staining (Fig.
2B), hepatic lobules in the ANIT model group were demonstrated
to have a markedly larger population of collagen fibers than the
control group. Thick yellow and red fibers were distributed around
or star-shaped around the central vein, distributed around the
clearance of hepatic sinus and portal area, or stretched into the
lobule to formed interlobular separations. A few slender green
fibers were mainly distributed in the portal area, with small blood
vessels and the central vein also demonstrating discontinuous
distribution around the clearance of the hepatic sinus.
Compared with the ANIT model group UDCA treatment
group with H&E stained had a lower degree of hepatic fibrosis
(Fig. 1C), with no significant
formation of pseudolobuli and with a small amount of neutrophil and
eosinophil infiltrations. Scattered necrotic debris was visible.
With Sirius red-saturated picric acid stained, too, there were only
few thick yellow and red fibers found in UDCA group, and the
distributed of fibers was not so clear (Fig. 2C) compared with the ANIT model
group.
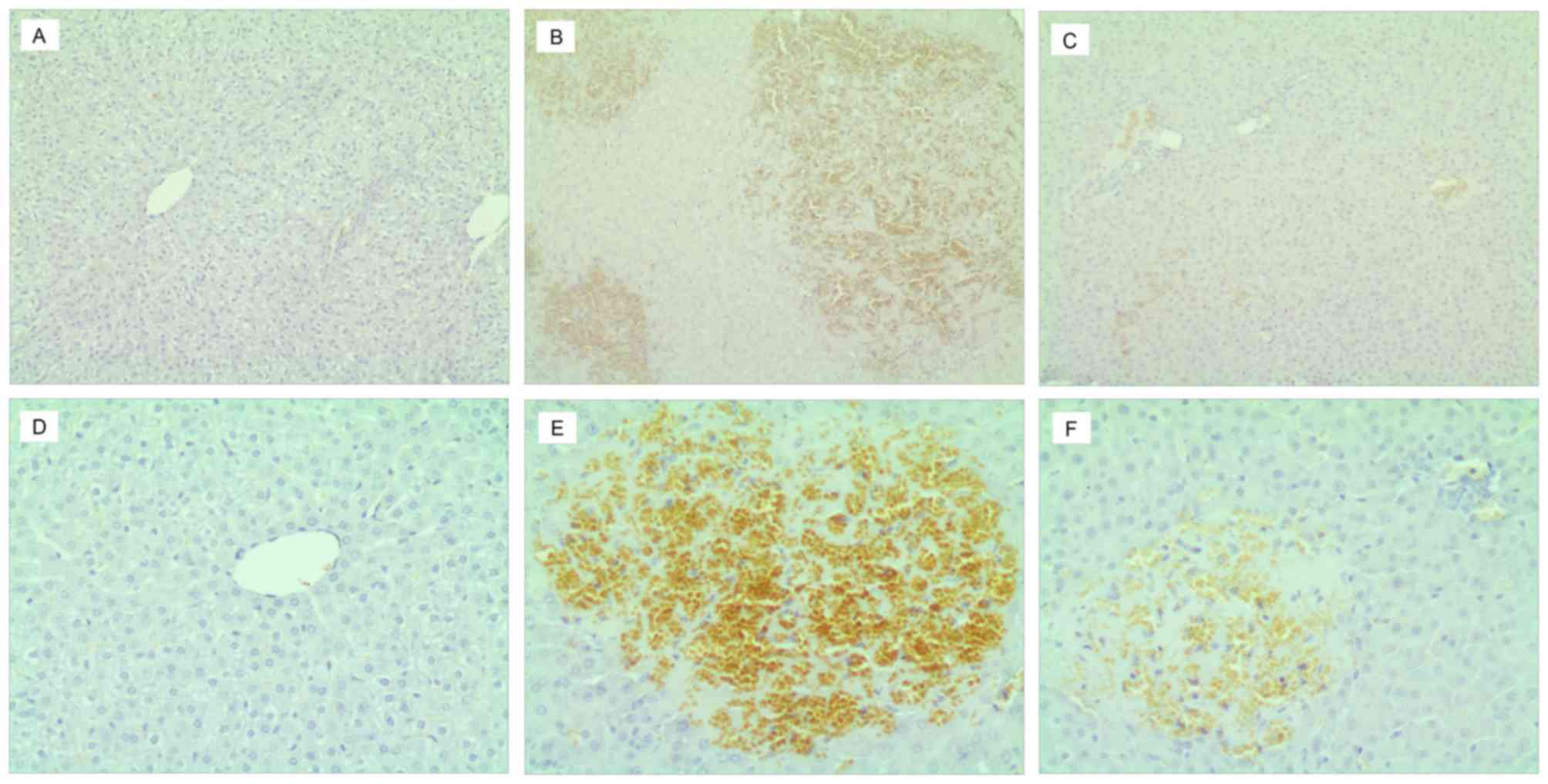
Immunohistochemical staining
Immunohistochemical staining is presented in
Fig. 3. A small amount of cIV and
LN deposition was observed in the sinusoidal and vessel walls of
liver tissues in the blank control (Fig. 3A and D), which indicated a
continuous linear positive reaction. In the ANIT model and UDCA
treatment groups, the brown granules of LN staining (Fig. 3E and F) were thicker and darker
than the cIV staining (Fig. 3B and
C). The regions of brown granule deposition were most marked in
the sinusoid and portal areas, and in area of necrotic hepatocytes,
showing visible necrotic cell debris. Positive cIV and LN staining
was stronger in the ANIT model group (Fig. 3B and E) than in the other groups,
exhibiting as coarse and linear positively stained fibers to form a
fibrous septa extending into the hepatic lobules to separate the
hepatic parenchyma. In the necrotic area, the positively-stained
region was interwoven into a network with varying amounts of
immersed inflammatory cells. Regardless of cIV and LN staining, the
brown granule deposition in the positively stained region of the
UDCA treatment group was markedly lower than in the ANIT model
group. The portal area and hepatic parenchyma as well as the
necrotic region with mild debris indicated weak and irregular
thickness of linear positively staining interwoven into a network.
Each type of collagen staining was not uniform and no obvious
difference between the groups was observed.
Discussion
ANIT is a hepatic toxic substance that induces acute
cholesteric liver injury (18). It
is able to induce epithelial cell swelling and necrosis in the bile
duct, which results in bile canaliculus hyperplasia and
interlobular inflammatory reactions surrounding the bile ducts,
which further induces cholestasis, damage to hepatic parenchyma
cells and incidence of haptic fibrosis (19). ANIT-induced reversible acute
intrahepatic cholestasis has previously been demonstrated as a
successful animal model of intrahepatic cholestasis (20,21).
Results of the present study demonstrated that following 48 h of
ANIT administration in infant SD rats, serum activities of ALT,
AST, TBIL, DBIL, IBIL, and γ-GT, and TBA were significantly raised
compared with the control group. In addition, LN and cIV in the
serum and hepatic tissue were significantly higher in the ANIT
model group than that in the control group. The structure of normal
liver tissue, predominantly the portal area of the infant rats was
destroyed or disappeared in the ANIT model group, suggesting that
the modeling of cholestatic hepatic fibrosis was successful.
The present results also demonstrated that,
following UDCA treatment, serum γ-GT and TBA of the infant SD rats
were significantly reduced, compared with the ANIT model group.
Serum γ-GT, which is predominantly produced by the mitochondria in
hepatocytes, localizes in cytoplasm and epithelium of intrahepatic
bile duct, and is excreted through bile duct into the duodenum.
When extrahepatic or intrahepatic obstruction and cholestasis
occur, excretion of γ-GT is blocked, and concentrations of serum
γ-GT increase (22,23). TBA is the part of bile acid spilled
into the bloodstream and taken up by hepatocytes through intestinal
reabsorption. When cholestasis or liver function is impaired, bile
discharge is blocked and in the presence of secretion and/or
ingestion blockage(s), TBA is also increased (24). It has previously been demonstrated
that serum TBA is specific, sensitive, and stable enough to reflect
liver function changes (25). A
previous study by the present authors reported that serum γ-GT and
TBA of infants with cholestasis were significantly higher than in
healthy infants (8). Previous
studies have indicated that UDCA exhibited good clinical
therapeutic effects against cholestatic hepatitis, liver cirrhosis,
and infant cholestatic liver diseases. For example, it has been
demonstrated that UDCA significantly reduced TBA (26) and lowered hepatotoxicity of bile
acids (27). However, only
preliminary treatment efficacy has been reported in clinical
practice (28,29). Fang et al (9) recently studied the liver function
indices of infants with cholestatic liver diseases that were
treated by UDCA, and they demonstrated that TBIL, DBIL, ALT, γ-GT
and TBA were significantly reduced when compared with pretreatment
levels. In the present study, AST level were higher than that in
previous studies (9,30). A possible reason for this is that
the AST level in erythrocytes is 30 times higher than that in the
serum, whereas the ALT level in erythrocytes is only 7 times higher
than that in the serum. It is possible that hemolysis may have led
to high AST levels when rats were decapitated to harvest blood;
which is a limitation of the present study.
Hepatic fibrosis is a response reaction to excessive
collagen and proteoglycan deposition in the liver, and liver injury
caused by other extracellular matrix macromolecules (31). LN belongs to a structural
glycoprotein that was positively correlated with the degree of
activity of hepatic fibrosis and portal vein pressure. It has high
sensitivity in the diagnosis of hepatic fibrosis (32). Significant deposition of LN in
hepatic sinusoids and its excretion into the bloodstream typically
occurs in acute hepatic fibrosis, resulting in elevated serum LN
levels. cIV, as the primary component constituting the basement
membrane, is a marker for early hepatic fibrosis and is considered
as an indicator for collagen production rather than collagen
degradation (33). cIV and LN are
positively correlated with the degree of hepatic fibrosis (9). HA is typically ingested and
decomposed by sinusoidal endothelial cells. When liver tissue is
damaged, HA synthesized by hepatic stellate cells is increased, and
its degradation in sinusoidal endothelial cells is decreased,
resulting in elevated serum HA (34). HA is able to accurately and
sensitively indicate hepatocyte damages and the quantity of
intrahepatic fiber production (35–37).
PCIII is a precursor of type III collagen that is not only valuable
in the early diagnosis of hepatic fibrosis, but is also beneficial
for predicting the prognosis of chronic liver disease. The serum
PCIII quantity is consistent with the degree and activity of
hepatic fibrosis (38). In the
present study, LN and cIV expressions in the serum and liver
tissues of the infant rats with cholestatic hepatic fibrosis
following UDCA treatment were significantly reduced, and the UDCA
treated rats exhibited marked improvements in their fibrotic liver
tissues. No significant differences in serum HA and PCIII were
observed between the UDCA treatment group and the ANIT model group,
confirming that UDCA is able to reduce LN and cIV, thereby playing
a role in anti-hepatic fibrosis.
Recently, the molecular mechanisms underlying the
effects of UDCA in cholestatic hepatic fibrosis have been the focus
of an increasing number of studies (39,40).
Previous studies have typically focused on adult cholestasis;
however, in the present study, rats with cholestatic liver fibrosis
were chosen as the research object, through analyzing liver
function, four serum biomarkers of hepatic fibrosis, liver fibrosis
index and liver tissue pathological changes, to explore the
therapeutic effect of UDCA on cholestatic liver fibrosis in rats.
The aim was to identify an effective and early intervention method
for the treatment of cholestatic liver fibrosis in infants.
Limitations of the present study include the short duration of
modeling and treatment, and rats were administrated with UDCA prior
to ANIT modeling to achieve more observable effects of UDCA;
therefore, results cannot completely reflect the anti-hepatic
fibrosis effect of UDCA. It is hoped in a future study to clarify
the therapeutic effect of UDCA on children with cholestatic liver
disease, by improving the modeling method, the experimental design
and increasing the animal sample size. Although some experimental
results still require validation, the present study suggested that
UDCA was able to improve liver function through the reduction of
serum γ-GT and TBA, and improved hepatic fibrosis through the
reduction of LN and cIV expression to prevent liver cirrhosis.
Future studies should be performed to investigate
the role of UDCA in HSCs in children with cholestatic liver
fibrosis, in order to elucidate the mechanism of UDCA in resisting
hepatic fibrosis.
References
|
1
|
Fischler B and Lamireau T: Cholestasis in
the newborn and infant. Clin Res Hepatol Gastroenterol. 38:263–267.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catzola A and Vajro P: Management options
for cholestatic liver disease in children. Expert Rev Gastroenterol
Hepatol. 11:1019–1030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lindor KD, Gershwin ME, Poupon R, Kaplan
M, Bergasa NV and Heathcote EJ; American Association for Study of
Liver Diseases, : Primary biliary cirrhosis. Hepatology.
50:291–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
European Association for the Study of the
Liver, . EASL clinical practice guidelines: Management of
cholestatic liver diseases. J Hepatol. 51:237–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orso G, Mandato C, Veropalumbo C, Cecchi
N, Garzi A and Vajro P: Pediatric parenteral nutrition-associated
liver disease and cholestasis: Novel advances in
pathomechanisms-based prevention and treatment. Dig Liver Dis.
48:215–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu YL, Gong JY, Feng JY, Wang RX, Han J,
Liu T, Lu Y, Li LT, Zhang MH, Sheps JA, et al: Defects in myosin VB
are associated with a spectrum of previously undiagnosed low
γ-glutamyltransferase cholestasis. Hepatology. 65:1655–1669. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang N, Zhang Y, Liu Z, Fu T, Liang Q and
Ai X: Correlation analysis between four serum biomarkers of liver
fibrosis and liver function in infants with cholestasis. Biomed
Rep. 5:107–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang YQ, Lv DX, Jia W, Li J, Deng YQ, Wang
Y, Yu M and Wang GQ: Case-control study on prednisolone combined
with ursodeoxycholic acid and azathioprine in pure primary biliary
cirrhosis with high levels of immunoglobulin G and transaminases:
Efficacy and safety analysis. Medicine (Baltimore). 93:e1042014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rudic JS, Poropat G, Krstic MN, Bjelakovic
G and Gluud C: Ursodeoxycholic acid for primary biliary cirrhosis.
Cochrane Database Syst Rev. 12:CD0005512012.PubMed/NCBI
|
|
11
|
Simić D, Milojević I, Bogićević D,
Milenović M, Radlović V, Drasković B, Benka AU, Sindjić S and
Maksimović R: Preventive effect of ursodeoxycholic acid on
parenteral nutrition-associated liver disease in infants. Srp Arh
Celok Lek. 142:184–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatano R, Kawaguchi K, Togashi F, Sugata
M, Masuda S and Asano S: Ursodeoxycholic acid ameliorates
intrahepatic cholestasis independent of biliary bicarbonate
secretion in Vil2kd/kd mice. Biol Pharm Bull. 40:34–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou WC, Zhang QB and Qiao L: Pathogenesis
of liver cirrhosis. World J Gastroenterol. 20:7312–7324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizuochi T, Kimura A, Suzuki M, Ueki I,
Takei H, Nittono H, Kakiuchi T, Shigeta T, Sakamoto S, Fukuda A, et
al: Successful heterozygous living donor liver transplantation for
an oxysterol 7α-hydroxylase deficiency in a Japanese patient. Liver
Transpl. 17:1059–1065. 2011.PubMed/NCBI
|
|
15
|
Wang L, Wu G, Wu F, Jiang N and Lin Y:
Geniposide attenuates ANIT-induced cholestasis through regulation
of transporters and enzymes involved in bile acids homeostasis in
rats. J Ethnopharmacol. 20:178–185. 2017. View Article : Google Scholar
|
|
16
|
Tang N, Zhang Y, Liu Z, Ai X and Liang Q:
Correlation of four potential biomarkers of liver fibrosis with
liver function and grade of hepatic fibrosis in a neonatal
cholestatic rat model. Mol Med Rep. 16:415–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Knodell RG, Ishak KG, Black WC, Chen TS,
Craig R, Kaplowitz N, Kiernan TW and Wollman J: Formulation and
application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis.
Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang T, Zhou ZX, Sun LX, Li X, Xu ZM, Chen
M, Zhao GL, Jiang ZZ and Zhang LY: Resveratrol effectively
attenuates α-naphthyl-isothiocyanate-induced acute cholestasis and
liver injury through choleretic and anti-inflammatory mechanisms.
Acta Pharmacol Sin. 35:1527–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Connolly AK, Price SC, Connelly JC and
Hinton RH: Early changes in bile duct lining cells and hepatocytes
in rats treated with alpha-naphthylisothiocyanate. Toxicol Appl
Pharmacol. 93:208–219. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kossor DC, Meunier PC, Handler JA, Sozio
RS and Goldstein RS: Temporal relationship of changes in
hepatobiliary function and morphology in rats following
alpha-naphthylisothiocyanate (ANIT) administration. Toxico1 Appl
Pharmacol. 119:108–114. 1993. View Article : Google Scholar
|
|
21
|
Golbar HM, Izawa T, Yano R, Ichikawa C,
Sawamoto O, Kuwamura M, Lamarre J and Yamate J: Immunohistochemical
characterization of macrophages and myofibroblasts in
α-Naphthylisoth-iocyanate (ANIT)-induced bile duct injury and
subsequent fibrogenesis in rats. Toxicol Pathol. 39:795–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tarantino G, Finelli C, Colao A, Capone D,
Tarantino M, Grimaldi E, Chianese D, Gioia S, Pasanisi F, Contaldo
F, et al: Are hepatic steatosis and carotid intima media thickness
associated in obese patients with normal or slightly elevated
gamma-glutamyl-transferase? J Transl Med. 10:502012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tavian D, Degiorgio D, Roncaglia N,
Vergani P, Cameroni I, Colombo R and Coviello DA: A new splicing
site mutation of the ABCB4 gene in intrahepatic cholestasis of
pregnancy with raised serum gamma-GT. Dig Liver Dis. 41:671–675.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lurie Y, Webb M, Cytter-Kuint R,
Shteingart S and Lederkremer GZ: Non-invasive diagnosis of liver
fibrosis and cirrhosis. World J Gastroenterol. 21:11567–11583.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsui S, Yamane T, Takita T, Oishi Y and
Kobayashi-Hattori K: The hypocholesterolemic activity of Momordica
charantia fruit is mediated by the altered cholesterol- and bile
acid-regulating gene expression in rat liver. Nutr Res. 33:580–585.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soroka CJ, Mennone A, Hagey LR, Ballatori
N and Boyer JL: Mouse organic solute transporter alpha deficiency
enhances renal excretion of bile acids and attenuates cholestasis.
Hepatology. 51:181–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geenes V, Lövgren-Sandblom A, Benthin L,
Lawrance D, Chambers J, Gurung V, Thornton J, Chappell L, Khan E,
Dixon P, et al: The reversed feto-maternal bile acid gradient in
intrahepatic cholestasis of pregnancy is corrected by
ursodeoxycholic acid. PLoS One. 9:e838282014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poupon R: Ursodeoxycholic acid and
bile-acid mimetics as therapeutic agents for cholestatic liver
diseases: An overview of their mechanisms of action. Clin Res
Hepatol Gastroenterol. 36 Suppl 1:S3–S12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ozel Coskun BD, Yucesoy M, Gursoy S,
Baskol M, Yurci A, Yagbasan A, Doğan S and Baskol G: Effects of
ursodeoxycholic acid therapy on carotid intima media thickness,
apolipoprotein A1, apolipoprotein B, and apolipoprotein B/A1 ratio
in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol.
27:142–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MD, Kim SS, Cha HY, Jang SH, Chang DY,
Kim W, Suh-Kim H and Lee JH: Therapeutic effect of hepatocyte
growth factor-secreting mesenchymal stem cells in a rat model of
liver fibrosis. Exp Mol Med. 46:e1102014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friedman SL: Hepatic fibrosis-overview.
Toxicology. 254:120–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suk KT, Kim DY, Sohn KM and Kim DJ:
Biomarkers of liver fibrosis. Adv Clin Chem. 62:33–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Mezayen HA, Habib S, Marzok HF and Saad
MH: Diagnostic performance of collagen IV and laminin for the
prediction of fibrosis and cirrhosis in chronic hepatitis C
patients: A multicenter study. Eur J Gastroenterol Hepatol.
27:378–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HH, Seo YS, Um SH, Won NH, Yoo H, Jung
ES, Kwon YD, Park S, Keum B, Kim YS, et al: Usefulness of
non-invasive markers for predicting significant fibrosis in
patients with chronic liver disease. J Korean Med Sci. 25:67–74.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Shabrawi MH, Zein El Abedin MY, Omar N,
Kamal NM, Elmakarem SA, Khattab S, El-Sayed HM, El-Hennawy A and
Ali AS: Predictive accuracy of serum hyaluronic acid as a
non-invasive marker of fibrosis in a cohort of multi-transfused
Egyptian children with β-thalassaemia major. Arab J Gastroenterol.
13:45–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nath NC, Rahman MA, Khan MR, Hasan MS,
Bhuiyan TM, Hoque MN, Kabir MM, Raha AK and Jahan B: Serum
hyaluronic acid as a predictor of fibrosis in chronic hepatitis B
and C virus infection. Mymensingh Med J. 20:614–619.
2011.PubMed/NCBI
|
|
37
|
Nobili V, Alisi A, Torre G, De Vito R,
Pietrobattista A, Morino G, De Ville De Goyet J, Bedogni G and
Pinzani M: Hyaluronic acid predicts hepatic fibrosis in children
with nonalcoholic fatty liver disease. Transl Res. 156:229–234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li ZX, He Y, Wu J, Liang DM, Zhang BL,
Yang H, Wang LL, Ma Y and Wei KL: Noninvasive evaluation of hepatic
fibrosis in children with infant hepatitis syndrome. World J
Gastroenterol. 12:7155–7160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paumgartner G and Beuers U:
Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of
action and therapeutic use revisited. Hepatology. 36:525–531. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alaca N, Özbeyli D, Uslu S, Şahin HH,
Yiğittürk G, Kurtel H, Öktem G and Çağlayan Yeğen B: Treatment with
milk thistle extract (Silybum marianum), ursodeoxycholic acid, or
their combination attenuates cholestatic liver injury in rats: Role
of the hepatic stem cells. Turk J Gastroenterol. 28:476–484. 2017.
View Article : Google Scholar : PubMed/NCBI
|