Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the most common types of head and neck squamous cell carcinoma, the
incidence of which continues to rise. Despite the continuous
improvement in surgical, radiotherapeutic and chemotherapeutic
techniques, an efficient prolongation of the survival rate of
patients with LSCC has yet to be achieved (1,2). The
5-year survival rate of patients with LSCC is ~30% (3). Due to the specificity of the anatomic
location of the throat, patients with LSCC not only have to endure
painful surgery, but they also experience postoperative impairment
and loss of throat function. Subsequent physical and psychological
afflictions have a serious impact on the outcome of postoperative
rehabilitation and the quality of life of these patients (4). In recent years, cancer immunotherapy
has gradually become a subject undergoing intense study in cancer
research and has achieved satisfactory therapeutic efficacy in the
clinical treatment of cancers (5).
However, compared with other types of cancer treatment, progress in
the development of LSCC-related immunotherapy has been fairly slow.
One of the main reasons behind this slow progress is the lack of
effective molecular targets for immunotherapy. Therefore, it is
necessary to determine the immunoregulatory mechanisms underlying
LSCC and to identify factors that are critical for the success of
immunotherapy.
Regulatory T cells (Tregs) comprise a subpopulation
of T cells with unique immunomodulatory functions. Tregs are able
to suppress immune responses and therefore serve important roles in
the maintenance of immune homeostasis, tumor immunity and
transplant tolerance (6). Forkhead
box P3 (FOXP3) is a characteristic Treg surface marker, and a key
factor for Treg development and maintenance of function (7). Franco et al (8) silenced the expression of FOXP3, to
reduce the infiltration of FOXP3+ Tregs, and identified
that melanoma growth was significantly inhibited, and that the
immunosuppressive microenvironment was restored. Treg-secreted
cytokines, including interleukin (IL)-10, serve an important role
in the function of Tregs. These cytokines may initiate inhibitory
signals and suppress the activity of functional T lymphocytes,
which inhibits the tumor immune response (9). In addition, it has previously been
demonstrated that certain costimulatory molecules exhibit an
important influence on the role of Tregs in cancer immune evasion.
For example, the costimulatory molecule cluster of differentiation
(CD)274 (also known as programmed death-ligand 1) is abnormally
expressed in various tumor tissues. CD274 is an important molecule
that mediates cancer immune evasion (10). The expression status of CD274 is
closely associated with the prognosis of various tumors, including
ovarian cancer, bladder cancer and pancreatic cancer (11–13).
These previous findings suggest that Tregs positive for the tumor
immunity-associated molecules IL-10, CD274 and FOXP3 serve an
important role in tumor development and progression. However,
correlations among the expression of these three molecules, and the
association between these three molecules and the prognosis of LSCC
remain to be elucidated.
Numerous studies have demonstrated that the coding
and non-coding gene regulatory associations are extensive in tumor
immunity, and that they serve essential roles in tumor development
and progression. Liang et al (14) indicated that microRNA (miR)-22 was
able to impair antitumor immunity through targeting p38. In
addition, various regulatory associations exist between coding and
non-coding genes in complex gene regulatory networks. For instance,
miRNAs bind to sites in the 3′-termini of their target genes to
regulate gene expression, and long non-coding RNAs (lncRNAs) or
circular RNAs (circRNAs) act as competitive endogenous RNAs
(ceRNAs) to regulate target gene expression (15). Therefore, it is useful to identify
the non-coding genes that regulate IL-10, FOXP3 and CD274 in order
to understand the molecular mechanism underlying tumor immunity in
LSCC.
In the present study, immunohistochemistry (IHC) was
employed to examine the expression of IL-10, CD274 and FOXP3 in
tumor samples derived from 133 patients with LSCC. Subsequently,
associations between the expression of IL-10, CD274 and FOXP3 and
the clinical characteristics of LSCC were analyzed. In addition,
Spearman's rank correlation method was used to analyze the
correlations among IL-10, CD274 and FOXP3 protein expression. The
Kaplan-Meier (KM) method and the Cox regression model were employed
in combination with the clinical data to analyze the effects of the
expression of these three proteins on the survival rate of patients
with LSCC. The present study aimed to increase understanding
regarding the immune evasion mechanisms of LSCC and to aid the
development of LSCC immunotherapy.
Materials and methods
Collection of LSCC samples
The present study was approved by the Clinical
Research Ethics Committee of Beijing Tongren Hospital, Capital
Medical University (Beijing, China), and written informed consent
was obtained from the patients. This investigation was conducted
according to the Declaration of Helsinki. A total of 133 LSCC
samples were collected for use in the present study; these samples
consisted of paraffin-embedded tissue blocks obtained from patients
with primary LSCC that underwent surgical treatment between January
2012 and July 2013 at Beijing Tongren Hospital affiliated with
Capital Medical University. The patients received a definite
pathological diagnosis, and complete clinical information was
available. In addition, none of the patients received preoperative
radiotherapy or chemotherapy. The inclusion criteria were
established based on tumor size and location, invasion status of
the primary tumors and the status of lymph node metastasis. The
LSCC samples were subjected to histopathological classification and
tumor stage evaluation, which were conducted according to the
Koppel standards and the tumor-node-metastasis (TNM) staging
system, respectively. All pathological diagnoses were verified by
senior pathologists through a review of the tissue sections
(Table I). The normal laryngeal
mucosa tissue and vocal cord polyp tissue were derived from the
patients with recurrent cysts and vocal cord polyps who had
undergone surgery between January 2012 and July 2013 at Beijing
Tongren Hospital affiliated with Capital Medical University
(Beijing, China).
 | Table I.General information and clinical
characteristics of patients with LCSS. |
Table I.
General information and clinical
characteristics of patients with LCSS.
| Variable | Cases | % |
|---|
| Gender |
|
|
|
Male | 128 | 96.2 |
|
Female |
5 |
3.8 |
| Age |
|
|
| <60
years | 48 | 36.1 |
| ≥60
years | 85 | 63.9 |
| Tumor location |
|
|
|
Supraglottic | 30 | 22.6 |
|
Glottic | 101 | 75.9 |
|
Subglottic | 2 |
1.5 |
| TNM |
|
|
|
T1/T2 | 91 | 68.4 |
|
T3/T4 | 42 | 31.6 |
| LNM |
|
|
| No | 98 | 73.7 |
|
Yes | 35 | 26.3 |
| Clinical stage |
|
|
| I | 48 | 36.1 |
| II | 38 | 28.6 |
|
III | 26 | 19.5 |
| IV | 21 | 15.8 |
| Course of
disease |
|
|
| ≤6
months | 53 | 39.8 |
| 6–12
months | 28 | 21.1 |
| ≥12
months | 52 | 39.1 |
| Pathological stage
(differentiation) |
|
|
|
Well | 52 | 39.1 |
|
Moderate | 53 | 39.8 |
|
Poor | 28 | 21.1 |
Hematoxylin and eosin (H&E)
staining
Phosphate-buffered saline (PBS) was used to wash the
sections of the normal and tumor tissues for 5 min. The nucleus was
stained with hematoxylin for 5 min and was differentiated with
hydrochloric acid and ethanol for 30 sec. Tap water and distilled
water was used to wash the sections for 1 min. Eosin staining was
performed for 30 sec. The slices were washed with a 70% ethanol for
30 sec, a 90% ethanol rinse for 30 sec, a 95% ethanol rinse for 30
sec, two 100% ethanol rinses for 2 min each time and xylene
clearing twice for 5 min each time. Finally, an inverted light
microscope was used to observe the stained sections.
IHC staining
Paraffin-embedded tissue sections (4 µm) were
deparaffinized and rehydrated according to conventional methods.
The tissue sections were washed in PBS and were subjected to
antigen retrieval. EDTA buffer (pH 9.0) was used to retrieve
antigen as a repair solution. The antigen was placed in an
autoclave for 2 min. Once cool, the tissue sections were rinsed
with distilled water and then stored in Tris Buffered Saline (pH
7.4). Following antigen retrieval, each section was incubated with
50 µl 3% H2O2 at room temperature for 10 min
to block endogenous peroxidase activity. Anti-CD274 antibody
(NBP1-76769; 1:200) and anti-FOXP3 antibody (NB100-39002; 1:200)
were purchased from Novus Biologicals, LLC (Littleton, CO, USA),
and the anti-IL-10 antibody (BA1201-1; 1:200) was purchased from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Following
washing with PBS, the primary antibodies (1:200 dilution) were
added dropwise to the sections, followed by incubation at 4°C
overnight. Subsequently, the sections were washed again with PBS.
According to the protocol of an IHC two-step detection kit
(PV-6001; OriGene Technologies, Beijing, China), the section was
incubated with 100 µl antibodies at room temperature for 30 min.
Subsequently, the sections were exposed to the chromogenic
substrate 3,3′-diaminobenzidine (OriGene Technologies) and observed
under a light microscope for 3–10 min. The appearance of
brownish-yellow particles indicated positive staining. Following
washing with distilled water, the sections were counterstained with
hematoxylin at room temperature for 10 min, differentiated in 0.1%
hydrochloric acid and rinsed with tap water. The sections were then
dehydrated in a graded series of alcohol, cleared in xylene and
mounted in neutral gum. Negative controls were established by
replacing the primary antibodies with PBS. Gastric cancer tissues
from the tissue bank of Beijing Tongren Hospital were used as
positive controls. Uniformly stained sections that exhibited clear
tissue structure were subjected to semi-quantitative analysis. The
staining results were evaluated by two pathologists using the
double-blind method.
Assessment of the IHC results
The IHC results were scored based on the
semi-quantitative immunoreactivity scoring system developed by
Remmele and Stegnerde (16). For
each tissue section, 5 areas within the tumor were randomly
selected under ×200 magnification. The percentage of cells in the
selected areas that exhibited positive cytoplasmic/plasma membrane
staining was calculated and assigned a score between 0 and 4, as
follows: 0, No cells exhibited positive staining; 1, 1–10% of the
cells exhibited positive staining; 2, 11–50% of the cells exhibited
positive staining; 3, 51–75% of the cells exhibited positive
staining; and 4, >75% of the cells exhibited positive staining.
In addition, the staining intensity was scored as follows: 0, No
staining; 1, weak intensity (light yellow); 2, moderate intensity
(brownish yellow); and 3, strong intensity (dark brown). The two
scores were multiplied together to generate IHC scores, and the
samples were graded according to their IHC scores as follows:
Negative (−), 0; weakly positive (+), 1, positive (++), 2–3; and
strongly positive (+++), >3. This semi-quantitative scoring
system was used to determine staining in tumor-infiltrating
lymphocytes. Initially, the distribution characteristics of the
positive cells in whole tissue sections were examined under low
magnification (×40), which allowed cancer foci to be distinguished
from tumor stroma. To quantify the tumor-infiltrating lymphocytes,
the 5 areas richest in tumor-infiltrating lymphocytes were selected
from each tissue section under low magnification (×40), and the
tumor-infiltrating lymphocytes were counted under a high-power
field (×400). The number of tumor-infiltrating lymphocytes was
expressed as the average number of cells in the five fields of
view. The distribution characteristics of the CD274-, FOXP3- and
IL-10-positive and -negative cells in whole tissue sections were
examined under low magnification. The appearance of brownish-yellow
precipitate in the cytoplasm and/or the cell membrane indicated
positive staining. The staining results were evaluated using a
semi-quantitative scoring system as described above. The
infiltrating lymphocytes were classified into the following four
categories based on the IHC staining scores for CD274, FOXP3 and
IL-10: (−), <1; (+), 1–5; (++), 6–19; and (+++), >20. In
addition, (−) and (+) indicated low expression, whereas (++) and
(+++) indicated high expression. These scores were determined
independently by two pathologists and were averaged and subjected
to statistical analysis.
KM survival rate analysis and Cox
regression model
The KM model was used to assess the effects of
FOXP3, IL-10 and CD274 expression on the survival rate of patients
with LSCC. Due to the advantages associated with the KM model,
including the accurate assessment of the survival rates and the
median survival time, and the pairwise comparison of survival time
distributions between numerous groups, this model has been widely
adopted. In addition, the Cox regression model was employed to
analyze and compare the effects of various factors on the survival
time of patients with LSCC (17).
The Cox regression model is commonly used to analyze the prognosis
of tumors and other chronic diseases. The equation used in the Cox
regression analysis is as follows: h(t/X)=h0(t) exp (β1 X1 + β2 X2
+ … + βp Xp), where h(t/X) is the baseline hazard function, namely,
the hazard function at time t when all independent variables assume
a value of zero. The influencing factors (variables) are denoted by
X1, X2, …, Xp, whereas the regression coefficients are represented
by β1, β2, …, βp.
Identification of miRNA, lncRNA and
circRNA that regulate FOXP3, IL-10 and CD274
To identify the potential mechanism underlying the
correlations among the three genes, the miRTarBase database
(http://mirtarbase.mbc.nctu.edu.tw/)
was used to predict the miRNAs of FOXP3, IL-10 and CD274. Based on
the miRNAs, StarBase version 2.0 database (http://starbase.sysu.edu.cn/) was used to identify the
lncRNAs and circRNAs that regulate the miRNAs. These interactions
were confirmed by experiments including western blot analysis and
PCR. Based on these interactions, Cytoscape software (http://www.cytoscape.org/) was used to establish the
target gene-miRNA-lncRNA and the target gene-miRNA-circRNA
networks.
Statistical analysis
The data were statistically analyzed using SPSS
software version 21.0 (IBM Corp., Armonk, NY, USA). Comparisons
between the two groups were performed using the χ2 test.
A correlation analysis was conducted using Spearman's rank
correlation test. The KM method and log-rank test were used to
analyze survival rates. The association between the expression
levels of CD274, Foxp3 and IL-10 and various clinicopathological
characteristics was subjected to a rank transformation and entered
into Spearman rank correlation test tests. P<0.05 was considered
to indicate a statistically significant difference.
Results
CD274, FOXP3 and IL-10 expression in
normal laryngeal mucosa, vocal cord polyp and LSCC
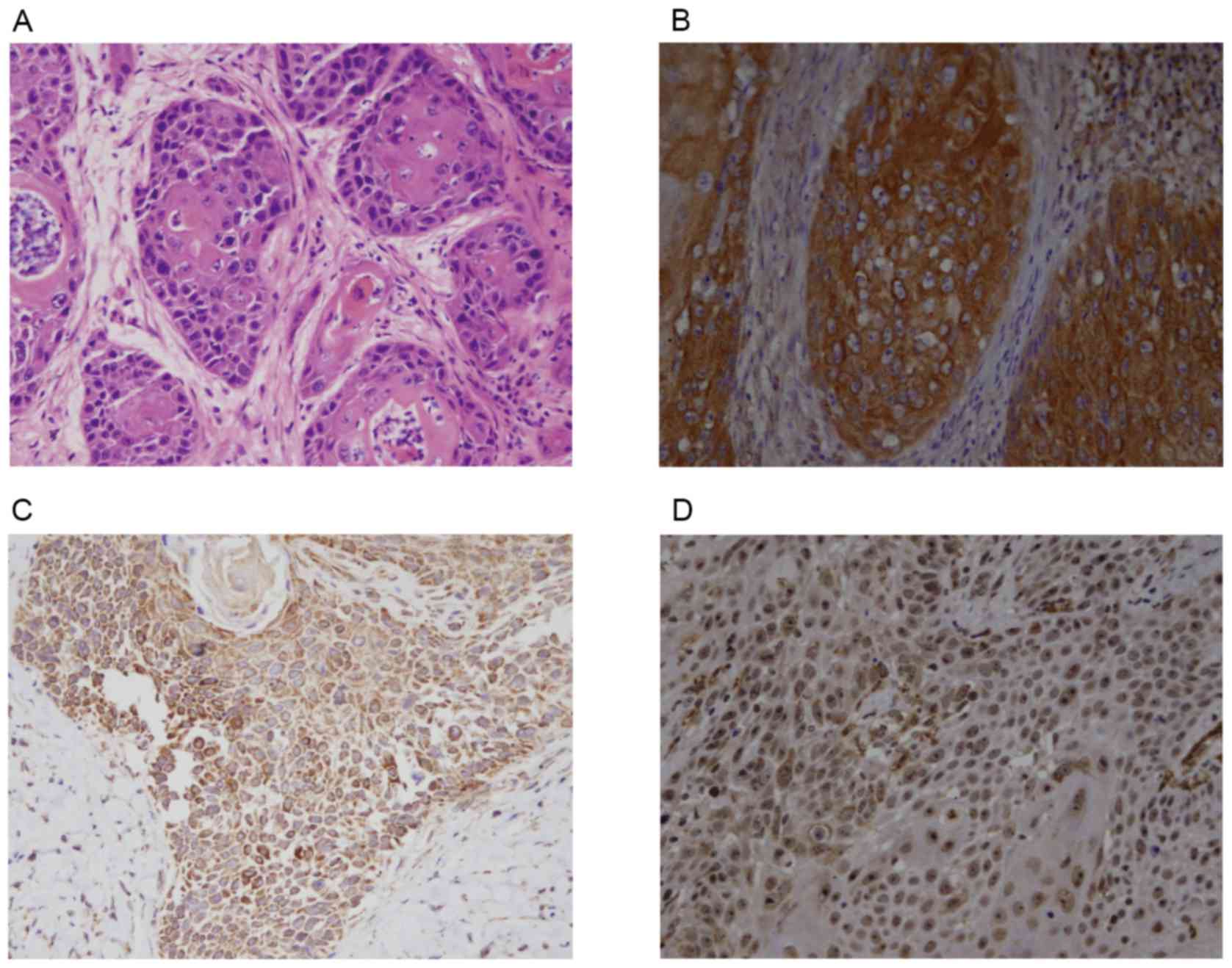
The hematoxylin and eosin staining of LSCC tissues
is presented in Fig. 1A. The
results of IHC demonstrated that the positive expression rate of
CD274 was 58.6% (Fig. 1B), the
positive expression rate of IL-10 was 73.7% (Fig. 1C) and the positive expression rate
of FOXP3 was 68.4% (Fig. 1D) in
the 133 LSCC tissues. However, their expression in normal laryngeal
mucosa and vocal cord polyp tissues was negative or at a low level
(Table II).
 | Table II.Expression of CD274, FOXP3 and IL-10
in three tissue types. |
Table II.
Expression of CD274, FOXP3 and IL-10
in three tissue types.
|
|
| CD274 | FOXP3 | IL-10 |
|---|
|
|
|
|
|
|
|---|
| Tissue type | Cases | + | − | χ2 | P-value | + | − | χ2 | P-value | + | − | χ2 | P-value |
|---|
| NLM | 15 | 0 | 0 | / | / | 3 | 12 | 31.10 | <0.001 | 2 | 13 | 38.07 | <0.001 |
| VCP | 15 | 0 | 0 |
|
| 1 | 14 |
|
| 2 | 13 |
|
|
| LCSS | 133 | 78 | 55 |
|
| 91 | 42 |
|
| 98 | 35 |
|
|
Analysis of the correlations between
the clinicopathological features and CD274, FOXP3 and IL-10
expression
In patients with LSCC, CD274 expression demonstrated
no correlation with tumor location, TNM stage, clinical stage or
pathologic grade (P>0.05). However, CD274 expression was
significantly correlated with lymph node metastasis, disease course
prior to hospital admission, the age of the patients and prognosis
(P<0.05). FOXP3 expression appeared to exhibit no correlation
with the age of the patients, tumor location, T stage, clinical
stage or pathological grade (P>0.05). However, FOXP3 expression
exhibited clear correlations with disease course prior to hospital
admission, lymph node metastasis and prognosis (P<0.05). IL-10
expression demonstrated correlations with all factors, with the
exception of patient age and tumor location (P<0.05; Table III).
 | Table III.CD274, FOXP3 and IL-10 expression in
LCSS. |
Table III.
CD274, FOXP3 and IL-10 expression in
LCSS.
|
| CD274 | FOXP3 | IL-10 |
|---|
|
|
|
|
|
|---|
| Variable | − | + | P-value | − | + | P-value | − | + | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
|
| ≤60
years | 14 | 34 | 0.032 | 16 | 32 | 0.744 | 14 | 34 | 0.575 |
| >60
years | 41 | 44 |
| 26 | 59 |
| 21 | 64 |
|
| Tumor location |
|
|
|
|
|
|
|
|
|
|
Supraglottic | 11 | 19 | 0.497 | 9 | 21 | 1.000 | 6 | 24 | 0.453 |
|
Glottic | 44 | 57 |
| 33 | 68 |
| 29 | 72 |
|
|
Subglottic | 0 | 2 |
| 0 | 2 |
| 0 | 2 |
|
| TNM |
|
|
|
|
|
|
|
|
|
|
T1/T2 | 41 | 50 | 0.202 | 33 | 58 | 0.087 | 31 | 60 | 0.003 |
|
T3/T4 | 14 | 28 |
| 9 | 33 |
| 4 | 38 |
|
| LNM |
|
|
|
|
|
|
|
|
|
| No | 48 | 50 | 0.003 | 37 | 61 | 0.010 | 32 | 66 | 0.005 |
|
Yes | 7 | 28 |
| 5 | 30 |
| 3 | 32 |
|
| Clinical stage |
|
|
|
|
|
|
|
|
|
| I | 24 | 25 | 0.570 | 17 | 32 | 0.579 | 20 | 29 | 0.008 |
| II | 14 | 23 |
| 13 | 24 |
| 10 | 27 |
|
|
III | 10 | 16 |
| 8 | 18 |
| 4 | 22 |
|
| IV | 7 | 14 |
| 4 | 17 |
| 1 | 20 |
|
| Course of
disease |
|
|
|
|
|
|
|
|
|
| ≤6
months | 13 | 40 | 0.004 | 7 | 46 | <0.001 | 8 | 45 | 0.024 |
| 6–12
months | 13 | 15 |
| 9 | 19 |
| 7 | 21 |
|
| ≥12
months | 29 | 23 |
| 26 | 26 |
| 20 | 32 |
|
| Pathological stage
(differentiation) |
|
|
|
|
|
|
|
|
|
|
Well | 26 | 26 | 0.169 | 20 | 32 | 0.076 | 18 | 34 | 0.026 |
|
Moderate | 21 | 32 |
| 18 | 35 |
| 15 | 38 |
|
|
Poor | 8 | 20 |
| 4 | 24 |
| 2 | 26 |
|
| Prognosis |
|
|
|
|
|
|
|
|
|
|
Recurrence (−) | 45 | 25 | <0.001 | 34 | 36 | <0.001 | 30 | 40 | <0.001 |
|
Recurrence (+) | 10 | 53 |
| 8 | 55 |
| 5 | 58 |
|
Correlation of CD274, FOXP3 and IL-10
expression in LSCC
The results of a Spearman analysis demonstrated that
there was a positive expression correlation between CD274 and FOXP3
(rs=0.767, P<0.001), between CD274 and IL-10
(rs=0.640, P<0.001), and between IL-10 and FOXP3
(rs=0.637, P<0.001; Fig.
2).
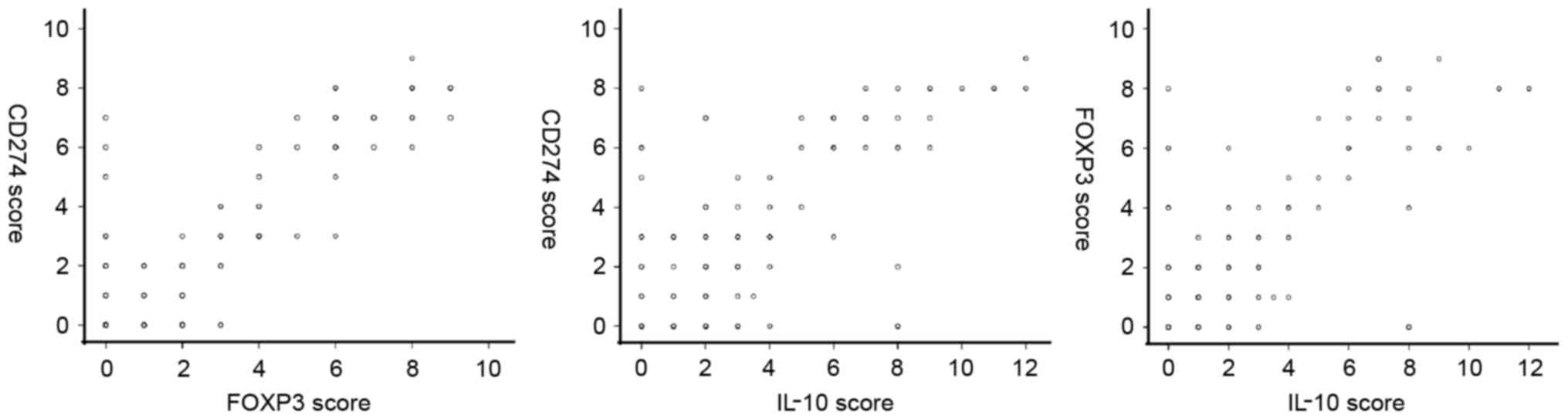 | Figure 2.Correlation of CD274, FOXP3 and IL-10
expression in LSCC. Spearman analysis revealed that there was a
positive expression correlation between CD274 and FOXP3
(rs=0.767, P<0.001), between CD274 and IL-10
(rs=0.640, P<0.001), and between IL-10 and FOXP3
(rs=0.637, P<0.001). CD, cluster of differentiation;
FOX, forkhead box; IL, interleukin; LSCC, laryngeal squamous cell
carcinoma. |
Correlation between CD274, FOXP3 and
IL-10 expression and the prognosis of patients with LSCC
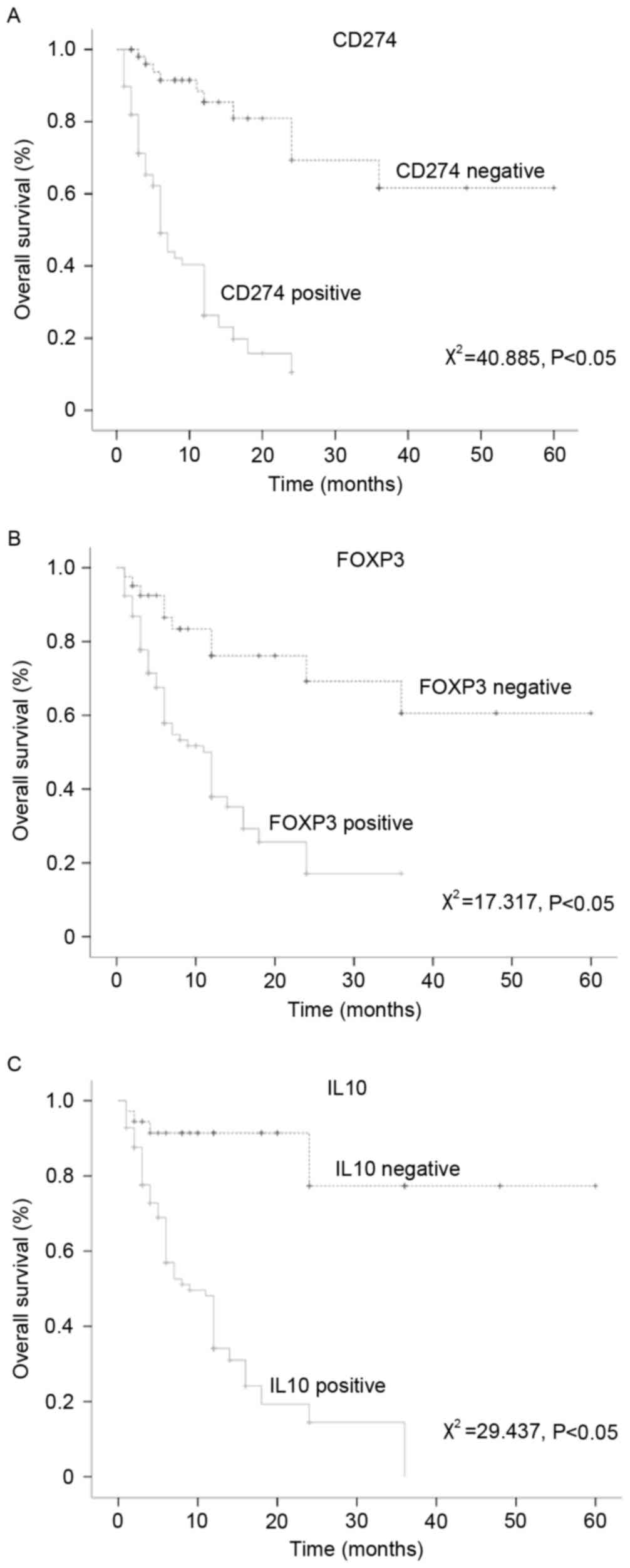
The KM analysis revealed that the CD274-, FOXP3- and
IL-10-negative patients with LSCC experienced a significantly
prolonged mean survival time compared with the CD274-, FOXP3- and
IL-10-positive patients with LSCC (P<0.05; Fig. 3). A multivariate Cox regression
analysis of the effects of the expression of CD274, FOXP3 and IL-10
on the prognosis of LSCC yielded hazard ratios >1 and P<0.05,
which indicated that the expression of these three proteins was a
significant risk factor for LSCC (Table IV).
 | Table IV.Analysis of prognostic factors in
LSCC. |
Table IV.
Analysis of prognostic factors in
LSCC.
| Factor | B | SE | Wald | P-value | HR | 95% CI |
|---|
| CD274 | 0.109 | 0.045 | 5.986 | 0.014 | 1.115 | 1.022 | 1.217 |
| FOXP3 | 0.132 | 0.042 | 9.777 | 0.002 | 1.141 | 1.050 | 1.239 |
| IL-10 | 0.076 | 0.037 | 4.239 | 0.040 | 1.079 | 1.004 | 1.160 |
CD274, FOXP3 and IL-10-associated
ceRNA networks
Using the miRTarBase and StarBase v2.0 databases, it
was established that lncRNA- and circRNA-associated ceRNA networks
regulated CD274, FOXP3 and IL-10 (Figs. 4 and 5). The two networks revealed that the
three genes can be regulated by specific miRNAs including
hsa-miR-106a-5p, which regulated IL-10; hsa-miR-31-5p and
hsa-miR-210-3p, which regulated FOXP3; and hsa-miR-124-3p,
hsa-miR-103a-3p, hsa-miR-214-3p, hsa-miR-16-5p, hsa-miR-326,
hsa-miR-708-5p and hsa-miR-29c-3p, which regulated CD274 (Fig. 4). The number of neighboring nodes
directly connected to the node indicates the importance of the node
in the network. The degree values of the network were then analyzed
using Cytoscape software. The results demonstrated that miRNA
nodes, including hsa-miR-16-5p, hsa-miR-106a-5p and hsa-miR-103a-3p
in the network, exhibited larger degree values (Figs. 4B and 5B). Based on the network, 35
IL-10-associated lncRNAs [including long intergenic non-protein
coding RNA 116, X-inactive specific transcript (XIST) and zinc
ribbon domain containing 1-AS1], 12 FOXP3-associated lncRNAs
(including membrane-associated guanylate kinase, WW, and PDZ
domain-containing protein 2-AS3, small nucleolar RNA host gene 16
and DNA polymerase δ-interacting protein 2) and 134
CD274-associated lncRNAs (including long intergenic non-protein
coding RNA 662, H19 and HOX transcript antisense RNA) were
identified. These lncRNAs may regulate the three genes through
their ceRNA effects. The results demonstrated that lncRNA nodes in
the network, including XIST, RP11-467L20.9 and AC084219.4,
exhibited larger degree values. (Fig.
4C). In addition to lncRNAs, 408 circRNAs that regulate the
three genes in LSCC were identified (Fig. 5A). Among them, it was identified
that ISY1 splicing factor homolog hsa_circ_001859, tubulin γ
complex associated protein 3 hsa-circ_001373 and tubulin α 1b
hsa_circ_002179 in the network exhibited larger degree values
(Fig. 5C).
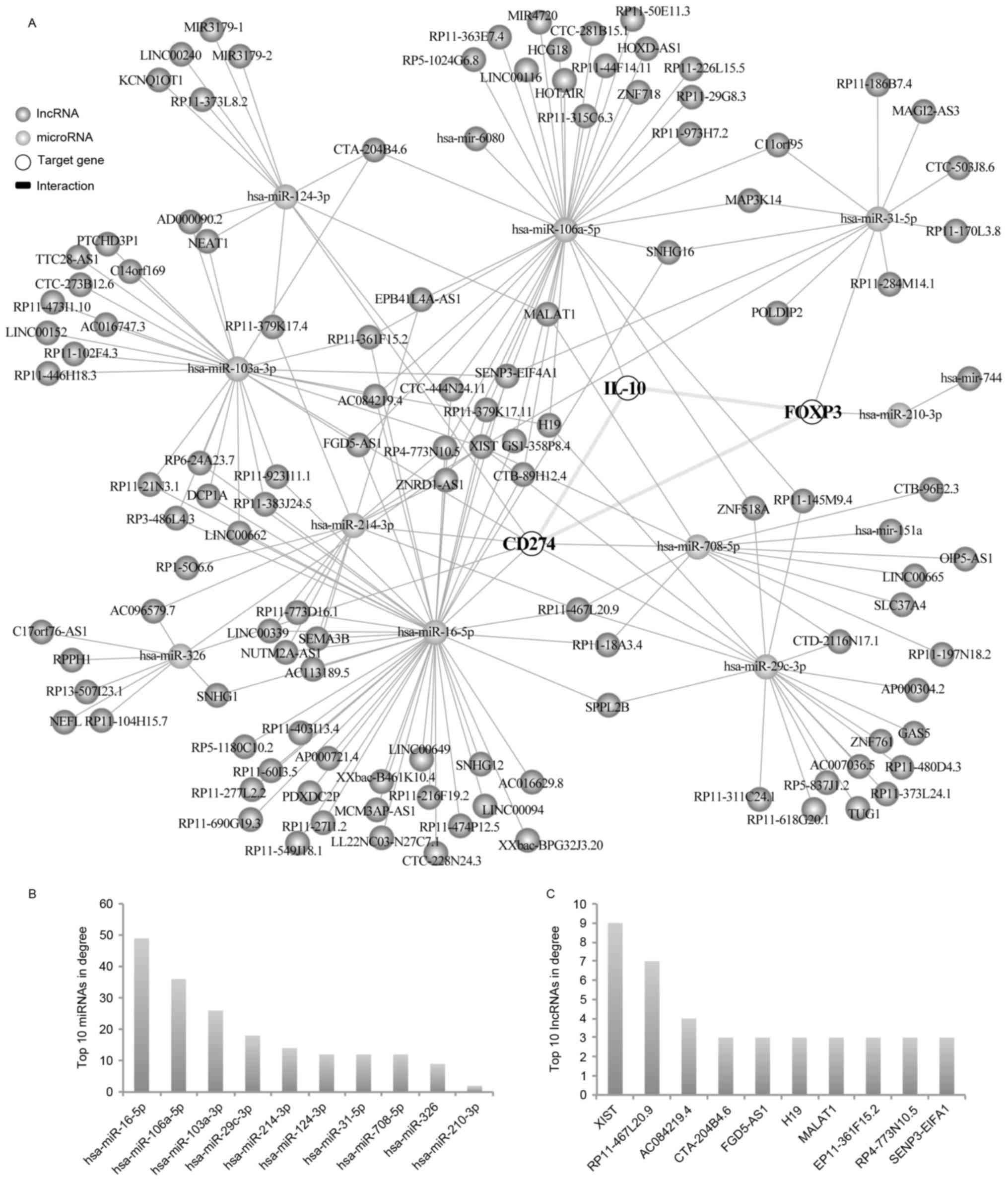 | Figure 4.lncRNA-associated ceRNA network of
CD274, FOXP3 and IL-10 in LSCC. (A) Based on the association among
lncRNA, miRNA and mRNA, databases were used to establish the ceRNA
network. The number of neighboring nodes directly connected to the
node indicates the importance of the node in the network. The
degree values of the network were then analyzed using Cytoscape
software. (B) Top 10 miRNAs, according to degree values. (C) Top 10
lncRNAs, according to degree values. CD, cluster of
differentiation; ceRNA, competitive endogenous RNA; FOX, forkhead
box; IL, interleukin; LSCC, laryngeal squamous cell carcinoma;
lncRNA, long non-coding RNA; miRNA, microRNA. |
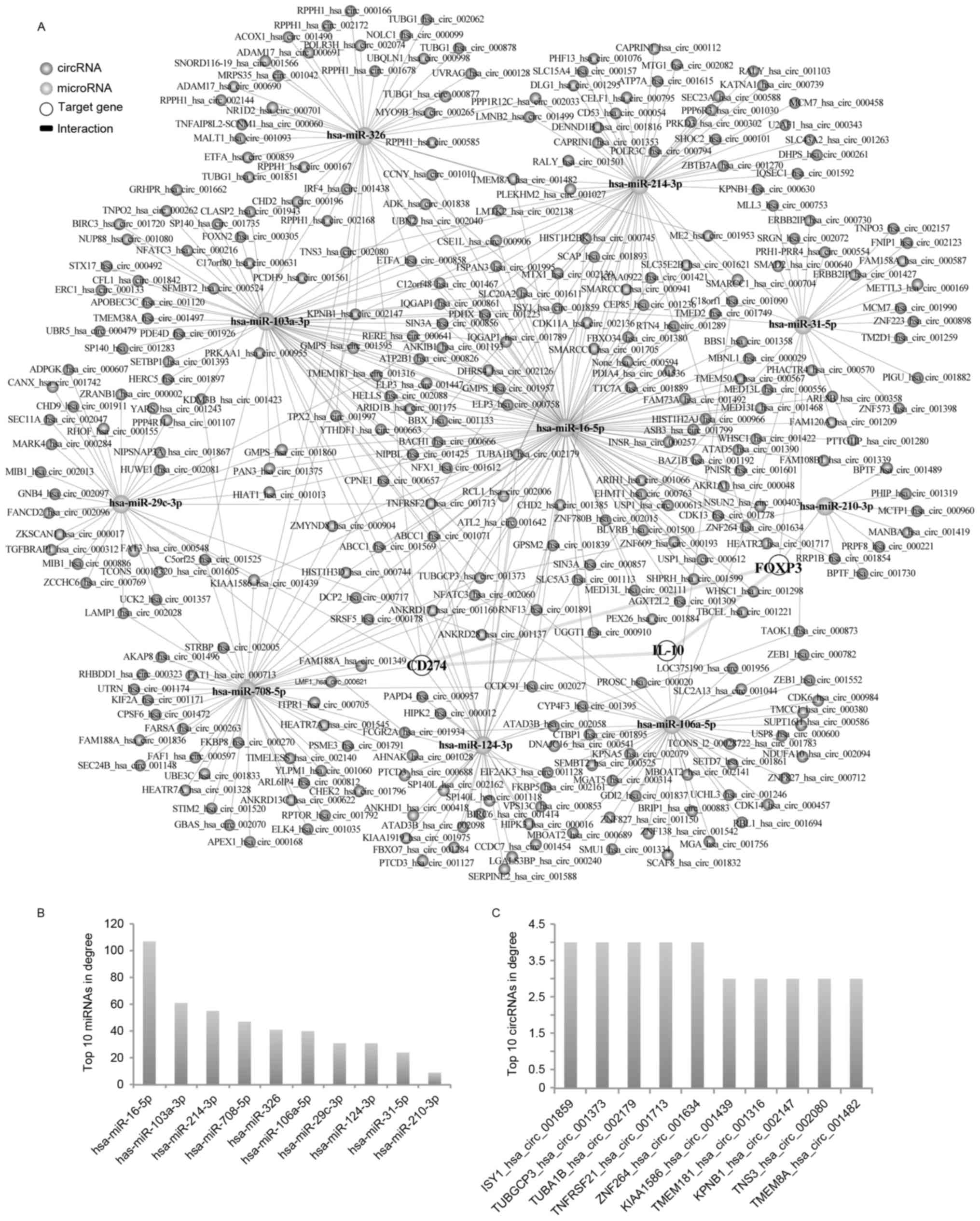 | Figure 5.circRNA-associated ceRNA network of
CD274, FOXP3 and IL-10 in LSCC. (A) Based on the association among
circRNA, miRNA and mRNA, databases were used to establish the ceRNA
network. The number of neighboring nodes directly connected to the
node indicates the importance of the node in the network. The
degree values of the network were then analyzed using Cytoscape
software. (B) Top 10 miRNAs, according to degree values. (C) Top 10
circRNAs, according to degree values. CD, cluster of
differentiation; circRNA, circular RNA; FOX, forkhead box; IL,
interleukin; LSCC, laryngeal squamous cell carcinoma; miRNA,
microRNA. |
Discussion
As an important mechanism of tumorigenesis, immune
evasion has been a subject undergoing intense study in cancer
research. Tumor development and progression depend on the tumor
microenvironment. The infiltration and function of T cells in the
tumor microenvironment determine the outcome of tumor
immunosurveillance. The recently identified Tregs are a
subpopulation of T cells that possess immunoregulatory functions.
Tregs possess two major functions: Induction of immune incompetence
and immunosuppression (18,19).
FOXP3 is a characteristic surface marker of Tregs. It has
previously been identified that the proportion of FOXP3+
Tregs is increased in the peripheral blood of patients with cancer,
including lung, breast, pancreatic and colon cancer (20–23).
These findings indicated that FOXP3+ Tregs are closely
associated with tumor development. The present study identified
that FOXP3 expression was not only increased in LSCC but was also
negatively correlated with the survival rate of patients with LSCC.
Such a finding indicated that FOXP3+ Tregs may promote
the malignant progression of LSCC. The correlation analysis of
clinical factors revealed that the expression levels of FOXP3 were
significantly elevated in patients with a long disease course and
in those who developed lymph node metastasis. These results
demonstrated that FOXP3 was closely associated with the degree of
malignancy of LSCC.
The promoter effect of Tregs on cancer immune
evasion depends on the expression of inhibitory molecules,
including IL-10, which are secreted by Tregs themselves (24). Previous studies have demonstrated
that Tregs initiate inhibitory signals through the secretion of
IL-10 and other cytokines, which inhibits the activity of
functional T lymphocytes and suppresses tumor immune responses
(9,25). IL-10 not only induces immune
tolerance through the inhibition of the dendritic cell-mediated
transformation of T cells into cytotoxic T lymphocytes, but also
induces immunosuppression through the inhibition of the
antigen-presenting function of antigen-presenting cells (26–28).
In certain patients with cancer, IL-10 expression may serve as an
independent negative prognostic factor (29,30).
The present study identified that IL-10 was highly expressed in
LSCC. In addition, the expression levels of IL-10 exhibited a
significant negative correlation with the survival rate of patients
with LSCC. These findings indicated that IL-10 may be closely
associated with the malignant progression of LSCC. Analysis of the
clinicopathological factors demonstrated that the expression levels
of IL-10 were significantly positively correlated with the TNM
stage of cancer, lymph node metastasis, course of the disease and
the pathological grade of cancer in patients with LSCC. The results
further demonstrated that IL-10 may be involved in the malignant
progression of LSCC. In addition, a significant correlation between
IL-10 and FOXP3 expression was identified, which indicated that
FOXP3+ Tregs may affect the malignant progression of
LSCC through the secretion of IL-10.
It has previously been demonstrated that the
expression of negative costimulatory molecules in the tumor
microenvironment is involved in tumor escape from the immune system
(10). Negative costimulatory
molecules are important factors that constitute the tumor
microenvironment. CD274 is one of the primary members of the B7
family of negative costimulatory molecules, which is closely
associated with the progression of various tumors, including
melanoma and glioma (11,31). Previous studies have reported that
CD274 exerts its effects on tumors through the regulation of tumor
immunity. For example, CD274 suppresses type 1 T helper cell-based
immune responses and induces T cell apoptosis in vitro via
the inhibitory receptor programmed cell death protein 1, which aids
the escape of tumor cells from the immune system (32,33).
The present study demonstrated that the expression levels of CD274
in LSCC were positively correlated with the TNM stage of cancer,
lymph node metastasis and the pathological stage of cancer. In
addition, the survival rate was significantly reduced in patients
with high CD274 expression compared with those with low CD274
expression. These findings indicated that CD274 may be involved in
the malignant progression of LSCC. In addition, CD274 and FOXP3
co-expression was detected in LSCC tissues, thus indicating that
CD274 not only inhibits the proliferation of activated T cells but
also exerts an immunosuppressive effect through an increase in the
number of FOXP3+ Tregs, which promotes the progression
of LSCC. Therefore, CD274 may serve as a potential target for LSCC
immunotherapy, whereas CD274 expression may serve as an important
prognostic indicator of LSCC. Furthermore, in addition to the
finding that CD274 was co-expressed along with FOXP3, CD274
expression was also positively correlated with that of IL-10. Based
on the aforementioned results, the following hypothesis was
proposed: A high level of CD274 expression increases the number of
FOXP3+ Tregs, and, as a result, the expression of the
immunosuppressive molecule IL-10 is increased, ultimately promoting
LSCC immune evasion and enhancing the degree of malignancy of LSCC.
This hypothesis is likely to represent an important mechanism
underlying LSCC immune evasion; however, this hypothesis needs to
be verified in the future through large-scale scientific
studies.
To understand the potential molecular mechanism
underlying the correlation among these three genes in LSCC,
miRTarBase and StarBase databases were used to identify the miRNAs
and lncRNAs that regulated the three genes. The results
demonstrated that certain miRNAs in the network exhibited larger
degree values, including hsa-miR-16-5p and hsa-miR-103a-3p.
Previous studies have demonstrated that miRNAs may serve important
roles in cancer progression. Hudcova et al (34) identified that reduced expression of
miR-29c-3p in tumor tissue was associated with worse relapse-free
survival rate in head and neck cancer. Ye et al (35) indicated that miR-106a-5p was
associated with the expression of circulating exosomes and immune
escape in human nasopharyngeal carcinoma. In addition to miR-29c-3p
and miR-106a-5p, the association between other miRNAs and head and
neck cancer is not clear; however, their roles in other cancers
have been reported. Li et al (36) reported that miR-16-5p is able to
control the malignant development of osteoarthritis in
chondrocycytes. Wang et al (37) demonstrated that miR-326 can
regulate cell proliferation and migration in lung cancer. In
addition, miR-106a-5p can inhibit the proliferation and migration
of astrocytoma cells and promote apoptosis (38). Based on these findings, it may be
hypothesized that these miRNAs exhibit the potential to serve
important roles in LSCC.
Salmena et al (15) introduced the concept of ceRNAs in
2011, which states that all types of RNA molecules (e.g., lncRNA
and circRNA) are able to mutually regulate one another by
competitively binding to miRNAs as long as they share common
miRNA-binding sites. In the present study, the effects of ceRNAs in
the 1ncRNA-miRNA-mRNA regulatory network in LSCC were evaluated.
The results demonstrated that certain lncRNAs in the network
exhibited larger degree values, including XIST, H19 and metastasis
associated lung adenocarcinoma transcript 1 (MALAT1). Numerous
studies have identified that these lncRNAs are associated with the
process of cancer. Huang et al (39) demonstrated that a reduction in XIST
expression in breast tissue can upregulate Akt phosphorylation and
tumor cell viability. Li et al (40) reported that the lncRNA H19 can
promote cell invasion in nasopharyngeal carcinoma. In addition, it
has been identified that MALAT1 can serve roles in numerous types
of cancer, including breast and pancreatic cancer (41,42).
Chen et al (43) revealed
that MALAT1 is an important target of cisplatin and paclitaxel, and
may have the potential as a novel molecular target for the
treatment of patients with LSCC. Based on these findings, it may be
hypothesized that these lncRNAs exercise their ceRNA effects to
regulate LSCC through upregulating IL-10, FOXP3 or CD274. The
lncRNAs predicted in the present study may serve as a basis for new
studies regarding the mechanism of tumor immunity in LSCC. In
addition, the circRNA-related ceRNA network identified 408 circRNAs
that regulated the three genes. It has been identified that some of
these genes bind with miRNAs and act as natural miRNA sponges to
inhibit the activities of associated miRNAs. For instance, Xie
et al (44) demonstrated
that circ_001569 can promote the proliferation and invasion of
colorectal cancer via its ceRNA effect. The present study
identified circ_001569 in the ceRNA network, thus suggesting that
circ_001569 may serve roles in LSCC. As research on circRNA remains
in the early stages, the effects of more circRNAs on cancer are yet
to be elucidated. However, the present study indicated that these
circRNAs may bind with the miRNAs that regulate CD274, FOXP3 and
IL-10. Based on these findings, it may be hypothesized that these
circRNAs exhibit the potential to be ceRNAs of the three genes that
regulate the progression of LSCC.
In conclusion, the present study reported that the
expression levels of CD274, FOXP3 and IL-10 were higher in LSCC
tissue compared with their expression levels in normal tissues.
Notably, there was a strong positive correlation among the
expression of these three proteins. In addition, it was identified
that the expression of the three proteins was closely correlated
with the clinical characteristics of LSCC, and the expression
levels of FOXP3, IL-10 and CD274 had a negative association with
the survival rate of patients with LSCC. The results of the Cox
regression model suggested that the three proteins were prognostic
risk factors for LSCC. In addition, the ceRNA network of the three
genes was established. Data generated from the present study
provided novel information, which may increase understanding
regarding the immune evasion mechanisms of LSCC, and aid the
development of LSCC immunotherapy.
Acknowledgements
The present study was supported by the Inner
Mongolia Natural Science Foundation (grant nos. 2014MS0856 and
2017MS0838), the Natural Science Foundation of China (grant no.
81473499) and the General Research Projects of the Affiliated
Hospital of Inner Mongolia Medical University (grant no. NYFY YB
027).
References
|
1
|
Seiferlein E, Haderlein T, Schuster M,
Gräßel E and Bohr C: Correlation between coping strategies and
subjective assessment of the voice-related quality of life of
patients after resection of T1 and T2 laryngeal tumours. Eur Arch
Otorhinolaryngol. 269:2091–2096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:pp. 386–396. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olsen KD: Reexamining the treatment of
advanced laryngeal cancer. Head Neck. 32:1–7. 2010.PubMed/NCBI
|
|
4
|
Moser SE: Laryngeal problems. Prim Care.
41:99–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naldini L: Gene therapy returns to centre
stage. Nature. 526:351–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arce-Sillas A, Álvarez-Luquín DD,
Tamaya-Domínguez B, Gomez-Fuentes S, Trejo-García A, Melo-Salas M,
Cárdenas G, Rodríguez-Ramírez J and Adalid-Peralta L: Regulatory T
cells: Molecular actions on effector cells in immune regulation. J
Immunol Res. 2016:17208272016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeuchi Y and Nishikawa H: Roles of
regulatory T cells in cancer immunity. Int Immunol. 28:401–409.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franco A, Almanza G, Burns JC, Wheeler M
and Zanetti M: Endoplasmic reticulum stress drives a regulatory
phenotype in human T-cell clones. Cell Immunol. 266:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaudhry A, Samstein RM, Treuting P, Liang
Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller
W and Rudensky AY: Interleukin-10 signaling in regulatory T cells
is required for suppression of Th17 cell-mediated inflammation.
Immunity. 34:566–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-driven NSCLC:
Implication for optional immune targeted therapy for NSCLC patients
with EGFR mutation. J Thorac Oncol. 10:910–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Budczies J, Bockmayr M, Denkert C,
Klauschen F, Gröschel S, Darb-Esfahani S, Pfarr N, Leichsenring J,
Onozato ML, Lennerz JK, et al: Pan-cancer analysis of copy number
changes in programmed death-ligand 1 (PD-L1, CD274)-associations
with gene expression, mutational load, and survival. Genes
Chromosomes Cancer. 55:626–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian Y, Deng J, Geng L, Xie H, Jiang G,
Zhou L, Wang Y, Yin S, Feng X, Liu J, et al: TLR4 signaling induces
B7-H1 expression through MAPK pathways in bladder cancer cells.
Cancer Invest. 26:816–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Winograd R, Byrne KT, Evans RA, Odorizzi
PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry
EJ and Vonderheide RH: Induction of T-cell immunity overcomes
complete resistance to PD-1 and CTLA-4 blockade and improves
survival in pancreatic carcinoma. Cancer Immunol Res. 3:399–411.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang X, Liu Y, Mei S, Zhang M, Xin J,
Zhang Y and Yang R: MicroRNA-22 impairs anti-tumor ability of
dendritic cells by targeting p38. PLoS One. 10:e01215102015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
17
|
Abd El Hafeez S, Torino C, D'Arrigo G,
Bolignano D, Provenzano F, Mattace-Raso F, Zoccali C and Tripepi G:
An overview on standard statistical methods for assessing
exposure-outcome link in survival analysis (Part II): The
Kaplan-Meier analysis and the Cox regression method. Aging Clin Exp
Res. 24:203–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YM, Ghali J, Zhang GY, Hu M, Wang Y,
Sawyer A, Zhou JJ, Hapudeniya DA, Wang Y, Cao Q, et al: Development
and function of Foxp3(+) regulatory T cells. Nephrology (Carlton).
21:81–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paust S and Cantor H: Regulatory T cells
and autoimmune disease. Immunol Rev. 204:195–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kryczek I, Liu R, Wang G, Wu K, Shu X,
Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, et al: FOXP3
defines regulatory T cells in human tumor and autoimmune disease.
Cancer Res. 69:3995–4000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto T, Yanagimoto H, Satoi S,
Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S and Kwon AH:
Circulating CD4+CD25+ regulatory T cells in patients with
pancreatic cancer. Pancreas. 41:409–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe MA, Oda JM, Amarante MK and Cesar
Voltarelli J: Regulatory T cells and breast cancer: Implications
for immunopathogenesis. Cancer Metastasis Rev. 29:569–579. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan F, Du R, Wei F, Zhao H, Yu J, Wang C,
Zhan Z, Ding T, Ren X, Chen X and Li H: Expression of TNFR2 by
regulatory T cells in peripheral blood is correlated with clinical
pathology of lung cancer patients. Cancer Immunol Immunother.
64:1475–1485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakaguchi S, Sakaguchi N, Shimizu J,
Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M and
Takahashi T: Immunologic tolerance maintained by CD25+ CD4+
regulatory T cells: Their common role in controlling autoimmunity,
tumor immunity, and transplantation tolerance. Immunol Rev.
182:18–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maynard CL, Hatton RD, Helms WS, Oliver
JR, Stephensen CB and Weaver CT: Contrasting roles for all-trans
retinoic acid in TGF-beta-mediated induction of Foxp3 and Il10
genes in developing regulatory T cells. J Exp Med. 206:343–357.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dennis KL, Blatner NR, Gounari F and
Khazaie K: Current status of interleukin-10 and regulatory T-cells
in cancer. Curr Opin Oncol. 25:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tucci M, Stucci S, Passarelli A, Giudice
G, Dammacco F and Silvestris F: The immune escape in melanoma: Role
of the impaired dendritic cell function. Expert Rev Clin Immunol.
10:1395–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raker VK, Domogalla MP and Steinbrink K:
Tolerogenic dendritic cells for regulatory T cell induction in man.
Front Immunol. 6:5692015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai T, Mazzoli S, Meacci F, Tinacci G,
Nesi G, Zini E and Bartoletti R: Interleukin-6/10 ratio as a
prognostic marker of recurrence in patients with intermediate risk
urothelial bladder carcinoma. J Urol. 178:1906–1912. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta M, Han JJ, Stenson M, Maurer M,
Wellik L, Hu G, Ziesmer S, Dogan A and Witzig TE: Elevated serum
IL-10 levels in diffuse large B-cell lymphoma: A mechanism of
aberrant JAK2 activation. Blood. 119:2844–2853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF,
Qu YM and Han S: The PD-1/B7-H1 pathway modulates the natural
killer cells versus mouse glioma stem cells. PLoS One.
10:e01347152015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kubo S, Yamada T, Osawa Y, Ito Y, Narita N
and Fujieda S: Cytosine-phosphate-guanosine-DNA induces CD274
expression in human B cells and suppresses T helper type 2 cytokine
production in pollen antigen-stimulated CD4-positive cells. Clin
Exp Immunol. 169:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hudcova K, Raudenska M, Gumulec J, Binkova
H, Horakova Z, Kostrica R, Babula P, Adam V and Masarik M:
Expression profiles of miR-29c, miR-200b and miR-375 in tumour and
tumour-adjacent tissues of head and neck cancers. Tumour Biol.
37:12627–12633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS,
Zhang XS, Cui J, Zeng YX and Li J: Tumor-derived exosomes promote
tumor progression and T-cell dysfunction through the regulation of
enriched exosomal microRNAs in human nasopharyngeal carcinoma.
Oncotarget. 5:5439–5452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Jia J, Liu X, Yang S, Ye S, Yang W
and Zhang Y: MicroRNA-16-5p controls development of osteoarthritis
by targeting SMAD3 in chondrocytes. Curr Pharm Des. 21:5160–5167.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang R, Chen X, Xu T, Xia R, Han L, Chen
W, De W and Shu Y: MiR-326 regulates cell proliferation and
migration in lung cancer by targeting phox2a and is regulated by
HOTAIR. Am J Cancer Res. 6:173–186. 2016.PubMed/NCBI
|
|
38
|
Zhi F, Zhou G, Shao N, Xia X, Shi Y, Wang
Q, Zhang Y, Wang R, Xue L, Wang S, et al: miR-106a-5p inhibits the
proliferation and migration of astrocytoma cells and promotes
apoptosis by targeting FASTK. PLoS One. 8:e723902013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang YS, Chang CC, Lee SS, Jou YS and
Shih HM: Xist reduction in breast cancer upregulates AKT
phosphorylation via HDAC3-mediated repression of PHLPP1 expression.
Oncotarget. 7:43256–43266. 2016.PubMed/NCBI
|
|
40
|
Li X, Lin Y, Yang X, Wu X and He X: Long
noncoding RNA H19 regulates EZH2 expression by interacting with
miR-630 and promotes cell invasion in nasopharyngeal carcinoma.
Biochem Biophys Res Commun. 473:913–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang NS, Chi YY, Xue JY, Liu MY, Huang S,
Mo M, Zhou SL and Wu J: Long non-coding RNA metastasis associated
in lung adenocarcinoma transcript 1 (MALAT1) interacts with
estrogen receptor and predicted poor survival in breast cancer.
Oncotarget. 7:37957–37965. 2016.PubMed/NCBI
|
|
42
|
Jiao F, Hu H, Yuan C and Wang L, Jiang W,
Jin Z, Guo Z and Wang L: Elevated expression level of long
noncoding RNA MALAT-1 facilitates cell growth, migration and
invasion in pancreatic cancer. Oncol Rep. 32:2485–2492. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Xin Y, Zhou L, Huang JM, Tao L,
Cheng L and Tian J: Cisplatin and paclitaxel target significant
long noncoding RNAs in laryngeal squamous cell carcinoma. Med
Oncol. 31:2462014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ and Liang L: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016.PubMed/NCBI
|