Introduction
Respiratory syncytial virus (RSV) remains one of the
most common pathogens in the world among young children (1,2). It
has been previously reported that RSV is a serious threat to
children with chronic lung disease, congenital heart disease or
prematurity compared with healthy children (3,4). Due
to the high incidence of RSV infection and its potential for
negative outcomes, it is important to investigate, identify and
prioritize children who are at high risk of developing
RSV-associated acute lower respiratory infection.
Toll-like receptor 4 (TLR4) is a primary receptor
for Gram-negative lipopolysaccharides (LPS) (5). The structure of the RSV fusion
protein is different from LPS; however, it may also trigger TLR4
signaling similarly to LPS (6,7). It
has been previously suggested that RSV infection is markedly
reduced in mice with TLR4 mutations (8,9).
Early lung nuclear factor-κB (NF-κB) response to RSV has been
previously reported to be TLR4-dependent, indicating that enhanced
susceptibility to RSV may induce an appropriate inflammatory
response by regulating TLR4 (10).
Therefore, elucidating the underlying mechanism of TLR4 regulation
may aid in identifying the treatment of RSV infection in
children.
Maternally expressed gene 3 (MEG3), a long
non-coding RNA (lncRNA), has been extensively identified in various
normal tissues (11). In various
types of tumor, loss of MEG3 expression enhances the progression of
tumors; therefore, ectopic expression of MEG3 may suppress cancer
cell proliferation (12,13). Reduced MEG3 expression has been
previously identified in non-small cell lung cancer (NSCLC) tissues
and may be involved in p53 activation, subsequently prompting the
progression of NSCLC (14).
However, the expression level and functional role of MEG3 in the
progression of RSV infection remains to be elucidated.
The present study demonstrated that the level of
MEG3 was reduced in the nasopharyngeal (NPA) samples of patients
infected with RSV. Furthermore, overexpression of MEG3 may
upregulate the expression level of TLR4, subsequently suppressing
RSV infection-induced inflammatory responses.
Materials and methods
NPA samples for mRNA analysis
NPA samples were collected from RSV bronchiolitis
patients within 24 h after being admitted in the hospital (n=104)
or from healthy controls (n=40) in Wuhan Children's Hospital
(Wuhan, China) from December 2015 to May 2016. The exclusion
criteria were as previously described (15), including corticosteroid use in the
past 48 h, significant congenital heart or lung disease and
immunodeficiency, or the presence of one of 15 different viral
pathogens. The clinical characteristics are presented in Table I. NPAs were collected from the
nostrils by deep nasal suctioning. RSV infection was detected using
a rapid antigen test (BinaxNOW RSV Card; Alere, Inc., Waltham, MA,
USA) and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed in the hospital. The respiratory
secretions were subsequently removed from the NPAs. Nasal
epithelial cells were collected from each nostril by rotating a
cytology brush (Medscand Medical Cytobrush Plus; CooperSurgical,
Inc., Trumbull, CT, USA) over the anterior nasal mucosa.
Immediately afterwards, the brushes were immersed in RNA
stabilization reagent RNAlater (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) and epithelial cells were detached from the
brushes and stored at −80°C in RNAlater for subsequent mRNA
analysis.
 | Table I.Clinical characteristics of control
and RSV-positive patients included in the quantitative polymerase
chain reaction analysis. |
Table I.
Clinical characteristics of control
and RSV-positive patients included in the quantitative polymerase
chain reaction analysis.
| Characteristic | Control | RSV patients |
|---|
| Number of
participants | 40 | 104 |
| Age (years), median
(IQR) | 6.5±4.1 | 6.1±3.8 |
| Male gender, n
(%) | 22
(55%) | 52
(50%) |
| Weight (g) mean
(SD) | 5,873 (1,647) | 6,998 (2,350) |
| Duration of symptoms
(days), median (IQR) | NA | 4.6 (3–6) |
| Admission, n (%) | NA | 84 (80.8%) |
| Length of stay,
median (IQR) | NA | 0 (0–0) |
| Length of stay >3
days, n (%) | NA | 0 (0%) |
Cell culture
BEAS-2B human bronchial epithelial cells were
purchased from The American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% (v/v) horse serum (GE Healthcare, Logan, UT,
USA), 100 U/ml penicillin (Invitrogen; Thermo Fisher Scientific,
Inc.), and 0.1 mg/ml streptomycin (GE Healthcare) at 37°C in a
humidified atmosphere with 5% CO2.
RSV propagation and titer
determination
Viral stock of human RSV A2-strain was purchased
from the Viral Laboratory at Beijing Children's Hospital (Capital
University of Medical Sciences, Beijing, China). RSV and
ultraviolet light-inactivated RSV was prepared as previously
described (16). Multiplicity of
infection was 10 in the subsequent experiments.
RNA extraction
Whole blood samples (5 ml) were collected in tubes
containing EDTA. The total RNA from the blood samples or the
epithelial cells was isolated using RNAVzol LS (Vigorous
Biotechnology, Beijing, China) in accordance with the
manufacturer's protocol. The concentration and the purity of the
RNA samples were determined at 260–280 nm.
RT-qPCR
For synthesis of cDNA of the specific mRNA, 1 µg of
the total RNA was reverse transcribed using the PrimeScript RT
reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China) with
specific primers. To quantify the relative mRNA expression levels,
a qPCR assay was performed using SYBR Green supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) on a Bio-Rad iCycleriQ
Real-Time PCR detection system. The PCR amplifications were
performed in a 10 µl total reaction volume, containing 5 µl
SYBR-Green supermix, 0.4 µl forward primer, 0.4 µl reverse primer,
2.2 µl ddH2O and 2 µl template cDNA (1 µg). Initial
denaturation occurred at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min. The relative expression level
of mRNA was determined using the 2−∆∆Cq method (17). GAPDH was chosen as the internal
control. The primers used in the present study were listed as
follows: MEG3 forward, 5′-CCACCCCTCTTGCTTGTCTT-3′ and reverse,
5′-CTTTGTGAGCGTGCTTCCAC-3′; TNF-α forward,
5′-CTGGGCAGGTCTACTTTGGG-3′ and reverse, 5′-CTGGAGGCCCCAGTTTGAAT-3′;
IL-8 forward, 5′-GCCACCATCTTACCTCACAGT-3′ and reverse,
5′-CACAGCACTACCAACACAGC-3′; TLR4 forward, ATCCCTCCCCTGTACCCTTC and
reverse TCAAGGAGCATTGCCCAACA; GAPDH forward, TCCTCCGGGTGATGCTTTTC
and reverse, TTCCCGTTCTCAGCCTTGAC.
Construction of adenovirus associated
virus (AAV)-MEG3
The present study used AAV subtype 9. AAV-MEG3 was
constructed by GeneChem Co., Ltd., (Shanghai, China) as previously
described (18). The
single-stranded AAV vectors were generated by cloning the green
fluorescent protein or human MEG3 coding sequences under control of
the ubiquitous CAG hybrid promoter (CMV enhancer, chicken β-actin
promoter) into AAV backbone plasmids. AAV8 were produced by triple
transfection of 293 cells and purified by an optimized CsCl-based
gradient method that renders high purity vectors preps as
previously described (19). Vector
titers were verified using qPCR.
Transient transfection
Cells were seeded at a density of 6×105
cells/well in 6-well plates with 2 ml DMEM culture medium
containing 10% (v/v) horse serum and antibiotics as aforementioned.
A small interfering RNA (siRNA) targeting TLR4
(CCAGGUGCAUUUAAA-GAAATT) or a negative control
(UUCUCCGAACGUGUCACGUTT) were mixed with HiperFect transfection
reagent (Qiagen GmbH, Hilden, Germany) and incubated at room
temperature for 10 min. Subsequently, each complex was transfected
into BEAS-2B cells and the cells were collected for other
experiments 48 h post-transfection.
Western blotting
Cell protein was extracted using
radioimmunoprecipitation lysis buffer (Beijin Solarbio Science
& Technology Co., Ltd., Beijing, China) and was collected
following centrifugation at 12,000 × g. A bicinchoninic protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used to
determine the protein concentration. A total of 15 µg protein was
loaded per lane, separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 8% non-fat dry milk at 4°C overnight. Following three washes
with PBS with Tween 20 (5 min/wash), the membranes were incubated
with the following primary antibodies at 4°C overnight: TLR4 (cat.
no. 14358; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), NF-κB (cat. no. 8801; 1:1,000; Cell Signaling Technology,
Inc.), phosphorylated (p)-NF-κB (cat. no. 3033; 1:1,000; Cell
Signaling Technology, Inc.), p38MAPK (cat. no. 8690; 1:1,000; Cell
Signaling Technology, Inc.), p-p38MAPK (cat. no. 4511; 1:1,000;
Cell Signaling Technology, Inc.), GAPDH (cat. no. 5174; 1:1,000;
Cell Signaling Technology, Inc.) were used. Following several
washes with TBST, blots were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit and anti-mouse IgG secondary
antibodies (cat no. TA130023 and TA140002; 1:5,000; OriGene
Technologies, Inc., Beijing, China) for 2 h at room temperature.
Blots were washed again and bands were subsequently visualized with
enhanced chemiluminescent substrate (EMD Millipore, Billerica, MA,
USA). GAPDH was used as an internal control. ImageJ software
(version 1.43b; National Institutes of Health, Bethesda, MD, USA)
was used for densitometry.
Statistical analysis
Data are presented as the mean ± standard deviation.
Two-tailed unpaired Student's t-test were used for comparisons of
two groups. Analysis of variance multiple comparisons test followed
by Tukey's post-hoc test was used for comparisons of more than two
groups. All statistical analysis was performed using SPSS version
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
lncRNA MEG3 expression level is
reduced following RSV infection
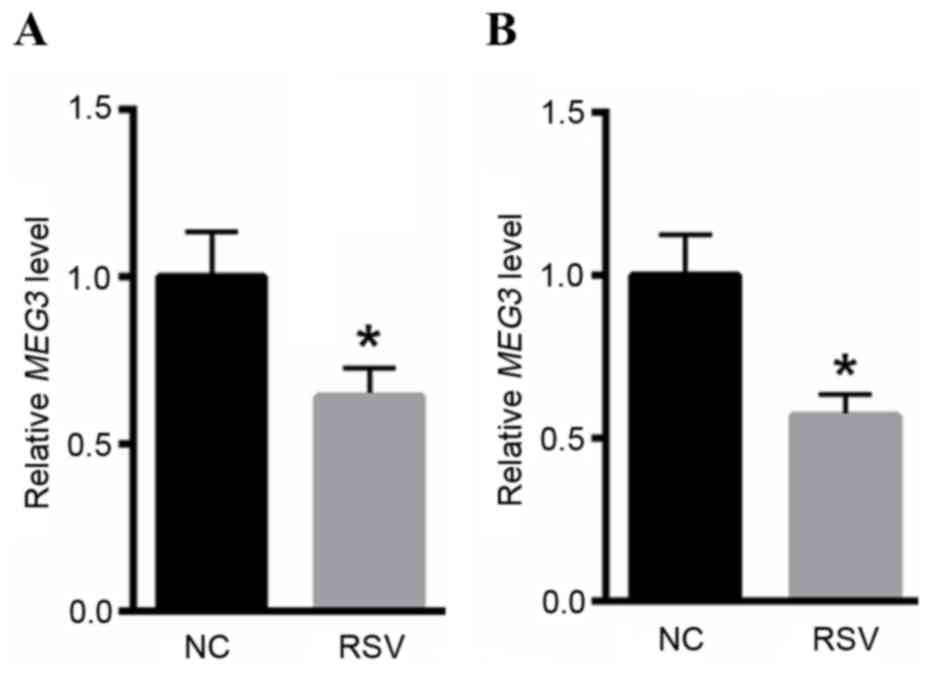
The expression level of MEG3 was markedly reduced in
the NPAs of RSV-infected patients (Fig. 1A) when compared with healthy
controls. BEAS-2B cells were infected with RSV for 24 h.
Subsequently, RNA was extracted from BEAS-2B cells, compared with
normal controls, the expression level of lncRNA MEG3 was reduced in
RSV-infected BEAS-2B cells (Fig.
1B). These data revealed the involvement of lncRNA MEG3 in the
progression of RSV infection-associated disease.
RSV infection enhances TLR4, TNFα and
IL-8 mRNA expression levels
The mRNA expression levels of TLR4, TNFα and IL-8 in
RSV infected samples from patients were quantified. As presented in
Fig. 2A-C, the expression levels
of TLR4, TNFα and IL-8 were significantly increased in patients
with RSV compared with healthy controls. Subsequently the TLR4 mRNA
expression level in BEAS-2B cells transfected with RSV was
determined. RT-qPCR revealed that the expression level of TLR4 was
increased by ~1.67, ~2.56 and ~3.89-fold in RSV-infected cells at
6, 12 and 24 h, respectively (Fig.
2D). The mRNA expression levels of inflammatory factors,
including TNFα and IL-8 were quantified. As presented in Fig. 2E and F, infection with RSV
significantly increased TNFα and IL-8 mRNA levels of compared with
the control. Additionally, the present study also investigated the
protein expression of TLR4 following infection of RSV. Western blot
analysis indicated that the TLR4 protein levels were significantly
increased following RSV infection for 6, 12 and 24 h (Fig. 2G).
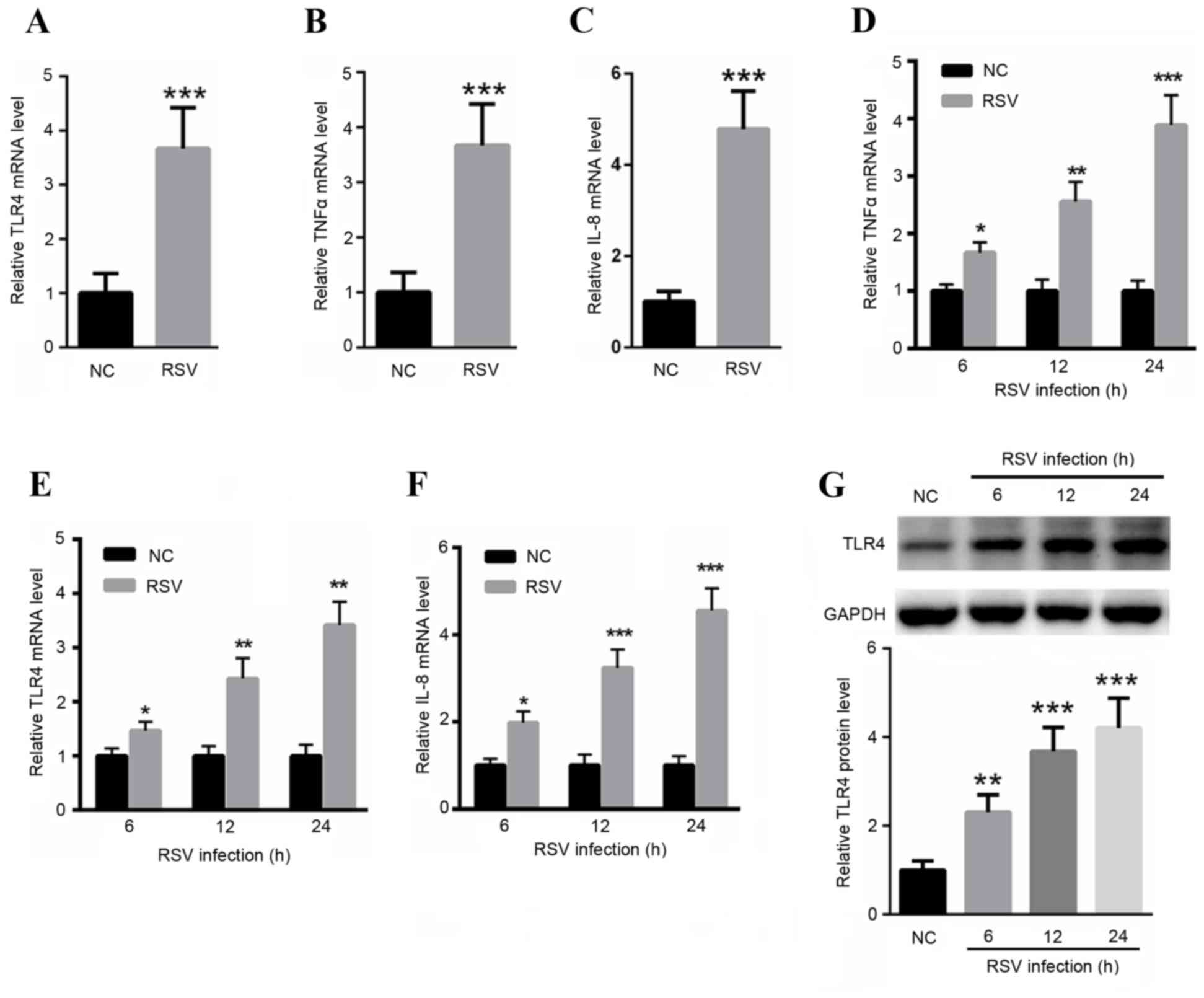 | Figure 2.RSV infection increased mRNA
expression levels of TLR4, TNFα and IL-8. The mRNA expression
levels of (A) TLR4, (B) TNF-α and (C) IL-8 in RSV infected samples
from patients were detected. RSV infection enhanced the mRNA levels
of (D) TLR4, (E) TNFα and (F) IL-8 as assessed by reverse
transcription-quantitative polymerase chain reaction analysis in
BEAS-2B cells at different time points. (G) Western blot analysis
indicated that the protein levels of TLR4 were significantly
increased following RSV infection for 6, 12 and 24 h. *P<0.05,
**P<0.01 and ***P<0.001 vs. control. NC, healthy control;
RSV, respiratory syncytial virus; TLR4, toll-like receptor 4; TNFα,
tumor necrosis factor-α; IL-8, interleukin-8. |
LncRNA MEG3 suppresses TLR4, TNFα and
IL-8 mRNA expression levels
In order to investigate the role of lncRNA MEG3 in
RSV infection-induced inflammatory responses, the present study
overexpressed lncRNA MEG3 in RSV-infected BEAS-2B cells. RT-qPCR
analysis indicated that transfection with AAV-MEG3 significantly
increased the level of lncRNA MEG3 at 6, 12 and 24 h in BEAS-2B
cells (Fig. 3A). It is of note
that an upregulation of lncRNA MEG3 was able to partially reduce
RSV infection-induced TLR4 upregulation at 6, 12 and 24 h, as
indicated by RT-qPCR and western blot analysis (Fig. 3B and C). Additionally, RSV-induced
upregulation of TNFα and IL-8 may also be reduced by ectopic
expression of lncRNA MEG3 (Fig. 3D and
E), indicating the protective role of lncRNA MEG3 in RSV
infection.
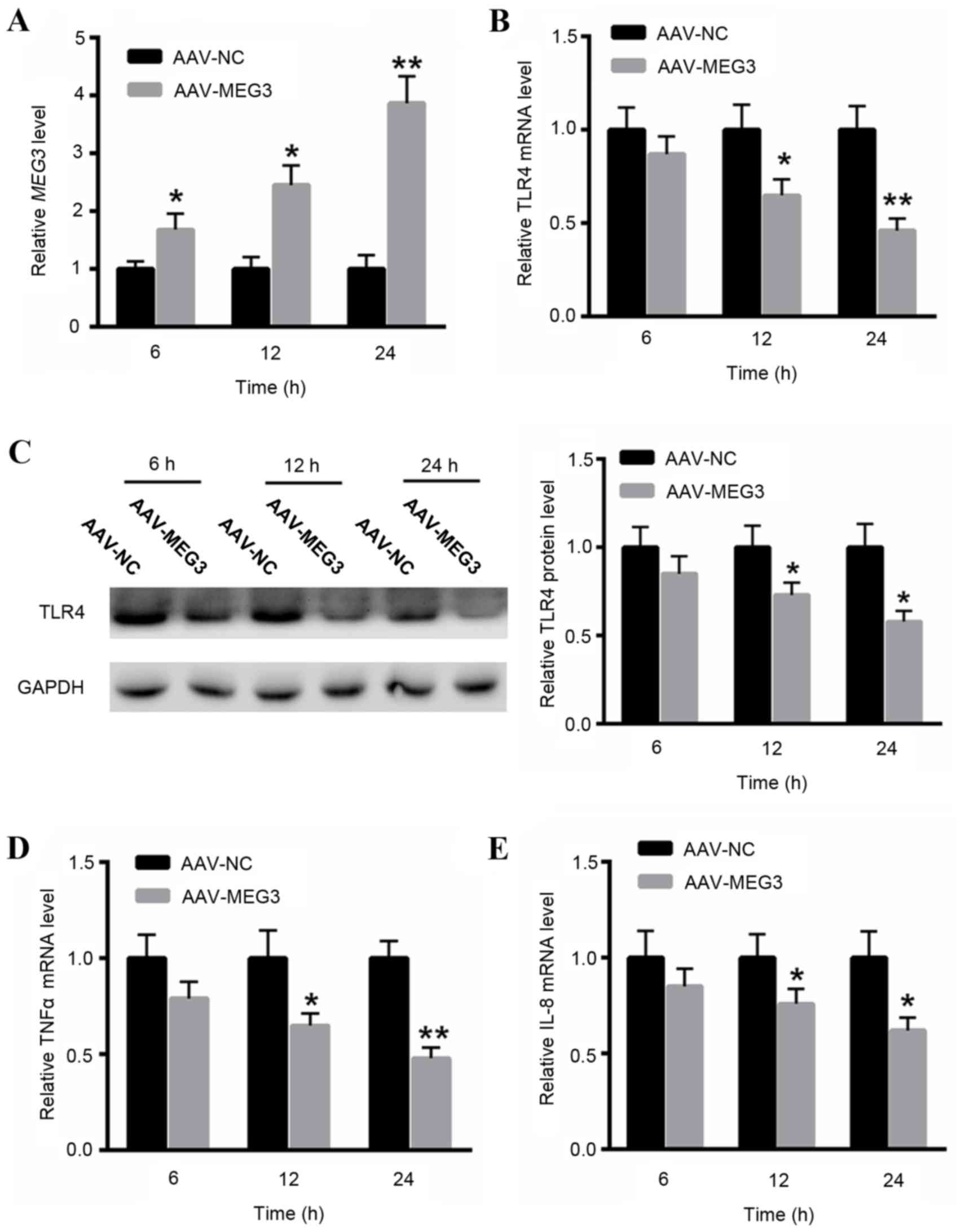 | Figure 3.LncRNA MEG3 suppressed the mRNA
expression level of TLR4, TNFα and IL-8. (A) qPCR analysis
indicated that transfection with AAV-MEG3 significantly increased
the level of lncRNA MEG3 at 6, 12, 24 h in BEAS-2B cells.
Upregulation of lncRNA MEG3 was able to reduce RSV
infection-induced TLR4 upregulation at 6, 12, 24 h, as indicated by
(B) qPCR and (C) western blot analysis. RSV-induced upregulation of
(D) TNFα and (E) IL-8 was also reduced by ectopic expression of
lncRNA MEG3. *P<0.05, **P<0.01 vs. control. AAV,
adenovirus-associated virus; NC, healthy control; RSV, respiratory
syncytial virus; MEG3, maternally expressed gene 3; TLR4, toll-like
receptor 4; TNFα, tumor necrosis factor-α; IL-8, interleukin-8;
lncRNA, long noncoding RNA; qPCR, quantitative polymerase chain
reaction. |
LncRNA MEG3 reduces the activation of
NF-κB and p38 mitogen-activated protein kinase (MAPK)
signaling
Previous studies have indicated the key role of
NF-κB and p38 MAPK signaling in the activation of virus
internalization (20,21); therefore, the present study
investigated the effect of lncRNA MEG3 on the NF-κB and p38 MAPK
pathways. Western blot analysis revealed that RSV infection
significantly increased the activation of NF-κB and p38 MAPK
signaling (Fig. 4A). Conversely,
overexpression of lncRNA MEG3 may significantly suppress RSV
infection-induced NF-κB and p38 MAPK activation (Fig. 4A). In order to verify whether MEG3
protected human airway epithelial cells from RSV infection
primarily by suppressing TLR4-dependent p38 MAPK and NF-kB
signaling, the present study silenced TLR4 in BEAS-2B cells. As
presented in Fig. 4B, silencing of
TLR4 did not affect the expression level of MEG3 in cells
transfected with or without RSV. Western blotting revealed that
knockdown of TLR4 suppressed p38 MAPK and NF-κB signaling (Fig. 4C). However, RSV infection-induced
activation of p38 MAPK and NF-κB signaling may be partially reduced
by knockdown of TLR4 (Fig. 4C).
These findings confirmed that TLR4 has a key role in MEG3-mediated
NF-κB and p38 MAPK activation by RSV infection.
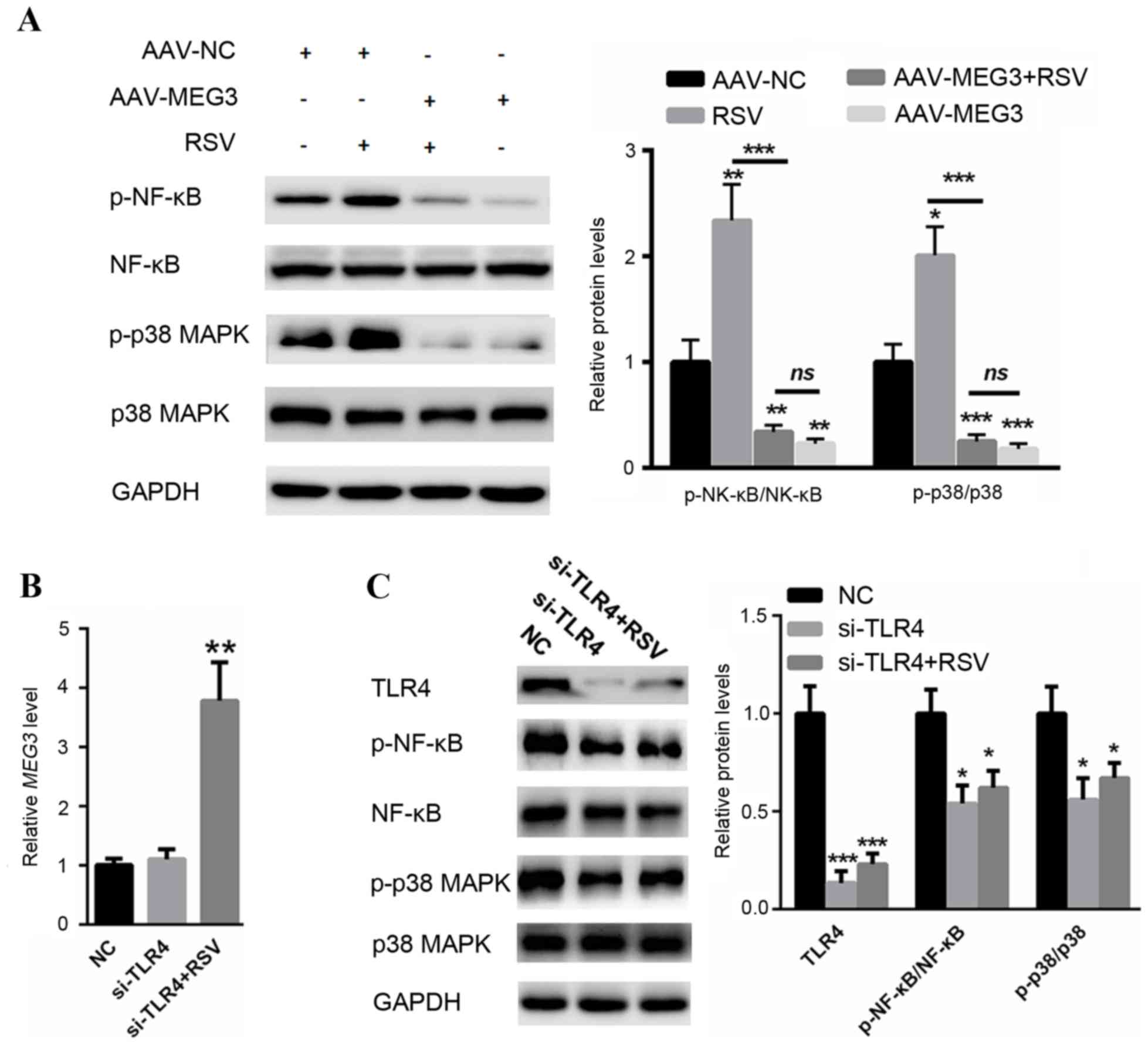 | Figure 4.(A) Long noncoding RNA MEG3 reduced
the activation of NF-κB and p38 MAPK signaling. (B) Silencing of
TLR4 did not affect the level of MEG3 in samples with or without
RSV infection. (C) Western blot assay revealed that RSV
infection-induced activation of p38 MAPK and NF-κB signaling may be
partially abolished by knockdown of TLR4. *P<0.05, **P<0.01
and ***P<0.001 vs. control. AAV, adenovirus-associated virus;
NC, healthy control; RSV, respiratory syncytial virus; MEG3,
maternally expressed gene 3; p-, phosphorylated; NF-κB, nuclear
factor-κB; p38 MAPK, p38 mitogen-activated protein kinase; TLR4,
toll-like receptor 4; si, small interfering; lncRNA, long noncoding
RNA. |
Discussion
RSV is frequently identified in the environment and
it may lead to serious illness through its interaction with
respiratory epithelial cells (22). There are 11 genes and encoded
proteins involved in the infection process of RSV (23). The fusion protein is one of the
most important viral attachment protein, primarily exerting its
function through TLR4 signaling (23,24).
It has been previously suggested that in the early RSV response,
TLR4 signaling is an important component, enhancing the
inflammatory responses in sequelae to RSV infection phenotype
(25).
A previous study revealed that RSV infection
enhances the airway epithelial cell receptors, which may enhance
the binding of inhaled environmental agents (26). The current study revealed that the
level of TLR4 was increased by ~1.67-, ~2.56- and ~3.89-fold in RSV
infected cells at 6, 12 and 24 h, respectively. Additionally, the
inflammatory factors, such as TNFα and IL-8, were markedly
increased following RSV infection compared with the controls. These
findings indicate that increased TLR4 expression may lead to the
activation of inflammatory cytokine production in human airway
epithelial cells.
Genome-wide surveys have previously demonstrated
that lncRNAs are ubiquitously involved in diverse biological
processes, including cell cycle control and cell differentiation
through distinct mechanisms (27,28).
Various lncRNAs have been previously identified in cancer biology
(29,30). However, the involvement of lncRNAs
in RSV infection remains to be determined. The lncRNA MEG3
expression has been reported to be significantly reduced in primary
bladder tumors, which increased cancer cell metastasis (31). To highlight the impact of MEG3 in
RSV infection, the present study investigated the expression level
of lncRNA MEG3 in the NPA samples of RSV-positive patients. The
expression level of MEG3 was markedly reduced in the NPAs of
RSV-infected patients compared with the healthy controls.
Furthermore, the expression level of lncRNA MEG3 was reduced in
RSV-infected BEAS-2B cells. These findings suggest that reduced
MEG3 expression level may promote RSV infection in BEAS-2B
cells.
It is of note that the current findings revealed
that lncRNA MEG3 suppressed the mRNA expression level of TLR4, TNFα
and IL-8, indicating a protective role of lncRNA MEG3 in the
progression of RSV-associated disease. TLR4, a signaling receptor
for structurally diverse microbe-associated molecular patterns, has
been previously revealed to be activated by the RSV fusion protein
(32). Furthermore, TLR4-mediated
activation of MAPK and NF-κB signaling has been extensively
reported in multiple previous studies (33,34).
For example, Ugonin M, a Helminthostachys zeylanica
constituent, has been found to prevent LPS-induced acute lung
injury by suppressing the TLR4-mediated MAPK and NF-κB signaling
pathways (33). The p38 MAPK
signaling activated by pattern recognition receptors has been
previously reported as part of the antiviral immune response
(35). It has been previously
reported that RSV infection activates cellular MAPKs, which
enhances host-cell targeting and virus-cell fusion (20). In addition, TLR4-mediated
inflammatory response may be, in part, dependent on NF-κB
activation (25). Additionally,
TLR4 signaling has been previously identified to induce functional
nerve growth factor receptor p75NTR in mouse dendritic cells
through p38 MAPK and NF-κB pathways (36). The present study validated that
infection with RSV significantly increased the activation of p38
MAPK and NF-κB signaling which was in agreement with the previous
studies. By contrast, ectopic expression of MEG3 by transfection
partially blocked p38 MAPK and NF-κB activation. These findings
suggest the involvement of MEG3 in p38 MAPK and NF-κB signaling to
suppress RSV infection.
In conclusion, to the best of our knowledge, the
present study for the first time, identified reduced lncRNA MEG3
expression levels in patients with RSV infection. The current study
also demonstrated that MEG3 protected human airway epithelial cells
from RSV infection primarily by suppressing TLR4-dependent p38 MAPK
and NF-κB signaling.
References
|
1
|
Othman HT, Abu Elhamed WA, Hassan DM,
Soliman MS and Abdel Baset RW: Respiratory syncytial virus and
human metapneumovirus in severe lower respiratory tract infections
in children under two. J Infect Dev Ctries. 10:283–289. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Relić T, Ilić N, Kostić G, Jovanović D,
Tambur Z and Lazarević I: Respiratory syncytial virus infection and
bronchial hyperreactivity in children up to two years of age in
correlation with atopy. Vojnosanit Pregl. 73:59–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simões EA: The outpatient burden of
respiratory syncytial virus infections in older children. J Infect
Dis. 215:1–3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan XL, Li YN, Tang YJ, Xie ZP, Gao HC,
Yang XM, Li YM, Liu LJ and Duan ZJ: Clinical characteristics and
viral load of respiratory syncytial virus and human metapneumovirus
in children hospitaled for acute lower respiratory tract infection.
J Med Virol. 89:589–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogel SN, Fitzgerald KA and Fenton MJ:
TLRs: Differential adapter utilization by toll-like receptors
mediates TLR-specific patterns of gene expression. Mol Interv.
3:466–477. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurt-Jones EA, Popova L, Kwinn L, Haynes
LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT,
Anderson LJ and Finberg RW: Pattern recognition receptors TLR4 and
CD14 mediate response to respiratory syncytial virus. Nat Immunol.
1:398–401. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröder NW and Arditi M: The role of
innate immunity in the pathogenesis of asthma: Evidence for the
involvement of Toll-like receptor signaling. J Endotoxin Res.
13:305–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abe T, Hemmi H, Miyamoto H, Moriishi K,
Tamura S, Takaku H, Akira S and Matsuura Y: Involvement of the
Toll-like receptor 9 signaling pathway in the induction of innate
immunity by baculovirus. J Virol. 79:2847–2858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haynes LM, Moore DD, Kurt-Jones EA,
Finberg RW, Anderson LJ and Tripp RA: Involvement of toll-like
receptor 4 in innate immunity to respiratory syncytial virus. J
Virol. 75:10730–10737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haeberle HA, Takizawa R, Casola A, Brasier
AR, Dieterich HJ, Van Rooijen N, Gatalica Z and Garofalo RP:
Respiratory syncytial virus-induced activation of nuclear
factor-kappaB in the lung involves alveolar macrophages and
toll-like receptor 4-dependent pathways. J Infect Dis.
186:1199–1206. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chunharojrith P, Nakayama Y, Jiang X, Kery
RE, Ma J, De La Hoz Ulloa CS, Zhang X, Zhou Y and Klibanski A:
Tumor suppression by MEG3 lncRNA in a human pituitary tumor derived
cell line. Mol Cell Endocrinol. 416:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Li C, Liu C, Yu S and Zhang Y:
Expression of the long non-coding RNAs MEG3, HOTAIR, and MALAT-1 in
non-functioning pituitary adenomas and their relationship to tumor
behavior. Pituitary. 18:42–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang NQ, Luo XJ, Zhang J, Wang GM and Guo
JM: Crosstalk between Meg3 and miR-1297 regulates growth of
testicular germ cell tumor through PTEN/PI3K/AKT pathway. Am J
Transl Res. 8:1091–1099. 2016.PubMed/NCBI
|
|
14
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Templeton KE, Scheltinga SA, Beersma MF,
Kroes AC and Claas EC: Rapid and sensitive method using multiplex
real-time PCR for diagnosis of infections by influenza a and
influenza B viruses, respiratory syncytial virus, and parainfluenza
viruses 1, 2, 3, and 4. J Clin Microbiol. 42:1564–1569. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monick MM, Yarovinsky TO, Powers LS,
Butler NS, Carter AB, Gudmundsson G and Hunninghake GW: Respiratory
syncytial virus up-regulates TLR4 and sensitizes airway epithelial
cells to endotoxin. J Biol Chem. 278:53035–53044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Tang Y, Wu L, Mo F, Wang X, Li H, Qi
R, Zhang H, Srivastava A and Ling C: The hepatocyte-specific
HNF4α/miR-122 pathway contributes to iron overload-mediated hepatic
inflammation. Blood. 130:1041–1051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayuso E, Mingozzi F and Bosch F:
Production, purification and characterization of adeno-associated
vectors. Curr Gene Ther. 10:423–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marchant D, Singhera GK, Utokaparch S,
Hackett TL, Boyd JH, Luo Z, Si X, Dorscheid DR, McManus BM and
Hegele RG: Toll-like receptor 4-mediated activation of p38
mitogen-activated protein kinase is a determinant of respiratory
virus entry and tropism. J Virol. 84:11359–11373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marr N and Turvey SE: Role of human TLR4
in respiratory syncytial virus-induced NF-κB activation, viral
entry and replication. Innate Immun. 18:856–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Zhang X, Liu S, Wang Z, Chen Q, Wu
Y, He Z and Huang Z: Genetic association of TLR4 Asp299Gly, TLR4
Thr399Ile, and CD14 C-159T polymorphisms with the risk of severe
RSV infection: A meta-analysis. Influenza Other Respir Viruses.
10:224–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cyr SL, Angers I, Guillot L,
Stoica-Popescu I, Lussier M, Qureshi S, Burt DS and Ward BJ: TLR4
and MyD88 control protection and pulmonary granulocytic recruitment
in a murine intranasal RSV immunization and challenge model.
Vaccine. 27:421–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shirey KA, Pletneva LM, Puche AC, Keegan
AD, Prince GA, Blanco JC and Vogel SN: Control of RSV-induced lung
injury by alternatively activated macrophages is IL-4R alpha-,
TLR4-, and IFN-beta-dependent. Mucosal Immunol. 3:291–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caballero MT, Serra ME, Acosta PL, Marzec
J, Gibbons L, Salim M, Rodriguez A, Reynaldi A, Garcia A, Bado D,
et al: TLR4 genotype and environmental LPS mediate RSV
bronchiolitis through Th2 polarization. J Clin Invest. 125:571–582.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Gao L, Wang X and Xing Y:
Respiratory syncytial virus infection inhibits TLR4 signaling via
up-regulation of miR-26b. Cell Biol Int. 39:1376–1383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Zhang M, An G and Ma Q: LncRNA TUG1
acts as a tumor suppressor in human glioma by promoting cell
apoptosis. Exp Biol Med (Maywood). 241:644–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Botti G, Marra L, Malzone MG, Anniciello
A, Botti C, Franco R and Cantile M: LncRNA HOTAIR as prognostic
circulating marker and potential therapeutic target in patients
with tumor diseases. Curr Drug Targets. 18:27–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao T, Qu N, Shi RL, Guo K, Ma B, Cao YM,
Xiang J, Lu ZW, Zhu YX, Li DS and Ji QH: BRAF-activated LncRNA
functions as a tumor suppressor in papillary thyroid cancer.
Oncotarget. 8:238–247. 2017.PubMed/NCBI
|
|
30
|
Liu L, Yue H, Liu Q, Yuan J, Li J, Wei G,
Chen X, Lu Y, Guo M, Luo J and Chen R: LncRNA MT1JP functions as a
tumor suppressor by interacting with TIAR to modulate the p53
pathway. Oncotarget. 7:15787–15800. 2016.PubMed/NCBI
|
|
31
|
Ying L, Huang Y, Chen H, Wang Y, Xia L,
Chen Y, Liu Y and Qiu F: Downregulated MEG3 activates autophagy and
increases cell proliferation in bladder cancer. Mol Biosyst.
9:407–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rallabhandi P, Phillips RL, Boukhvalova
MS, Pletneva LM, Shirey KA, Gioannini TL, Weiss JP, Chow JC,
Hawkins LD, Vogel SN and Blanco JC: Respiratory syncytial virus
fusion protein-induced toll-like receptor 4 (TLR4) signaling is
inhibited by the TLR4 antagonists Rhodobacter sphaeroides
lipopolysaccharide and eritoran (E5564) and requires direct
interaction with MD-2. MBio. 3:pii: e00218–12. 2012. View Article : Google Scholar
|
|
33
|
Wu KC, Huang SS, Kuo YH, Ho YL, Yang CS,
Chang YS and Huang GJ: Ugonin M, a Helminthostachys zeylanica
constituent, prevents LPS-induced acute lung injury through
TLR4-mediated MAPK and NF-κB signaling pathways. Molecules.
22(pii): E5732017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nair AR, Elks CM, Vila J, Del Piero F,
Paulsen DB and Francis J: A blueberry-enriched diet improves renal
function and reduces oxidative stress in metabolic syndrome
animals: Potential mechanism of TLR4-MAPK signaling pathway. PLoS
One. 9:e1119762014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kopp E and Medzhitov R: Recognition of
microbial infection by Toll-like receptors. Curr Opin Immunol.
15:396–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang Y, Chen G, Zheng Y, Lu L, Wu C,
Zhang Y, Liu Q and Cao X: TLR4 signaling induces functional nerve
growth factor receptor p75NTR on mouse dendritic cells via p38MAPK
and NF-kappa B pathways. Mol Immunol. 45:1557–1566. 2008.
View Article : Google Scholar : PubMed/NCBI
|