Introduction
Obesity is a worldwide public health concern, the
prevalence of which has continuously increased over the past
decades (1–3). In 2014, the World Health Organization
estimated that ~2 billion people were overweight and ~600 million
of these were obese (4). Obesity
is an independent risk factor for renal dysfunction and chronic
kidney disease, and may directly lead to kidney injury, known as
obesity-related glomerulopathy (ORG) (3,5–7). As
the number of patients with obesity rises, the incidence rate of
ORG is also rapidly increasing. A previous study reported that
among 6,818 patients that underwent renal biopsy, the percentage of
patients with ORG increased from 0.2% in 1986–1990 to 2% in
1996–2000 (6). Another study
reported that among the renal biopsy cases at a Chinese nephrology
center, patients with ORG accounted for 3.80% in 2006–2008 and
9.85% in 2010–2015 (7). Therefore,
an increasing number of studies are investigating ORG.
The pathological features of ORG include
glomerulomegaly with or without focal segmental glomerulosclerosis
detectable under light microscopy, and decreased podocyte density
and number with increased foot-process width observable under
electron microscopy (6–9). The severity of podocyte injury has
been correlated with the degree of proteinuria and renal
dysfunction; therefore, podocyte injury is considered a hallmark of
ORG and serves a pivotal role in the initiation and progression of
ORG (9–11).
Previous studies have demonstrated that
mineralocorticoid receptor (MR) was expressed in in vitro
cultured podocytes as well as in in vivo glomerular
podocytes (12–16), and elevated serum aldosterone
(ALDO) levels were also reported in the patients with metabolic
syndrome as well as in the rat models of metabolic syndrome
(12,16). Therefore, it is necessary to
determine whether ALDO exposure may exert direct harmful effects on
podocytes by binding with MR in the pathogenesis of metabolic
syndrome. A study from 2006 reported that a spontaneously
hypertensive rats/NDmcr-cp (SHR/cp) rat model of metabolic syndrome
exhibited increased proteinuria and podocyte injury (12). Notably, elevated serum ALDO levels
were observed in the SHR/cp rats and a positive correlation was
determined between serum ALDO levels and proteinuria. Furthermore,
administration of eplerenone, a selective MR antagonist,
effectively reduced proteinuria and alleviated podocyte injury.
These data indicated that ALDO may be involved in the pathogenesis
of kidney damage in the metabolic syndrome model (12,14,15).
However, renal histological changes were not apparent in this
model, and the glomerular diameters were not measured in this
study; therefore, the kidney damage in this metabolic syndrome
model may not be considered as ORG.
Wnt/β-catenin signaling in podocytes serves a
crucial role in integrating cell adhesion, motility,
differentiation and survival (17). Podocyte injury and proteinuria were
observed in the mouse model of Adriamycin-induced nephropathy
(18–20), a mouse model of transforming growth
factor (TGF)-β-driven kidney damage (21). In a previous study, it was
demonstrated that patients with focal and segmental
glomerulosclerosis induced podocyte injury and proteinuria
(18). Additionally, in patients
with diabetic nephropathy and in mice models of diabetic kidney
disease, the proteinuria and podocyte injury was observed (17,19,22).
Additionally, accumulating evidence has suggested that activation
of Wnt/β-catenin signaling pathway induces proteinuria and podocyte
dysfunction, such as diabetic nephropathy and adriamycin-induced
nephropathy (8,23,24).
Our previous study demonstrated the activation of
Wnt/β-catenin signaling in podocytes in the ORG mouse model
(25); however, the pathogenic
role of ALDO and the relationship between ALDO and the activation
of Wnt/β-catenin signaling in this ORG model have not been
explored.
The present study used in vivo and in
vitro experiments to investigate the pathogenic roles of ALDO
in podocyte injury of ORG and the possible role of activated
Wnt/β-catenin signaling in mediating podocyte injury. It was
demonstrated that ALDO was involved in pathogenesis of ORG and
podocyte lesion. Podocyte injury were observed in ORG model and
cultured podocytes were stimulated with ALDO. These alterations
were alleviated by Spironolactone and eplerenone (EPL). In
addition, the Wnt inhibitory factor Dickkopf protein-1 (DKK1), not
only inhibited Wnt/β-catenin signaling activity, but also protected
ALDO-induced podocyte injury. The present study suggested that
activation of Wnt/β-catenin signaling pathway is involved in
podocyte lesion caused by ALDO and is involved in ORG and podocyte
injury.
Materials and methods
Animal model and grouping
Male C57BL/6J mice (n=18; age, 6 weeks; weight, 20±2
g; Sibeifu Biology Technology Co., Ltd., Beijing, China) were
housed in a 50~60% humidity and temperature (20–26°C)- and light
(light/dark cycle: 12 h light, 12 h dark)-controlled animal room of
specific-pathogen-free cleanliness grade. Mice were randomly
divided into the following 3 groups: i) Control group (n=6), which
were fed a common diet ad libitum that contained fat
accounting for 10% kcal (Beijing Huafukang Biological Technology
Co., Ltd., Beijing, China); ii) ORG model group (n=6), which were
fed a high-fat diet that contained fat accounting for 60% kcal
(Research Diet, Inc., New Brunswick, NJ, USA) as described by us
previously (26); and iii)
Spironolactone intervention group (n=6), which also were the
high-fat diet, and at week 9 received daily subcutaneous injections
of spironolactone (20 mg/kg/d; catalog no. Y0001295; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), a non-selective MR antagonist,
that was dissolved in 60% propylene glycol; mice in the Control
group and in the ORG model group received daily subcutaneous
injections of 60% propylene glycol only. All mice were sacrificed
at the end of 12th week and the kidneys collected. Mice were
scarified by 100% carbon dioxide. The filling rate was 20% of the
volume of the chamber per minute to achieve animal unconsciousness
rapidly and reduce animal pain. The sign of death is the lack of
respiration and fading of the eye-color. Once these two phenomena
were observed, mice were removed from the box. A portion of the
kidney tissue was fixed in 4% neutral formaldehyde solution for 48
h in room temperature, for light microscopy; the remaining of the
kidney tissue was rapidly frozen in liquid nitrogen for use in
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blotting assays.
All animal care and experimental protocols complied
with the US National Institutes of Health Guide for the Care and
Use of Laboratory Animals (publication no. 85–23, 1996) and were
approved by the Institutional Animal Care and Use Committee of
Capital Medical University (Beijing, China).
Cell culture and experimental
protocols
A conditionally immortalized mouse podocyte cell
line was kindly provided by Professor Maria Pia Rastaldi (San Carlo
Hospital, University of Milan, Italy) and cultured as described
previously (25). Briefly,
podocytes were grown in RPMI-1640 with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Waltham, MA, USA) with mouse
recombinant interferon-γ (20 U/ml (IFN-γ; Sigma-Aldrich; Merck
KGaA) at 33°C, and subsequently differentiated in RPMI-1640 without
interferon-γ at 37°C. Results from preliminary experiments were
similar to previously described results of podocytes cultured in
serum-containing medium and serum-free medium with 2% FBS (data not
shown) following aldosterone and/or eplerenone stimulation
(27–29). Based on these date, serum-free
medium was used for podocyte culture for subsequent experiments
post-stimulation.
In the first set of experiments, podocytes were
incubated in RPMI-1640 medium only, in medium containing ALDO (cat.
no. A9477, Sigma-Aldrich; Merck KGaA), in medium containing
eplerenone (cat. no. E6657, Sigma-Aldrich; Merck KGaA) or in medium
containing both eplerenone and ALDO (cells in this group were
pre-incubated with eplerenone for 30 min at 37°C, and subsequently
incubated with both eplerenone and ALDO). The concentration of ALDO
and eplerenone in the media was 10−7 and 10−5
mol/l, respectively. Cells were incubated for 12 or 24 h at 37°C
and harvested for RT-qPCR or western blotting assays,
respectively.
In the second set of experiments, podocytes were
incubated in RPMI-1640 medium only, in medium containing ALDO, in
medium containing Dickkopf-related protein 1 (DKK1, a well-known
inhibitor of Wnt/β-catenin signaling; cat. no. 5897-DK, R&D
Systems, Inc., Minneapolis, MN, USA) or in medium containing DKK1
and ALDO (cells in this group were pre-incubated with DKK1 for 1 h
at 37°C, followed by incubation with DKK1 and ALDO). Cells were
incubated and harvested for RT-qPCR and western blotting as
aforementioned.
Biological parameters
Mouse body weight was measured every 4 weeks and
body length was measured at week 12. Nocturnal 12 h urine samples
were collected at weeks 0 and 12 to measure urinary protein using a
Bradford Protein Assay kit (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer's protocol. Blood
and urine samples were collected at week 12 to measure serum
triglycerides, serum cholesterol and blood glucose levels, as well
as serum and urine creatinine levels using an Olympus AU5400
Chemistry Analyzer (Olympus Corporation, Tokyo, Japan). Following
sacrifice, the weight of visceral fat mass was measured as
previously described (30) and the
weight of kidney was also measured.
The follows formulas were used: Lee's obesity
index=[(Body weight (g) ×1,000)1/3]/Body length (cm) (31,32);
abdominal visceral fat index=[abdominal visceral fat mass (g)/body
weight (g)] × 100% (30); and
creatinine clearance rate (ml/min)=[(urine creatinine (µmol/l) ×
urine volume (ml/min)]/serum creatinine (µmol/l) (33).
Histological examination
Fixed kidney cortical tissues were dehydrated,
embedded, sectioned (3 µm) and stained with periodic acid-Schiff
reagent. Paraffin production: A portion of kidney tissues were
fixed in 4% neutral formaldehyde solution for 48 h. Subsequently,
they were dehydrated with 70, 80, 90, 95, and 100% ethanol
gradient, xylene transparent and 60°C dipping wax. Paraffin
embedding machine made wax, then making paraffin wax slicing
machine. PAS slice thickness was 3 µm, and 58°C for 1 h. PAS
staining: 3 µm thick paraffin sections were dewaxed, washed with 1%
periodic acid for 10 min, then were rinsed using tap water for 5
times and washed once with distilled water. Subsequently, Schiff
reagent (cat. no. TR1337, ZSGB-BIO; OriGene Technologies, Inc.,
Beijing; China) was added for 30 min in the dark, rinsed with tap
water for 5 times and washed 3 times with distilled water.
Subsequently, hematoxylin Mayer dye was added for 5 min, rinsing
with tap water for 5 times and washed 3 times with distilled water,
then using hydrochloric acid alcohol differentiation for 10 sec,
and rinsing with ethanol 3 times. Finally, xylene was transparent
and neutral resin sheet was produces. A total of 20 images of
glomerular maximal profiles with vascular pole and/or urinary pole
were captured using a confocal microscope (×400 magnification). The
two longest perpendicular diameters of the glomerular capillary
tufts were measured using the Nikon NIS-Elements Basic Research
Image Analysis software (Nikon NIS-Elements Basic Research Image
Analysis software 3.0; Nikon Corporation, Tokyo, Japan), and the
mean value was calculated (25,26,34).
In addition, images of 20 random visual fields containing only
tubules and interstitium were captured (×200 magnification), and
the relative area of tubules displaying vacuolar degeneration of
cytoplasm in each visual field was assessed using a
semi-quantitative method, and then their mean value was calculated.
A semi-quantitative method described as: ‘Each slice randomly
selected with 20 visual fields, the area of vacuolated tubules that
accounted for <25, 26–50, 51–75 or >75% of the visual field
were given lesion scores of 1, 2, 3 and 4, respectively’ (35). Calculating mean value in every
slice, then calculating the mean value of every group.
Reverse transcription and RT-qPCR
Total RNA (50 µg) was extracted from the renal
cortex of mouse kidney or 30–40,000 cultured podocytes/dish using
the TRIzol RNA isolation system (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. RNA (2 µg) was reverse transcribed to cDNA with Moloney
Murine Leukemia Virus reverse transcriptase (Beijing TransGen
Biotech Co., Ltd., Beijing, China) as follows: Incubation for 30
min at 42°C, inactivation in 85°C for 5 min, followed by incubation
at 12°C. The synthetic cDNA is preserved at −20°C. qPCR was
performed using SYBR-Green labelled kit (TransGen Biotech, Beijing,
China) under a Rotor-Gene 6000 thermocycler (Qiagen GmbH, Hilden,
Germany). The thermocycling profile was as denaturation follows:
Initial denaturation at 95°C for 60 sec, followed by 40 cycles of
at 95°C (15 sec), annealing at 60°C (15 sec) and extension at 72°C
(45 sec). Gene-specific primers were designed using GenBank
(www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome)
and synthesized by Beijing SBS Genetech Co., Ltd. (Beijing, China)
(Table I). A no template control
was used as a negative control. mRNA expression levels of various
genes were calculated following normalizing with GAPDH expression.
Reactions were performed in triplicate and quantification cycle
(Cq) numbers were averaged. Relative mRNA expression levels were
calculated according to the formula: 2−(target gene Cq -
control gene Cq) ×103 (36).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| Nephrin | F:
TATCGCCAAGCCTTCACAGG |
|
| R:
GCCACCTGGTCGTAGATTCC |
| Podocin | F:
CAGAAGGGGAAAAGGCTGCT |
|
| R:
GATGCTCCCTTGTGCTCTGT |
| Podocalyxin | F:
AGCCTGTGGATTCTTCACCG |
|
| R:
GTGTGGAGACGGGCAATGTA |
| Podoplanin | F:
AGGGAGGGACTATAGGCGTG |
|
| R:
GCTGAGGTGGACAGTTCCTC |
| Wnt1 | F:
CGAACGACCGTGTTCTCTGA |
|
| R:
GCTCCAGGCGCAGCAG |
| Wnt2b | F:
GATGGGGCCAATTTCACAGC |
|
| R:
AGTTGTGTCATACCCTCGGC |
| Wnt6 | F:
CAACTGGCTCTCCAGATGCT |
|
| R:
TGGCACTTACACTCGGTGC |
| β-catenin | F:
ACTGGAGCTCTCCACATCCT |
|
| R:
GTGGCTCCCTCAGCTTCAAT |
| GAPDH | F:
TGTGAACGGATTTGGCCGTA |
|
| R:
GATGGGCTTCCCGTTGATGA |
Western blot analysis
The tissue was removed from the liquid nitrogen and
placed on ice and into the homogenizer, where Ripa lysis buffer
(R0010-100 ml; Beyotime Institute of Biotechnology) with protease
inhibitors was added following homogenization. Subsequently, the
suspension was transferred to the sterilization of the EP tube
(Eppendorf AG, Hamburg, Germany), and centrifuged at a low
temperature high speed centrifuge at 10,000 × g for 10 min, the
supernatant was transferred to the EP tube. All incubations were
performed on ice. Total protein was extracted from mouse renal
cortex or cultured podocytes (200 µg) using Cell Lysis Buffer (Cell
Signaling Technology, Inc., Danvers, MA, USA). Lysate samples were
boiled for 5 min, and proteins (40 µg) were separated by 10%
SDS-PAGE followed by transferred to nitrocellulose membranes (GE
Healthcare Life Sciences, Little Chalfont, UK). Following blocking
with 5% skim milk in PBS containing 0.1% Tween-20 for 1 h at room
temperature, the membranes were incubated with various primary
antibodies at 4°C overnight, followed by incubation with the
corresponding fluorophore-labeled secondary antibodies at room
temperature for 1 h. The primary and secondary antibodies are
listed in Table II. The blotted
proteins were quantified using an Odyssey Infrared Imaging System
(LI-COR Biosciences, Lincoln, NE, USA). β-actin was used as
internal loading control. The relative expression level of each
target protein was normalized to β-actin. All assays were performed
at least in triplicate. The relative level of phosphorylated
(p)-β-catenin was expressed as the ratio of p-β-catenin/total
β-catenin using image J 1.51j8 (Image J, National Institutes of
Health, Bethesda, MD, USA).
 | Table II.Primary and corresponding secondary
antibodies used for western blotting. |
Table II.
Primary and corresponding secondary
antibodies used for western blotting.
| Primary antibody
(dilution) | Company (location;
catalog no.) | Secondary
antibody | Company (location;
catalog no.) |
|---|
| Rabbit anti-nephrin
pAb (1:200) | Abcam (Cambridge,
UK; ab58968) | IRDye
800-conjugated goat anti-rabbit IgG pAb (1:5,000) | Rockland
Immunochemicals, Inc. (Pottstown, PA, USA; 211–1102) |
| Rabbit
anti-podocin | Sigma-Aldrich | IRDye
800-conjugated |
|
| pAb (1:750) | (Merck KGaA;
P0372) | goat anti-rabbit
IgG pAb (1:5,000) |
|
| Rabbit
anti-podocalyxin | Santa Cruz
Biotechnology, Inc. | IRDye 800 goat
anti-rabbit |
|
| pAb (1:200) | (Dallas, TX, USA;
M300) | IgG pAb
(1:5,000) |
|
| Rabbit
anti-podoplanin | Santa Cruz
Biotechnology, | IRDye
800-conjugated |
|
| pAb (1:750) | Inc. (M172) | goat anti-rabbit
IgG pAb (1:5,000) |
|
| Rabbit
anti-phosphorylated | Cell Signaling
Technology, | IRDye
800-conjugated goat |
|
| β-catenin pAb
(1:1,000) | Inc. (9561s) | anti-rabbit IgG pAb
(1:5,000) |
|
| Mouse
anti-β-catenin | BD Biosciences (San
Jose, CA, | IRDye
680-conjugated goat | Rockland
Immunochemicals, |
| pAb (1:1,000) | USA; 610153) | anti-mouse IgG pAb
(1:5,000) | Inc.
(110–1102) |
| Mouse anti-mouse
β-actin | Sigma-Aldrich | IRDye
680-conjugated goat |
|
| mAb (1:10,000) | (Merck KGaA;
A5441) | anti-mouse IgG pAb
(1:5,000) |
|
Statistical analysis
All data of continuous variables were expressed as
the mean ± standard deviation and analyzed using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance followed by the Dunnett's test was performed
to evaluate the significant differences among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Spironolactone improves biological
parameters of ORG model mice
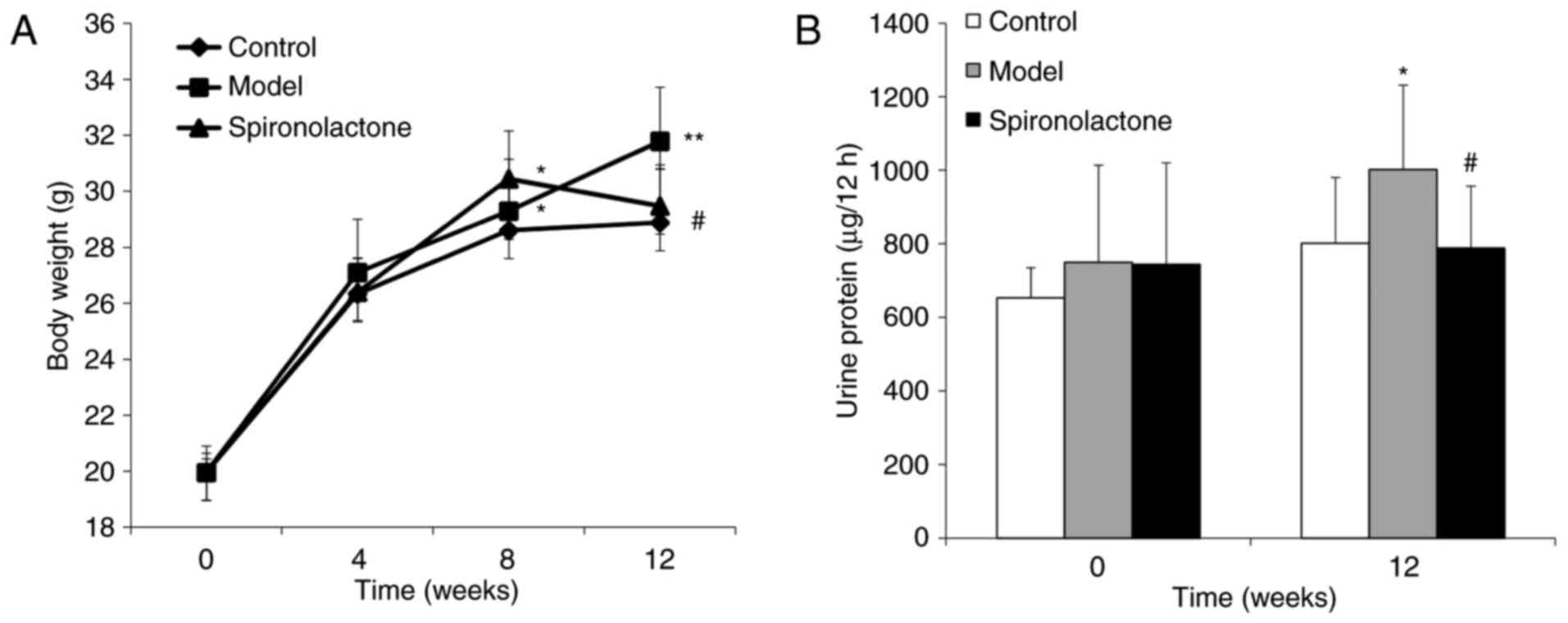
No significant differences in body weight were
identified among the three groups week 0 (P>0.05; Fig. 1A). At week 4, there were no
difference in any group (P>0.05). At week 8, body weights in the
Model group and the Spironolactone group were significantly heavier
compared with the Control group (P<0.05), whereas no significant
difference was identified between the Model group and the
Spironolactone group w (P>0.05). However, at week 12, body
weight in the Spironolactone group was significantly lower compared
with the Model group (P<0.05), and the difference between
Spironolactone group and Control group was not significant
(P>0.05).
At week 12, Lee's index, abdominal visceral fat
index and kidney weight were significantly increased in the Model
group compared with the measurements of these parameters in the
Control group (P<0.01 or P<0.05; Table III), whereas these parameter
measurements were significantly decreased in the Spironolactone
group compared with the Model group (P<0.01 or P<0.05).
 | Table III.Physicochemical parameters of mice at
12 weeks old. |
Table III.
Physicochemical parameters of mice at
12 weeks old.
| Parameter | Control
groupa | Model
groupa | Spironolactone
groupa |
|---|
| Abdominal visceral
fat index (%) | 1.60±0.50 |
4.90±1.60c |
3.30±0.30d |
| Lee's index | 16.86±0.38 |
17.64±0.56c |
16.73±0.29e |
| Kidney weight
(g) | 0.34±0.03 |
0.37±0.02b |
0.35±0.03e |
| Serum triglycerides
(mmol/l) | 0.54±0.13 |
0.85±0.39b |
0.59±0.21d |
| Serum cholesterol
(mmol/l) | 2.22±0.44 |
3.63±0.28c |
3.13±0.56d |
| Blood glucose
(mmol/l) | 9.34±0.91 | 9.12±1.02 | 9.10±0.77 |
| Creatinine
clearance (ml/min) | 0.41±0.14 |
0.72±0.28b |
0.48±0.18d |
Also at week 12, the levels of serum triglyceride
and cholesterol in the Model group were significantly increased
compared with the Control group (P<0.05 and P<0.01,
respectively; Table III),
whereas these parameters were significantly decreased in the
Spironolactone group compared with the Model group (P<0.05). No
significant difference was identified for blood glucose levels
among the three groups (P>0.05; Table III).
There was no significant difference identified for
the nocturnal 12 h urinary protein excretion among the three groups
at week 0 (P>0.05; Fig. 1B);
however, at week 12, urinary protein excretion in the Model group
was significantly higher compared with the Control group
(P<0.05), and mice treated with spironolactone exhibited a
significantly lower level of urine protein compared with the Model
group (P<0.05). In addition, creatinine clearance rate in the
model group was significantly higher compared with the Control
group (P<0.05; Table III),
whereas this level was significantly reduced in the Spironolactone
group compared with mice in the Model group (P<0.05).
Spironolactone alleviates renal
histological lesions in ORG model mice
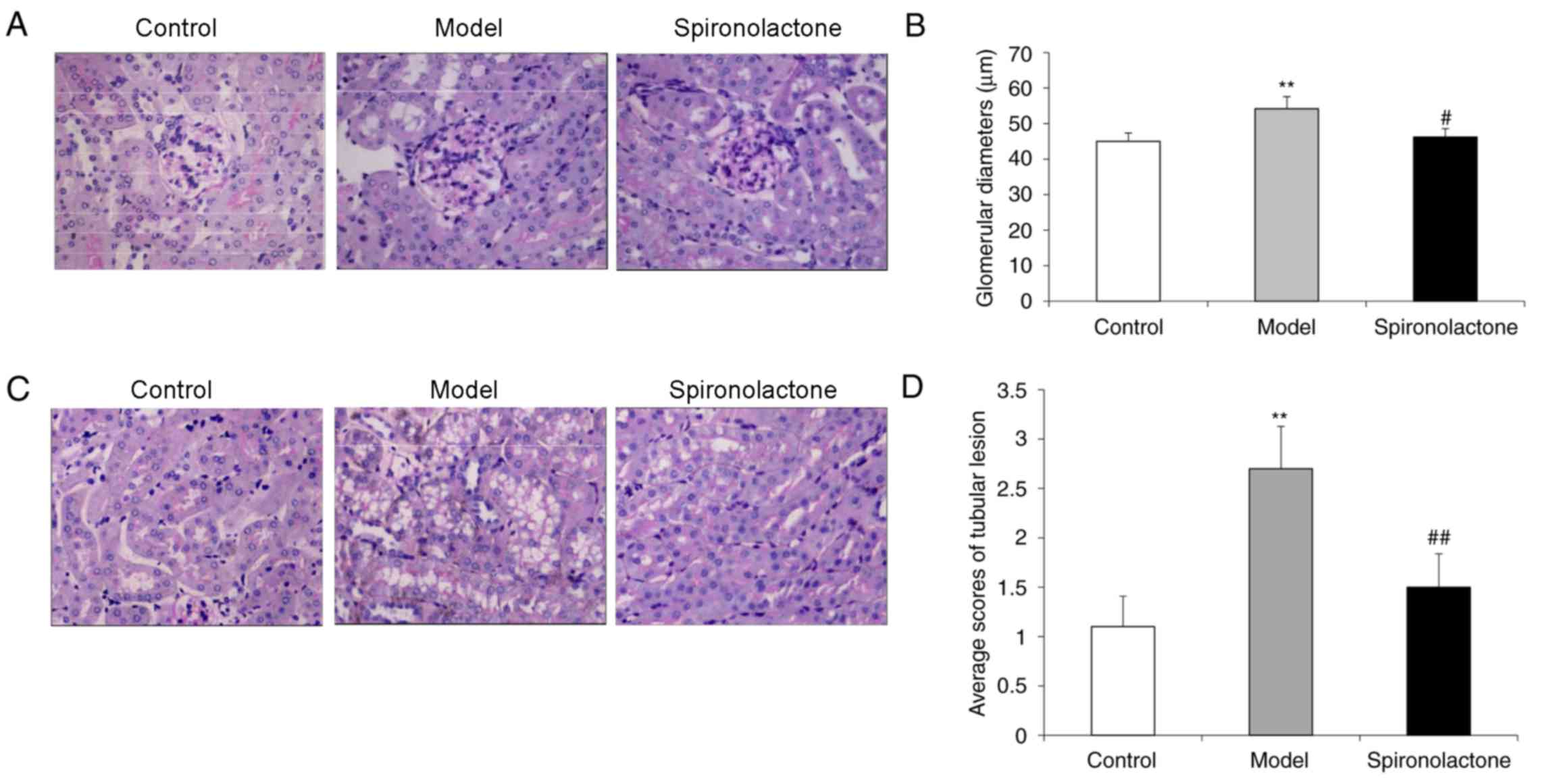
The average glomerular diameter in kidneys from mice
in the Model group was significantly larger compared with the
Control group (P<0.01; Fig. 2A and
B), and the glomerular diameter in the Spironolactone group was
significantly smaller compared with the Model group (P<0.05).
The average tubular lesion score in the kidneys from Model group
mice was significantly higher compared with mice in the Control
group (P<0.01; Fig. 2C and D),
whereas the lesion score in the Spironolactone group was
significantly lower compared with the Model group (P<0.01).
Spironolactone improves the expression
of podocyte-associated molecules in ORG model mice
The mRNA and protein expression levels of
podocyte-associated molecules, including nephrin, podocin,
podoplanin and podocalyxin, in the kidney cortical tissue was
significantly downregulated in Model group mice compared with mice
in the Control group (Fig. 3A and
B, respectively). Treatment with spironolactone significantly
upregulated the mRNA and protein expression of these molecules,
compared with the model group.
Spironolactone inhibits the activation
of Wnt/β-catenin signaling in ORG mice
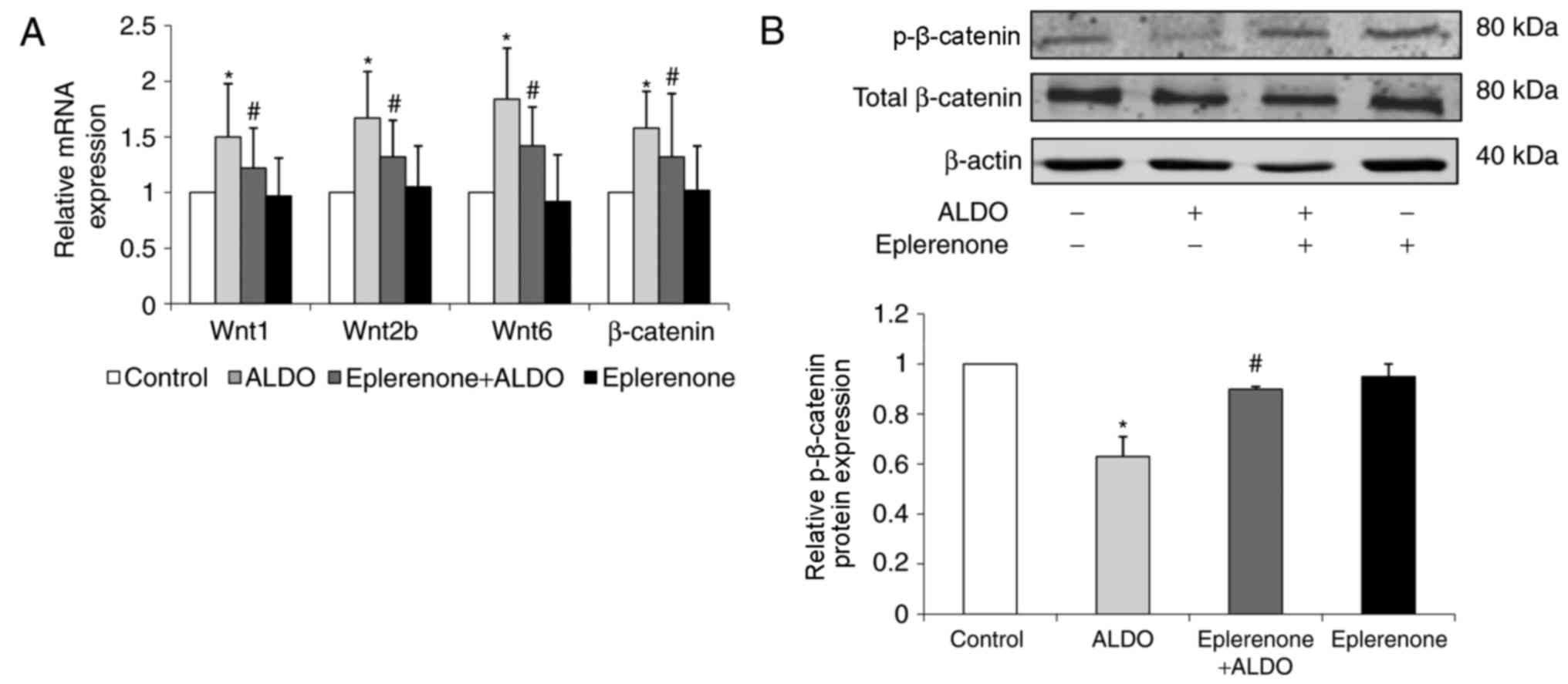
The mRNA expression levels of Wnt1, Wnt2b, Wnt6 and
β-catenin in the kidney cortical tissue were significantly
upregulated in ORG model mice compared with the respective
expression levels in the Control group (P<0.05; Fig. 4A). ORG model mice treated with
spironolactone exhibited significantly downregulated expression
levels of Wnt1, Wnt2b, Wnt6 and β-catenin compared untreated mice
in the Model group (P<0.05).
The expression level of p-β-catenin protein was
significantly decreased in Model group mice compared with
expression in the Control group (P<0.01; Fig. 4B); p-β-catenin protein expression
level was significantly increased in Spironolactone group mice
compared mice in the Model group (P<0.05).
ALDO-induced activation of
Wnt/β-catenin signaling and expression of podocyte-associated
molecules are reversed by eplerenone treatment
The mRNA expression levels of Wnt1, Wnt2b, Wnt6 and
β-catenin in cultured podocytes were significantly upregulated in
the ALDO-treated group compared with the respective expression
levels in the untreated Control group (P<0.05; Fig. 5A). ALDO-treated cells that were
co-treated with eplerenone exhibited significantly downregulated
expression levels of these podocyte-associated molecules compared
with the ALDO-only group (P<0.05). The level of p-β-catenin
protein expression in cultured podocytes was significantly
decreased in the ALDO group compared with the control group
(P<0.05; Fig. 5B) and
significantly increased in the eplerenone + ALDO group compared
with the ALDO-only group (P<0.05).
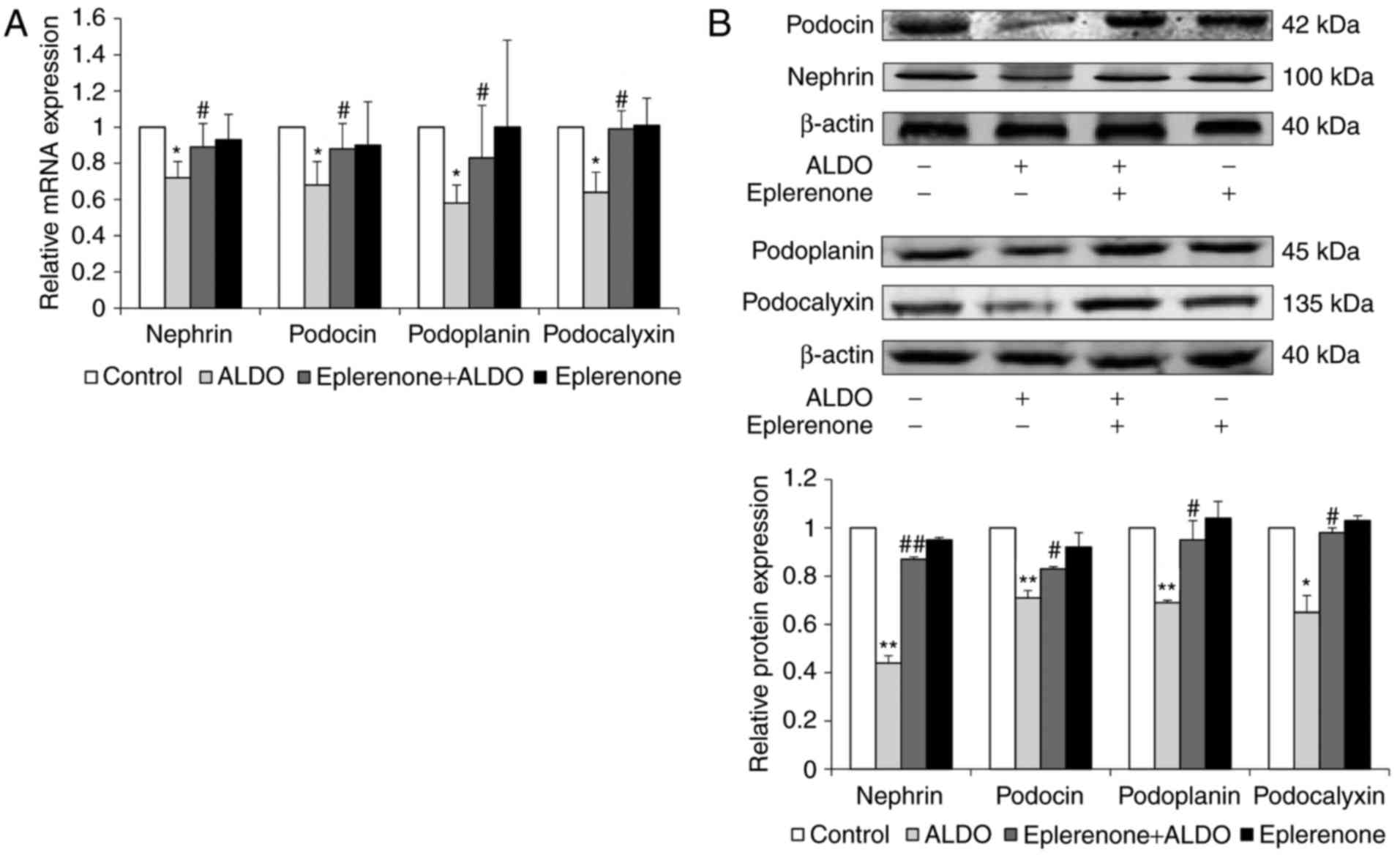
The mRNA and protein expression levels of additional
podocyte-associated molecules, including nephrin, podocin,
podoplanin and podocalyxin in cultured podocytes were significantly
reduced in the ALDO group compared with expression levels in the
Control group (P<0.05, P<0.01; Fig. 6A and B, respectively); the mRNA and
protein expression levels of these molecules were significantly
upregulated in cells co-treated with eplerenone and ALDO, compared
with the respective expression levels in the ALDO-only treatment
group (P<0.05, P<0.01).
ALDO-induced downregulation of
p-β-catenin protein expression and podocyte-associated molecules
expression levels are reversed by treatment with DKK1
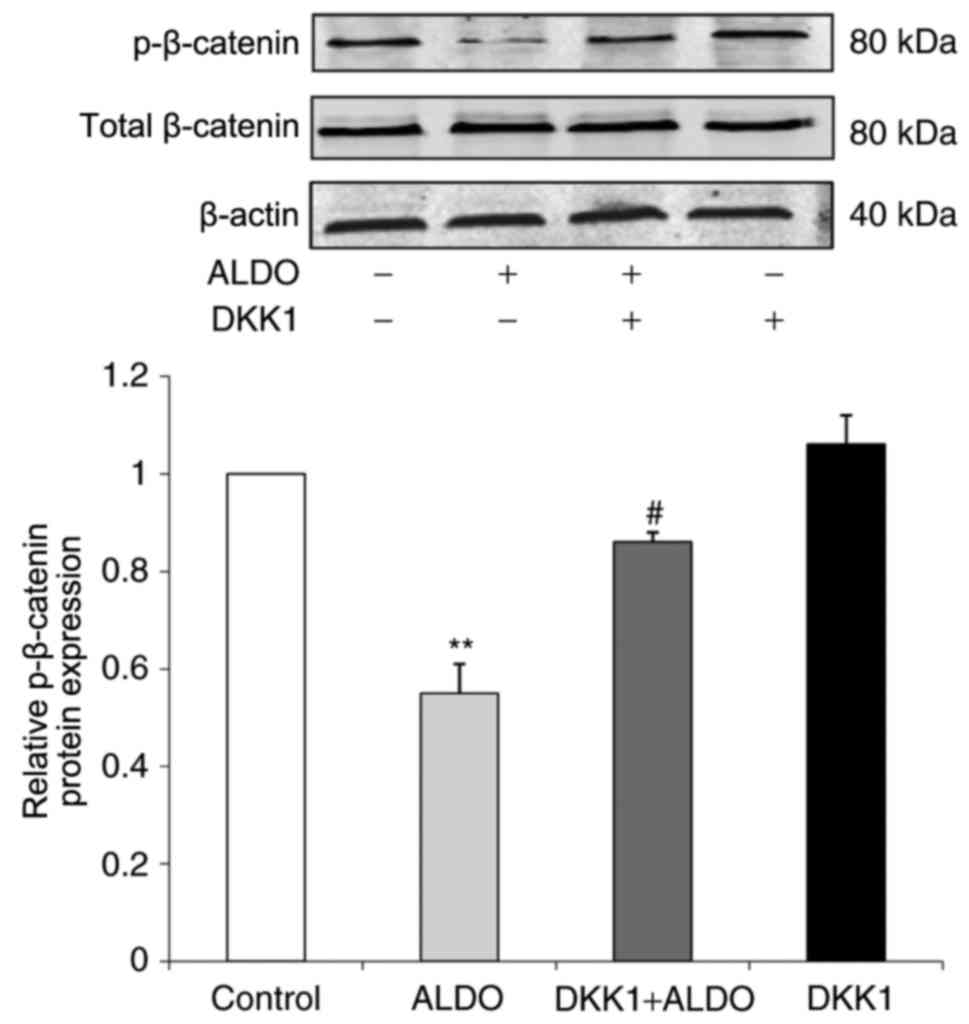
The expression level of p-β-catenin protein in
cultured podocytes was significantly decreased in the ALDO group
compared with the Control group (P<0.01; Fig. 7), and p-β-catenin protein
expression was significantly increased in cells co-treated with
ALDO and DKK1 compared with the expression level of those in the
ALDO-only treatment group (P<0.05).
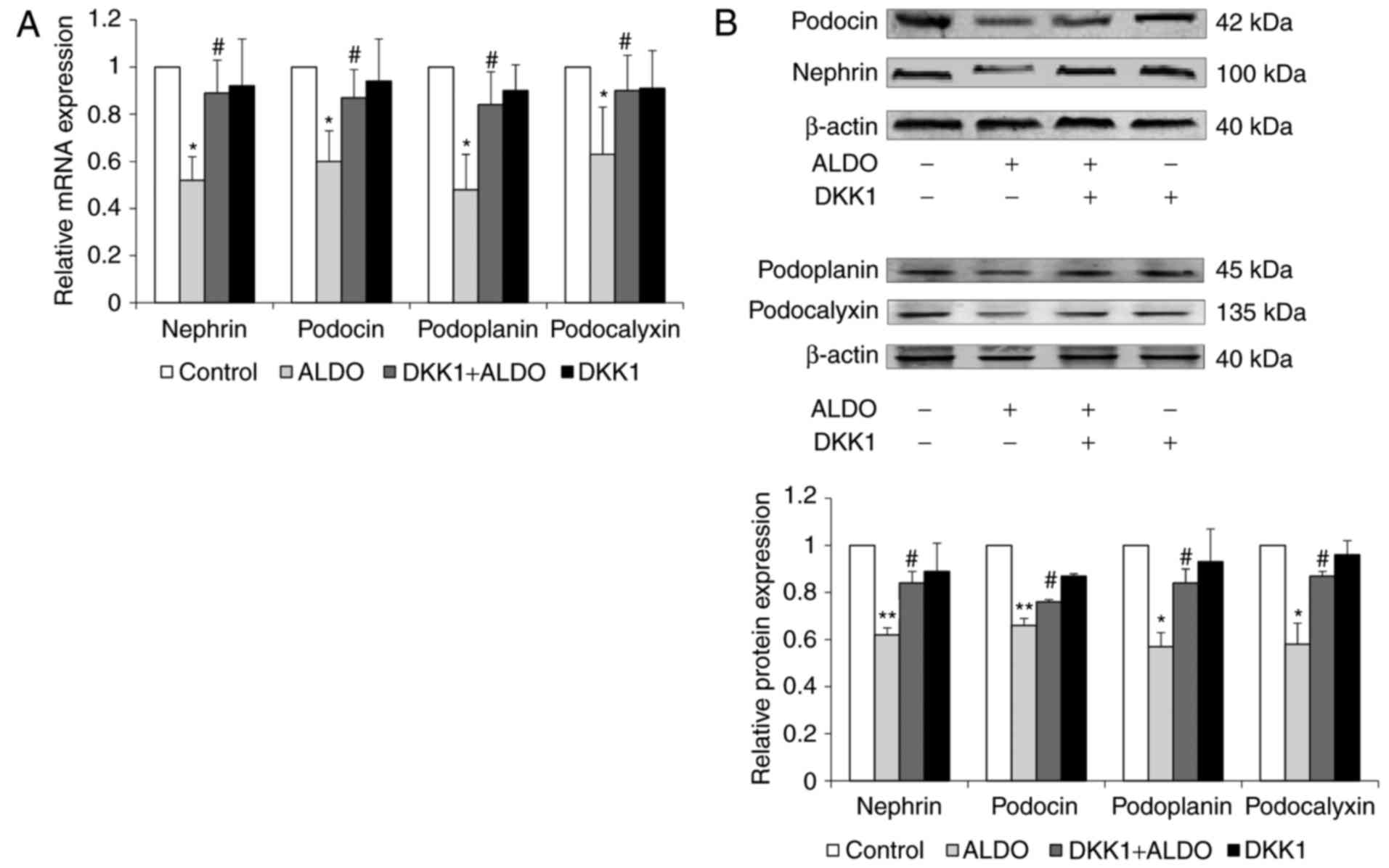
The mRNA and protein expression levels of the
podocyte-associated molecules nephrin, podocin, podoplanin and
podocalyxin in cultured podocytes were significantly reduced in
cells treated with ALDO compared with untreated cells in the
Control group (P<0.05, <0.01; Fig. 8A and B, respectively); the
expression levels of these molecules was significantly upregulated
in cells co-treated with ALDO and DKK1 compared with the expression
levels in those cells in the ALDO-only treatment group
(P<0.05).
Discussion
A number of structural changes of podocytes have
been observed under electron microscope in patients with ORG and in
animal models of ORG, including hypertrophied podocytes, decreased
podocyte density and number, and increased foot-process width on
the peripheral basement membrane are the most common manifestations
(8,12). In addition, podocyte detachment
from basement membrane may also be seen in obesity-associated focal
and segmental glomerulosclerosis (9–11,37).
Recently, it has been reported that an increase in the number of
podocalyxin-positive cells in the urine may be used as a biomarker
for detecting early kidney damage in obese patients, owing to the
possible shedding of podocytes from the glomerular basement
membrane into urine during ORG pathogenesis (38). Based on these data, podocyte injury
is considered the hallmark of ORG. Therefore, the present study
selected podocytes to be used as the principal research object.
The pathogenic mechanism of ORG has not been fully
elucidated, and there are many factors that may result in podocyte
injury and dysfunction in ORG (8,11).
Obese patients (16,35–37)
and obese rats (12) often exhibit
elevated levels of circulating ALDO. Adipocytes are able to produce
a number of ALDO-releasing substances, which stimulate adrenal ALDO
secretion independently of angiotensin II (4,11,16,39–42).
In addition, adipocytes themselves are able to synthesize and
secrete ALDO (39,43). Previous studies have demonstrated
that both circulating ALDO and adipocyte-derived ALDO participate
in the development of obesity-related hypertension and
cardiovascular complications (39,40,42,43).
Therefore, the present study conducted a preliminary exploration
into whether ALDO participated in ORG pathogenesis.
ORG model mice in the present study exhibited
increased urine protein excretion and elevated creatinine clearance
rate, glomerulomegaly and podocyte injury, as indicated by the
downregulated mRNA and protein expression levels of
podocyte-associated molecules. However, ORG model mice treated with
spironolactone exhibited a significant reduction of these effects.
These results suggested that ALDO may be involved, at least in
part, in the pathogenesis of ORG. In cultured podocytes, ALDO
treatment significantly reduced the mRNA and protein expression
levels of podocyte-associated molecules, whereas co-treatment with
eplerenone reversed these effects. These results suggested that
ALDO treatment may exert a direct harmful effect on podocytes,
perhaps through binding to and activating MRs in podocytes, which
may lead to subsequent podocyte injury. Foot processes can be
divided into 3 specific membrane zones: basal, lateral and apical
regions. Besides nephrin and podocin, the salivary protein
podocalyxin in the podocytes can maintain the negative charge. The
basal aspect adheres to the glomerular basement membrane firmly via
podoplanin. Through the analysis of podocyte proteins in these
different membrane regions, it will help understand the podocyte
injury (7,8).
It has been suggested that Wnt/β-catenin signaling
serves a pivotal role in causing podocyte injury and dysfunction.
For example, the activation of Wnt/β-catenin signaling has been
observed in a wide range of proteinuric kidney diseases, including
in animal models of Adriamycin-induced nephropathy, diabetic kidney
disease and TGF-β-driven kidney damage, as well as in patients with
focal and segmental glomerulosclerosis or diabetic kidney disease
(18–22). The Wnts are part of a family
comprising 19 secretory proteins. Upon binding to cell membrane
receptors, Wnts induce a series of downstream signaling events,
which lead to the dephosphorylation of β-catenin. The activated
β-catenin in cytoplasm is subsequently translocated into nucleus,
where it regulates the transcription of Wnt target genes, thus
controlling myriad biological processes (20,44–46).
Therefore, the present study investigated the role of Wnt/β-catenin
signaling in ALDO-induced podocyte injury in ORG.
Previous studies have failed to address how
Wnt/β-catenin signaling is activated in the ORG model mice and
ALDO-induced podocyte injury. Results from the present in
vivo and in vitro experiments demonstrated that
Wnt/β-catenin signaling was activated in the ORG model mice and in
the ALDO-stimulated podocytes, as indicated by the upregulated mRNA
expression levels of Wnt1, Wnt2, Wnt6 and β-catenin, as well as the
reduction in p-β-catenin protein expression, whereas administration
of MR antagonists (spironolactone or eplerenone) significantly
improved Wnt/β-catenin signaling activation. In addition, the
activation of Wnt/β-catenin signaling was accompanied by podocyte
injury, which suggested that activated Wnt/β-catenin signaling may
lead to podocyte injury. In order to verify this inference, DKK1,
an inhibitor of Wnt signaling, was added in the culture medium of
podocytes containing ALDO. The experimental result revealed that
DKK1 intervention was able to alleviate ALDO-induced podocyte
injury, which further indicated that activated Wnt/β-catenin
signaling may be involved in ALDO-induced podocyte injury in
ORG.
In conclusion, results from the present study
demonstrated that ALDO exerted a direct injury effect on podocytes
in vitro and participated, at least in part, in ORG
pathogenesis, which caused podocyte injury and proteinuria in
vivo. Our studies also show that the activation of
Wnt/β-catenin signaling plays a pivotal role in the ALDO-induced
podocyte injury. So, In view of the above, it can be concluded that
ALDO is involved in the pathogenesis of ORG via activation of
Wnt/β-catenin signaling in podocytes.
Acknowledgements
This study was supported by The Beijing Municipal
Natural Science Foundation (grant nos. 7112047 and 7152048). The
authors thank Dr Xiao-Yi Xu, Dr Yan-Yan Wang, Dr Yuan-Yuan Pei and
Dr Li-Jun Sun for their help in conducting experiments.
Glossary
Abbreviations
Abbreviations:
|
ALDO
|
aldosterone
|
|
DKK1
|
Dickkopf-related protein 1
|
|
MR
|
mineralocorticoid receptor
|
|
ORG
|
obesity-related glomerulopathy
|
References
|
1
|
Wang Y and Beydoun MA: The Obesity
epidemic in the United States-gender, age, socioeconomic,
racial/ethnic, and geographic characteristics: A systematic review
and meta-regression analysis. Epidemiol Rev. 29:6–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arroyo-Johnson C and Mincey KD: Obesity
epidemiology worldwide. Gastroenterol Clin North Am. 45:571–579.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wickman C and Kramer H: Obesity and kidney
disease: Potential mechanisms. Semin Nephrol. 33:14–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie D and Bollag WB: Obesity, hypertension
and aldosterone: Is leptin the link? J Endocrinol. 230:F7–F11.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu CY, McCulloch CE, Iribarren C,
Darbinian J and Go AS: Body mass index and risk for end-stage renal
disease. Ann Intern Med. 144:21–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kambham N, Markowitz GS, Valeri AM, Lin J
and D'Agati VD: Obesity-related glomerulopathy: An emerging
epidemic. Kidney Int. 59:1498–1509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng H, Chen YP, Zhang C, Fang J, Dong
HR, Li WG and Zou WZ: Comparative study of clinicopathological
features between two kinds of obesity-related glomerulopathy. Clin
J Nephrol. 25:261–264. 2009.
|
|
8
|
D'Agati VD, Chagnac A, de Vries AP, Levi
M, Porrini E, Herman-Edelstein M and Praga M: Obesity-related
glomerulopathy: Clinical and pathologic characteristics and
pathogenesis. Nat Rev Nephrol. 12:453–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HM, Liu ZH, Zeng CH, Li SJ, Wang QW
and Li LS: Podocyte lesions in patients with obesity-related
glomerulopathy. Am J Kidney Dis. 48:772–779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zoccali C and Mallamaci F: Obesity,
diabetes, adiponectin and the kidney: A podocyte affair. Nephrol
Dial Transplant. 23:3767–3770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camici M, Galetta F, Abraham N and Carpi
A: Obesity-related glomerulopathy and podocyte injury: A mini
review. Front Biosci (Elite Ed). 4:1058–1070. 2012.PubMed/NCBI
|
|
12
|
Nagase M, Yoshida S, Shibata S, Nagase T,
Gotoda T, Ando K and Fujita T: Enhanced aldosterone signaling in
the early nephropathy of rats with metabolic syndrome: Possible
contribution of fat-derived factors. J Am Soc Nephrol.
17:3438–3446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata S, Nagase M, Yoshida S, Kawachi H
and Fujita T: Podocyte as the target for aldosterone: Roles of
oxidative stress and Sgk1. Hypertension. 49:355–364. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagase M and Fujita T: Aldosterone and
glomerular podocyte injury. Clin Exp Nephrol. 12:233–242. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagase M: Activation of the
aldosterone/mineralocorticoid receptor system in chronic kidney
disease and metabolic syndrome. Clin Exp Nephrol. 14:303–314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibata S and Fujita T: Mineralocorticoid
receptors in the pathophysiology of chronic kidney diseases and the
metabolic syndrome. Mol Cell Endocrinol. 350:273–280. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato H, Gruenwald A, Suh JH, Miner JH,
Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB and
Susztak K: Wnt/β-catenin pathway in podocytes integrates cell
adhesion, differentiation, and survival. J Biol Chem.
286:26003–26015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai C, Stolz DB, Kiss LP, Monga SP,
Holzman LB and Liu Y: Wnt/β-catenin signaling promotes podocyte
dysfunction and albuminuria. J Am Soc Nephrol. 20:1997–2008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heikkilä E, Juhila J, Lassila M, Messing
M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J and Holthofer
H: β-catenin mediates adriamycin-induced albuminuria and podocyte
injury in the adult mouse kidneys. Nephrol Dial Transplant.
25:2437–2446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou L and Liu Y: Wnt/β-catenin signalling
and podocyte dysfunction in proteinuric kidney disease. Nat Rev
Nephrol. 11:535–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang D, Dai C, Li Y and Liu Y: Canonical
Wnt/β-catenin signaling mediates transforming growth
factor-β1-driven podocyte injury and proteinuria. Kidney Int.
80:1159–1169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao L, Wang M, Yang S, Liu F and Sun L: A
glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling
in diabetic nephropathy. Biomed Res Int. 2013:9870642013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Xu J, Xu P, Liu S and Yang Z:
Wnt/β-catenin signalling pathway mediates high glucose induced cell
injury through activation of TRPC6 in podocytes. Cell Prolif.
46:76–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He W, Kang YS, Dai C and Liu Y: Blockade
of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria
and kidney injury. J Am Soc Nephrol. 22:90–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu BL, Chen YP, Cheng H, Wang YY, Rui HL,
Yang M, Dong HR, Han DN and Dong J: The Protective Effects of
Curcumin on obesity-related glomerulopathy are associated with
inhibition of Wnt/β-catenin signaling activation in podocytes. Evid
Based Complement Alternat Med. 2015:8274722015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pei YY, Yang M, Zuo HL, Wang YY, Dong HR,
Cheng H and Zuo ZZ: The establishment of obesity-related
glomerulopathy mouse model. Chin J Integr Tradit West Nephrol.
15:110–113. 2014.(In Chinese).
|
|
27
|
Toyonaga J, Tsuruya K, Ikeda H, Noguchi H,
Yotsueda H, Fujisaki K, Hirakawa M, Taniguchi M, Masutani K and
Iida M: Spironolactone inhibits hyperglycemia-induced podocyte
injury by attenuating ROS production. Nephrol Dial Transpalnt.
26:2475–2484. 2011. View Article : Google Scholar
|
|
28
|
Huang H, You Y, Lin X, Tang C, Gu X, Huang
M, Qin Y, Tan J and Huang F: Inhibition of TRPC6 signal pathway
alleviates podocyte injury induced by TGF-β1. Cell Physiol Biochem.
41:163–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji ZZ and Xu YC: Melatonin protects
podocytes from angiotensin II-induced injury in an in vitro
diabetic nephropathy model. Mol Med Rep. 14:920–926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rebuffé-Scrive M, Surwit R, Feinglos M,
Kuhn C and Rodin J: Regional fat distribution and metabolism in a
new mouse model (C57BL/6J) of non-insulin-dependent diabetes
mellitus. Metabolism. 42:1405–1409. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang XX, Ye T, Li M, Li X, Qiang O, Tang
CW and Liu R: Effects of octreotide on hepatic glycogenesis in rats
with high fat diet-induced obesity. Mol Med Rep. 16:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Yang J, Zhu Y, Liu Y, Shi X and Yang
G: Mouse maternal high-fat intake dynamically programmed mRNA
m6A modifications in adipose and skeletal muscle tissues
in offspring. Int J Mol Sci. 17(pii): E13362016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oksay T, Yunusoğlu S, Calapoğlu M, Aydın
Candan I, Onaran İ, Ergün O and Özorak A: Protective impact of
resveratrol in experimental rat model of hyperoxaluria. Int Urol
Nephrol. 49:769–775. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng H, Dong HR, Lin RQ, Sun LJ and Chen
YP: Determination of normal value of glomerular size in Chinese
adults by different measurement methods. Nephrology (Carlton).
17:488–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Darouich S, Goucha R, Jaafoura MH, Zekri
S, Ben Maiz H and Kheder A: Clinicopathological characteristics of
obesity-associated focal segmental glomerulosclerosis. Ultrastruct
Pathol. 35:176–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suwanpen C, Nouanthong P, Jaruvongvanich
V, Pongpirul K, Pongpirul WA, Leelahavanichkul A and Kanjanabuch T:
Urinary podocalyxin, the novel biomarker for detecting early renal
change in obesity. J Nephrol. 29:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dinh Cat AN, Friederich-Persson M, White A
and Touyz RM: Adipocytes, aldosterone and obesity-related
hypertension. J Mol Endocrinol. 57:F7–F21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawarazaki W and Fujita T: The role of
aldosterone in obesity-related hypertension. Am J Hypertens.
29:415–423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ehrhart-Bornstein M, Lamounier-Zepter V,
Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H,
McCann SM, Scherbaum WA and Bornstein SR: Human adipocytes secrete
mineralocorticoid-releasing factors. Proc Natl Acad Sci USA.
100:pp. 14211–14216. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luther JM: Aldosterone in vascular and
metabolic dysfunction. Curr Opin Nephrol Hypertens. 25:16–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Briones AM, Nguyen Dinh Cat A, Callera GE,
Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE,
Gomez-Sanchez EP, et al: Adipocytes produce aldosterone through
calcineurin-dependent signaling pathways: Implications in diabetes
mellitus-associated obesity and vascular dysfunction. Hypertension.
59:1069–1078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|