Introduction
Pseudoaneurysm refers to the focal enlargement of
the vascular lumen due to partial or complete disruption of the
arterial wall and a contained bleed (1,2).
Various etiological factors have been described with regards to the
formation of pseudoaneurysms, including iatrogenic injury, trauma,
infection, numerous punctures and local inflammation. As the number
of drug abusers worldwide has increased (16–38 million) (3), so has the prevalence of
trauma-induced pseudoaneurysm in drug abusers. FA pseudoaneurysm
accounts for ~0.14% of complications associated with the
intravenous injection of drugs (4). A previous study estimated that the
annual prevalence of mycotic pseudoaneurysm in intravenous drug
abusers was 0.03% in San Francisco (5). Pseudoaneurysm in drug abusers is
predominantly caused by repeated trauma to the arterial walls in
response to intravenous puncture. In addition to painful and
pulsatile swelling in the femoral region, drug abusers with
pseudoaneurysm present with abscess, cellulitis and endocarditis.
In general, small pseudoaneurysms (<2 cm) can be restored
spontaneously, whereas large and complex pseudoaneurysms require
appropriate treatment (6,7). Pseudoaneurysms should be treated as
soon as possible after diagnosis, due to the tendency of various
complications, including rupture and compression of the adjacent
femoral vein or the femoral nerve (8–11).
In the past, surgical treatment involving ligation,
grafting or bypassing was considered the most reliable therapeutic
option for the treatment of PA. In addition, several nonsurgical
methods have been used as alternatives, including close
observation, ultrasound-guided thrombin injection and compression.
Recently, the use of endovascular stent-graft repair has garnered
attention as a surgical reconstructive technique, particularly for
drug abuse patients with bleeding pseudoaneurysm (12,13).
The first case of treating pseudoaneurysm with the implantation of
a covered stent (Palmaz P294 stent; autogenous vein-covered and
balloon-expanded) was reported by McGraw et al in 1998
(14), and numerous studies
regarding the use of covered stents in the treatment of
pseudoaneurysm have been conducted since (1,15,16).
These previous studies only contain information on one or two
cases; however, they have indicated that percutaneous endovascular
treatment using a covered stent may be a safe and feasible method
for the treatment of pseudoaneurysm. The present study recruited 29
drug abuse patients with superficial FA (SFA) pseudoaneurysm from a
single institution and percutaneously repaired the pseudoaneurysms
using polyethylene terephthalate-covered stents, in order to
further evaluate their clinical efficacy.
Materials and methods
Patients
The present study was approved by the Xijing
Hospital Institutional Review Board (Xi'an, China) and informed
written consent was obtained from each patient, following a
discussion of treatment options, and of the endovascular operation
with its risks and benefits.
Over 2 years, between January 2012 and May 2014, a
total of 29 suspected pseudoaneurysm patients (21 men and 8 women;
age range, 25–52 years; mean age 40.38 years) with 5–10 years of
intravenous drug abuse history were presented to the emergency
department, due to the hemorrhage and rupture of their
pseudoaneurysms. Following simple application of a compression
bandage, they were admitted to the angiographic suite of the Xijing
Hospital (Xi'an, China).
All patients were noted to be undergoing an
inflammatory flare-up and pigmentation was detected in the inguinal
region following numerous punctures. The patients exhibited pain at
the puncture site, due to repeated trauma of the arterial wall, and
a progressively enlarged pulsatile mass was palpated in each case.
A vascular bruit, or dual-phase (systolic and diastolic) vascular
bruit, was noted in all patients. Preoperational physical
examination also revealed that dorsalis pedis pulse was faint in 9
cases (31.03%) and not palpable in 14 cases (48.28%) with bimanual
palpation. Active hemorrhage was noted in 21 cases (72.41%).
Furthermore, 3 cases (10.34%) were positive for hepatitis B virus,
5 cases (17.24%) were positive for hepatitis C virus, 2 cases
(6.70%) were positive for syphilis and 1 case (3.45%) was positive
for human immunodeficiency virus-3 (Table I).
 | Table I.Demographic characteristics of the
patients with superficial femoral artery pseudoaneurysm. |
Table I.
Demographic characteristics of the
patients with superficial femoral artery pseudoaneurysm.
| Characteristic
(n=29) | n (%) |
|---|
| Age, year | 40.38±9.88 |
| Male | 21 (72.41) |
| BMI,
kg/m2 | 25 ± 8 |
| Medical history |
|
|
Hypertension | 2 (6.70) |
|
Diabetes | 2 (6.70) |
|
Smoking | 19 (65.52) |
| Physical
examination |
|
| Dorsalis
pedis pulse |
|
|
Faint | 9 (31.03) |
|
Non-palpable | 14 (48.28) |
| Active
hemorrhage | 21 (72.41) |
| Laboratory
examination |
|
| Hepatitis
B virus, + | 3 (10.34) |
| Hepatitis
C virus, + | 5 (17.24) |
| Syphilis,
+ | 2 (6.70) |
| HIV3,
+ | 1 (3.45) |
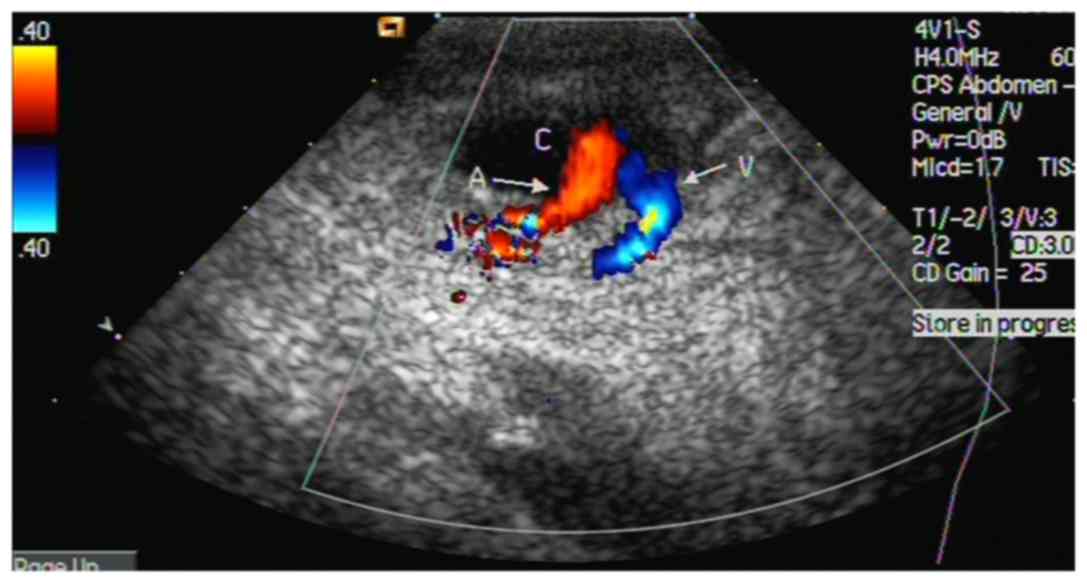
Sonography
The diagnosis of a pseudoaneurysm was confirmed by
color Doppler sonography (LOGIQ P5; GE Healthcare, Waukesha, WI,
USA). The operator had >10 years of experience with sonography.
The diagnosis was based on detecting an extravascular false lumen
connected to the FA at the puncture site. Doppler sonography
detected 2-way blood flow signals (pansystolic flow from the FA to
the neck of the pseudoaneurysm, and pandiastolic reverse flow in
the neck of the pseudoaneurysm) in all 29 patients (Fig. 1). These signals were located in the
SFA. The pseudoaneurysm neck was first localized using a color
Doppler technique, which exhibited abnormal color flow at the
lesion site (2-way blood flow signals). The ultrasound system was
then shifted to the gray scale mode without moving the probe. The
widest part of the lesion was marked and measured.
CT protocol
To detect the morphology, origin and neck of the PA,
multidetector computed tomographic (MDCT) angiography was
performed. All patients were scanned on a dual-source MDCT scanner
(SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany)
following administration of an iodinated contrast agent (70–100 ml
Ultravist 370) at 3 ml/sec. All patients were scanned from the
level of the thoracic inlet to the level of the mid-thigh region
using a nonelectrocardiographically gated helical mode of
acquisition with the following parameters: Gantry rotation time,
330 msec; beam collimation, 24×1.2 mm; tube voltage, 120 kVp;
reference tube current, 170 effective mAs with anatomic based tube
current modulation; beam pitch, 0.6. Images were reconstructed with
3-mm slice thickness.
Procedure
The operations were performed by interventional
radiologists in a dedicated endovascular suite with a fixed imaging
unit. The patients were maintained under local anesthesia in a
supine position. The contralateral FA was punctured using modified
Seldinger's technique, and selective angiography was performed by
insertion of a Cobra sheath into the common iliac artery or
external iliac artery, in order to identify the location and size
of the pseudoaneurysm. Furthermore, angiography was performed to
check for the inflow vessels and significant outflow vessels, and
the compression extent of adjacent structures was also evaluated.
The suitable site was confirmed following FA angiography in
multi-angle image. Under roadmap guidance, the sheath and guidewire
were introduced into the distal SFA, and the guidewire was then
exchanged with a 0.035-inch stiff guidewire using an angiographic
catheter. Finally, a 9 F introducer sheath (William Cook Europe,
Bjæverskov, Denmark) was placed over the stiff guidewire. Following
confirmation of pseudoaneurysm by the utilization of selective
catheters and angiograms, a self-expandable stent graft (Fluency
Plus Vascular Stent Graft; Bard GmbH, Karlsruhe, Germany) of a
suitable diameter and length was passed over the stiff guidewire
and deployed at the site with roadmap guidance, with attention not
to cover the orifice of the SFA. In some patients, minimal
extravasation from the upper and lower end of the stent segment was
revealed by control angiograms. Subsequently, the stent was
expanded by dilatation of a balloon catheter of the same diameter.
Finally, the control angiogram detected no signs of pseudoaneurysm
filling and the stent was patent; therefore, the sheath and the
wire were removed.
As study medication, all patients received 70 IU/kg
heparin during the procedure. Patients were discharged from the
hospital 2 or 3 days after the operation and were administered 100
mg aspirin and 75 mg Clopidogrel Hydrogen Sulphate Tablets once a
day for 1 month, in order to ensure stent patency.
The follow-up patient visits were scheduled at 1 and
9 months post-treatment, and included physical examination,
computed tomography angiography (CTA) examination and assessment of
the ankle-brachial index (ABI) (17).
Statistical analysis
The continuous variables of age and other baseline
conditions were expressed as the mean ± standard deviation. Other
discrete variables were summarized as count and percentage. The
results of ABI were compared using one-way analysis of variance
followed by the Student-Newman-Keuls test. P<0.05 was considered
to indicate a statistically significant difference. All analyses
were performed using SPSS software (v19.0; SPPS Inc., Chicago, IL,
USA).
Results
Radiological findings
Suspected Enterobacter aerogenes infection
with air in the peripheral soft tissue was detected in 5 cases
(17.24%), extensive pseudoaneurysm cavity thrombosis was detected
in 11 cases (37.93%) and arteriovenous fistula was present in 1
case (3.45%) (Table II). The mean
length and width of the pseudoaneurysms were 8.4±3.5 and 6.0±2.9
cm, respectively, and the mean entry tear size was 5.7±2.4 mm, as
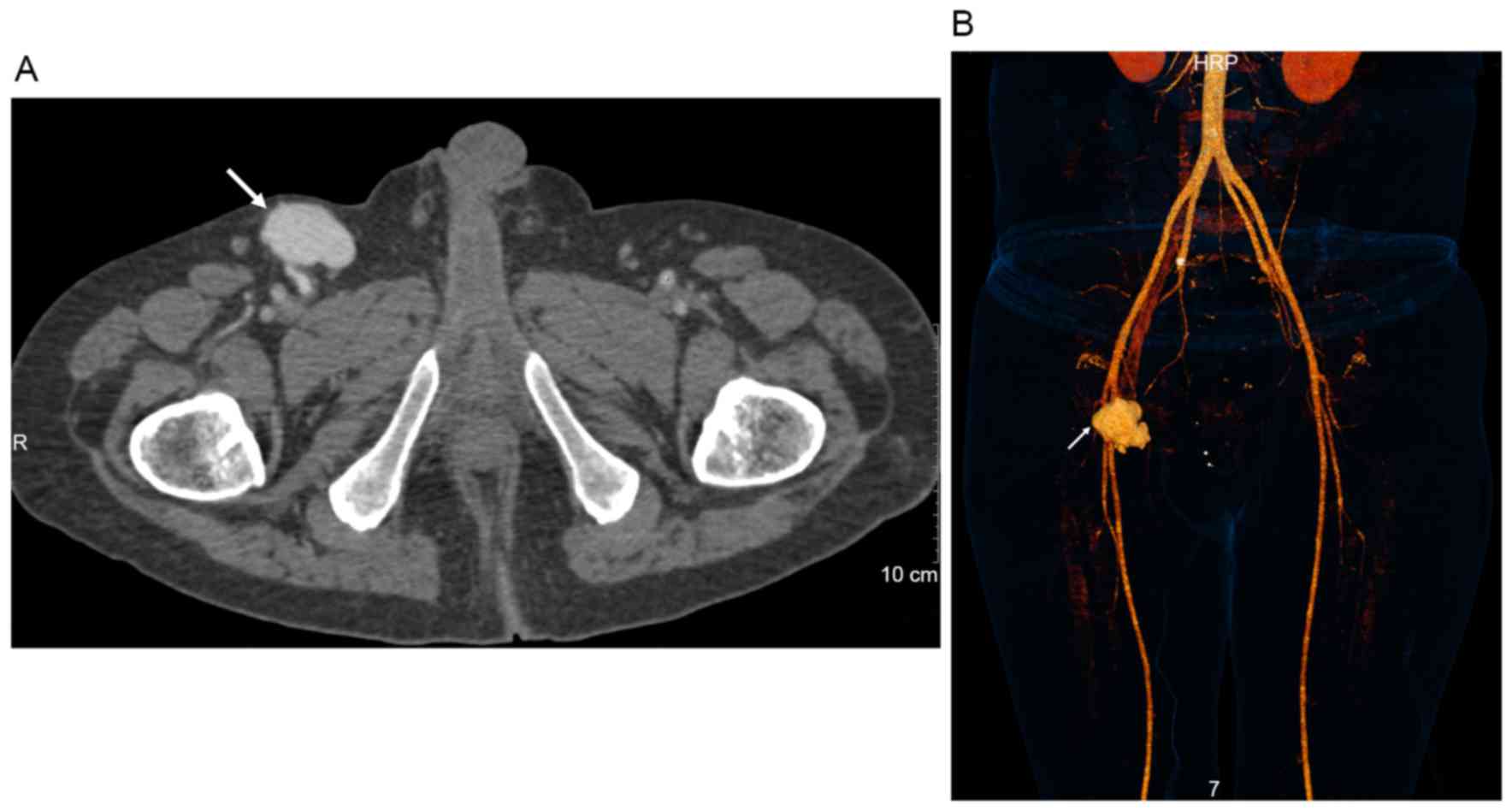
confirmed by sonography. Lower extremity CTA in the arterial and
venous phases demonstrated the presence of a pseudoaneurysm of the
upper SFA in all patients (Fig.
2). The pseudoaneurysm was spherical in shape. The
corresponding FAs in 29 cases were constricted, due to large
pseudoaneurysm compression.
 | Table II.Preoperational diagnostic
findings. |
Table II.
Preoperational diagnostic
findings.
| Radiological
finding | n (%) |
|---|
| Air in peripheral
soft tissue | 5 (17.24) |
| Thrombus in
pseudoaneurysm | 11 (37.93) |
| Arteriovenous
fistula | 1 (3.45) |
| Pseudoaneurysm
length (cm) | 8.4±3.5 |
| Pseudoaneurysm
width (cm) | 6.0±2.9 |
| Entry tear size
(mm) | 5.7±2.4 |
| Stenosis of femoral
artery caused by compression | 29 (100) |
Outcomes
Placement of the covered stents was technically
successful in all 29 patients. When a corresponding stent was
deployed to cover the entry tear of the pseudoaneurysm, abnormal
flow was restored immediately. All the ruptured pseudoaneurysms
were successfully sealed, with no cases of intraprocedural
mortality. Contrast medium extravasation into the lumen of the
pseudoaneurysm was detected in 16 cases (55.17%); however, the
extravasation was controlled immediately by distending the balloon
catheter. In addition, in 1 case, follow-up angiography was
performed 24 h after stent placement, in order to check the patency
of the FA due to soreness. As a result, stent thrombosis was
detected in this case, and blood flow was successfully
reestablished following thrombolytic therapy.
Furthermore, dorsalis pedis pulse was easily
palpated at the completion of the operation. Signs of active
hemorrhage subsided and the patients' condition improved rapidly;
the vascular bruit immediately disappeared and pain in the majority
of patients was obviously alleviated following placement of the
covered stent (Table III).
 | Table III.Outcomes of endovascular
treatment. |
Table III.
Outcomes of endovascular
treatment.
| Outcome | n (%) |
|---|
| Technical success
of stent deployment | 29 (100) |
| Outcomes of
intervention |
|
| Normal
dorsalis pedis pulse | 29 (100.00) |
| Active
hemorrhage | 0 (0.00) |
|
Vascular bruit | 0 (0.00) |
| Additional
treatmenta | 1 (3.45) |
| Stent length
(mm) | 43.75±14.08 |
| Stent diameter
(mm) | 7.25±1.39 |
| Operation time
(min) | 70±15 |
| Duration of
hospitalization (days) | 2±1.20 |
Based on the preoperative CTA measurement and the
arteriography confirmation of these findings, the chosen stent
diameter varied between 6 and 10 mm. The length of the stent ranged
between 40 and 80 mm (Table
III). The average operative time was 70±15 min (range, 45–90
min). Finally, all patients remained asymptomatic, apyrexial and
hemodynamically stable postoperation, and the average
hospitalization time was 2±1.20 days.
Follow-up
A total of 8 patients had to be excluded from
follow-up analysis due to major protocol violations: 5 patients
continued to inject at the stent site and 3 patients withdrew their
informed consent after discharge. The pulsatile mass in 29 cases
and pain in 13 cases in the inguinal region did not immediately
subside after treatment; however, these symptoms were alleviated
over time. At 1-month postoperation, pulsatile mass was still noted
in 18 cases (62.07%) and pain was still noted in 5 cases (17.24%).
After 9 months, no pulsatile mass or pain was detected (Table IV). In addition, stent thrombosis
was not detected. Furthermore, the patency rate of the stents was
100%, with no stent migration, restenosis, fracture, occlusion or
infections 9 months postoperation.
 | Table IV.Follow-up results in patients with
pseudoaneurysm in the femoral artery. |
Table IV.
Follow-up results in patients with
pseudoaneurysm in the femoral artery.
| Follow-up
result | Postoperation
(n=29) | Prior to hospital
discharge (n=29) | 1 month follow-up
(n=21) | 9 months follow-up
(n=21) |
|---|
| Pulsatile mass
(%) |
|
|
|
|
|
Decreased in size | 29 (100) | 29 (100) | 18 (85.71) | 0 (0) |
|
None | 0 (0) | 0 (0) | 3 (14.29) | 21 (100) |
| Pain (%) |
|
|
|
|
| Without
alleviation | 13 (44.83) | 6 (20.69) | 0 (0) | 0 (0) |
|
Alleviation | 16 (55.17) | 15 (51.72) | 5 (23.81) | 0 (0) |
| Without
pain | 0 (0) | 8 (27.59) | 16 (76.19) | 21 (100) |
| Stent thrombosis
(%) | 1 (3.45) | 0 (0) | 0 (0) | 0 (0) |
| Patency of distal
femoral artery (%) | 29 (100) | 29 (100) | 21 (100) | 21 (100) |
| ABI | 0.52±0.09 |
0.92±0.05a |
0.96±0.27a |
0.97±0.37a |
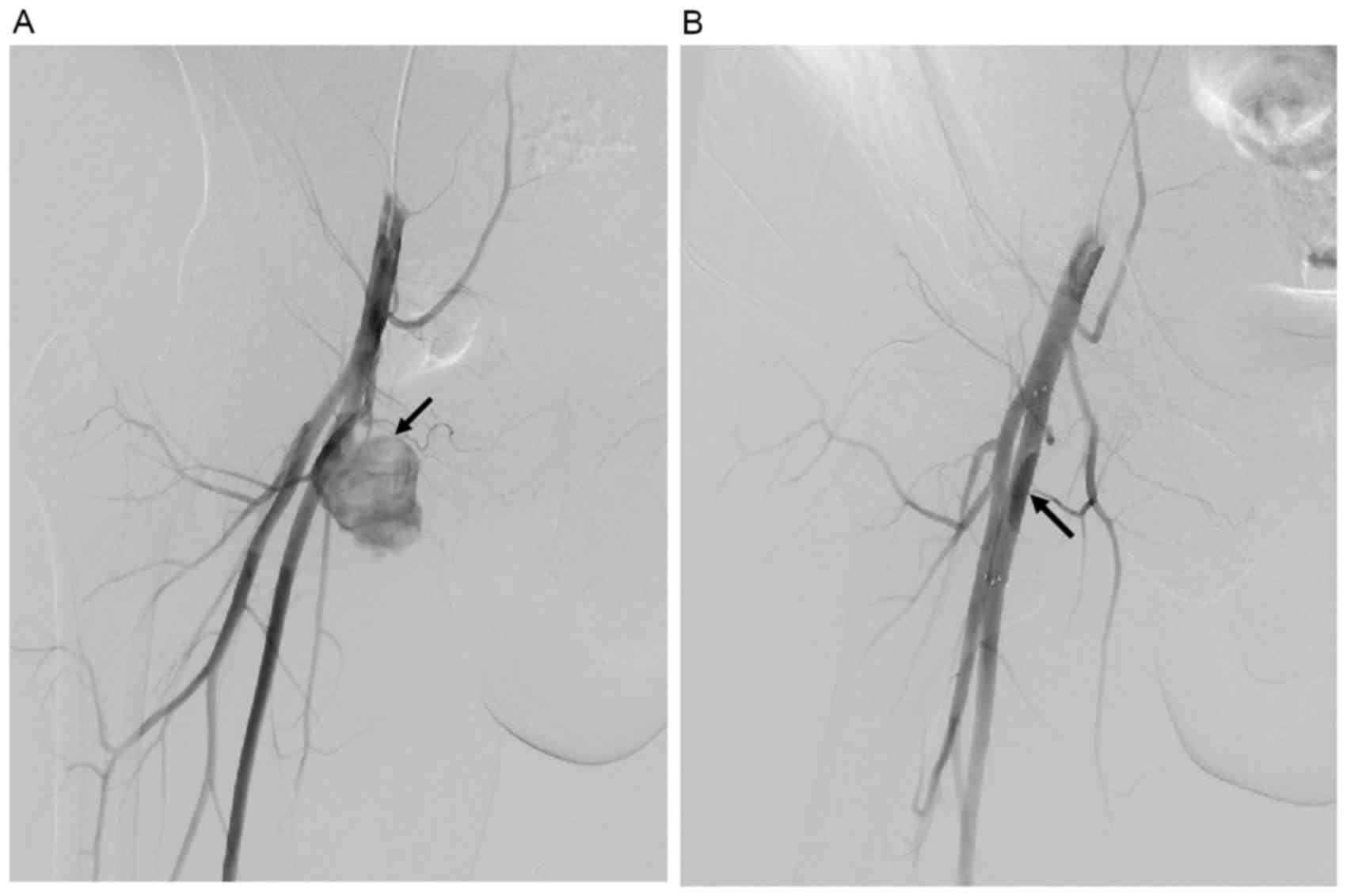
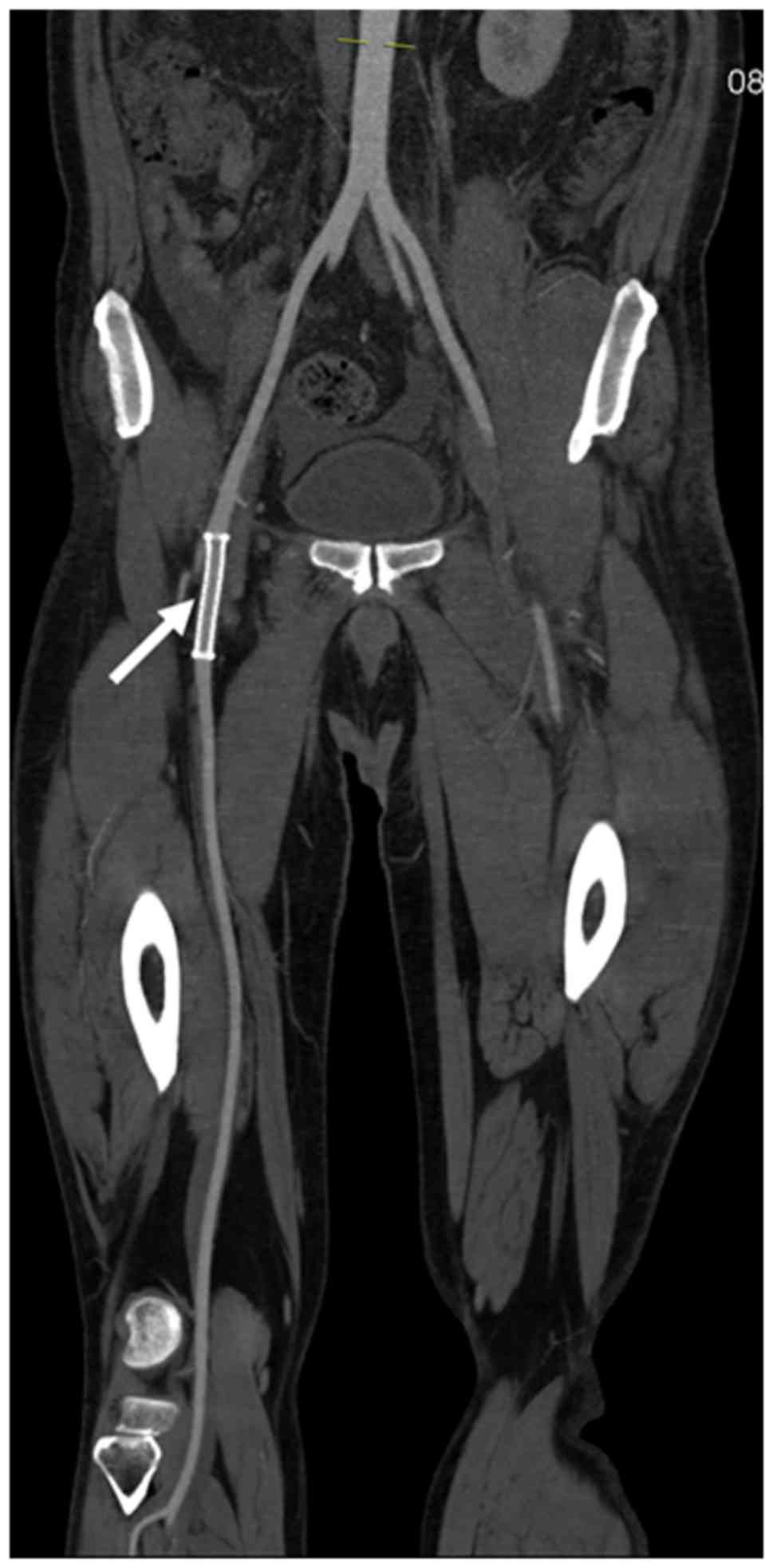
Clinical examinations and CTA performed at 1 and 9
months postoperation confirmed normal blood flow in the lower
extremity, which reflected the patency of the stent, and no blood
flow in the pseudoaneurysm was detected. Furthermore, no complaint
or complication was reported during follow-up (Figs. 3 and 4).
The ABI in these subjects increased from 0.52±0.09
immediately post-operation to 0.92±0.05 prior to discharge
(P<0.01). At 1 month follow-up the ABI was 0.96±0.27 and after 9
months it was 0.97±0.37. ABI values at discharge, and 1 and 9
months postoperation were all significantly increased compared with
ABI post-operation; however, there was no significant difference
between these three time points (Table IV).
Discussion
The results of the present study validated that
endovascular repair using a covered stent may be considered a
promising therapeutic option for the treatment of SFA
pseudoaneurysm in drug abusers. In the present study, 29 drug abuse
patients with SFA pseudoaneurysms were successfully treated with
covered stents. In addition, the follow-up data indicated promising
short-term outcomes.
FA pseudoaneurysm represents a common complication
following FA overuse in diagnostic, therapeutic and parenteral drug
use applications (18–20). Pseudoaneurysm rupture in drug
abusers is a limb- and life-threatening condition, which
necessitates emergency operation. Prior to therapy, radiological
examinations should be performed in order to make accurate
diagnoses (21). Color duplex
ultrasonography is an easy, non-invasive diagnostic method that can
be used to detect pseudoaneurysms and obtain essential information
concerning the size and length of the cavity, the presence of a
thrombus, the diameter of the neck of a pseudoaneurysm and the
possible compression on surrounding tissues (22). However, confusing images may be
obtained due to the complex anatomic configuration of this area.
Therefore, in the present study, a conclusive and precise CT
angiogram was performed for pretreatment evaluation as well as
postoperative assessment.
During therapy, hemorrhage-associated complications
caused by open surgical repair and invasive traditional treatment
may increase the likelihood of mortality (up to 7.5% within 1 year)
(23). Furthermore, surgical
treatment for pseudoaneurysm is not recommended in patients with
ischemia of surrounding tissues, caused by vascular compromise,
nerve compression, deep venous thrombosis, bleeding or infection of
the pseudoaneurysm. Although ultrasound-guided compression is
highly efficient, it is only applicable for pseudoaneurysms with a
narrow neck, which are slow growing, small in size (<6 cm in
diameter) and located below the inguinal ligament (24). In the present study, all
pseudoaneurysms were bleeding and large in size (>6 cm in
diameter); therefore, endovascular repair using a covered stent was
the primary therapeutic option (25–27).
Interventional stenting is an alternative to open
surgery, which has been reported to demonstrate an improvement over
percutaneous transluminal angioplasty alone for the treatment of
aortoiliac and femoral occlusive disease since 1969 (28). Compared with angioglasty, stent
placement yields similar complication rates; however, the technical
success rate of stenting is often higher and the risk of long-term
failure may be reduced (29–31).
Therefore, stenting is considered an established therapeutic
modality for the treatment of iliac artery stenosis and occlusion.
Stents can prevent late vessel remodeling by mechanically
scaffolding the vessel wall. By testing various types of stents,
uncovered stents may secure intimal flaps and seal the dissected
vessel wall, and can treat acute or threatened vessel closure
following unsuccessful balloon angioplasty. However, long-term
results in large lesions may be compromised by restenosis. Despite
this, covered stents lined with expanded polytetrafluoroethylene
have the potential to overcome this limitation by providing a
barrier against neointimal hyperplasia, thus potentially increasing
stent patency (32).
Due to the non-invasive nature of the treatment
described in the present study, the mean operation time was 70±15
min, and the hospitalization time was 2±1.32 days due to the lower
rate of wound and infection. The durations of the operation and
hospitalization associated with covered stent placement were
significantly shorter compared with those associated with surgical
operation (3±0.2 h and 15.9±14.7 days, respectively) (33). Furthermore, such a minimally
invasive therapeutic approach avoids the physiological burden of
surgical repair and its associated risks, particularly in
cachectic, immunocompromised patients with poor general health and
nutritional status (12).
Furthermore, due to lack of associated restenosis and reduced wound
rate, ABI values were markedly increased during the follow-up. In a
previous study, 9 months postoperation, ABI values were stable, and
no significant differences were reported compared with in normal
legs (34).
The primary aim of treating ruptured pseudoaneurysms
is to reduce the risk of mortality and reserve lower extremity
functionality, particularly in young patients who have no atheroma
and therefore no collateral vessels (33). According to clinical experience, FA
reservation is a prerequisite to blood flow to the lower extremity.
Covered stent implantation excludes the aneurysm sac from the
circulation while preserving sufficient blood flow to distal
organs. Furthermore, the stents were implanted away from the origin
of SFA with great caution in case of subsequent ischemia of the
lower extremity. Therefore, the length of the covered stent should
be relatively short, in order to reduce the influence of hip joint
motion and lessen thrombotic formation. However, internal leakage
can be caused if the stent is too short. Combining our clinical
experience with the findings of previous study (35), the diameter of the stent used in
the present study depended on, and was slightly larger than, the
diameter of the native artery (10–20% larger). The length of the
stent was longer than the injured artery, and the margins were no
more than 1 cm into the superior and inferior segment of the normal
artery (35).
Although endovascular repair of a pseudoaneurysm
using a covered stent exhibited promising results, there remain
some limitations to the present study. Firstly, the present study
is a single-center study, which requires confirmation in larger
multicenter studies. Secondly, all patients in the present study
were drug abusers with traumatic pseudoaneurysms that were admitted
to the emergency department; therefore, preoperation clinical data
were incomplete and the type of patients was limited. In addition,
due to the low compliance of drug abusers, the follow-up only
reached 9 months. Finally, the application of covered stents was
limited by the disadvantages of the stent itself and the location
of the lesion. For example, the FA originates close to the inguinal
ligament and repetitive hip flexion during ambulation may result in
stent compression, and consequently lead to poor blood flow of the
deep FA. Therefore, during the operation, stents should be
delivered in an appropriate place so that the proximal FA entry
tear is covered by the middle of the stent as soon as possible. The
long-term clinical efficacy and safety of this technique, as well
as its applicability in other types of pseudoaneurysm, still
require investigation.
In conclusion, although further clinical trials are
required, these findings are encouraging, and the results indicated
that covered stents may be an effective, safe and minimally
invasive option for the treatment of SFA pseudoaneurysms.
Acknowledgements
The authors of the present study would like to thank
Dr Hua He, Dr Wenlong Zhang and Dr Xiaobin Yang (Xijing Hospital,
Xi'an, China) for acquisition of image data and thoughtful
suggestions.
Glossary
Abbreviations
Abbreviations:
|
ABI
|
ankle-brachial index
|
|
CTA
|
computed tomography angiography
|
|
FA
|
femoral artery
|
|
MDCT
|
multidetector computed tomographic
|
|
SFA
|
superficial femoral artery
|
References
|
1
|
Siani A, Flaishman I, Siani LM, Mounayergi
F, Zaccaria A, Schioppa A and Baldassarre E: Spontaneous rupture of
the superficial femoral artery treated via an endovascular
approach. Tex Heart Inst J. 35:66–68. 2008.PubMed/NCBI
|
|
2
|
Dhillon MS, McCafferty I, Davies AM and
Tillman RM: Intra-osseous pseudoaneurysm following curettage of an
aneurysmal bone cyst. Skeletal Radiol. 36 Suppl 1:S46–S49. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Drug Report 2010. United Nations
Office on Drugs and Crime (UNODC). United Nations Publication Sales
No.E.10.XI.13.
|
|
4
|
Tsao JW, Marder SR, Goldstone J and Bloon
AI: Presentation, diagnosis, and management of arterial mycotic
pseudoaneurysms in injection drug users. Ann Vasc Surg. 16:652–662.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karkos CD, Kalogirou TE, Giaqtzidis IT and
Papazoglou KO: Ruptured mycotic common femoral artery
pseudoaneurysm: Fatal pulmonary embolism after emergency
stent-grafting in a drug abuser. Tex Heart Inst J. 41:634–637.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imsand D and Hayoz D: Current treatment
options of femoral pseudoaneurysms. Vasa. 36:91–95. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kronzon I: Diagnosis and treatment of
iatrogenic femoral artery pseudoaneurysm: A review. J Am Soc
Echocardiogr. 10:236–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin J, Huang L, Li AM, Song YM, Jin J, Yu
XJ, Zhou XB, Lin CM, Gao YH, et al: Comparison of ultrasound-guided
thrombin injection and compression repair in treatment of
iatrogenic femoral arterial pseudoaneurysms. J Med Coll PLA.
21:261–267. 2006.
|
|
9
|
Lönn L, Olmarker A, Geterud K and Risberg
B: Prospective randomized study comparing ultrasound-guided
thrombin injection to compressionin the treatment of femoral
pseudoaneurysms. J Endovasc Ther. 11:570–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Ayala M, Smith R, Zanieski G, Fahoum B
and Tortólani AJ: Acute arterial occlusion after ultrasound-guided
thrombin injection of a common femoral artery pseudoaneurysm with a
wide, short neck. Ann Vasc Surg. 22:473–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmad F, Turner SA, Torrie P and Gibson M:
Iatrogenic femoral artery pseudoaneurysms: A review of current
methods of diagnosis and treatment. Clin Radiol. 63:1310–1316.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antoniou GA, Papas TT, Tsagkos I,
Trachanellis S, Antoniou SA, Tsanis A and Bessias N: Endovascular
stent-graft repair of bleeding common femoral artery pseudoaneurysm
in intravenous drug users: A bridge to surgical reconstruction.
Vasa. 43:473–476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karkos CD, Kalogirou TE, Giagtzidis IT and
Papazoglou KO: Ruptured mycotic common femoral artery
pseudoaneurysm: Fatal pulmonary embolism after emergency
stent-grafting in a drug abuser. Tex Heart Inst J. 41:634–637.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGraw JK, Patzik SB, Gale SS, Dodd JT and
Boorstein JM: Autogenous vein-covered stent for the endovascular
management of a superior mesenteric artery pseudoaneurysm. J Vasc
Interv Radiol. 9:779–782. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramus JR, Gibson M, Magee T and Torrie P:
Spontaneous rupture of the superficial femoral artery treated with
endovascular stent grafting. Cardiovasc Intervent Radiol.
30:1016–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samara O, Saleh AI, Alomari A, AI Ryalat
N, Hadidy A and Alsmady M: Giant spontaneous femoral artery
pseudoaneurysm treated with covered stents: Report of a rare
presentation and review of literature. Sultan Qaboos Univ Med J.
13:E472–E475. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lammer J, Zeller T, Hausegger KA, Schaefer
PJ, Gschewendtner M, Muller-Huelsbeck S, Rand T, Funovics M, Wolf
F, Rastan A, et al: Heparin-bonded covered stents versus bare-metal
stents for complex femoropopliteal artery lesions: The randomized
VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive
surface [VIA] versus bare nitinol stent in the treatment of long
lesions in superficial femoral artery occlusion disease). J Am Coll
Cardiol. 62:1320–1327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alsmady M, Abdallah F, Shanti H and Samara
O: Spontaneous femoral artery pseudoaneurysm in a young patient. J
Surg Case Rep. 2012:182012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McIlroy MA, Reddy D, Markowitz N and
Saravolatz LD: Infected false aneurysms of the femoral artery in
intravenous drug addicts. Rev Infect Dis. 11:578–585. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ting AC and Cheng SW: Femoral
pseudoaneurysms in drug addicts. World J Surg. 21:783–787. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Starnes BW and Arthurs ZM: Endovascular
management of vascular trauma. Perspect Vasc Surg Endovasc Ther.
18:114–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Posner SR, Wilensky J, Dimick J and Henke
PK: A true aneurysm of the profundafemoris artery: A case report
and review of the English language literature. Ann Vasc Surg.
18:740–746. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dzijan-Horn M, Langwieser N, Groha P,
Bradaric C, Linhardt M, Böttiger C, Byrne RA, Steppich B, Koppara
T, Gödel J, et al: Safety and efficacy of a potential treatment
algorithm by using manual compression repair and ultrasound-guided
thrombin injection for the management of iatrogenic femoral artery
pseudoaneurysm in a large patient cohort. Circ Cardiovasc Interv.
7:207–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Latic A, Delibegovic M, Pudic I, Latic F,
Samardzic J and Karmela R: Non-invasive ultrasound guided
compression repair of post puncture femoral pseudoaneurysm. Med
Arh. 65:113–114. 2011.PubMed/NCBI
|
|
25
|
Riesenman PJ, Farber MA, Rich PB, Sheridan
BC, Mendes RR, Marston WA and Keagy BA: Outcomes of surgical and
endovascular treatment of acute traumatic thoracic aortic injury. J
Vasc Surg. 46:934–940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ott MC, Stewart TC, Lawlor DK, Gray DK and
Forbes TL: Management of blunt thoracic aortic injuries:
Endovascular stents versus open repair. J Trauma. 56:565–570. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kasarajan K, Heffernan D and Langsfeld M:
Acute thoracic aortic trauma: A comparison of endoluminal stent
grafts with open repair and nonoperative management. Ann Vasc Surg.
17:589–595. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blum U, Gabelmann A, Redecker M, Nöldge G,
Dornberg W, Grosser G, Heiss W and Langer M: Percutaneous
recanalization of iliac artery occlusions: Results of a prospective
study. Radiology. 189:536–540. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puech-Leão P, Wolosker N, Zerati AE and
Nascimento LD: Impact of endovascular technique in vascular surgery
training at a large university hospital in Brazil. J Surg Educ.
68:19–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wolosker N, Mendes Cde A, Jacob CE,
Woloker AM and Puech Leão P: Endovascular infrarenal aortic
aneurysm repair combined with laparoscopic cholecystectomy. Clinics
(Sao Paulo). 65:743–744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferreira J, Canedo A, Brandão D, Maia M,
Braga S, Chaparro M, Barreto P and Vaz G: Isolated iliac artery
aneurysms: Six-year experience. Interact Cardiovasc Thorac Surg.
10:245–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Virmani R, Kolodgie FD, Dake MD, Silver
JH, Jones RM, Jenkins M and Gillespie DL: Histopathologic
evaluation of an expanded polytetrafluoroethylene-nitinol stent
endoprosthesis in canine iliofemoral arteries. J Vasc Interv
Radiol. 10:445–456. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Devecioglu M, Settembre N, Samia Z,
Elfarra M and Malikov S: Treatment of arterial lesion in drug
addicts. Ann Vasc Surg. 28:184–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duda SH, Bosiers M, Pusich B, Hüttl K,
Oliva V, Müller-Hülsbeck S, Bray A, Luz O, Remy C, Hak JB and
Beregi JP: Endovascular treatment of peripheral artery disease with
expanded PTFE-covered nitinol stents: Interim analysis from a
prospective controlled study. Cardiovasc Intervent Radiol.
25:413–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Z, Zhao L, Wang K, Cheng J, Zhao Y and
Ren W: Characteristics and treatment of vascular injuries: A review
of 387 cases at a Chinese center. Int J Clin Exp Med. 7:4710–4719.
2014.PubMed/NCBI
|