Introduction
Combined neuraxial and general anesthesia markedly
reduces the sedative/anesthetic requirements of general anesthetics
(1–3). Similar sensorial levels with
neuraxial anesthesia reduce the need for propofol in similar
amounts (4), and the high spinal
blocking is associated with increased sedation (5). Previous studies have indicated that
the sensorial levels of epidural and spinal anesthesia serve a key
role in de-afferentation-dependent sedation. These findings have
been verified by further studies, demonstrating that neuraxial
anesthesia affects the degree of subcortical arousal via peripheral
de-afferentation (6,7). This may occur principally through the
ablation of ascending nociceptive information transmission, which
reduces arousal from surgical stimuli. A second subtle mechanism of
neuraxial anesthesia may involve the blockade of motor and sensory
activities, leading to reduced arousal even in the absence of any
noxious stimuli (8–10).
Local anesthetics (LAs) including bupivacaine may
not only block sodium channels, however may also affect synaptic
transmission, modulate neurotransmitter release and interact with
numerous other membrane proteins (11–13).
These actions of LAs suggest that they may exert effects on
glutamatergic synaptic transmission (11). Although sensory de-afferentation in
the spinal cord may explain the sedative effect of neuraxial
anesthesia, the neurochemical basis of de-afferentation-dependent
sedation remains unknown. There is a lack of data on the
neurochemical alterations that occur in the spinal cord following
subarachnoid blockade with bupivacaine, particularly regarding
synaptic transmission, which may be important for
de-afferentation-dependent sedation during spinal anesthesia
(14). In the transmission of
somatosensory activity from the periphery to the brain, the
N-methyl-D-aspartate (NMDA) subtype of glutamate receptors is
considered to be essential (15,16).
In the present study, subarachnoid bupivacaine
blockade was hypothesized to produce a sensory de-afferentation by
downregulating the N-methyl-D-aspartate receptor 2B
subunit/calcium-calmodulin-dependent protein kinase II α/cAMP
response element-binding protein (NR2B/CaMKIIα/CREB) excitatory
signaling pathway in the spinal cord. To test this hypothesis, the
effect of intrathecal bupivacaine at clinically relevant
concentrations was investigated on the expression levels of NR2B,
CaMKIIα/p-CaMKIIα, and CREB/p-CREB in rat lumbar spinal cord
samples. The NR2B subunit of the NMDA receptor was investigated as
it is considered to be specifically required for nociceptive
afferents (16).
Materials and methods
Animals
The present study was approved by the Ethics
Committee of Ningxia Medical University (Yinchuan, China). A total
of 47 adult male Sprague-Dawley (SD) rats (weight, 280–320 g; age,
7–8 weeks) were obtained from the Animal Center of Ningxia Medical
University. All of the procedures were performed in accordance with
the National Institute of Health guidelines on animal care. Rats
were housed in separate plastic cages with unlimited access to
water and food, and kept in temperature-controlled rooms (20–24°C,
relative humidity 50–60%) under a 12/12 h light/dark cycle (with
the dark cycle beginning at 7:00 p.m.). All experiments were
conducted during light hours.
Catheter modification and intrathecal
catheter placement
Rats were allowed to acclimate to the laboratory
environment for 3 days. A rat model of intrathecal catheterization
was established as previously described (17). To construct the intrathecal
catheter, sections of polyethylene tubing of sizes PE-20 (8 cm) and
PE-10 (2 cm) were connected to each other to produce a catheter
with a decreasing diameter profile. Animals were anesthetized with
an intraperitoneal (i.p.) injection of pentobarbital sodium at a
dose of 60 mg kg−1. A longitudinal 1.5-cm incision was
made in the lumbar L4-L5 vertebra of each rat
placed in a prone position. One end of the small profiled catheter
was inserted into the subarachnoid space through the incision,
while the other was tunneled subcutaneously toward the occiput,
exposing the distal tip out of the neck skin. Cefazolin sodium (100
mg) was injected intramuscularly to prevent infection. Following 24
h of catheterization, all rats with any sign of neurological
deficit, infection, catheter displacement, or clogging, and those
without bilateral lower extremity paralysis following intrathecal
injection of 15 µl 2% lidocaine (Yinhu Shiyao Pharmaceutical Co,
Ltd., Yuncheng, Shanxi, China) within 5 min, were excluded from the
study.
Treatment groups
Following catheterization, 36 male SD rats were
included and assigned to a normal saline (NS) group (n=18) or a
bupivacaine (Bup) group (n=18). Each rat was intrathecally
administered once with 20 µl saline solution or 0.5% bupivacaine
(Shanghai Zhaohui Pharmaceutical Co, Ltd., Shanghai, China) and
injected for 10 sec. From each group, samples from six rats were
used for western blotting, samples from another six rats were used
for reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), and samples from the remaining six rats were used for
immunohistochemical (IHC) analysis.
Western blot analysis
Following 10 min of intrathecal administration of
bupivacaine or saline, the rats were sacrificed by spinal
dislocation. The lumbar enlargement of the spinalrd, which was
estimated to be in the L1-L5 region (18), was collected and homogenized in
lysis buffer (Nanjing KeyGEN Biotech, Co., Ltd., Nanjing, China).
The homogenate was centrifuged at 4°C and 14,000 × g for 15 min.
The supernatant (50 µg per sample, as quantified by a bicinchoninic
acid protein assay kit (Nanjing KeyGEN Biotech, Co., Ltd.) was
separated on 8% SDS-PAGE and transferred onto nitrocellulose
membranes (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Following incubation in 5% non-fat milk for 2 h at room
temperature, membranes were incubated with primary antibodies
against GAPDH (10494–1-AP; 1:2,000; ProteinTech Group, Inc.,
Chicago, IL, USA), NR2B (21920–1-AP; 1:1,000; ProteinTech Group,
Inc.), CaMKIIα (13730–1-AP; 1:1,000; ProteinTech Group, Inc.), CREB
(9197; 1:900; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated (p)-CaMKIIα (11278; 1:700; Signal-Aldrich; Merck
KGaA, Darmstadt, Germany), and p-CREB (9198; 1:600; Cell Signaling
Technology, Inc., USA) at 4°C overnight. Following washing, the
membranes were incubated at room temperature for 2 h with secondary
antibody (goat anti-rabbit IgG, SA00001-2; 1:2,000; ProteinTech
Group, Inc.). Following washing with TBS with Tween-20, blots were
visualized with an enhanced chemiluminescence kit (32109; Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and subsequently
analyzed densitometrically with a western blotting detection system
(Quantity One software version 4.6.2; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Results were normalized to the protein level of
GAPDH in each sample.
Quantification of the spinal cord mRNA
expression levels
RT-qPCR analysis was conducted following a
previously described method (19).
Total RNA from the spinal cord (L1-L5) tissue
was isolated with a Total RNA Miniprep Kit (Axygen A; Corning
Incorporated, Corning, NY, USA) and reverse transcribed into cDNA
(TransGen Biotech, China). Primer sequences used for PCR are
presented in Table I. PCR was
performed in a Light Cycler system (qTOWER 2.0; Analytic Jena AG,
Jena, Germany), using SYBR Green I dye (TransGen Biotech, Beijing,
China) to detect the mRNA expression levels of NR2B, CaMKIIα, CREB,
and GAPDH. The cDNA products (3.5 µl), 10 µM forward primer (1 µl),
10 µM reverse primer (1 µl), and 2XTransStart Top Green qPCR
SuperMix (12.5 µl) were mixed and added with double distilled water
to obtain a total reaction volume of 25 µl. The reaction conditions
were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 30 sec at
94°C and 30 sec at 60°C. Data were analyzed by the
2−ΔΔCq method, using GAPDH as a reference gene (20).
 | Table I.Primer sequences and annealing
temperatures. |
Table I.
Primer sequences and annealing
temperatures.
| Primer | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) | Annealing
temperature (°C) |
|---|
| GAPDH |
ACAGCAACAGGGTGGTGGAC |
TTTGAGGGTGCAGCGAACTT | 59 |
| NR2B |
CCTTCCTGCCAGAGTGAGAG |
CCTCTTCTCGTGGGTGTTGT | 60 |
| CaMKII |
CTGAACCCTCACATCCACCT |
ACACGGGTCTCCTCTGACTG | 59 |
| CREB |
CATGGACTCTGGAGCAGACA |
CTGGGCTAATGTGGCAATCT | 57 |
IHC analysis
Under deep anesthesia with sodium pentobarbital at a
dose of 60 mg kg−1, six male SD rats from each
experimental group were transcardially perfused with 500 ml NS,
followed by 500 ml 4% paraformaldehyde in PBS. The spinal cord was
removed and post-fixed in 10% formalin solution for 12 h at 4°C.
All samples were embedded in paraffin and transversely cut into
4-µm thick sections with a sliding microtome. Following
deparaffinization and hydration, sections were blocked in 3%
H2O2 for 10 min at room temperature, and were
incubated with primary antibodies, including anti-NR2B (ab216621;
1:250; Abcam, Cambridge, UK), anti-p-CaMKIIα (ab5683; 1:300;
Abcam), and anti-p-CREB (9198; 1:700; Cell Signaling Technology,
Inc.), for 2 h at 37°C. Slides were washed with 0.1 M PBS 3 times
for 2 min and subsequently incubated with a goat anti-rabbit
secondary antibody (PV-9001; 1:500; ZSGB-BIO; OriGene Technologies,
Inc., Rockville, MD, USA) for 30 min at 37°C, then stained with
diaminobenzidine (DAB kit; ZSGB-BIO; OriGene Technologies, Inc.)
and counterstained with hematoxylin for 30 sec at room temperature.
Images were captured with a light microscope (Olympus Corporation,
Tokyo, Japan). Quantitative image analysis of the relative optical
density (OD) was performed using Image Pro Plus software version
6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All analyses were performed with SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). All parametric data
were presented as the mean ± standard deviation. Comparisons
between the two groups were performed with independent samples
t-tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Of the 47 SD rats intrathecally catheterized, 36
were included in the experiments. Of the rats excluded, five
exhibited a neurological deficit and six exhibited failed
catheterization.
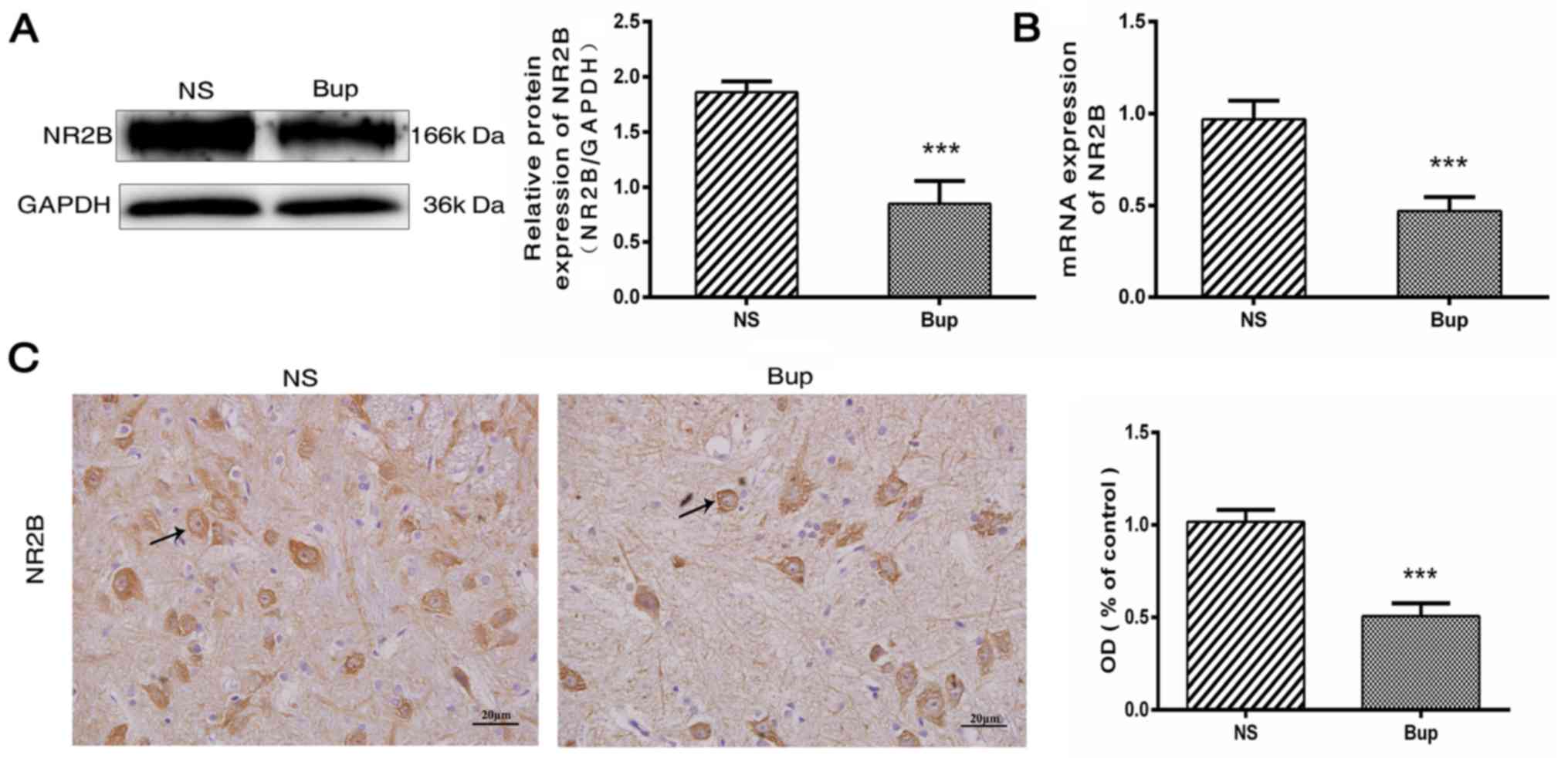
Intrathecal bupivacaine decreases NR2B
expression in the spinal cord
Following the intrathecal administration of
bupivacaine, protein and mRNA expression levels of NR2B were
decreased by ~54 and 51%, respectively, compared with the NS rat
group (Fig. 1A and B; P<0.001).
This was further verified by IHC analysis, which demonstrated that
the NR2B protein expression was decreased by ~50% in the spinal
cord tissues following bupivacaine treatment (Fig. 1C; P<0.001). Together these
observations indicated that the decreased expression of NR2B is
associated with subarachnoid bupivacaine blockade.
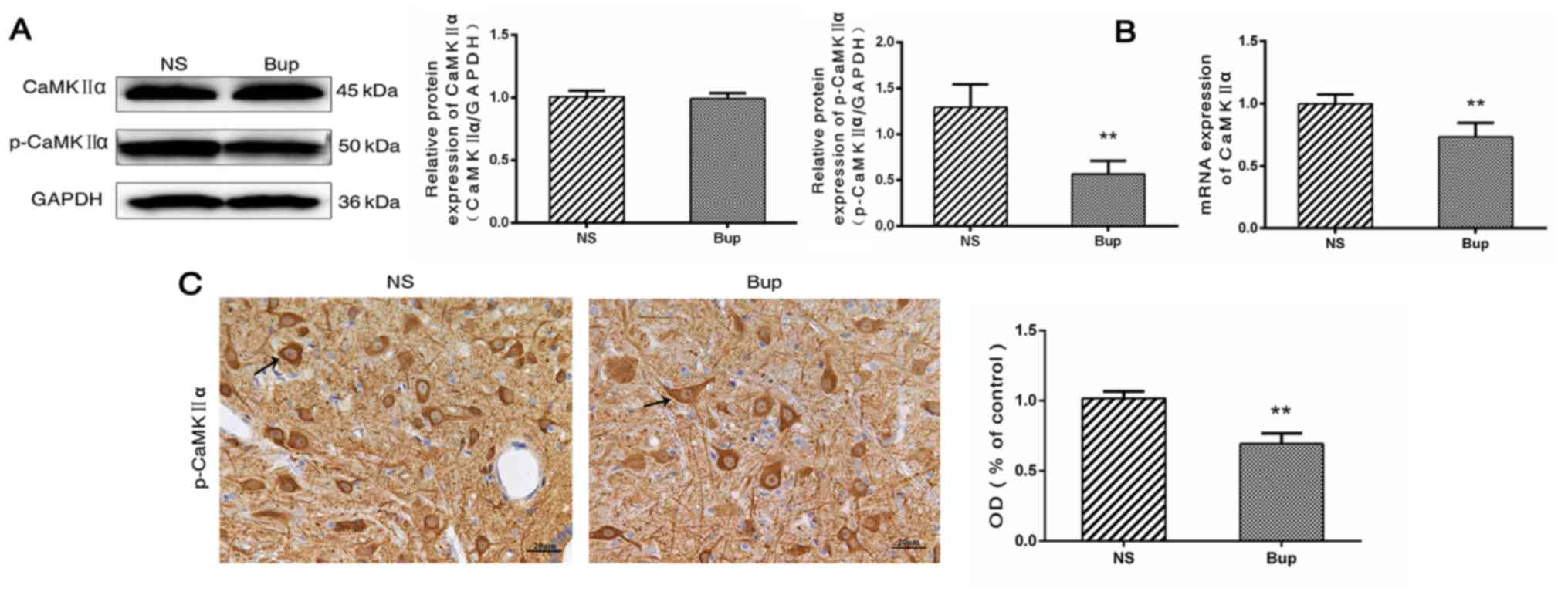
Effect of intrathecal bupivacaine on
CaMKIIα and p-CaMKIIα levels in the spinal cord
Western blotting revealed that the protein
expression level of total CaMKIIα in the spinal cord did not differ
between the Bup and NS groups (Fig.
2A; P>0.05). Compared with the NS group, the mRNA level of
CaMKIIα was reduced by ~36% in the Bup group (Fig. 2B; P<0.01). Western blotting and
IHC indicated that the protein expression levels of p-CaMKIIα were
decreased by ~56 and 32%, respectively in the Bup group compared
with the NS group (Fig. 2A and C;
P<0.01).
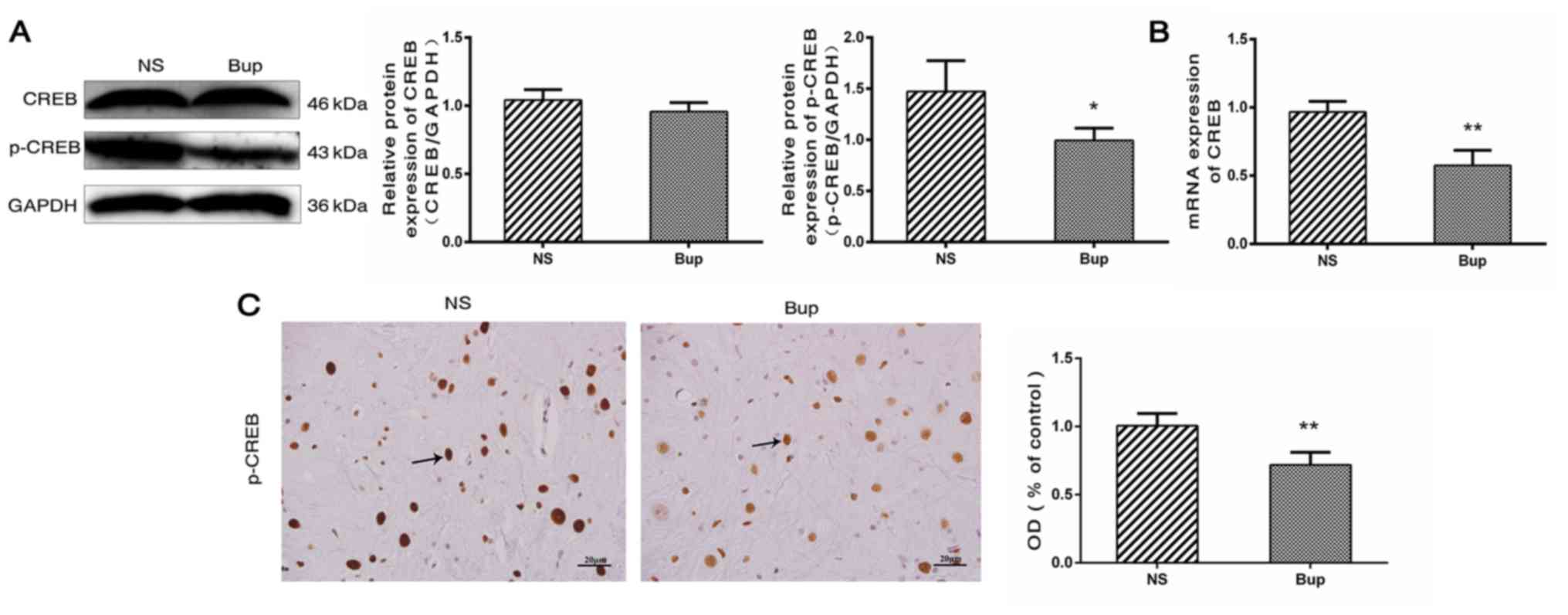
Effect of intrathecal bupivacaine on
CREB and p-CREB levels in the spinal cord
Based on the results of western blot analysis, the
alterations in the expression of CREB in the spinal cord were
similar to those of CaMKIIα (Fig.
3A; P>0.05). Compared with the NS group, mRNA levels of CREB
were reduced by ~41% in the Bup group (Fig. 3B; P<0.01). The protein
expression level of p-CREB was indicated to be decreased by ~33 and
28% by western blotting and IHC, respectively in the Bup group,
compared with the NS group (Fig. 3A
and C; P<0.05 and P<0.01).
Discussion
Sedation during neuraxial blockade was highlighted
in 1994 when Tverskoy et al (21) reported that subarachnoid blockade
with bupivacaine reduces the hypnotic requirements of midazolam and
thiopental. In animal (22) and
clinical studies (23,24), these findings were verified, and
neuraxial blocking was reported to elicit sedative effects. While
the neurochemical mechanism underlying the reduced requirement for
general anesthetic remains poorly understood at the spinal level,
the systemic general anesthetic effects of absorbed local
anesthetics (25) and
de-afferentation (26) have been
revealed to be mechanisms that may explain the interaction between
local and general anesthetics. The afferentation theory suggests
that tonic sensory and muscle-spindle activities maintain a state
of wakefulness (26,27). For de-afferentation, neuraxial
anesthesia reduces the spinal afferent input and thus affects the
level of consciousness, enabling decreases in subsequent doses of
the agent to achieve a defined level of sedation (27). The most speculated mechanism for
sedation during neuraxial anesthesia is the de-afferentation
phenomenon, which affects sensitivity to sedative/anesthetic drugs.
Additionally, afferent somatosensory information, particularly
nociceptive information, considerably affects brain activity
(28,29). Complete spinal cord transection
immediately slows down cortical electroencephalogram activity
(30,31). Similarly, the degree of subcortical
arousal is significantly affected when spinal and epidural blocking
reduce or prevent afferent inputs from the blocked region of the
body to the brain. However, the potential neurochemical mechanisms
involved remain unknown.
The effect of subarachnoid bupivacaine blockade on
the NMDA receptor subunit NR2B and its associated signal
transduction pathway were investigated in the lumbar spinal cord of
rats, using bupivacaine at clinically relevant concentrations.
NR2B, p-CaMKIIα, and p-CREB were considerably downregulated
following intrathecal administration of bupivacaine. Similar
alterations in expression were observed by IHC analysis. Based on
the current findings, it may be suggested that the sedative effect
of subarachnoid bupivacaine blockade is associated with a decrease
in spinal afferent input, possibly due to the downregulation of
NR2B, p-CaMKIIα, and p-CREB expression levels in the lumbar spinal
cord.
Glutamate acts as an excitatory neurotransmitter in
primary afferent terminals and serves a critical role in spinal
synaptic transmission through its activation of glutamate receptors
(32). NMDA receptors are
glutamate receptors in the central nervous system. The binding of
glutamate to the NMDA receptor results in an influx of
extracellular Ca2+, which controls membrane excitability
and synaptic transmission (33).
CaMKIIα is a multifunctional serine/threonine protein kinase, which
is activated when cytosolic Ca2+ increases and in the
presence of peripheral pain stimuli, CaMKIIα is phosphorylated at
Thr286 (p-CaMKIIα) in the spinal cord (34). The p-CaMKIIα then translocates to
the nucleus where it phosphorylates CREB at Ser133 in the dorsal
horn of the spinal cord. Subsequently, p-CREB initiates gene
transcription and translation and is thus possibly involved in the
ascending transmission of nociceptive information. Peripheral
nociceptive information, including visceral, inflammatory, and
neuropathic pain, may activate NR2B, p-CaMKIIα, and p-CREB and may
facilitate nociceptive transmission in the spinal cord. Therefore,
reduced expression of NR2B, p-CaMKIIα, and p-CREB may suppress
various types of hyperalgesia, including inflammatory pain
(35,36), neuropathic pain (36–39),
opioid-induced hyperalgesia (40),
visceral pain (41–43), and central pain (44–46).
A key step in the transmission of nociceptive information from the
spinal cord to the brain is the activation of NMDA receptors in the
spinal dorsal horn neurons (47).
This suggests that ascending nociceptive transmission may be
reduced by downregulating NMDA receptor expression and the
associated signal transduction pathways. Furthermore, bupivacaine
inhibits NMDA-induced glutamatergic transmission in rat dorsal horn
neurons (48,49). This result is concordant with the
present results. In the present study, spinally administered
bupivacaine was indicated to inhibit NMDA-induced glutamatergic
transmission in the spinal cord through downregulation of NR2B,
p-CaMKIIα, and p-CREB.
Several important limitations were noted in the
current study. Firstly, although protein and mRNA expression levels
of NR2B, p-CaMKIIα, and p-CREB were all downregulated following
intrathecal bupivacaine, the results indirectly indicate that the
NR2B/CaMKIIα/CREB signaling pathway is involved in sedation during
spinal anesthesia. Therefore, future studies are necessary in order
to verify the association between sedation during spinal anesthesia
and NR2B/CaMKIIα/CREB signaling by using agonists or antagonists.
Secondly, the results demonstrated that expression levels of total
CaMKIIα, and CREB mRNA were decreased, although the levels of their
protein expression were not altered following intrathecal
bupivacaine. The association between mRNA and protein expression is
not strictly linear, however has a more intrinsic and complex
dependence (50). Therefore, this
variation may be due to post-transcriptional and post-translation
regulation and experimental errors (50,51).
Thirdly, the upstream and downstream molecules of NR2B/CaMKIIa/CREB
were not detected. Future studies may elucidate the function of
these molecular components.
In conclusion, sedation during spinal anesthesia
from decreased spinal afferent input may be associated with the
downregulation of NR2B, p-CaMKIIα, and p-CREB expression levels in
the spinal cord. Therefore, stimulatory input to the brain may be
indirectly reduced by inhibiting the NR2B/CaMKIIα/CREB signaling
pathway in spinal neurons, with this inhibition rendering the brain
susceptible to the effects of sedative drugs. The present findings
may prompt anesthesiologists to plan appropriate dosage guidelines
for the prevention of anesthetic overdose and investigate the
mechanism of sedation during neuraxial anesthesia.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660198) and
Ningxia Natural Science Foundation (grant. no. NZ15137).
References
|
1
|
Kim SH, Chun DH, Chang CH, Kim TW, Kim YM
and Shin YS: Effect of caudal block on sevoflurane requirement for
lower limb surgery in children with cerebral palsy. Paediatr
Anaesth. 21:394–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiang Y, Chen CQ, Chen HJ, Li M, Bao FP
and Zhu SM: The effect of epidural lidocaine administration on
sedation of propofol general anesthesia: A randomized trial. J Clin
Anesth. 26:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Zhang W and Li B: The effect of
epidural anesthesia with different concentrations of ropivacaine on
sevoflurane requirements. Anesth Analg. 104:984–986. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sentürk M, Gücyetmez B, Ozkan-Seyhan T,
Karadeniz M, Dinçer S, Akpir D, Sengül T and Denkel T: Comparison
of the effects of thoracic and lumbar epidural anaesthesia on
induction and maintenance doses of propofol during total i.v.
anaesthesia. Br J Anaesth. 101:255–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gentili M, Huu PC, Enel D, Hollande J and
Bonnet F: Sedation depends on the level of sensory block induced by
spinal anaesthesia. Br J Anaesth. 81:970–971. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pollock JE, Neal JM, Liu SS, Burkhead D
and Polissar N: Sedation during spinal anesthesia. Anesthesiology.
93:728–734. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang W, Geng Y, Liu Y, Li A, Liu J, Xing J
and Li W: Comparison of effects of thoracic epidural and
intravenous administration of lidocaine on target-controlled
infusion of propofol and tracheal intubation response during
induction of anesthesia. J Cardiothorac Vasc Anesth. 27:1295–1300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hodgson PS and Liu SS: Epidural lidocaine
decreases sevoflurane requirement for adequate depth of anesthesia
as measured by the Bispectral Index monitor. Anesthesiology.
94:799–803. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doufas AG, Wadhwa A, Shah YM, Lin CM,
Haugh GS and Sessler DI: Block-dependent sedation during epidural
anaesthesia is associated with delayed brainstem conduction. Br J
Anaesth. 93:228–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antognini JF, Atherley R and Carstens E:
Isoflurane action in spinal cord indirectly depresses cortical
activity associated with electrical stimulation of the reticular
formation. Anesth Analg. 96:999–1003. 2003.PubMed/NCBI
|
|
11
|
Lin TY, Chung CY, Lu CW, Huang SK, Shieh
JS and Wang SJ: Local anesthetics inhibit glutamate release from
rat cerebral cortex synaptosomes. Synapse. 67:568–579. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cherng CH, Wong CS, Wu CT and Yeh CC:
Glutamate release and neurologic impairment after intrathecal
administration of lidocaine and bupivacaine in the rat. Reg Anesth
Pain Med. 36:452–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishizawa N, Shirasaki T, Nakao S, Matsuda
H and Shingu K: The inhibition of the N-methyl-D-aspartate receptor
channel by local anesthetics in mouse CA1 pyramidal neurons. Anesth
Analg. 94:325–330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lanier WL, Iaizzo PA, Milde JH and
Sharbrough FW: The cerebral and systemic effects of movement in
response to a noxious stimulus in lightly anesthetized dogs.
Possible modulation of cerebral function by muscle afferents.
Anesthesiology. 80:392–401. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhai QZ and Traub RJ: The NMDA receptor
antagonist MK-801 attenuates c-Fos expression in the lumbosacral
spinal cord following repetitive noxious and non-noxious colorectal
distention. Pain. 83:321–329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong CK and MacDermott AB: Synaptic GluN2A
and GluN2B containing NMDA receptors within the superficial dorsal
horn activated following primary afferent stimulation. J Neurosci.
34:10808–10820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito Y, Kaneko M, Kirihara Y, Sakura S
and Kosaka Y: Interaction of intrathecally infused morphine and
lidocaine in rats (part I): Synergistic antinociceptive effects.
Anesthesiology. 89:1455–1463. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oklinski MK, Lim JS, Choi HJ, Oklinska P,
Skowronski MT and Kwon TH: Immunolocalization of water channel
proteins AQP1 and AQP4 in rat spinal cord. J Histochem Cytochem.
62:598–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HL, Li YX, Niu YT, Zheng J, Wu J, Shi
GJ, Ma L, Niu Y, Sun T and Yu JQ: Observing anti-inflammatory and
anti-nociceptive activities of glycyrrhizin through regulating
COX-2 and pro-inflammatory cytokines expressions in mice.
Inflammation. 38:2269–2278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tverskoy M, Shagal M, Finger J and Kissin
I: Subarachnoid bupivacaine blockade decreases midazolam and
thiopental hypnotic requirements. J Clin Anesth. 6:487–490. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eappen S and Kissin I: Effect of
subarachnoid bupivacaine block on anesthetic requirements for
thiopental in rats. Anesthesiology. 88:1036–1042. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal A, Pandey R, Dhiraaj S, Singh PK,
Raza M, Pandey CK, Gupta D, Choudhury A and Singh U: The effect of
epidural bupivacaine on induction and maintenance doses of propofol
(evaluated by bispectral index) and maintenance doses of fentanyl
and vecuronium. Anesth Analg. 99:1684–1688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ingelmo PM, Ferri F and Fumagalli R:
Interactions between general and regional anesthesia. Minerva
Anestesiol. 72:437–445. 2006.PubMed/NCBI
|
|
25
|
Inagaki Y, Mashimo T, Kuzukawa A, Tsuda Y
and Yoshiya I: Epidural lidocaine delays arousal from isoflurane
anesthesia. Anesth Analg. 79:368–372. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foffani G, Humanes-Valera D,
Calderon-Muñoz F, Oliviero A and Aguilar J: Spinal cord injury
immediately decreases anesthetic requirements in rats. Spinal Cord.
49:822–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hodgson PS, Liu SS and Gras TW: Does
epidural anesthesia have general anesthetic effects? A prospective,
randomized, double-blind, placebo-controlled trial. Anesthesiology.
91:1687–1692. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Antognini JF and Carstens E: Isoflurane
blunts electroencephalographic and thalamic-reticular formation
responses to noxious stimulation in goats. Anesthesiology.
91:1770–1779. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antognini JF, Carstens E, Sudo M and Sudo
S: Isoflurane depresses electroencephalographic and medial thalamic
responses to noxious stimulation via an indirect spinal action.
Anesth Analg. 91:1282–1288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aguilar J, Humanes-Valera D,
Alonso-Calviño E, Yague JG, Moxon KA, Oliviero A and Foffani G:
Spinal cord injury immediately changes the state of the brain. J
Neurosci. 30:7528–7537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alonso-Calviño E, Martinez-Camero I,
Fernández-López E, Humanes-Valera D, Foffani G and Aguilar J:
Increased responses in the somatosensory thalamus immediately after
spinal cord injury. Neurobiol Dis. 87:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aanonsen LM and Wilcox GL: Nociceptive
action of excitatory amino acids in the mouse: Effects of spinally
administered opioids, phencyclidine and sigma agonists. J Pharmacol
Exp Ther. 243:9–19. 1987.PubMed/NCBI
|
|
33
|
Tang Q, Bangaru ML, Kostic S, Pan B, Wu
HE, Koopmeiners AS, Yu H, Fischer GJ, McCallum JB, Kwok WM, et al:
Ca2+-dependent regulation of Ca2+ currents in
rat primary afferent neurons: Role of CaMKII and the effect of
injury. J Neurosci. 32:11737–11749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeitz KP, Giese KP, Silva AJ and Basbaum
AI: The contribution of autophosphorylated alpha-calcium-calmodulin
kinase II to injury-induced persistent pain. Neuroscience.
128:889–898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakanishi M, Hata K, Nagayama T, Sakurai
T, Nishisho T, Wakabayashi H, Hiraga T, Ebisu S and Yoneda T: Acid
activation of Trpv1 leads to an up-regulation of calcitonin
gene-related peptide expression in dorsal root ganglion neurons via
the CaMK-CREB cascade: A potential mechanism of inflammatory pain.
Mol Biol Cell. 21:2568–2577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Descalzi G, Fukushima H, Suzuki A, Kida S
and Zhuo M: Genetic enhancement of neuropathic and inflammatory
pain by forebrain upregulation of CREB-mediated transcription. Mol
Pain. 8:902012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miletic G, Pankratz MT and Miletic V:
Increases in the phosphorylation of cyclic AMP response element
binding protein (CREB) and decreases in the content of calcineurin
accompany thermal hyperalgesia following chronic constriction
injury in rats. Pain. 99:493–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma W, Hatzis C and Eisenach JC:
Intrathecal injection of cAMP response element binding protein
(CREB) antisense oligonucleotide attenuates tactile allodynia
caused by partial sciatic nerve ligation. Brain Res. 988:97–104.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katano T, Nakazawa T, Nakatsuka T,
Watanabe M, Yamamoto T and Ito S: Involvement of spinal
phosphorylation cascade of Tyr1472-NR2B, Thr286-CaMKII, and
Ser831-GluR1 in neuropathic pain. Neuropharmacology. 60:609–616.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Ma W, Chabot JG and Quirion R:
Calcitonin gene-related peptide as a regulator of neuronal
CaMKII-CREB, microglial p38-NFκB and astroglial ERK-Stat1/3
cascades mediating the development of tolerance to morphine-induced
analgesia. Pain. 151:194–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Wu J, Lin Q, Nauta HJ, Yue Y and
Fang L: Effects of general anesthetics on visceral pain
transmission in the spinal cord. Mol Pain. 4:502008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan X, Chen J, Wang W, Chen L, Wang L, Ma
Q, Zhang J, Chen L, Wang G, Zhang M, et al: Resveratrol-induced
antinociception is involved in calcium channels and
calcium/caffeine-sensitive pools. Oncotarget. 8:9399–9409.
2017.PubMed/NCBI
|
|
43
|
Miranda A, Mickle A, Bruckert M,
Kannampalli P, Banerjee B and Sengupta JN: NMDA receptor mediates
chronic visceral pain induced by neonatal noxious somatic
stimulation. Eur J Pharmacol. 744:28–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Crown ED, Gwak YS, Ye Z, Yu Tan H, Johnson
KM, Xu GY, McAdoo DJ and Hulsebosch CE: Calcium/calmodulin
dependent kinase II contributes to persistent central neuropathic
pain following spinal cord injury. Pain. 153:710–721. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang L, Wu J, Zhang X, Lin Q and Willis
WD: Calcium/calmodulin dependent protein kinase II regulates the
phosphorylation of cyclic AMP-responsive element-binding protein of
spinal cord in rats following noxious stimulation. Neurosci Lett.
374:1–4. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mitsikostas DD, Knight YE, Lasalandra M,
Kavantzas N and Goadsby PJ: Triptans attenuate capsaicin-induced
CREB phosphorylation within the trigeminal nucleus caudalis: A
mechanism to prevent central sensitization? J Headache Pain.
12:411–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu H, Zhang Y, Qi D and Li W:
Downregulation of the spinal NMDA receptor NR2B subunit during
electro-acupuncture relief of chronic visceral hyperalgesia. J
Physiol Sci. 67:197–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Paganelli MA and Popescu GK: Actions of
bupivacaine, a widely used local anesthetic, on NMDA receptor
responses. J Neurosci. 35:831–842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Furutani K, Ikoma M, Ishii H, Baba H and
Kohno T: Bupivacaine inhibits glutamatergic transmission in spinal
dorsal horn neurons. Anesthesiology. 112:138–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
de Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
51
|
Fraser HB, Hirsh AE, Giaever G, Kumm J and
Eisen MB: Noise minimization in eukaryotic gene expression. PLoS
Biol. 2:e1372004. View Article : Google Scholar : PubMed/NCBI
|